Abstract
The bioavailability of 22 heavy metals was investigated at 19 sampling sites in Tieguanyin tea garden in Anxi County, Fujian Province, southeastern China. Heavy metal concentrations were determined by inductively coupled plasma-mass spectrometry (ICP-MS) and evaluated by geo-accumulation index (I geo ). Dilute nitric acid extraction was used to evaluate biological activity. Cu, Pb, and Cd were highly bioavailable and most easily absorbed by tea trees. Heavy metal bioavailability in the surface soil was as the ratio of the effective state to the total amount. Cd had the highest I geo values, and the respective samples and sites were classified as moderately/strongly contaminated. Cd element is considered the main factor of heavy metal pollution in the tea garden in Anxi. The other heavy metals studied were present in lower concentrations; thus, the samples were classified as uncontaminated or slightly contaminated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Tieguanyin tea, produced mainly in Anxi County, Fujian Province, China, is a type of Oolong tea. As one of China’s ten most famous teas, this type is in high demand by consumers owing to its pleasant aroma and high contents of amino acids, vitamins, minerals, tea polyphenols, and alkaloids in addition to a variety of other nutritional ingredients. The tea industry plays an important role in Quanzhou’s economy, culture, and tourism. However, the considerable economic development in recent years has been tainted by wastewater irrigation, atmospheric deposition, industrial waste emissions, and the application of pesticides and chemical fertilizers that have caused soil pollution. Heavy metal pollution, in particular, is becoming an increasingly serious issue. High concentrations of heavy metals in the soil can endanger human health through the food chain, and pose a substantial threat to the ecosystem.
Although it is an important indicator of soil pollution, total heavy metal content is only part of the story; heavy metal migration in soil is complicated by partial absorption of the metals. Therefore, content does not correspond to bioavailability of heavy metals (Alloway 2013; Maderova and Paton 2013). An increasing amount of recent environmental research has been devoted to methods for determining heavy metal bioavailability in soils.
It is widely accepted that the bioavailability of heavy metals is the amount absorbed by organisms at different levels of toxicity. Many studies have shown that although the total content of heavy metals in soil can be used to evaluate pollution status, the bioavailability of heavy metals in a specific environment is closely related to its peculiarities (Gao et al. 2014). At present, single extraction and sequential extraction are the most commonly used methods for extracting heavy metals from soil. In the single extraction protocol, which is simple and effective, chemical reagents such as dilute nitric acid and dilute hydrochloric acid are used to evaluate the mechanism of heavy metal pollution. Many countries have adopted the single extraction method for evaluating heavy metals, whereas others employ multistage extraction. The European Community Bureau of Reference (BCR) sequential extraction method, which is characterized by adequate stability, reproducibility, and precision, has also been extensively applied (Mossop and Davidson 2003).
In this study, we determined the bioavailability of 22 heavy metals at 19 sampling sites in Tieguanyin tea garden, Anxi County. The geo-accumulation index (I geo ) was used to evaluate the total heavy metal content, and bioavailability was calculated as the ratio of dilute nitric acid single extraction to the total amount of heavy metals. The aim of this study was to provide a scientific basis for effective prevention and control of heavy metal pollution in Tieguanyin tea garden.
2 Materials and methods
2.1 Sampling and preparation
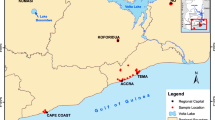
Nineteen soil samples from 0 to 20 cm depth were collected from 19 sites in Tieguanyin tea garden in June and October 2016 (Fig. 1). All samples were carefully stored in clean plastic vessels prior to processing and analysis and were labelled with their sampling location, date, number, and the name of the sample collector. In the laboratory, the soil samples were air-dried in a controlled clean environment, and all litter, roots, animal residues, and other debris were removed. The soil was then spread into a thin layer and repeatedly turned over to accelerate drying. The samples were then ground with an agate mortar and pestle and sieved with a 200-mesh nylon sieve. The material under the sieve was stored in sealed polyethylene bags for future analysis, and the sieve materials were returned to the original bag.
2.2 Sample analysis
2.2.1 Measurement of total heavy metal content
All soil samples were analyzed by microwave digestion with HCl–HNO3–HF (Wysocka and Vassileva 2016). Fe and Mn were analyzed by atomic absorption spectrometer (AAS; TAS-986, Beijing Purkinje General Instrument Co., Ltd.) and other metals were analyzed by inductively coupled plasma-mass spectrometry (ICP-MS). The following procedure was applied for preparing the blend solutions. About 0.1 g of soil sample and adequate volumes of the spike solutions—4 mL of HNO3, 0.1 mL of HCl, and 1 mL of HF—were subsequently added to each Teflon vessel, and sample digestion realized in a closed microwave system. Rhodium was used as the internal standard. The sample solutions and reagent blanks were analyzed for metals by ICP-MS (ELAN9000; PerkinElmer, Massachusetts, USA) at the Chinese Academy of Sciences. Background corrections and matrix interference were monitored throughout the analyses. All experiments involving the soil samples were conducted in duplicate. A parallel sample analysis of soil GSS-7 series standard (GBW07407) showed 94%–106% recovery of each element.
2.2.2 Measurement of heavy metal speciation
Briefly, 1 g of soil was weighed in a 50-mL plastic centrifuge tube and mixed with 15 mL of 0.5 mol/L HNO3. Next, the sample was oscillated at 220 rpm for 24 h and then centrifuged at 4000 rpm for 10 min. The constant volume was adjusted to 50 mL and placed in a refrigerator at 4 °C. The concentrations of heavy metals were determined by ICP-MS.
3 Results and discussion
3.1 Total heavy metal levels
Descriptive statistics of heavy metals in soil samples in Tieguanyin tea garden are shown in Table 1. The ratios of mean to background values greater than 1.00 were Cd (7.62) > In (2.65) > Zn (2.52) > Sb (1.8) > Cs (1.63) > Bi (1.59) > Sr (1.57) = Cu (1.57) = Sc (1.57) > V (1.45) > U (1.44) > Be (1.36) > Tl (1.29) > W (1.23) > Th (1.14). The geological accumulation index method was used for further evaluation.
In 1969, Muller (1969) proposed the utilization of I geo to assess the pollution of heavy metals in sediments; subsequently, this method was used to determine soil pollution (Loska and Wiechula 2003). Here, I geo is calculated by the formula:
where C n is the concentration of a trace element in the soil, B n is the geochemical background content (Liu 1995), and 1.5 is the background matrix correction factor owing to lithogenic effects. I geo was classified into five grades, as shown in Table 2.
The results of I geo are shown in Fig. 2. Most I geo classes were comprised of uncontaminated or slightly contaminated soils, with a few moderately contaminated by heavy metals. Overall, Cd had the highest I geo value, classifying as moderately/strongly contaminated. In previous studies, P fertilizers have been considered the main source of Cd pollution (Gray et al. 1999).
In fact, the various forms of heavy metals present in the acid-extractable and residual phases have different mobility and toxicity (Gao et al. 2014). In this study, extraction of all soil samples was performed with 0.5 mol/L HNO3 (Zhang et al. 2015), and bioavailability based on the results.
3.2 Bioavailability of heavy metals
The bioavailability of heavy metals can be expressed as the rate of extraction or activation—the ratio of the effective state of heavy metals to the total amount (Hu et al. 2013). Heavy metal bioavailability was calculated as:
where A c is the bioavailability of a given trace element in the soil, A ci the HNO3-extractable content of the trace element, and B ci the total content of the trace element. Higher Ac indicates increased bioavailability of heavy metals in the ecological environment, which leads to more pronounced negative effects on the ecosystem (Wu et al. 2016). As shown in Fig. 3, the ratios of W, Mo, Rb, Li, Sb, Cs, Cr, Tl, Bi, Th, Sc, and V were less than 25%, indicating that their bioavailability was low. Low bioavailability suggests low activity and thus reduced absorption by the roots of tea plants. The proportions of acid-extractable concentrations of certain heavy metal elements such as Ba, Zn, U, In, Be, and Ni were close to 40% at some sites, indicating the presence of biological activity. The ratios of acid-extracted Cu, Pb, and Cd were higher at the S13 sampling site, and the proportion of the acid-extracted forms of the three heavy metals was almost 80%. These results indicate easy absorption of the heavy metals by the tea plants.
4 Conclusions
Cd had the highest I geo ; thus, the respective sampling site was classified as moderately/strongly contaminated. This heavy metal was identified as the main pollution factor in the tea garden in Anxi. Fertilizer containing P was the main source of Cd contamination; therefore, its application should be justified and carefully controlled. The dilute nitric acid extraction method was employed to evaluate biological availability. Cu, Pb, and Cd showed high bioavailability and were easily absorbed by tea trees. Although the total amount of Pb and Cu did not indicate severe pollution, their high biological activities warrant considerable attention to their concentrations.
References
Alloway BJ (2013) Heavy metals in soils: trace metals and metalloids in soils and their bioavailability, 3rd edn. Springer, Berlin
Gao X, Zhou F, Chen CA (2014) Pollution status of the Bohai Sea: an overview of the environmental quality assessment related trace metals. Environ Int 62:12–30
Gray CW, McLaren RG, Roberts AC (1999) The effect of long-term pho-sphatic fertiliser applications on the amounts and forms of cadmium in soils under pasture in New Zealand. Nutr Cycl Agroeco Syst 54:267–277
Hu GR, Yu RL, Lin YP et al (2013) Assessment of toxicity of heavy metal contaminated dustfall in Quanzhou City by toxicity characteristic leaching procedure. Acta Mieralogica Sin 33:1–9 (in Chinese)
Liu YQ (1995) Study and Application of the soil environmental background values in Fujian coastal zone. Mar Environ Sci 14:68–73 (in Chinese)
Loska K, Wiechula D (2003) Application of principal component analysis for the estimation of source of heavy metal contamination in surface sediments from the Rybnik Reservoir. Chemosphere 51:723–733
Maderova L, Paton GI (2013) Deployment of microbial sensors to assess zinc bioavailability and toxicity in soils. Soil Biol Biochem 66:222–228
Mossop K, Davidson C (2003) Comparison of original and modified BCR sequential extraction procedures for the fractionation of copper, iron, lead, manganese and zinc in soils and sediments. Anal Chim Acta 478:111–118
Muller G (1969) Index of geoacccumulation in sediments of the Rhine River. Geochem J 2:108–118
Wu JY, Fang FM, Lin YH et al (2016) Contamination characteristics and bioavailability of heavy metals in campus dusts of Huainan City. Environ Chem 35:1346–1353 (in Chinese)
Wysocka I, Vassileva E (2016) Determination of cadmium, copper, mercury, lead and zinc mass fractions in marine sediment by isotope dilution inductively coupled plasma mass spectrometry applied as a reference method. Microchem J 128:198–207
Zhang CC, Hu GR, Yu RL, Liu Y (2015) Speciation and bioavailability of heavy metals in sediments from tidal reach of the Jinjiang River. Environ Chem 34:505–513 (in Chinese)
Acknowledgements
This work was supported by the National Science Foundation of China (21177043, 21377042), the National Science Foundation of Fujian Province (2015J01147), Fujian Provincial Key Laboratory of Ecology-Toxicological Effects and Control for Emerging Contaminants (PY16006), and the Research Program of Science and Technology of Quanzhou City Government (2012Z86, 2014Z130). The authors wish to express their thanks to those colleagues who participated in the sampling work. Thanks are also extended to the anonymous reviewers for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
11th International Symposium on Geochemistry of the Earth’s Surface.
Rights and permissions
About this article
Cite this article
Sun, J., Yu, R., Hu, G. et al. Bioavailability of heavy metals in soil of the Tieguanyin tea garden, southeastern China. Acta Geochim 36, 519–524 (2017). https://doi.org/10.1007/s11631-017-0224-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-017-0224-9