Abstract
Cheilanthes argentea (S.G. Gmel.) Kunze is a highly valued indigenous/endemic Korean bracken species used for ornamental purposes. In this study, we developed a practical proliferation method for mass production of C. argentea plants using tissue culture. The gametophyte proliferation using the chopping method produced gametophytes that were morphologically identical to those obtained from in vitro spore germination. This new method increased plant fresh weight by more than 40-fold, from 300 mg to 12.7 g, in Murashige and Skoog medium. A blending method was used to produce 74.3 sporophytes in 7.5-cm2 plastic pots using 1 g of gametophytes. Furthermore, addition of exogenous gibberellin promoted sporophyte development and growth, suggesting the possibility of controlling the number of sporophytes formed. However, further studies are needed to explain the effect of exogenous gibberellin, which is closely related to the sex-determining hormone antheridiogen, on the number of sporophytes and its mechanism of action. Our new tissue culture system is capable of mass proliferation of C. argentea gametophytes in vitro and the formation of sporophytes by ex vitro preparation using the generated gametophytes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cheilanthes argentea (S.G. Gmel.) Kunze, of the family Pteridaceae, is an evergreen perennial plant that grows on rock crevices in sunny places in the mountains. The leaf surface is green, while the abaxial surface is covered with a white or silver-white powder, which esthetically contrasts with the color of the leaf surface. The Korean name “Bu-sit-git-go-sa-ri” was given because the white color of the abaxial surface is the same as that of tinder (Lee 2003), while its English name is “silver cloak fern” (Lee and Lee 2018). In addition, it is called “Tong-Kyung-Cho” in oriental medicine, where it is used for cough suppression, for treating menstrual irregularity, and for some diseases in women (KBIS 2018). Because of its unique morphology, C. argentea is used both as a potted plant and a ground cover plant.
Fern plants propagate by spore germination and develop into gametophytes after germination, followed by a series of processes in which sporophytes are formed (Moran 2004). This process requires suitable environmental conditions for spore germination (Fernandez and Revilla 2003) and a prolonged period of plant growth. An alternative propagation method involves the collection of rhizomes in their natural habitat and activation of the root buds by burying the rootstock in the soil. This shortens the process of spore germination, gametophyte development, and sporophyte formation and allows plants to grow quickly; however, it requires considerable amounts of labor. Although both spore and rhizome propagation methods produce plants, they are not suitable for mass production of uniform plants; thus, practical methods for industrialization need to be developed.
To address this problem, we devised an alternative tissue culture method that enables fern mass production throughout the year. We used a culture method whereby gametophytes obtained from in vitro spore germination proliferate under conditions that induce sporophyte formation. Additionally, the method allows the cultured material to be maintained over an extended period through subculture. Among available methods, chopping/blending involves finely chopping the gametophytes using a scalpel/blender to produce numerous clones from the fragments (Miller 1968; Jang et al. 2017, 2019); by this means, it is possible to mass produce uniform gametophytes in vitro, in a short time (Sheffield and Attree 1983). Furthermore, the method can be used to efficiently mass produce sporophytes by transplanting the cultured gametophytes obtained ex vitro (Fernandez et al. 1999; Lee 2004). However, nutrient requirements for the growth of gametophytes in vitro vary depending on the species; similarly, the suitable soil conditions required for transplantation ex vitro also differ. Species such as Equisetum arvense, Dryopteris affinis sp. affinis, and Adiantum capillus-veneris are grown as gametophytes in Murashige and Skoog (MS) medium, which is rich in nutrients (Kuriyama et al. 1989; Fernandez et al. 1996; Kuriyama et al. 2004). However, gametophytes of Osmunda regalis show excellent growth in Knop medium, which is not as rich in nutrients as the MS medium (Fernandez et al. 1997). Thus, the propagation method using tissue culture is highly effective for annual mass production of fern plants; however, culture conditions suitable for growth and sporophyte formation of gametophytes must be investigated.
Therefore, this study aimed to develop a mass proliferation method using tissue culture for the commercial production of C. argentea (S.G. Gmel.) Kunze and to determine suitable conditions for gametophyte propagation and sporophyte formation. The information obtained in this study provides a highly applicable and practical propagation method for C. argentea for ornamental and/or medicinal purposes.
2 Materials and methods
2.1 Plant material
Plants were collected from Dosan-myeon, Andong-si, Gyeongsangbuk-do, South Korea (lat. 36°43′33.5″N, long. 128°50′13.0″E) in February 2014 and transplanted into a plastic-film greenhouse at Chungbuk National University, Cheongju-si, Korea (lat. 36°37′29.0″N, long. 127°27′18.4″E). Sporophylls were harvested on June 8, 2014, dried in a paper box at 25 ± 1 °C for 7 days, and passed through a 100-μm sieve (Chunggye Co., Korea) to obtain the spores. Spore germination was performed according to methods described by Jang et al. (2019). The spores were cultured in MS medium (Murashige and Skoog 1962) supplemented with 3.0% (w/v) sucrose and 0.8% (w/v) agar and adjusted to pH 5.8 before autoclaving. The spores were germinated at 25 ± 1 °C under a 16/8-h photoperiod, under a light intensity of 30 ± 1.0 μmol m−2 s−1. Gametophytes obtained from germinated spores were subcultured in MS medium at 8-week intervals and then used for experimental gametophyte proliferation and sporophyte formation.
2.2 Gametophyte proliferation
The gametophytes grown from germinated spores were uniformly subcultured in MS medium and used in the following experiments. We examined the culture conditions suitable for gametophyte proliferation using the chopping method of Jang et al. (2017). MS (1/8×, 1/4×, 1/2×, 1×, and 2×), Knop medium, different concentrations of sucrose (0%, 1%, 2%, 3%, and 4%), and activated charcoal (0%, 0.2%, 0.4%, and 0.8%) were prepared. All the media, except those containing specific sucrose concentrations, were supplemented with 3% sucrose. For each of the treatments, 30 mL of the corresponding medium was dispensed into a 200-mL culture bottle and then 300 mg of uniformly chopped gametophytes was spread on the medium surface to induce fragmentation and 1 mL of sterile water was added. Cultures were incubated at 25 ± 1 °C under a light intensity of 30 ± 1.0 μmol m−2 s−1 and 16/8-h photoperiod for 8 weeks; thereafter, prothallus morphogenesis and proliferation rate were examined.
2.3 Sporophyte production and effect of exogenous gibberellic acid (GA3) treatment
We determined suitable substrate conditions for sporophyte formation using the blending method of Jang et al. (2019). In-vitro-cultured gametophytes were used after sterilization for 1 h with a 1000 × fungicide solution (Hymexazol 30%; Tachigaren, Dongbu Agrotech, Korea). Sterilized gametophytes were washed five times with distilled water. Sterilized gametophytes (1.0 g) were placed in 25 mL of distilled water in a plastic beaker and ground for 10 s using a hand blender as described by Jang et al. (2019). Eight soil mixtures were prepared using horticultural substrates (substrate: cocopeat, peat moss, vermiculite, perlite, zeolite, humic acid, and Hanareum no. 2; Shinsung Mineral Co., Ltd., Korea), peat moss (Sunshine; Sun Gro Hort., Canada), perlite (Newpershine no. 2; GFC. Co., Ltd., Korea), and decomposed granite (2 mm; Samgye Masato, Korea). Pots (75 × 75 × 75 mm) were filled with the soil mixtures prepared and placed in a plastic box (503 × 335 × 195 mm). Ground gametophytes were then spread uniformly on top of the soil mixtures. These cultures were incubated in a glass-plate-covered plastic box for 14 weeks at 25 ± 1 °C, under a 16/8-h photoperiod at a light intensity of 43 ± 2.0 μmol m−2 s−1, and with subirrigation conditions and a relative humidity of 72% ± 2%. After prothallus formation was observed, water was sprayed daily on the surface of the prothallus to facilitate fertilization.
We investigated the effect of exogenous gibberellic acid (GA3, Sigma-Aldrich, CAS No. 77-06-5) on the production of sporophytes using soil composition and gametophytes selected from the experiment described above. To improve sporophyte growth, gametophytes sterilized under the same conditions as described above were treated by soaking in various concentrations of GA3 (0, 50, 100, 200, and 500 mg L−1) for 1 h. GA3-treated gametophytes were washed three times with distilled water, ground using the blending method, and cultured under the same conditions and methods described above.
2.4 Sporophyte acclimatization
Seedlings obtained from the sporophyte formation experiment were transplanted into a plastic box tray containing horticultural substrates and acclimated for 3 weeks ex vitro. The environmental acclimatization conditions were as follows: 25 ± 1 °C, 43 ± 2.0 μmol m−2 s−1 light intensity, and 16/8-h photoperiod. Next, seedlings were cultivated in the plastic-film greenhouse at Chungbuk National University.
2.5 Data collection and statistical analysis
Gametophyte development was observed using a stereoscopic microscope (SZ51, Olympus, Japan). To confirm the effect of sporophyte production, the number of sporophytes, fresh weight, leaf length, leaf width, number of leaves, root length, number of roots, chlorophyll content (SPAD-502, Minolta, Japan), and growth were measured. All experiments were performed in a completely randomized design. Four replicates were used to investigate gametophyte proliferation, and ten replicates were used to investigate of sporophytes production. SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) was used to calculate the mean ± standard error for each treatment, and a factorial analysis was performed using Duncan’s multiple range test, with a significance level of p < 0.05.
3 Results
3.1 Effect of culture media on gametophyte proliferation
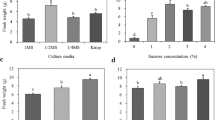
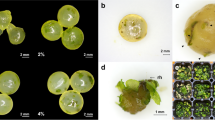
We recorded the changes in fresh weight of gametophytes cultured under various medium conditions (Fig. 1). Fresh weight peaked at 12.7 g, in the 1 × MS medium, and decreased with decreasing MS concentration. However, growth was suppressed in the 2 × MS medium. On the other hand, the fresh weight of gametophytes cultured in Knop medium was similar to that recorded in the 1/2 × MS medium. Specifically, gametophytes cultured in 1/4 × and 1/8 × MS media showed browning with age (Fig. 2f, g). In addition, gametophyte cells cultured in the 2 × MS medium did not proliferate normally and were necrotized in a clump form (Fig. 2c). On the other hand, gametophytes developed normally in 1 × MS, 1/2 × MS, and Knop media.
Culture response and sexual organ formation of Cheilanthes argentea (S.G. Gmel.) Kunze gametophytes cultured in different media. a and b Gametophyte after 8 weeks of culture; c–g, 2×, 1×, 1/2×, 1/4×, and 1/8× Murashige and Skoog (MS) media, respectively; h Knop medium; an, antheridium; ap, apical notch; br, browning; ce, cell clump; hg, heart-shaped gametophyte; rh, rhizoid; th, thin thallus
3.2 Effect of sucrose and charcoal on gametophyte proliferation
To investigate the effect of sucrose and activated charcoal in the medium, gametophytes were cultured for 8 weeks in 1 × MS medium containing different concentrations of sucrose and activated charcoal. Results revealed that fresh weight reached 15.6 g in the 2% sucrose treatment, which showed maximum growth (Fig. 3). In contrast, growth of gametophytes was substantially lower in the treatment without sucrose addition. On the other hand, in the media supplemented with 0.2–0.8% of activated charcoal, gametophyte fresh weight increased significantly relative to the control, but there was no significant difference among the concentrations tested (Fig. 4).
Effect of sucrose concentration on the fresh weight of Cheilanthes argentea (S.G. Gmel.) Kunze gametophytes cultured for 8 weeks. Vertical bars represent mean ± standard error (n = 4). Bars with a different lowercase letter are significantly different as per Duncan’s multiple range test at 5% probability level
Effect of charcoal concentration on the fresh weight of Cheilanthes argentea (S.G. Gmel.) Kunze gametophytes cultured for 8 weeks. Vertical bars represent mean ± standard error (n = 4). Bars with a different lowercase letter are significantly different as per Duncan’s multiple range test at 5% probability level
3.3 Production of sporophytes and effect of exogenous GA3 treatment
The mixture containing a 2:1 (v/v) ratio of horticultural substrate and perlite increased sporophyte formation to 74.3 sporophytes/pot (Table 1), which was 9.91 sporophyte/cm2. The 2:1 mixtures of the horticultural substrate and decomposed granite, and horticultural substrate alone induced an average development of 37.8 and 15.8 sporophytes, respectively. In contrast, peat moss tended to suppress the formation of gametophytes and sporophytes. A soil mixture of horticultural substrate and perlite at a 2:1 (v/v) ratio promoted excellent sporophyte growth, as shown by the measurements of fresh weight, leaf length, leaf width, and chlorophyll content (Table 1).
The 100 mg L−1 GA3 treatment yielded the greatest number of sporophytes (165.5), but as the concentration of gibberellin increased, the number of sporophytes that formed decreased (Table 2). Growth variables, such as fresh weight of the aerial body, leaf length, leaf width, and root length, registered an outstanding performance. All the cultured sporophytes were successfully transplanted into the plastic-film greenhouse after a 3-week acclimation.
4 Discussion
This study was carried out to establish optimal propagation conditions for mass production of sporophytes from gametophytes of Korean native C. argentea (S.G. Gmel.) Kunze. Gametophytes obtained from germinated spores were uniformly subcultured in 1 × MS medium and used in subsequent experiments; spore germination was observed 8 days after sowing (data not shown). Spore germination initiates from cell division and is known to be confirmed morphologically by protrusion of rhizoids and observation of initial filaments (Dyer 1979; Raghavan 1989). Meristematic cells of the gametophyte are known to develop into a heart-shaped or spatulate-shaped gametophyte by transverse and longitudinal division (Racusen 2002; Li et al. 2013). Gametophytes used as the starting material in this study also developed from spores into heart-shaped gametophytes (data not shown).
In contrast to conventional culture methods, where a part of the cultured cells is transplanted to induce proliferation, the chopping method used for multiplying the gametophytes involves chopping and fragmenting cultured cells with a scalpel. This method enables mass propagation of uniform gametophytes even with a small amount of gametophytic tissues. The fragmented gametophyte produces numerous clones from the fragments (Sheffield and Attree 1983), and the meristematic cells promote regeneration of the fragments and develop into normal gametophytes (Miller 1968). Gametophytes cultivated using the chopping method showed a heart or spatulate shape similar to the gametophytes obtained using spore germination (Fig. 2). However, the proliferation of gametophytes differed depending on the media components and concentrations. Specifically, 1 × MS medium showed a tendency to increase the plant fresh weight as the concentration of constituents increased (Fig. 1), but proliferation was inhibited in the 2 × MS due to the high concentration of nutrients. These results are illustrated in Fig. 2a–h. We speculate that cell division in the fragmented prothallus was inhibited, thereby leading to necrosis in the 2 × MS medium.
In vitro cultures should supply a carbon source to replace the carbohydrates produced by photosynthetic reactions due to the characteristic heterotrophic nature of tissue cultures. The carbon supply in the medium is used as an energy source for the formation of plant cells, tissues, and organs (Yaseen et al. 2013). Among common sources, sucrose is a representative soluble sugar added to media and is known as an essential nutrient source for long-term culture of plants (de Paiva Neto and Otoni 2003). Sucrose has also been reported to actively participate in cell division (Sami et al. 2016). In this study, proliferation of the gametophytes significantly differed according to the concentration of sucrose in the medium during the 8-week culture period, and proliferation of gametophytes in the medium without sucrose was below detection.
In the early stage of culture, toxic substances and phenolic compounds released from the tissue culture plants lead to browning and tissue necrosis by the medium (Pan and Staden 1998). To solve this problem, activated charcoal is added to culture media. According to Thomas (2008), activated charcoal has a fine network of pores with a large inner surface area, which shows excellent adsorption of various chemical substances (toxic substances, phenolic compounds, and plant growth regulators). It has been reported that activated charcoal affects morphogenesis, cell division, and sex determination of the gametophytes of Platycerium bifurcatum, E. arvense, and Blechnum spicant (Kuriyama et al. 1990; Teng 1997; Menéndez et al. 2006). For C. argentea, the fresh weight of the gametophytes was increased in the medium supplemented with activated charcoal. This observation suggests that cell division of the fragmented gametophyte was promoted.
The blending method used in the sporophyte formation experiment is designed to produce many sporophytes from a small number of gametophytes (Jang et al. 2017, 2019). In this study, the substrate supplemented with peat moss did not form any sporophytes. This could be attributed to the physical properties of the soil substrate and the irrigation method used in this study. Horticultural soil refers to a substrate that provides moisture, oxygen, and nutrients for plant growth and has controlled physical and chemical properties (Verdonck et al. 1983; Wilson 1984; Kang et al. 2001). Horticultural soil is composed of light soil, such as organic peat moss, cocopeat, inorganic perlite, vermiculite, and zeolite (Kim and Kim 2011). Inorganic soils change the substrate properties depending on the shape, particle size, and material, which increase pore space in the soil, thereby improving drainage and aeration (Mastalerz 1977). Among the inorganic substances, perlite imparts high drainage and aeration (Kang et al. 2001), and decomposed granite is reported to have relatively large and coarse particles, with low moisture and nutrient retention, and low nitrogen content (Matsuo and Nishida 1968; Fukumoto 1990; Masuda 1992). In contrast, peat moss is a substrate with high water content (Heiskanen 1995). The subirrigation method used in this study provides a continuous supply of water to the rhizosphere by capillary action. Therefore, the water content in the soil pore space was maintained constantly high because of the peat moss, which seems to have adversely affected the formation and growth of sporophytes.
The gametophyte develops from spore germination from antheridia and archegonia in sexual reproductive organs and basically in the form of a hermaphrodite. Several previous studies have reported that light (Chang et al. 2007), phytochrome (Kamachi et al. 2007), and an antheridium-inducing hormone (antheridiogen) affect sex determination of gametophytes. Antheridiogen, a hormone with a similar skeletal structure to that of gibberellin, is secreted by hermaphrodite gametophytes and is known to induce male organ (antheridium) development in juvenile gametophytes (Menéndez et al. 2006). Yamane (1998) reported that the addition of exogenous gibberellin induces the formation of antheridium. We hypothesized that exogenous gibberellin treatment of the gametophytes, followed by blending and sowing, would also affect the formation of reproductive organs and promote the formation of sporophytes. In this study, sporophyte formation was significantly promoted by GA3 treatment, and the most sporophytes formed in the 100 mg L−1 GA3-treated group. On the other hand, treatment with high concentrations of GA3 (200–500 mg L−1) suppressed the formation or growth of sporophytes; therefore, it is necessary to select a suitable concentration of GA3. In this study, exogenous GA3 treatment likely regulated sporophyte formation from gametophytes. However, further studies are needed to clarify whether exogenous gibberellin controls the formation of male reproductive organs or replaces antheridiogen.
5 Conclusion
We developed an efficient proliferation method for mass production of C. argentea (S.G. Gmel.) Kunze. Our results provide substantial information on a novel tissue culture propagation method and the development of gametophytes, as well as on the formation of sporophytes in C. argentea, which eventually produced many plants. Furthermore, the chopping method enabled mass propagation using only a few gametophytes, while the blending method mass-produced sporophytes using a few gametophytes. The application of these two propagation methods to tissue culture is very practical and suitable for industrialization and could be used for mass production of various fern plants besides C. argentea.
References
Chang HC, Agrawal DC, Kuo CL, Wen JL, Chen CC, Tsay HS (2007) In vitro culture of Drynaria fortunei, a fern species source of Chinese medicine “Gu-Sui-Bu”. In Vitro Cell Dev Biol Plant 43:133–139
de Paiva Neto VB, Otoni WC (2003) Carbon sources and their osmotic potential in plant tissue culture: does it matter? Sci Hortic 97:193–202
Dyer AF (1979) The culture of fern gametophytes for experimental investigation. In: Dyer AF (ed) The experimental biology of ferns. Academic Press, London, pp 254–305
Fernandez H, Revilla MA (2003) In vitro culture of ornamental ferns. Plant Cell Tissue Organ Cult 73:1–13
Fernandez H, Bertrand AM, Sanchez-Tames R (1996) Influence of tissue culture conditions on apogamy in Dryopteris affinis sp. affinis. Plant Cell Tissue Organ Cult 45:93–97
Fernandez H, Bertrand AM, Sanchez-Tames R (1997) Gemmation in cultured gametophytes of Osmunda regalis. Plant Cell Rep 16:358–362
Fernandez H, Bertrand AM, Sanchez-Tames R (1999) Biological and nutritional aspects involved in fern multiplication. Plant Cell Tissue Organ Cult 56:211–214
Fukumoto T (1990) A grading equation for decomposed granite soil. Soils Found 30:27–34
Heiskanen J (1995) Physical properties of two-component growth media based on Sphagnum peat and their implications for plant-available water and aeration. Plant Soil 172:45–54
Jang BK, Cho JS, Lee KC, Lee CH (2017) Culture conditions affecting spore germination, prothallus propagation and sporophyte formation of Dryopteris nipponensis Koidz. Hortic Sci Technol 35:480–489
Jang BK, Cho JS, Kwon HJ, Lee CH (2019) Optimal conditions for spore germination and gametophyte and sporophyte production in the autumn fern Dryopteris erythrosora. Hortic Environ Biotechnol 60:115–123
Kamachi H, Iwasawa O, Hickok LG, Nakayama M, Noguchi M, Inoue H (2007) The effects of light on sex determination in gametophytes of the fern Ceratopteris richardii. J Plant Res 120:629–634
Kang JY, Lee HH, Kim KH (2001) Physical and chemical properties of inorganic horticultural substrates used in Korea. Acta Hortic 644:231–235
Kim HS, Kim KH (2011) Physical properties of the horticultural substrate according to mixing ratio of peatmoss, perlite and vermiculite. Korean J Soil Sci Fert 44:321–330
Korea Biodiversity Information System (KBIS) (2018) Korea National Arboretum. Pocheon, Korea. http://www.nature.go.kr/kbi/idx/searchIndex.do. Accessed 05 Jul 2018
Kuriyama A, Hojoh T, Sugawara Y, Matsushima H, Takeuchi M (1989) A method for the rapid growth in culture of gametophytes of Equisetum arvense with antheridia. Plant Cell Physiol 30:1189–1192
Kuriyama A, Takeuchi M, Ueno S, Mitsuda H (1990) Enhancement of the division of Equisetum arvense protoplasts in culture by activated charcoal and their further development. Plant Cell Physiol 31:999–1004
Kuriyama A, Kobayashi T, Hayashi S, Maeda M (2004) Medium composition for the production of sporophytes of the fern Adiantum capillus-veneris. J Jpn Soc Hortic Sci 73:580–582
Lee TB (2003) Coloured flora of Korea. Hyangmunsa, Seoul, p 46
Lee CH (2004) Propagation and technique of masspropagation of Pteridophyta native to Korea. Korean Wild Res Assn 3:91–96
Lee CS, Lee KH (2018) Pteridophytes of Korea: lycophytes and ferns, 2nd edn. Geobook, Seoul, p 181
Li X, Fang YH, Yang J, Bai SN, Rao GY (2013) Overview of the morphology, anatomy, and ontogeny of Adiantum capillus-veneris: an experimental system to study the development of ferns. J Syst Evol 51:499–510
Mastalerz JW (1977) The greenhouse environment: the effect of environmental factors on the growth and development of flower crops. Wiley, New York
Masuda T (1992) Studies on the characteristics of Masa soil as a medium for tree growth and methods for its improvement. Landsc Res Jpn 56:138–145
Matsuo SI, Nishida K (1968) Physical and chemical properties of decomposed granite soil grains. Soils Found 8:10–20
Menéndez V, Revilla MA, Bernard P, Gotor V, Fernández H (2006) Gibberellins and antheridiogen on sex in Blechnum spicant L. Plant Cell Rep 25:1104–1110
Miller JH (1968) Fern gametophytes as experimental material. Bot Rev 34:361–440
Moran RC (2004) A natural history of ferns. Timber Press, Portland, pp 34–89
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Pan MJ, Staden JV (1998) The use of charcoal in in vitro culture—a review. Plant Growth Regul 26:155–163
Racusen RH (2002) Early development in fern gametophytes: interpreting the transition to prothallial architecture in terms of coordinated photosynthate production and osmotic ion uptake. Ann Bot 89:227–240
Raghavan V (1989) Developmental biology of fern gametophytes. Cambridge University Press, Cambridge, pp 168–177
Sami F, Yusuf M, Faizan M, Faraz A, Hayat S (2016) Role of sugars under abiotic stress. Plant Physiol Biochem 109:54–61
Sheffield E, Attree SM (1983) Further experiments with ferns in culture: regeneration. J Biol Educ 17:183–184
Teng WL (1997) Activated charcoal affects morphogenesis and enhances sporophyte regeneration during leaf cell suspension culture of Platycerium bifurcatum. Plant Cell Rep 17:77–83
Thomas TD (2008) The role of activated charcoal in plant tissue culture. Biotechnol Adv 26:618–631
Verdonck O, Penninck R, de Boodt M (1983) The physical properties of different horticultural substrates. Acta Hortic 150:155–160
Wilson GCS (1984) The physico-chemical properties of horticultural substrates. Acta Hortic 150:19–32
Yamane H (1998) Fern antheridiogens. Int Rev Cytol 184:1–32
Yaseen M, Ahmad T, Sablok G, Standardi A, Hafiz IA (2013) Review: role of carbon sources for in vitro plant growth and development. Mol Biol Rep 40:2837–2849
Acknowledgements
This study was financially supported by the project “Development of Mass propagation and Production Techniques on Useful Exploration Plant Resources” of the Korea National Arboretum, Project No. KNA1-2-15, 11-6. This study was also financially supported by the project “Development of Mass Propagation Protocol for the Establishment of Application Base Using Forest Plants” of the Korea National Arboretum, Project No. KNA1-2-25, 16-3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jang, B.K., Cho, J.S. & Lee, C.H. Propagation methods for gametophyte proliferation and sporophyte formation in silver cloak fern (Cheilanthes argentea). Hortic. Environ. Biotechnol. 60, 435–442 (2019). https://doi.org/10.1007/s13580-019-00128-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-019-00128-6