Abstract
This study evaluates the extent to which humans may be exposed to health risk from heavy metals in surface soils of public parks in Southern Ghana during outdoor activities. The study investigated surface soils of 56 public parks from seven metropolitan cities in Southern Ghana. Heavy metals (Cd, Cr, Cu, Hg, Mn, Ni, Pb, and Zn) were determined using microwave-assisted HNO3-HF acid digestion and atomic absorption spectroscopy with flame, graphite furnace, and cold vapor options. All parks studied registered the presence of heavy metals with mean concentrations following the order: Mn > Zn > Cr > Pb > Cu > Ni > Cd > Hg. Whereas the mean concentrations of Zn (221.92 mg/kg) and Cr (107.01 mg/kg) respectively exceeded the Canadian (200 mg/kg; 64 mg/kg) and the EU (100–200 mg/kg; 50–100 mg/kg) standards, the ranges of Cu (14.27–138.85 mg/kg) and Pb (6.46–628.31 mg/kg) also exceeded their EU range of 50–100 mg/kg. The results indicated that there was no immediate risk to Ni and Hg on the public parks studied; however, Pb, Cd, Zn, Cu, and Cr may pose some adverse effects as they exceed their respective guideline limits in soil. The ranges of non-carcinogenic risk for adults and children were 0.0186–0.0787 and 0.0197–0.0850 respectively while the corresponding ranges for carcinogenic risks were 3.75 × 10−7–1.28 × 10−6 and 4.17 × 10−7–1.31 × 10−6. Even though risk assessment suggested low and acceptable health risk levels to patrons, there is the need for close monitoring since Pb, Cd, Zn, Cu, and Cr have shown tendency of accumulating beyond acceptable limit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Some heavy metals which occur in the soil are toxic or poisonous to human health and could be detrimental especially to children who are exposed as a result of intentional or accidental ingestion of soil and inhalation of dust by playing in contaminated parks (Mostert 2008). Sources of contamination of soil by heavy metals are varied and may include mine tailings, smelter emissions, waste incineration, and atmospheric deposition (Mostert 2008). However, for public parks, soil contamination by heavy metals may be attributed largely to vehicular emissions (vehicle exhaust, tire and brake wear, and weathered street surface particles), industrial (coal combustion, metallurgical, chemical industry, etc.), domestic emissions, weathering of building, and pavement surface and waste incinerators (Kuzmanoski et al. 2014; Wei and Yang 2010).
As the most industrialized and economically significant metropolitan cities in Southern Ghana, Tema, Accra, Cape Coast, Takoradi, Ho, and Kumasi have witnessed heavy anthropogenic activities due to rapid economic development and urbanization (Bortey-Sam 2011). The human population as well as the number of vehicles in these cities had increased exponentially over the past decades (Tetteh-Addison 2012). The indiscriminate and open burning of electronic wastes, coupled with fuel leakages, smoke production from the exhaust of automobiles, and industry chimneys are but few that regularly introduce heavy metals into the atmosphere. These heavy metals, as a result of atmospheric deposition, eventually settle and accumulate on surface soils, including that of public parks.
Contamination of soil by heavy metal is of great concern to many researchers due to the toxicity of these heavy metals. Nickel can cause several unwanted effects such as the development of cancers of the lung, nose, larynx, and prostate (Shivhare and Sharma 2012) and exposure to cadmium can lead to kidney diseases, lung damage, fragile bones, and stomach irritation (Martin and Griswold 2009). Lead is reported to be a probable human carcinogen, exposure to which may severely damage the brain and kidneys, and ultimately cause death (Shivhare and Sharma 2012). Martin and Griswold (2009) noted that lead causes low sperm production in males and miscarriages in pregnant women, and interferes with the development of the nervous system, particularly in children, causing potentially permanent learning and behavior disorders. Mercury is no exception. Apart from the fact that metallic mercury vapor causes lung damage, Food and Nutrition Board/Institute of Medicine (2001) indicated that overexposure to mercury permanently damages the brain, kidneys, and developing fetuses. The board also noted that chromium, another known carcinogen and toxin, causes nose ulcers, breathing problems, asthma, cough, shortness of breath, or wheezing cough when exposed, especially to its hexavalent form. Manganese is a naturally occurring element and an essential nutrient; however, high levels of manganese are toxic. Manganese toxicity can result in permanent neurological disorders known as manganism (Lange and Condello 2016). Impotence and loss of libido are common symptoms in male workers affected with clinically identifiable signs of manganism (Flora 2014). Acute or intermediate exposure to excess manganese also affects the respiratory system. High levels of zinc disrupt the homeostasis of other essential elements. Large doses of zinc have been observed to cause gastrointestinal effects in humans (Nriagu 2011). Copper on the other hand commonly exhibits adverse health effect as gastrointestinal distress; higher doses can however cause liver damage (Food and Nutrition Board/Institute of Medicine 2001).
Heavy metals in contaminated soils not only find their way into human systems but they also have the ability to pollute groundwater supplies as well as accumulating in plants, thus contaminating the food chain and affecting human health (Kuzmanoski et al. 2014; Selinus et al. 2005; Saether et al. 1997). Resuspended metals from urban activities can undergo atmospheric transport and deposited at all places. The urban soil is therefore subjected to a significant level of heavy metal pollution (Kuzmanoski et al. 2014) and since heavy metals are persistent, they tend to accumulate in the environment. Parks and gardens which are fast becoming prominent features of urban development, thus become inevitable receptive grounds of such pollutants, especially roadside and residential park soils (Wang et al. 2017). This is of much concern since children in particular are more affected by soil contamination due to frequent hand-to-mouth activity, as well as higher dermal absorption rate in comparison with adults (Marjonovic et al. 2009). While much investigation has been conducted into a risk analysis of metals in agricultural soils, agricultural products, riverbed sediments, fishery products, and all manner of flora and fauna, virtually nothing is known about heavy metal pollution in Ghanaian parks. This presents a knowledge gap in the holistic understanding of risks posed by heavy metals in the environmental compartments of the nation. Furthermore, since several parks over the globe have been found to be associated with heavy metal health hazards, there is the need to investigate such parks in Ghana also to ascertain the health risks associated with them, if there exists any at all.
This research, being one of the pilot studies in the country, has therefore focused on Southern Ghana where industrialization and rapid urbanization is taking place, to provide baseline data on heavy metal pollution in parks. The study investigated soils from 56 public parks and aimed at the following: (1) identification of priority heavy metal pollutants of park soils and their background concentrations (2) using the carcinogenic and non-carcinogenic assessment models to evaluate the health risks posed by the heavy metals. It is predicted that heavy metal levels in them will be significant enough to raise health concerns and also to elicit further research attention. On the basis of findings, therefore, this study will be helpful for pollution control in relation to human health risk possibly associated with the use of public parks.
Materials and method
Study area
The study area accounts for 57.6 % of the total population of Ghana (Ghana Statistical Service 2012). Majority of these parks are bare with patches of grass. A good number of them are cited in densely populated communities while the rest are mostly situated near paved roads. As of March 2012, a total of 1,425,900 vehicles were registered in Ghana (Tetteh-Addison 2012). Out of this, over 74.5% were registered in the urban centers of Southern Ghana. About 97% of registered vehicles plying roads in these urban centers are considered old and barely roadworthy (Tetteh-Addison 2012). These have undoubtedly contributed to worsen the ambient air quality by the release from their malfunctioning exhaust pipes. Public parks in close proximity to roads will experience wet or dry atmospheric depositions form such vehicular emissions. Furthermore, open space public dumps are regular features of all the metropolises, serving as means of waste disposal for about 37.7% of households (Ghana Statistical Services 2012). Metallic as well as electronic and electrical wastes constitute major components of these dumps and these are burnt openly on regular basis, polluting the atmosphere. Other features commonly encountered are fuel filling stations and auto-mechanic fitting and repair shops. While it is a common knowledge that both pollute the soil through fossil fuel leakages, the latter are particularly noted for their intentional indiscriminate discharge of “black oil” (used up engine oil) as well as metallic pollution of the environment since metallic welding invariably constitute part of their daily engagements.
Geological setting
Southern Ghana is a geologically complex area (Anani et al. 2018). The Accra-Tema metropolis falls within the Devonian rocks of the Accraian Group in Ghana which overlies the Dahomeyan basement complex (Kesse 1985). The Accraian rocks consist of quartz grits, gently folded sandstones, shales, and mudstones (McCallien 1962). The southeastern segment of the study area where Ho and Koforidua metropolises are situated lies within the Dahomeyide Oregon of the West African Craton (WAC), comprising partly of exotic rocks that form the granitoid gneiss complexes (Attoh and Nude 2008), whereas Kumasi and Cape Coast fall within the Birimian Supergroup and Tarkwaian rocks which are the Early Proterozoic crystalline rocks. They possess a metaluminous character and are generally granodioritic and tonalitic in composition (Mahu et al. 2018). Takoradi part of the study area lies within the Sekondian Group crop out along the western and central coast of Ghana which is composed of sandstones and sandy shales resting unconformably on Paleoproterozoic basement rocks (Asiedu et al. 2005)
Materials
Certified reference materials, which were individual metal standards of cadmium (Cd), chromium (Cr), copper (Cu), iron (Fe), mercury (Hg), manganese (Mn), nickel (Ni), lead (Pb), and zinc (Zn), were purchased from Sigma Aldrich, certified to be within ± 0.5% of the reported value (1000 mg/L).
Nitric acid, hydrochloric acid, and hydrofluoric acid were of ultra-pure trace metal grade purchased from Fisher Scientific. Deionized water used for dilution was of ultra-pure grade.
A set of analyte calibration standard solutions (0.2, 0.4, 0.8, 2.0, 5.0, and 10.0 μg/L for graphite analysis) and (0.5, 1.0, 2.0, 4.0, 8.0, and 10 mg/L for flame analysis) were prepared from serial dilutions of the prepared analyte stock solutions, into 100-mL volumetric flask using ultra-pure deionized water.
Sampling
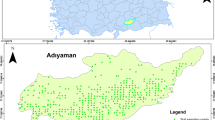
Fifty-six public parks from seven metropolitan areas in Ghana, namely Accra, Tema, Kumasi, Ho, Koforidua, Cape Coast, and Takoradi were chosen for this study (Appendix Table 8). The selection of the public parks for the investigations was based on four criteria. These are (a) popularity of the park (well-known, based on activities there), (b) potential source of pollutants of interest (development versus population of the Metropolis), (c) patronage or frequency of use of the park for activities, and (d) parks have to be accessible for sampling.
Three rounds of surface soil samples were collected during the period of September 2013 to March 2015, at 6-month intervals. The parks vary in size, and depending on the size of the park, 16 to 30 sampling points (spaced about 15-m apart) in a grid plan format were selected at random. Soil samples were taken between the depths of 0 and 10 cm. In each park, soil from all the sampling points was put together, properly homogenized in a big plastic container and 1-km composite sample taken into a zip-lock polypropylene sampling bag, labeled and sealed accordingly. A small handheld garden spade was used in taking the samples and after each sample was taken, the spade was thoroughly washed with water and cleaned with tissue paper to avoid cross-contamination. In all, 168 composite samples were taken, as shown in Table 2. All samples were transported to the Pesticide Residues Laboratory of Ghana Standards Authority in a cool, ice chest and were stored in a dark cabinet until further processing. Figure 1 shows a map indicating the sampling locations.
Sample preparation
The samples collected from each sampling site were transferred into individually clean containers and air-dried. Each soil sample was thoroughly mixed and sieved through 2-mm mesh and labeled. Each homogeneous soil sample was sieved with the aid of a mechanical shaker and stacked stainless steel sieves with mesh sizes of 63 μm, 125 μm, 250 μm, 500 μm, 1 mm, and 2 mm. A pan was placed beneath which collected the soil fractions smaller than 63 μm. Each sieved fraction was quantitatively weighed and recorded. This was used to obtain the information for computing the percentage of the different particle size fractions and was used to ascertain the effect of particle size on contamination loading. Each soil fraction was transferred into a separate bag, labeled accordingly, and then stored in a cool cabinet until further processing.
Sample digestion
The USEPA method 3052 was employed for sample digestion for the heavy metal determination. Soil samples of 0.5 ± 0.01 g were weighed into Teflon microwave vessels, and 9 mL of concentrated HNO3 and 4 mL concentrated HF were added. These vessels were then sealed after all initial visible reactions ceased in a fume hood and were placed in a microwave system. The vessels were connected appropriately, and the power settings for the microwave oven programmed steadily over 45 min to 120 psi at 100% power and then held constant at this pressure for 10 min. The vessels were allowed to cool, depressurized, and the system was vented and opened.
The digest was then filtered with the aid of Whatman no. 1 filter paper and polypropylene funnel into 100-mL volumetric flask, and 20 mL of 10 % HNO3 in deionized water was used to rinse the vessel and transferred onto the filter paper. The flask was finally made up to the mark with ultra-pure deionized water. Each was then transferred into plastic sample bottles with caps, labeled accordingly, and stored until analysis.
Instrumentation
A Varian model AA240/GTA120 atomic absorption spectrometer equipped with a specific hollow cathode lamp operated at a current recommended for the lamp by the manufacturer, an automatic background correction accessory, a computerized read-out, coupled with flame and graphite furnace techniques, and an automated flow injection analysis system (FIAS) adaptable to the AAS was used for (Cd, Cr, Cu, Mn, Ni, Pb, and Zn) determinations. The elements were measured by the optimum operating conditions with air acetylene for the flame analysis of Mn, Cu, and Zn, while the graphite furnace technique was used for the analysis of Cd, Cr, Pb, and Ni.
For mercury, a Varian model VGA77 mercury vapor accessory was coupled to the atomic absorption spectrometer equipped with Hg hollow cathode lamp operated at a current recommended by the lamp manufacturer, an automatic background correction accessory, a computerized read-out, and an automated flow injection analysis system adaptable to the AAS was used. A flow-controlled argon stream was used as an inert carrier to transport Hg vapor into the cell. Time-controlled addition of tin (II) chloride reducing solution in combination with automatic start of the read signal of the spectrometer was in place.
In both calibration and test solution measurements, at the start of each metal determination, the system was optimized with the selection of the correct analyte and wavelength from library.
Quality assurance
For metal determination, solvents and reagents were used; nitric acid, hydrofluoric acid, hydrochloric acid, tin (II) chloride, and deionized water were all of ultra-pure quality. Individual metal standards were from Sigma Aldrich certified to be within ± 0.5% of the reported value (1000 mg/L).
The Teflon microwave vessels and all glassware were soaked in 10% nitric acid for at least 12 h, rinsed thoroughly with the ultra-pure deionized water before use. Ultra-pure deionized water was used as blank. Samples were analyzed in three replicates. For each batch of 50 samples, two quality control samples (1-mg/kg spiked soil samples) were analyzed to determine efficiency (recovery) of results. One quality control soil sample was analyzed at the start and end of each analytical batch in order to verify the efficiency of both the preparative and analytical procedures. Verification of the calibration standards was also performed after fifty aspirations to ensure that the instrument continues to meet acceptable sensitivity and linearity requirements. The linearity of the calibration curve was maintained with regression coefficient (r2) greater than 0.996.
Data analysis
The mean and median of trace metal concentrations in samples was calculated. Ranges were compiled and corresponding standard deviations were determined using SPSS version 22 software for windows. Pearson correlation was used to assess the source of the metals in the study sites. All other calculations were performed using Microsoft Excel. The assumption of normality was assessed using Shapiro-Wilk test and, consequently, Kruskal-Wallis test was performed to test differences of means.
Human health risk assessment
In exposure analysis, there are three main exposure pathways considered as far as soil contamination is concerned. This includes exposure via ingestion, inhalation, and dermal contact. Ingestion of soil or dust refers to the direct intake of soil or dust through the oral route by hand-to-mouth mode. This intake is assumed to be greatest especially for children (Norwegian Pollution Control Authority 1999). Exposure via dermal contact refers to the contaminant attaching itself to the skin and getting absorbed by the skin surface into the blood. The exposure algorithms are based on the amount of soil per skin surface area, the area of skin exposed, the time of exposure, and the uptake of contaminants through the skin. The next exposure pathway is inhalation via the nose or the mouth. This exposure pathway has been proved to be the least in magnitude compared with both ingestion and dermal exposures (Norwegian Pollution Control Authority 1999). In other words, for all contaminants, the contribution from inhalation of soil (dust) is found to be less than 1% of the total intake; however, the health danger of inhalation of chemical substances, especially toxic to the lungs, can be large (Norwegian Pollution Control Authority 1999).
The following algorithms are considered for the various exposure estimations:
where ADD is the average daily dose (ing, ingestion; der, dermal; and inh, inhalation), C being the concentration of contaminant in soil, IRs is the soil ingestion rate for receptor, FI is the fraction ingested from contaminated source, CF is the conversion factor, SA is the skin surface area available for exposure, AF is the soil to skin adherence factor for contaminant, ABS is the absorption factor for contaminant, IRa is the inhalation rate for receptor, PEF is the soil-to-air particulate emission factor, ET is the exposure time for receptor, ED is the exposure duration for receptor, EF is the exposure frequency, BW is the bodyweight of receptor, and AT is the averaging time. The above parameters have values as specified in Table 1.
However, for carcinogenic and non-carcinogenic risk, an extra factor is added to the algorithms (1, 2, and 3) above:
Thus, carcinogenic risk:
CSF is the cancer slope factor. The total risk is then obtained by summation of risks from each exposure pathway (oral + dermal + inhalation).
And for non-carcinogenic risk:
RfD is the reference dose, oral, or dermally adjusted while RfC is the reference concentration for inhalation.
The sum of the HQ values for all the metals in the soil denotes the hazard index (HI). The HI was used to assess the overall non-carcinogenic effects posed by multiple metals.
Results and discussion
Total heavy metals concentration in surface soils of public parks
For all the heavy metals of interest in this study, exposure to certain concentrations could have serious health consequences. Therefore, to help protect humans from exposures to contaminants of soil, guideline values are set for most heavy metals. The Canadian Council of Ministers of the Environment (CCME) set guideline limits for both organic and inorganic pollutants in the soil for various land uses (CCME 1991, 1997, 2015). For non-polluted soils, there exists a world range of guidelines for most contaminants of concerned. The European Commission (Ackah 2012) also has its guidelines. All these sets of guidelines serve as a guide for the protection of soil and the environment.
Table 2 presents a number of parks studied and samples taken per metropolis, while Table 3 shows the mean, median, and range of concentrations of heavy metals in soils from this study, compared with three international standards. It is observed that there are wide discrepancies in standard values set by these organizations for heavy metals. Even then, Ni and Hg levels from this study did not exceed the corresponding values of any of the three standard guidelines for soil. Thus, in terms of soil safety and protection, the values observed in this study suggest there is no immediate risk to human health as far as Ni and Hg are concerned.
Mean concentration of Cd, Cr, and Pb in this study was higher, compared with what was registered in Suzhou soils in China (Wang et al. 2017). However, the levels of most of the metals, viz Cu, Mn, Ni, and Cr measured in Belgrade park soils (Kuzmanoski et al. 2014) were higher than their values registered in this study. Meanwhile, the concentration levels of most of the metals measured earlier by Marjonovic et al. (2009) in Belgrade park soils were much lower. The order of decreasing concentration, as recorded by Wang et al. (2017) in the Belgrade park soil, is similar to that in urban park soils of Southern Ghana. The mean concentration values in this study follow the order: Mn > Zn > Cr > Pb > Cu > Ni > Cd > Hg.
Among the selected heavy metals, those perhaps most widely known to be very deleterious to human health are Cd and Pb (Mostert 2008). Even though Cd recorded narrower range with the means falling below the CCME guideline (Table 3), three out of the 56 public parks recorded mean Cd concentration exceeding both the EU and the World range standard guideline values for non-polluted soil (Appendix Table 8). Furthermore, based on mean concentrations observed in this study, there exist some issues of concerned for Pb on surface soils from public parks in Southern Ghana. About 7% of the public parks studied had mean Pb concentrations exceeding CCME guideline for soil, 14.3% above the EU set limit for Pb and 30.4% exceeding the World range standard for non-polluted soils.
Lead is used to be an additive for fuel, and it is also used as base for paints. Thus, its presence in surface soil samples from the study area could be attributed to atmospheric deposition as a result of vehicular emission of leaded fuels and degradation of surface paints containing Pb onto surface soils. Cadmium has been reported to be used in motor tires and oil (Mostert 2008). As such, its presence in some public parks in the study could be ascribed to atmospheric deposition from vehicular emissions as well.
Other important heavy metals of human health concern in this study are Cr, Cu, and Zn. Chromium recorded the highest number of exceeds in this study. Thirty-five out of the total 56 public parks surface soils investigated recorded mean Cr concentrations exceeding CCME guideline; twenty-seven above the EU limit while twenty-four park soils exceeded the World range for non-polluted soils. Zinc is an essential metal; however, at elevated concentrations could be harmful. About 35% of the observed mean for Zn in this study exceeded the guideline limit for non-polluted soils. Again, 19.6% of the Zn mean concentration exceeded both the EU and CCME guideline limit for soil.
The most common adverse health effect of Cu is gastrointestinal distress, but higher doses of Cu can also cause liver damage (Food and Nutrition Board/Institute of Medicine 2001). Five out of the fifty-six public parks surface soils investigated recorded mean Cu concentrations which exceeded the guideline limits set by EU, CCME, and the World range standard for non-polluted soil. The heavy metals having exceeded the various guideline limit sets give an indication of health concerned to visitors of those parks.
High standard deviation values (Appendix Table 8) indicate high variability within each metropolis. This type of distribution shows discontinuity and can only be explained by point sources of pollution such as releases from automobile fitting shops, metallic scrap yards, and open dumps. However, with the exception of Mn and to a lesser extent, Cd, concentration of metals was less variable among the metropolises. A Kruskal-Wallis test showed significant differences in means of Mn (H = 18.258, p = 0.006).
Heavy metal one-on-one associations in soil
In order to understand and explain whether the presence of heavy metal is from natural or anthropogenic sources, a one-on-one metal correlation was done. Apart from anthropogenic factors, most metals are geochemically associated with each other (Mostert 2008). The study area (Tema, Accra, Cape Coast, Takoradi, Ho and Kumasi) comprises of rocks such as the granitoids, Voltaian, Tarkwaian, and the Dahomeyan rocks. Thus, there exists the probability that the presence of some of the metal may be a result of weathering of these rock types. This also implies geochemical associations in rocks such as ultramafic (Cr-Co-Ni-Cu), granites (Ba-Li-W-Mo), dark shales (Cu-Pb-Zn-Cd-V-Mo-Ni-As), and phyllis (Cu-Co-Ni-Zn) could be expected in this study, if all heavy metal sources were to be of natural origins (Melcher 1995; Mostert 2008).
Table 4 shows the one-on-one metal correlation in this study. There exist significant positive correlations between Cu and Pb, and between Mn and Pb. This association existed in the study area for typical manganese crusts with submarine volcanics and massive sulfide deposits (Melcher 1995). The high level of Cu can be attributed to the extensive use of the metal in products like old pipelines, coinage, and earings, and also, as component of alloys in motor tires and other parts of vehicles.
Copper is also a component of most fungicides formulation used in the control of cocoa diseases and vegetable pests in Ghana (EPA Ghana 2015). Thus, apart from natural levels, there is the greatest possibility of atmospheric transfer of Cu from urban agricultural fields onto the general environment, as well as surface soils of the public parks.
There was a significant positive correlation between Cr and Cd, as well as between Cr and Ni (Table 4). Geologically, associations such as ultramafic are possible in the study area. This notwithstanding, Cr is highly used with other metals such as Ni, Fe, and Co, in alloys for steel production and chrome plating for vehicles. They are also preferred for use in pigments and paints. In the wood industry, Cr is used to treat woods against pest attack. These uses will then lead to their presence on surface soils as a result of atmospheric deposition. Chromium is an essential trace element; however, its elevated levels could be highly harmful to the human body and the environment (Trautmann 2005).
Cadmium was the least detected of the heavy metals and its presence had a significant positive correlation with Cr. Levinson (1974) reported that geochemically, Cd is strongly associated with Zn. This was not the case in this study. A similar scenario of non-correlation of Zn with Cd was also observed by Mostert (2008) in her study of children’s playground located in public parks of Queensland locality in Australia. This was understood to be an anomaly and, at best, could be attributed to different sources of the pollutants studied. Thus, apart from the contributions from natural sources, atmospheric deposition of Cd on public parks as a result of vehicular traffic emissions is highly probable, since Cd is reportedly a component of motor tires and oil (Mostert 2008).
Other significant correlations were observed between Mn and Cu as well as Mn and Pb (at p = 0.01). Similarly, nickel shows a positive correlation with Cu, but its association with Cr and Pb were less significant (at p = 0.05). Lead also showed a positive correlation with Cu, Hg, and Mn (significant at p = 0.01). However, it was weakly associated with Ni (Table 4). With the use of leaded fuels and its presence as a base in paints, the presence of Pb in surface soils of public parks in the study area was thus expected, since most of the parks are in urban cities and are in close proximity to motor roads. The strong correlation between Pb and Hg could be said to be an anomaly and probably could be from some unique anthropogenic sources. Similarly, even though Zn was among the metals which were detected in soils of all public parks studied, it was not strongly correlated with any of the metals studied. This is thus reported as an anomaly which needs further investigation.
Effect of particle size on heavy metals concentration
The particle size of the soil is relevant with respect to both mobility and amount of pollutants it can retain on its surface. Mobility of larger particle sizes into the human body via all the exposed routes is limited. They could be trapped by hairs in the nostril and they could be easily dislodged or brushed off the skin. However, finer particle sizes may pass through the hairs in the nostrils and may get to target organs. Smaller particle sizes accumulate greater concentrations of the pollutants than the larger particle sizes since they possess a larger surface area (Mostert 2008; Fang et al. 2004). Studies have shown that smaller particle sizes induce respiratory illness (Hertz-Picciotto et al. 2007). As observed in Fig. 2, the negative slope observed indicates an inverse relation between average total heavy metal concentrations and particle size class, establishing the fact that smaller soil particle sizes in the study have a higher accumulative capacity of heavy metals. The current study confirms the findings of other authors like Ajmone-Marsan et al. (2008), Abouelnasr (2010), and Yao et al. (2015) whose researches showed that accumulation factor of heavy metals is higher in the finer fraction of soil particles. In the case of Ajmone-Marsan et al. (2008); however, coarser fractions were found to have a higher accumulation of Ni and Cr and this was attributed mainly to lithogenic contribution. Apart from their large surface area, finer particle sizes constitute a higher risk component since they can easily be carried by atmospheric transport from their place of origin. To reduce risk of exposure to pollutants in these parks, measures such as those suggested in other studies (Mostert 2008; Herngren et al. 2005), mainly covering the surfaces (e.g., by grassing) or frequent wetting of the surfaces could be adopted as a matter of necessity.
Human health risk assessment from heavy metals in surface soil
Human health risk due to heavy metal exposure through the soil via ingestion, inhalation, and dermal was assessed by calculating first the total exposure (average daily dose) and corresponding cancer and non-cancer risks for adult human and child. The parameters used for estimating human cancer risks are as stated in Table 1.
The total average daily dose for the eight heavy metals (Cd, Cr, Cu, Mn, Ni, Pb, Zn, and Hg) ranged from 1.1 × 10−4 to 2.1 × 10−3 mg/kg day with a mean value of 4.7 × 10−4 mg/kg day for adults, and from 1.3 × 10−4 to 2.4 × 10−3 mg/kg day for children with mean dose of 5.2 × 10−4 mg/kg day (Table 5). These generated an average carcinogenic risk (Cr, Ni, and Pb) of 7.89 × 10−7 and 8.58 × 10−7 for adult and child, respectively (Table 6).
According to the New York State Department of Health (2007) and Man et al. (2013), five categorizations of risks could be identified in carcinogenic risks as presented in Table 7.
With the above classification, results from Table 6 indicate that carcinogenic risks associated with trace metal concentrations in all the parks were low. According to Wei et al. (2015) and USEPA (2000), an excess lifetime cancer risk of ≥ 10−6 but ≤ 10−4 has been consistently considered as insignificant, and > 10−4 is significant. Thus, it could be concluded from this study that only low, insignificant, and acceptable cancer risks to heavy metals existed in public parks in Southern Ghana.
For non-carcinogenic chemicals, an estimated additive hazard index of less than 1 is considered highly unlikely that significant additive or toxic interactions would occur, so no further evaluation was necessary. However, an additive hazard index greater than 1 implies that there may be a concern for a potential non-cancer health effect.
Even with the worst-case scenarios for heavy metals, the additive hazard indices, calculated for both adult and child for the three exposure pathways (ingestion, inhalation, and dermal contact), were far lower than 1 (Table 6). The same outcome was observed by Wang et al. (2017) for industrial urban soils of Suzhou in China. This implies only very low risk exist to the human population within the study area. Furthermore, as stipulated by literature, this study also confirms that children are most at risk to metal pollution from soils since their total exposure, hazard index, and non-carcinogenic risk are all higher compared with those of adults (Tables 5 and 6)
In terms of ranking, however, the CR values of metals in the study areas follow the order Kumasi > Tema > Takoradi > Accra > Koforidua > Ho > Cape Coast. Similarly, the HI ranking follows Kumasi > Takoradi >Tema > Koforidua > Ho > Accra > Cape Coast. Thus, whereas parks in Cape Coast present the least risk, those in Kumasi, Tema, and Takoradi represent the top three with the highest risk.
Conclusion
The main heavy metals encountered in all the parks were Cd, Ni, Zn, Pb, Cr, Cu, Mn, and Hg. These occurred with mean concentrations in the following ranking order: Mn > Zn > Cr > Pb > Cu > Ni > Cd > Hg. The mean concentrations of Zn and Cr exceeded the EU and the Canadian standards while the ranges of Cu and Pb also exceeded the EU range. Since Cu, Pb, Zn, Cd, and Cr have shown the tendency of bio-accumulating beyond acceptable limits, they need regular monitoring. There was a significant inverse correlation between soil particle size and heavy metal concentration implying finer soil particles were associated with higher heavy metal concentrations. Even though lithogenic sources might contribute to the presence of some of the metals, diffuse anthropogenic sources such as vehicular emissions, paved roads, and use of fungal pesticides, among others on one hand and anthropogenic point sources such as open dumps, metallic scrap yards, automobile fitting shops, coinage, and jewelry industry on the other hand constitute sources of these heavy metals in soils of public parks. Risk analyses in this study showed that the excess lifetime cancer risk level to both adult and child fell within 10−6 and 10−4 range, and potential non-cancer toxicity, the hazard index were less than 1; hence, heavy metal levels in the parklands pose only low risk to human population within the study area and are therefore of acceptable health risk levels. In order to minimize exposure, particularly through inhalation and dermal routes, there must be greening of parks as a matter of necessity. There is also the need to control the development and indiscriminate burning of open dumps as well as the use of leaded fuels.
References
Abouelnasr, D. M. (2010). The relationship between soil particle size and lead concentration. Proceedings of the Annual International Conference on Soils, Sediments, Water and Energy: Vol. 14, Article 8. Available at: http://scholarworks.umass.edu/soilsproceedings/vol14/iss1/8.
Ackah, J. E. (2012). Distribution of heavy metals in cocoa farm soils in the western region of Ghana. Legon: University of Ghana press.
Ajmone-Marsan, F., Biasioli, M., Kralj, T., Grcman, H., Davidson, C. M., Hursthouse, A. S., Madrid, L., & Rodrigues, S. (2008). Metals in particle-size fractions of the soils of five European cities. Environmental Pollution, 152(1), 73–81. https://doi.org/10.1016/j.envpol.2007.05.020.
Anani, C. Y., Kwayisi, D., Agra, A. N., & Asiedu, D. K. (2018). Provenance of shales and sandstones from the Devonian Accraian Group, southern Ghana. Geosciences Journal, 22(3), 393–405. https://doi.org/10.1007/s12303-017-0066-9.
Asiedu, D. K., Hegner, E., Rocholl, A., & Atta-Peters, D. (2005). Provenance of late Ordovician to early Cretaceous sedimentary rocks from southern Ghana, as inferred from Nd isotopes and trace elements. Journal of African Earth Sciences, 41, 316–328. https://doi.org/10.1016/j.jafrearssci.2005.05.003.
Attoh, K., & Nude, P. M. (2008). Tectonic significance of carbonatite and ultrahigh-pressure rocks in the Pan-African Dahomeyide suture zone, southeastern Ghana. Geological Society, London, Special Publications., 297, 217–231. https://doi.org/10.1144/SP297.10.
Bortey-Sam, N. (2011). Distribution of polycyclic aromatic hydrocarbons in air and surface soil: case study in Kumasi, Ghana. Kumasi: Kwame Nkrumah University of Science and Technology Press.
CCME (Canadian Council of Ministers of the Environment). (1991). Soil quality guidelines for the protection of environment and human health. CCME factsheets for PAHs, 1991; Winnipeg.
CCME (Canadian Council of Ministers of the Environment). (1997). Soil quality guidelines for the protection of environment and human health. CCME factsheets for Cr, 1997; Winnipeg.
CCME (Canadian Council of Ministers of the Environment). (2015). Soil quality guidelines for the protection of environment and human health. CCME factsheets for Ni, 2015; Winnipeg
EPA Ghana. (2015). Register of pesticides as at 31st December, 2014 under Part II of the environmental protection agency act, 1994 (Act 490).
Fang, G. C., Wu, Y. S., Chen, M. H., Ho, T. T., Huang, S. H., & Rau, J. Y. (2004). Polycyclic aromatic hydrocarbons study in Taichung, Taiwan, during 2002 – 2003. Atmospheric Environment, 38, 3385–3391.
Flora, S. J. S. (2014). Metals. In R. Gupta (Ed.), Biomakers in Toxicology (pp. 485–519). Elsevier Inc.
Food and Nutrition Board / Institute of Medicine (FOB /IOM). (2001). Dietary reference intakes for copper, manganese and zinc. Washington, D. C: National Academy Press.
Ghana Statistical Service. (2012) 2010 Population and housing census. Summary report of final results. pp. 1–103.
Herngren, L., Goonetilleke, A., & Ayoko, G. A. (2005). Understanding heavy metal and suspended solids relationships in urban stormwater using simulated rainfall. Journal of Environmental Management, 76, 149–158.
Hertz-Picciotto, I., Baker, R. J., Yap, P., Dostal, M., Joad, J. P., Lipselt, M., Greenfield, T., Herr, C. E. W., Benes, I., Shumway, R. H., Pinkerton, K. I., & Sram, R. J. (2007). Early childhood lower respiratory illness and air pollution. Environmental Health Perspectives, 115(10), 1510–1518.
Kesse, G. O. (1985). The mineral and rock resources of Ghana (p. 610). Rotterdam: A.A. Balkema.
Kuzmanoski, M. M., Todorović, M. N., Urošević, M. P. A., & Rajšić, S. F. (2014). Heavy metal content of soil in urban parks of Belgrade. Hemijska Industrija, 68(5), 643–651. https://doi.org/10.2298/HEMIND131105001K.
Lange, J. H., & Condello, A. V. (2016). Neurotoxicity of manganism: an emerging issue. Journal of Headache & Pain Management, 2(1), 1–2. https://doi.org/10.4172/2472-1913.100033.
Levinson, A. A. (1974). Introduction to exploration geochemistry. Calgary: Applied Publishing.
Mahu, E., Asiedu, D. K., Nyarko, E., Hulme, S., Coale, K. H., & Anani, C. Y. (2018). Provenance, paleo-weathering and -redox signatures of estuarine sediments from Ghana, Gulf of Guinea. Quaternary International, 493, 176–186.
Man, Y. B., Kang, Y., Wang, H. S., Lau, W., Li, H., Sun, X. L., Giesy, J. P., Chow, K. L., & Wong, M. H. (2013). Cancer risk assessments of Hong Kong soils contaminated by polycyclic aromatic hydrocarbons. Journal of Hazardous Materials, 261, 770–776.
Marjonovic, M. D., Vukcevic, M. M., Antonovic, D. G., Dimitrijevic, S. I., Jovanovic, D. M., Matavulj, M. N., & Risttic, M. D. (2009). Heavy metals concentrations in soils from parks and green areas in Belgrade. Journal of the Serbian Chemical Society, 74(6), 697–706.
Martin, S., & Griswold, W. (2009). Human health effects of heavy metals. Environmental Science and Technology Briefs for Citizens, Center for Hazardous Substance Research, 15, 1–6.
McCallien, J. W. (1962). The rocks of Accra: a guide to the coast along high street. Accra: University of Ghana publication board 74 p.
Melcher, F. (1995). Genesis of chemical sediments in Birimian greenstone belts: evidence from gondites and related manganese-bearing rocks from northern Ghana. Mineralogical Magazine, 59, 229–251.
Mostert, M. M. R. (2008). Levels of pollutants on surfaces of children’s playgrounds situated in public parks. Queensland: Queensland University of Technology, press.
New York State Department of Health. (2007). NYS DOH (New York State Department of Health), Hopewell precision area contamination: Appendix C – NYS DOH. In: Procedure for Evaluating Potential Health Risks for Contaminants of Concern.
Norwegian Pollution Control Authority. (1999). Guidelines for the risk assessment of contaminated sites. Report 99:06, TA – 1691/1999.
Nriagu, J. (2011). Zinc toxicity in humans. Encyclopedia of Environmental Health. pp 801-807. https://doi.org/10.1016/B978-0-444-52272-6.00675-9
Saether, O. M., Krog, R., Segar, D., & Storroe, G. (1997). Contamination of soil and ground water at former industrial site in Trondheim, Norway. Applied Geochemistry, 12, 327–332.
Selinus, O., Alloway, B., Centeno, J. A., Finkelman, R. B., Fuge, R., Lindh, U., & Smedley, P. (2005). Essentials of medical geology: impacts of the natural environment on public health. Cambridge: Academic Press.
Shivhare, L., & Sharma, S. (2012). Effect of toxic heavy metal contaminated soil on an ornamental plant Georgina wild (Dahlia). Journal of Environmental & Analytical Toxicology, 2, 156. https://doi.org/10.4172/2161-0525.1000156.
Tetteh-Addison, E. (2012). Vehicle Population and International Trend. Ministry of Transport, Ghana, Presentation (pp. 1–22).
Trautmann, N. (2005). The dose makes the poison – or does it? Action Bioscience. www.actionbioscience.org/environment/trautmann/html. Assessed on 15th November, 2016.
United States Environmental Protection Agency, USEPA. (2000). Methodology for deriving ambient water quality criteria for the protection of human health (2000), EPA-822-B-00-004, (pp 2–3).
Wang, G., Liu, H. Q., Gong, Y., Wei, Y., Miao, A. J., Yang, L. Y., & Zhong, H. (2017). Risk assessment of metals in urban soils from a typical industrial city, Suzhou, Eastern China. International Journal of Environmental Research and Public Health, 14. https://doi.org/10.3390/ijerph14091025.
Wei, B., & Yang, L. (2010). A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchemical Journal, 94, 99–107.
Wei, H., Le, Z., Shuxian, L., Dan, W., Xiaojun, L., Lan, J., & Xiping, M. (2015). Health risk assessment of heavy metals and polycyclic aromatic hydrocarbons in soil at coke oven gas plants. Environmental Engineering and Management Journal, 14(2), 487–496.
Yao, Q., Wang, X., Jian, H., Chen, H., & Yu, Z. (2015). Characterization of the particle size fraction associated with heavy metals in suspended sediments of the Yellow River. International Journal of Environmental Research and Public Health, 12(6), 6725–6744. https://doi.org/10.3390/ijerph120606725.
Acknowledgments
The authors would like to thank the Metallic Contaminants Laboratory Unit of the Ghana Standards Authority, for the immense support and to the Head of stores of the Ghana Standards Authority as well, for his support and cooperation throughout the entire research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Frimpong, S.K., Koranteng, S.S. Levels and human health risk assessment of heavy metals in surface soil of public parks in Southern Ghana. Environ Monit Assess 191, 588 (2019). https://doi.org/10.1007/s10661-019-7745-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7745-0