Abstract
Propagation of gametophytes and sporophytes using mechanical fragmentation has been considered a suitable method for mass production of ferns. This study aimed to develop a practical propagation method for Lemmaphyllum microphyllum C. Presl, which is a fern of significant ornamental and medicinal value. Gametophytes were obtained through in vitro spore germination and used for propagation experiments. The gametophyte was mechanically fragmented using a scalpel into small fragments, which were then used to investigate gametophyte proliferation. In addition, the gametophyte was fragmented using a blender and then used to study sporophyte formation. Optimal proliferation conditions of the gametophyte were determined using Murashige and Skoog (MS) basal medium (double-, full-, half-, quarter-strength), Knop medium, and medium components (sucrose, nitrogen sources, activated charcoal), at various concentrations. The fresh weight of the gametophyte was 14-fold higher than that of gametophytes (300 mg) used as culture material, when cultured on double-strength MS. Moreover, 1 g of the gametophyte fragmented in 25 mL of distilled water formed more than 430 sporophytes in a soil mixture in an area of 7.5 cm2. The sporophytes were successfully cultivated in the greenhouse after acclimation. A large-scale production method for L. microphyllum that can be easily implemented in a fern production farm is outlined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lemmaphyllum microphyllum C. Presl, from the family Polypodiaceae, is a warm and temperate evergreen perennial, which is epiphytic, and grows on rocks and tree trunks in mountains of China, Taiwan, Japan, South Korea, Vietnam, and NE India. Mature plants are very short, and the rhizome is long, and trails on the surface. There are two types of leaves, the trophophylls are orbicular or oval, and the sporophylls are broadly linear or narrowly oblanceolate, and longer than trophophylls. In South Korea, the spores mature between July and September. The sori are formed on both sides of the midrib of the sporophyll, and the spores mature without any membranes (Lee and Lee 2018). The sporophylls with mature sori (referred to as “green bean fern” in Korea), resemble a split bean and are referred to in the Korean language as “Kong-jja-gae-deong-gul.” In the English language, the species is known as green penny fern, and named after its small leaves. The species is used as bonsai and in landscaping because of its high ornamental value (Kawano 2015). In oriental medicine, whole plants are used to treat rheumatoid arthritis, sores, and cough (Zheng and Xing 2009).
The propagation process of ferns starts with spores that germinate into a prothallus (gametophyte), and the formation of the reproductive organs known as antheridium and archegonium (Dyer 1979; Raghavan 1989; Banks 1999; Moran 2004). The sperm is released from the antheridium and transported to the egg in the archegonium, where it fertilizes the egg and forms a zygote (Gabriel y Galán and Migliaro 2011). This zygote divides and develops into a young sporophyte, which matures and forms spores, and completes the propagation process. Gametophyte cells have a very high regenerative capacity; new, identical gametophytes can be regenerated from fragments of gametophyte tissues (Ito 1962; Miller 1968). Due to this regeneration ability of the gametophyte, it is possible to mass-proliferate gametophytes in a short time, and induce sporophyte production from the fragmented gametophyte (Knauss 1976; Cooke 1979; Maeda and Ito 1981; Fernández et al.1997). In South Korea, ferns (bracken), are grown by dividing 2- to 3-y-old roots. However, root colonization is difficult, time consuming, and labor intensive. The propagation method that uses the gametophyte is effective, because young sporophytes can be obtained in 10 to 14 wk from the fragmented gametophyte. Unlike the rhizome division method, the planned production of a sporophyte seedling is possible. In the present study, a mechanical fragmentation method is described, which uses a scalpel and hand blender to propagate gametophytes and produce sporophytes of L. microphyllum on a large scale. This method will enable production of this species throughout the year.
Materials and methods
Plant material
Mature leaves of Lemmaphyllum microphyllum C. Presl were collected from Hogeun-dong, Seogwipo-si, Jeju-do, Korea (33° 17′ 30.1″ N, 126° 31′ 47.1″ E), in November 2016. The leaves were dried in a paper box at 25 ± 1°C for 1 wk, and the spores were removed and sieved through a 75 μm sieve (Chunggye Sieve, Gunpo, Korea), to remove impurities.
Spore germination
Spore germination was conducted according to the methods described by Jang et al. (2019a). Spores (10 mg), were placed in a conical tube filled with 15 mL distilled water, and the tube was incubated at 4°C for 24 h. The spore solution was centrifuged (3 min, 1811×g), to remove the supernatant, sterilized with 1.4% (v/v) sodium hypochlorite (Yuhanrox; Yuhanclorox Co., Ltd., Hwaseong, Korea) for 13 min, and washed three times with sterilized water. Finally, the spore solution was diluted to 10 mg of spores per 30 mL of sterilized water. The spore solution (1 mL), was inoculated in Knop medium (Knop 1865), and spores were germinated at 25 ± 1°C, under a 16/8 h photoperiod, and light intensity of 30 ± 1.0 μmol m−2 s−1. Prothalli obtained from the spores were subcultured on Murashige and Skoog (MS) medium (Murashige and Skoog 1962), at 8-wk intervals, and used for prothallus proliferation and sporophyte formation.
Prothallus proliferation
For prothallus proliferation, the prothalli were chopped with a scalpel and cultured in various media (Jang et al.2019b, 2019c). To identify the optimal media for prothallus proliferation, prothallus fragments were cultured on quarter-, half-, full-, and double-strength MS, and in Knop medium. Next, the selected optimal culture medium (Fig. 2A, double-strength MS medium), was amended with various concentrations of sucrose (0%, 1%, 2%, 3%, and 4%; w/v), a total nitrogen source [NH4Cl (CAS 12125-02-9, Samchun Chemicals, Pyeongtaek, Korea) and KNO3 (CAS 7757-79-1, Wako Pure Chemical Industries, Ltd., Osaka, Japan), 1:2; 20:40 (60), 40:80 (120), and 80:160 (240) mM], and activated charcoal (CAS 7440-44-0, Junsei Chemical Co. Ltd., Tokyo Japan) (0%, 0.2%, 0.4%, and 0.8%, w/v), to confirm the prothallus proliferation. Next, the cultures were incubated at 25 ± 1°C, under a light intensity of 30 ± 1.0 μmol m−2 s−1, and 16/8 h photoperiod. Morphogenesis and the proliferation rate of the prothallus tissues were examined after incubation for 8 wk.
Sporophyte formation
For sporophyte formation, 1.0 g of prothallus was ground with a hand blender for 10 s in 25 mL distilled water in a beaker. The fragments were cultured in various soil substrates (Jang et al.2019b, 2019c). Five soil mixtures were prepared using horticultural substrate (Hs, Hanareum no. 2; Shinsung Mineral Co., Ltd., Goesan, Korea), peat moss (Pt, Sunshine; Sun Gro Horticulture, Vancouver, BC, Canada), perlite (Pr, Newpershine no. 2; GFC. Co., Ltd., Hongseong, Korea), and decomposed granite (D, 2 mm; Samgye Masato, Gimhae, Korea); pots (75 × 75 × 75 mm), were filled with this prepared soil and placed in a plastic box. The volume of each soil was measured at a 1:1 (v/v) ratio, and mixed according to five soil combinations. Hs alone, 2:1 mixtures of Hs and Pr, 2:1 mixtures of Hs and D, 1:1:1 mixtures of Hs, Pt and Pr, and a 1:1:1 mixture of Hs, Pt, and D. After sowing the ground prothalli over the soil, the box with the cultures was covered with a glass plate, and incubated at 25 ± 1°C, light intensity of 43 ± 2.0 μmol m−2 s−1, and 16/8 h photoperiod for 14 wk. The pots were sub-irrigated, and the humidity was maintained at 84 ± 1.4%. After prothallus formation was observed, water was sprayed on the surface of the prothallus to induce fertilization.
Seedlings obtained from the sporophyte formation experiment were transplanted into a plastic pot, which contained horticultural substrates, and acclimated for 3 wk at 25 ± 1°C, light intensity of 43 ± 2.0 μmol m−2 s−1, and 16/8 h photoperiod. Next, the seedlings were cultivated in the greenhouse located at the Chungbuk National University, Cheongju-si, Korea.
Data collection and statistical analysis
Germinated spores and prothallus development were observed using a microscope (CKX53, SZ61; Olympus, Tokyo, Japan). Spore and prothallus images were captured using a CMOS camera (eXcope F630; Dixi Sci., Daejeon, Korea), and eXcope 3.7.12277 software. The success of sporophyte production was assessed by determining the number of sporophytes, the fresh weight, leaf length, leaf width, number of leaves, root length, and number of roots. All experiments were performed in a completely randomized design. Four replicates were used to investigate prothallus proliferation, and four replicates with eight plants per replicate were used to assess sporophyte production. SAS version 9.4 (SAS Institute Inc., Cary, NC), was used to calculate the mean ± standard error for each treatment, and a factorial analysis was performed using Tukey’s honestly significant difference (HSD) test, with a significance level of p < 0.05.
Results
Spore germination and prothallus development
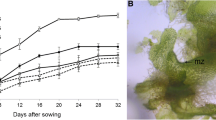
Spores obtained from the Lemmaphyllum microphyllum sporophyll collected in their natural habitat were germinated into gametophytes (Fig. 1A–D). The sowed spores were considered to be germinated after rhizoids and chloroplasts were observed. Spores germinated 9 d after sowing (Fig. 1B), and rhizoid elongation and primary filament development were observed 12 d after sowing (Fig. 1C). It also developed as a mature gametophyte 4 wk after sowing (Fig. 1D). The gametophytes that developed in spores (Fig. 1D), were morphologically identical to the subsequently cultured gametophytes, which were fragmented by scalpel (Fig. 1E). Afterwards, they were transferred to a full-strength MS medium rich in nutrients for rapid growth of the gametophytes and used as experimental material after subculture.
Life cycle of Lemmaphyllum microphyllum C. Presl through spore germination and propagation method: (A) mature plant found in natural habitat; (B) germinated spore; (C) filamentous gametophyte; (D), normal gametophyte; (E, F), gametophytes cultured for 8 wk, and obtained from gametophyte fragments with using scalpel; (G) young sporophytes cultured for 14 wk, and obtained from gametophyte fragments prepared using a hand blender; (H) sporophytes growing in greenhouse after 3 wk of acclimation.
Effect of medium components on prothallus proliferation
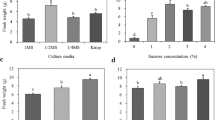
The L. microphyllum gametophyte was cut with a scalpel into fragments that were cultured on MS media of various strengths and Knop medium, to observe the changes in fresh weight of the regenerated prothallus. The fragments were able to effectively develop into many gametophyte clones in a short time (Fig. 1F). The fresh weight of the regenerating gametophytes increased with increasing concentrations of the MS medium; the highest fresh weight (4.3 g), was observed for gametophytes cultured on double-strength MS medium (Fig. 2A). Unlike spore germination, gametophyte proliferation required a high-concentration medium. The effect of the medium components was evaluated based on the effects on the control, which was double-strength MS medium. Nitrogen in the double-strength medium was present at a concentration of 120 mM. However, the fresh weight of the prothallus was higher when cultured in medium supplemented with 60 mM nitrogen than in medium with 120 mM nitrogen (Fig. 2B). The addition of sucrose increased the fresh weight of the gametophyte significantly compared with the medium without sucrose (Fig. 2C). Activated charcoal did not affect the fresh weight of regenerated gametophytes regardless of the concentration (Fig. 2D). The gametophyte cells, as observed with the naked eye, started browning or aging as the concentration of the MS medium decreased (Fig. 3A–E). Gametophyte cells cultured in the Knop medium also browned (Fig. 3A and F). As a result, L. microphyllum gametophyte proliferation was most effective in double-strength MS medium that contained 2% sucrose, 0% activated charcoal, and 60 mM of total nitrogen source.
Effect of medium components on Lemmaphyllum microphyllum C. Presl prothallus growth cultured for 8 wk: (A) type of culture media (quarter-strength Murashige and Skoog, 1/4 MS; half-strength, 1/2MS; full-strength, 1MS; and, double-strength, 2MS); (B) total nitrogen concentration; (C) sucrose concentration; (D) charcoal concentration. The columns represent means and vertical bars indicate standard error (n = 4).
Growth response and organ formation of Lemmaphyllum microphyllum C. Presl prothallus cultured in different media: (A) prothallus after 8 wk of culture (left to right: double-, full-, half-, and quarter-strength Murashige and Skoog [MS] media, and Knop medium); (B)–(F), double-, full-, half-, quarter-strength MS media and Knop medium, respectively. Scale bars are 1 mm.
Production of sporophyte seedlings
The mixture containing a 2:1 (v/v) ratio of horticultural substrate and decomposed granite, increased sporophyte formation to 430.0 sporophytes per pot, which corresponded to 57.3 sporophytes per cm2 (Table 1). Horticultural substrate alone and a 2:1 mixture of horticultural substrate and perlite, induced the development of 424.5 and 382.5 sporophytes per pot, respectively. No significant difference was observed for all three soil types. The number of roots was also 2.2 to 2.5, without a significant difference (Table 1). Growth of the formed sporophytes (Fig. 1G) was similar to that of natural habitat plants (Fig. 1A) in three soil types, and after 3 wk of acclimation, the sporophytes developed into mature plants (Fig. 1H). In contrast, unlike other soil substrates, peat moss-added substrates inhibited the regeneration of fragmented gametophytes, and no sporophytes were formed.
Discussion
This study examined gametophyte proliferation and sporophyte formation of Lemmaphyllum microphyllum cultured on in vitro and soil ex vitro conditions, to identify optimal conditions for the growth of regenerated prothallus, and to develop a practical method for mass production of this species throughout the year. Gametophytes or sporophytes can be easily propagated using homogenization culture methods (Knauss 1976; Cooke 1979; Fernández et al.1999; Fernández and Revilla 2003). In the present study, the gametophyte was mechanically fragmented with a scalpel and hand blender, and gametophyte proliferation and sporophyte formation were induced effectively by exploiting the high regenerative capacity of the fragmented gametophyte (Jang et al.2019a, b, c).
Ferns may have different nutritional requirements depending on the species (Menéndez et al.2010; Wu et al.2010), and the concentration of the medium appears to have a direct effect on gametophyte weight, which is indicative of its proliferation. Medium components, particularly nitrogen and sucrose, are essential for gametophyte growth and development (Miller 1968). Nitrogen supply affected spore germination and gametophyte development of Botrychium dissectum Spreng. and Psilotum nudum (L.) P. Beauv (Melan and Whittier 1990; Whittier 1990), and the reduction in total nitrogen concentration by 25%, produced the highest number of Adiantum capillus-veneris L. sporophytes (Kuriyama et al.2004). In the present study, the changes in gametophyte weight were caused by the concentration of the nitrogen source in the medium (Fig. 2B), and the optimal gametophyte growth was observed at low total nitrogen concentrations. In the early stages of tissue culture, due to the limited photosynthesis, an external energy source is required for healthy growth, proliferation, and differentiation (Kozai 1991; Waman et al.2014). Sucrose is the most commonly used energy source for tissue culture, as it promotes gametophyte growth, and is deeply involved in sex determination (Menéndez et al.2010). The fresh weight of the Dicksonia sellowiana Hook. gametophyte increased in MS medium supplemented with sucrose (Renner and Randi 2004), whereas the formation of the Microgramma vacciniifolia (Langsd. & Fisch.) Copel. sporophyte was progressively promoted with increasing sucrose concentrations (Hirsch 1975). In this study, sucrose increased the fresh weight of the sporophyte by promoting gametophyte proliferation and division (Fig. 2C). Sucrose is an essential factor for tissue culture regardless of its concentration. In contrast, activated charcoal is not essential for tissue culture, although it has often been used in tissue culture to promote cell growth and development (Pan and van Staden 1998). Activated charcoal is used in both liquid and solid media, and serves to adsorb substances that inhibit growth (Thomas 2008). Although the beneficial effects of activated charcoal were expected in the present study, there was no significant difference in fresh weight and development of the gametophyte cultured at different charcoal concentrations (Fig. 2D). In another study, activated charcoal did not affect the proliferation of Dryopteris erythrosora (D.C. Eaton) Kuntze gametophytes (Jang et al.2019a). In this study, mechanical fragmentation of the gametophyte with scalpels generated a large number of gametophytes. However, the fresh weight of the fragmented gametophyte was affected by medium components over time. Therefore, to increase the fresh weight of the fragmented gametophyte, optimal medium conditions should be developed.
Mechanical fragmentation of gametophytes using a hand blender is considered to be the most effective method for large-scale production of sporophytes (Fernández et al.1999; Jang et al.2019a, b). Young sporophytes were obtained from only 1 g of fragmented gametophyte that was sown on the surface of the soil substrate, which demonstrated that a large number of sporophytes can be obtained from small amounts of gametophytes. However, the number of sporophytes formed was affected by the type of soil substrate; sporophytes failed to form in soil that contained peat moss. Peat moss has high cation exchange capacity and water holding capacity, ample pore space, and high air permeability, which provides favorable conditions for plant growth (Nelson 1991; Robertson 1993; Stamps and Evans 1999). However, in the culture environment of this study, the soil substrate with a large amount of peat moss was very moist because of the continuous supply of water through sub-irrigation. Such a humid environment could act as an obstacle to the growth of fragmented gametophytes. In another study, very humid environments inhibited the formation of Dryopteris erythrosora (D.C. Eaton) Kuntze sporophytes in soil substrate with added peat moss (Jang et al.2019a). These results indicate that mechanical fragmentation of gametophytes can yield a large number of sporophytes; however, soil substrates and moist soils influence the formation of sporophytes. Therefore, soil substrates with good drainage and aeration should be used.
Conclusions
A secure proliferation method is proposed for gametophyte proliferation and sporophyte formation that is suitable for production of large quantities of L. microphyllum. Mechanical fragmentation using a scalpel and blender was the most effective way to produce a large number of gametophytes and sporophytes from small amounts of gametophyte. These results can be proposed commercially as a convenient cultivation method ex vitro. It can also be evaluated for propagation of other ferns that form gametophytes.
References
Banks JA (1999) Gametophyte development in ferns. Annu Rev Plant Physiol Plant Mol Biol 50:163–186
Cooke RC (1979) Homogenization as an aid in tissue culture propagation of Platycerium and Davallia. HortSci 14:21–22
Dyer AF (1979) The culture of fern gametophytes for experimental investigation. In: Dyer AF (ed) The experimental biology of ferns. Academic, London, pp 254–305
Fernández H, Bertrand AM, Sánchez-Tamés R (1997) Plantlet regeneration in Asplenium nidus L. and Pteris ensiformis L. by homogenization of BA-treated rhizomes. Sci Hortic 68:243–247
Fernández H, Bertrand AM, Sánchez-Tamés R (1999) Biological and nutritional aspects involved in fern multiplication. Plant Cell Tiss Org Cult 56:211–214
Fernández H, Revilla MA (2003) In vitro culture of ornamental ferns. Plant Cell Tiss Org Cult 73:1–13
Gabriel y Galán JM, Migliaro G (2011) Comparative study on the gametophyte morphology and development of three paramo species of Jamesonia (Pteridaceae, Polypodiopsida). Nor J Bot 29:249–256
Hirsch AM (1975) The effect of sucrose on the differentiation of excised leaf tissue into either gametophytes or sporophytes. Plant Physiol 56:390–393
Ito M (1962) Studies on the differentiation of fern gametophytes I. regeneration of single cells isolated from cordate gametophytes of Pteris vittata. Bot Mag Tokyo 75:19–27
Jang BK, Cho JS, Kwon HJ, Lee CH (2019a) Optimal conditions for spore germination and gametophyte and sporophyte production in the autumn fern Dryopteris erythrosora. Hortic Environ Biotechnol 60:115–123
Jang BK, Cho JS, Lee CH (2019b) Propagation methods for gametophyte proliferation and sporophyte formation in silver cloak fern (Cheilanthes argentea). Hortic Environ Biotechnol 60:435–442
Jang BK, Cho JS, Park KT, Lee CH (2019c) A methodology for large-scale Athyrium sheareri gametophyte proliferation and sporophyte production using tissue culture. In Vitro Cell Dev Biol--Plant 55:519–526. https://doi.org/10.1007/s11627-019-09991-5
Kawano (2015) Pteridophytes as active components in gardening, agricultural and horticultural ecosystems in Japan. Adv Hortic Sci 29:41–47
Knauss JF (1976) A partial tissue culture method for pathogen-free propagation of selected ferns from spores. Proc Fla State Hort Soc 89:363–365
Knop W (1865) Quantitative Untersuchungen uber die Ernahrungsprozesse der Pflanzen. Landwirtsch Vers Stn 7:93–107
Kozai T (1991) Photoautotrophic micropropagation. In Vitro Cell Dev Biol--Plant 27:47–51
Kuriyama A, Kobayashi T, Hayashi S, Maeda M (2004) Medium composition for the production of sporophytes of the fern Adiantum capillus-veneris. J Jpn Soc Hortic Sci 73:580–582
Lee CS, Lee KH (2018) Pteridophytes of Korea: Lycophytes and ferns, 2nd edn. Geobook, Seoul
Maeda M, Ito M (1981) Isolation of protoplasts from fern prothallia and their regeneration to gametophytes. Bot Mag Tokyo 94:35–40
Melan MA, Whittier DP (1990) Effects of inorganic nitrogen sources on spore germination and gametophyte growth in Botrychium dissectum. Plant Cell Environ 13:477–482
Menéndez V, Arbesú R, Somer M, Revilla A, Fernández H (2010) From spore to sporophyte: how to proceed in vitro. In: Fernández H, Kumar A, Revilla A (eds) Working with ferns: issues and applications. Springer, New York, pp 97–110
Miller JH (1968) Fern gametophytes as experimental material. Bot Rev 34:361–440
Moran RC (2004) A natural history of ferns. Timber Press, Portland
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nelson PV (1991) Greenhouse operation and management. Prentice Hall, North Carolina
Pan MJ, van Staden J (1998) The use of charcoal in in-vitro culture – a review. Plant Growth Regul 26:155–163
Raghavan V (1989) Developmental biology of fern gametophytes. Cambridge University Press, UK
Renner GDR, Randi AM (2004) Effects of sucrose and irradiance on germination and early gametophyte growth of the endangered tree fern Dicksonia sellowiana Hook (Dicksoniaceae). Acta Bot Bras 18:375–380
Robertson RA (1993) Peat, horticulture and environment. Biodivers Conserv 2:541–547
Stamps RH, Evans MR (1999) Growth of Dracaena marginata and Spathiphyllum ‘Petite’ in Sphagnum peat and coconut coir dust-based growing media. J Environ Hortic 17:49–52
Thomas TD (2008) The role of activated charcoal in plant tissue culture. Biotechnol Adv 26:618–631
Waman AA, Pooja B, Sathyanarayana BN (2014) Not all sugars are sweet for banana multiplication. In vitro multiplication, rooting and acclimatization of banana as influenced by carbon source-concentration interactions. In Vitro Cell Dev Biol--Plant 50:552–560
Whittier DP (1990) Effects of nitrogen source on spore germination and gametophyte growth in Psilotum. Bot Gaz 151:50–53
Wu H, Liu XQ, Ji H, Chen LQ (2010) Effects of light, macronutrients, and sucrose on germination and development of the endangered fern Adiantum reniforme var. sinense (Adiantaceae). Sci Hortic 125:417–421
Zheng X, Xing F (2009) Ethnobotanical study on medicinal plants around Mt. Yinggeling, Hainan Island, China. J Ethnopharmacol 124:197–210
Acknowledgments
This study was supported by the “useful plant collection and development of mass propagation protocol for the establishment of the foundation of convergence platform using forest plants (KNA1-2-25, 16-3)” project funded by Korea National Arboretum.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Editor: Masaru Nakano
Rights and permissions
About this article
Cite this article
Jang, B.K., Cho, J.S., Park, K. et al. Practical methodology for gametophyte proliferation and sporophyte production in green penny fern (Lemmaphyllum microphyllum C. Presl) using mechanical fragmentation. In Vitro Cell.Dev.Biol.-Plant 56, 318–324 (2020). https://doi.org/10.1007/s11627-020-10055-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-020-10055-2