Abstract
This article describes a fast, simple, and efficient plant regeneration protocol for Ocimum basilicum L. Two sets of experiments were performed. The first set was performed on Murashige and Skoog (MS) medium lacking zinc sulfate heptahydrate (ZnSO4·7H2O) and containing varying concentrations of indole-3-acetic acid (IAA). The second set used three different concentrations (8.6, 12.9, and 17.2 mg L−1) of ZnSO4·7H2O along with varying concentrations of IAA. In the first set, without zinc sulfate, an IAA concentration of 1.0 mg L−1 (MS4) was found to be most effective, producing a mean of 12.6 roots per hypocotyl explant, while shoots were not produced. In the second set, with zinc sulfate, a combination of 12.9 mg L−1 ZnSO4·7H2O + 1.0 mg L−1 IAA (MS11) produced significantly more shoots per explant (15 shoots) than a combination of 12.9 mg L−1 ZnSO4·7H2O + 0.5 mg L−1 IAA (MS10), which produced only six shoots. Later, the plantlets were successfully acclimatized (100%) and finally transferred to the greenhouse (ex vitro). In the O. basilicum plants grown using MS11 medium, total phenolic content and rosmarinic acid content were estimated from stem, shoot tip, and old leaf tissue of in vitro plantlets, ex vitro plantlets, and flowering plants. The highest amounts of total phenolic content (131.8 mg gallic acid equivalent g−1 DW) and rosmarinic acid (13.0 mg g−1 DW) were obtained in the old leaf tissue at flowering time. This rapid regeneration protocol for O. basilicum L. represents a major improvement over the conventional protocols for plant regeneration and propagation of this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ocimum basilicum L. (Lamiaceae) is an aromatic herb native to tropical Asia that is commonly used in Indian and Southeast Asian cuisine, especially in Thai stir-fries. O. basilicum is well-known as a source of essential oils and also as a spice ingredient, imparting flavor and delicacy to the prepared food. Many Indians consume small quantities of young basil leaves either as an offering after divine worship in temples or as a food additive (Archana and Namasivayam 2002). The domestic herb is much appreciated for its beauty and fragrance as an ornamental plant. The leaves of O. basilicum are rich in rosmarinic acid, a predominant phenolic acid detected in the methanolic extract of the leaves and stems (Hakkim et al. 2007), which have numerous dots of oil glands that secrete strongly scented volatile oils.
Regarding its medicinal properties, the plant is considered to be stomachic, anthelmintic, antipyretic, diaphoretic, expectorant, carminative, stimulant, and pectoral (Kirtikar and Basu 2003) as well as having antiseptic, antiallergic, and anticancer effects (Hakkim et al. 2007). It is also used to treat purulent discharge of the ear, bronchitis, hiccup, and diseases of the heart and brain (Siddique and Anis 2007). Several studies have established that the compounds present in basil oil have potent antioxidant, antiaging, anticancer, antiviral, and antimicrobial properties. In India, O. basilicum plants have been traditionally used for the supplementary treatment of stress, asthma, and diabetes (Dube et al. 1989).
The conventional method for the propagation of O. basilicum is via seeds. However, poor seed germination restricts its multiplication by this method. As a consequence of cross-pollination of the plant, the seedling progeny shows wide variation (Heywood 1993). The present article is the first report on the in vitro multiplication of O. basilicum through direct shoot regeneration techniques and thus offers an effective alternative method of propagation of this important multipurpose medicinal plant. Recent reports on plant tissue culture techniques have focused on facilitating plant propagation and production of Ocimum (Sahoo et al. 1997; Begum et al. 2002; Kintzios et al. 2003; Rady and Nazif 2005; Gopi and Ponmurugan 2006; Siddique and Anis 2007; Kiferle et al. 2011), but none of these reports used a combination of ZnSO4·7H2O plus indole-3-acetic acid (IAA) for direct plant regeneration.
IAA is the most common naturally occurring phytohormone, and it is predominantly produced in the cells of the apex (i.e., bud) and very young leaves. IAA synthesis can take place through several independent biosynthetic pathways. The most common route starts from tryptophan, but there is also a biosynthetic pathway independent of tryptophan (Zhao 2010). IAA has many different effects, such as inducing cell elongation and cell division, which ultimately contribute to plant growth and development. On a larger scale, IAA serves as a signaling molecule necessary for the development of plant organs and coordination of plant growth.
Micronutrients are essential requirements for the balanced growth and development of plants (Graham et al. 2001). Among the metals, zinc is one of the most essential and is supplied in the form of zinc sulfate heptahydrate (ZnSO4·7H2O). Zinc regulates many physiological and metabolic processes in plants (Ramesh et al. 2004). It plays a vital role in cell division, cell expansion, protein synthesis, and metabolism of carbohydrates, nucleic acids, and lipids (Lepp 1981). The most distinct zinc deficiency symptoms, namely stunted growth and small leaf size, are presumably related to disturbances in the metabolism of auxins and in the generation of IAA. It has been established that tryptophan is the most likely precursor for the biosynthesis of IAA (Hossain et al. 1997; Saeki et al. 2000), and there are several reports indicating that zinc is required for the synthesis of tryptophan (Oguchi et al. 2004a, b). In addition, zinc is a constituent of a metalloenzyme or a cofactor for several enzymes such as anhydrases, dehydrogenases, oxidases, and peroxidases and plays an important role in regulating nitrogen metabolism, cell multiplication, photosynthesis, and auxin synthesis in plants (Shier 1994). Further, zinc finger transcription factors (ZF-TFs) are required for plant morphogenesis and organogenesis (Kobayashi et al. 1998; Yanagisawa 2004). As zinc is considered to be an essential element for plant growth and development (Shier 1994; Welch and Shuman 1995), it must be present in low concentrations in all media used for in vitro plant regeneration. Optimization of zinc content has resulted in a very significant improvement in the regeneration of a range of monocots and dicots (Pande et al. 2000; Dahleen and Bregitzer 2002). Based on this literature, a systematic investigation of the effect of changing concentrations of zinc sulfate and IAA on the in vitro regeneration of O. basilicum was undertaken.
This report describes a simple and effective in vitro protocol for the direct regeneration of O. basilicum plantlets from hypocotyl segments, with an emphasis on total phenolic content and rosmarinic acid content of the plant.
Materials and Methods
Plant material, surface sterilization, and seed germination conditions
Commercially available seeds of O. basilicum L. were purchased from a local market in Istanbul, Turkey. The seeds were surface-disinfected with 100 mL of 20% commercial bleach (Domestos®, Unilever, Istanbul, Turkey) along with five drops of Tween 20 (Merck, Darmstadt, Germany) for 10 min by using a sonicator and then rinsed three times with sterile distilled water. An average of 20–25 seeds were aseptically cultured in 100-mm × 15-mm Petri dishes containing 30 mL of MS medium (Murashige and Skoog 1962; Duchefa Biochemie, Haarlem, The Netherlands) containing 3% (w/v) sucrose (Duchefa Biochemie, Haarlem, The Netherlands). The medium pH was adjusted to 5.8 using either 0.1 N HCl or 0.1 N KOH. The resultant medium was then solidified with 0.8% (w/v) agar (Duchefa Biochemie, Haarlem, The Netherlands) after autoclaving at 121°C and 1.06 kg cm−2 pressure for 15 min. The seeds were kept in the dark at 23 ± 1°C for 2 d and then transferred to a 16-h photoperiod provided by a cool-white fluorescent light (Philips Master, Warsaw, Poland) with irradiance at 50 μmol photons m−2 s−1 at a relative humidity of 60%.
Establishment of cultures
Hypocotyl segments (5–8 mm long) of O. basilicum excised from aseptically germinated 1-mo-old seedlings were used as explants for direct shoot regeneration. After 6 wk, plantlets with shoots and roots were transferred to capped bottles (Magenta™ B-cap, Sigma-Aldrich®, St. Louis) and observed for up to 8 wk. The explants were cultured on MS medium containing various concentrations of ZnSO4·7H2O and/or IAA (Duchefa Biochemie, Haarlem, The Netherlands) (Table 1). The cultures were incubated in a growth chamber under the conditions described above. Each experiment was performed three times, each using 15 replicates (i.e., a total of 45 explants per treatment). Both the frequency (%) of explants developing shoots with roots and the mean number of shoots with roots per explant were recorded after 8 wk of culture. After a period of 12 wk, the developed plantlets were placed in pots containing a mixture of soil/manure/moss/sand (1:2:2:1 [w/w/w/w]), incubated in the growth chamber for 15 d, and transferred to greenhouse (ex vitro) conditions. Samples were collected for the determination of total phenolic content and rosmarinic acid content from the stem, shoot tip, and old leaf tissue of in vitro and ex vitro, and plants at the flowering stage (after 8, 16, and 21 wk, respectively).
Sampling and extraction
Dried samples (stem, shoot tip, or old leaf) were collected from the MS11 treatment and then powdered using a mortar and pestle and screened through a 380-μm sieve. Fifty milligrams of the fine powder was extracted with 2 mL of methanol (Sigma-Aldrich®) and kept at room temperature (20 ± 3°C) for 2 d in dark condition. The resulting mixture was centrifuged at 13147×g (14,000 rpm) for 10 min. This extract was taken for total phenolic content determination.
For the purpose of rosmarinic acid content determination, 50 mg of the fine powdered was extracted twice with 2 mL of methanol in a 2.5-mL microcentrifuge tube using ultrasonication at room temperature (20 ± 3°C) for 30 min. The resulting mixture was centrifuged at 13147×g (14000 rpm) for 5 min. The supernatant was then transferred into a new 2.5-mL microcentrifuge tube. A 50-μL sample of extract was transferred into a new microcentrifuge tube, brought to a volume of 1000 μL with methanol, and mixed by vortexing for 1 min. The mixture was filtered through a 0.22-μm Millex Millipore syringe filter (Merck, Istanbul, Turkey).
Determination of total phenolic content
The total phenolic content in the extract was determined following a modified Folin–Ciocalteu method (Marigo and Boudet 1979). Briefly, a 20-μL aliquot of the extract was placed in a 2-mL microcentrifuge tube containing 1.58 mL of water and 100 μL of Folin–Ciocalteu reagent (AppliChem, Ankara, Turkey). The microcentrifuge tube was allowed to stand for 5 min, and then 300 μL 20% Na2CO3 (w/v) (Merck, Darmstadt, Germany) was added into the tube. After about 20 min at 40°C, the absorbance of the solution was measured using a spectrophotometer (Hitachi U-1900 UV–vis, Tokyo, Japan) at λ = 750 nm. The total phenolic content was expressed as (milligrams gallic acid equivalent)/(gram dry weight) or milligrams GAE per gram DW.
Determination of rosmarinic acid using HPLC
Three extracts were prepared from each sample and analyzed using HPLC (Agilent 1100 series, Waldbronn, Germany). A 20-μL aliquot of the filtrate (vide supra, “Sampling and extraction” section) was injected into the HPLC system for analysis of rosmarinic acid. The quantification of rosmarinic acid was done by comparing the sample to a pure standard (Sigma-Aldrich®, Steinheim, Germany). For the HPLC analysis, the selected wavelength was 320 nm for UV detection. Elution was carried out at a flow rate of 1.0 mL min−1 at 20°C using a binary pump solvent system. An autosampler (Waters Autosampler 717 Plus) was used for injecting 20 μL of the sample (the filtrate mentioned above) into an Inertsil ODS-3, 4.6-mm × 150-mm column (GL Sciences Inc., Tokyo, Japan). Two mobile phases, A and B, were used. Mobile phase A was 2% (v/v) aqueous acetic acid, while mobile phase B was 70% acetic acid:30% acetonitrile (7:3, [v/v]). Rosmarinic acid was eluted with gradients A and B as follows: 0–3 min: 30% B, 70% A; 3–9 min: 40% B, 60% A; 9–12 min: 50% A, 50% B, 12–24 min: 60% B, 40% A; and 24–35 min: 70% B, 30% A. All reagents used in the HPLC runs were obtained from Sigma-Aldrich®.
Statistical analysis
The collected data were statistically analyzed using a computer program (SPSS Statistics, version 17.0, SPSS Inc., Chicago, IL). The experimental results were subjected to an analysis of variance (ANOVA) (Maxwell and Delaney 2004) and Duncan’s multiple range test (Duncan 1955). The mean ± SE (standard error) were subjected to Duncan’s multiple range test at p < 0.05.
Results and Discussion
The present article describes the first systematic study that aims to unravel the effects of zinc sulfate heptahydrate (ZnSO4·7H2O) and IAA on total phenolic content and rosmarinic acid accumulation in in vitro cultures of Ocimum basilicum L. For this purpose, ZnSO4, IAA, and ZnSO4 + IAA combinations were tested for direct shoot induction.
Induction of direct organogenesis and plant regeneration
One week after culture initiation, microshoot-like structures were clearly visible from the cut end of the hypocotyl explants cultured on MS medium containing various concentrations and combinations of ZnSO4 and IAA. During the following 2 wk, small microshoot-like structures developed on the cut surface of the hypocotyl segments. Well-developed shoots were observed within 4 wk of culture initiation. The absence of any callus formation indicated that the process of shoot development was direct, with the appearance of root formation within 4 wk (Fig. 1a ). The presence of both ZnSO4 and IAA in the medium improved shoot and root formation simultaneously (Fig. 1b, c ), and finally, whole plantlets were developed (Fig. 1d ). These plantlets gradually formed many shoots after an additional 1-mo culture on the same media without any subculturing (Fig. 1e ).
Direct shoot and plant regeneration from hypocotyl explants of Ocimum basilicum L. (a) Shoot and root induction on MS11 medium (12.90 mg L−1 ZnSO4 plus 1.0 mg L−1 IAA) after 6 wk. (b–d) Simultaneous development of shoots and roots, enabling production of whole plantlets (after 8 wk). (e) Plantlets gradually formed many shoots after further culture on the same medium without subculturing (after 11 wk). (f, g) Plantlets growing in the soil (after 16 wk). (h) Well-developed plant (flowering time; after 21 wk). Inset shows flowering.
For those treatments that produced both shoots and roots, there were no appreciable differences in morphology of the plantlets induced by different concentrations of IAA in combination with ZnSO4 (Table 2). All shoots developed into normal plantlets when transferred to the same medium. Finally, plantlets were transferred to a mixture of soil, manure, moss, and sand (1:2:2:1 [w/w/w/w]), where they continued growing well and acclimating under the greenhouse conditions (Fig. 1f–h ). Eventually, all the plantlets were established in the field, with 100% survival.
Effect of ZnSO 4 alone
Hypocotyl segments of O. basilicum excised from 1-mo-old seedlings were cultured on MS medium in the presence or absence of ZnSO4. Following a 1-mo-old culture period on medium MS0 containing zinc sulfate but no IAA, hypocotyl explants became necrotic and no morphogenic change was observed. Variations of ZnSO4 concentration alone produced no effect on morphogenesis of hypocotyl explants (Table 2). Thus, it can be inferred that zinc sulfate in the absence of IAA is ineffective on the in vitro cultures of O. basilicum.
Effect of IAA alone
The highest number of roots was obtained with an IAA concentration of 1.0 mg L−1 (using medium MS4, no ZnSO4), which produced a mean of 12.6 roots per hypocotyl explant and mean root length was 0.9 cm (Table 2). In comparison, MS2 (no ZnSO4) and MS3 (no ZnSO4 + IAA) produced longer roots (1.5 cm long) but mean number of roots was much less (Table 2).
Effect of ZnSO 4 plus IAA
MS media supplemented with ZnSO4 (net concentration 12.9 mg L−1) plus IAA (0.1, 0.5, and 1.0 mg L−1; in media MS9, MS10 and MS11, respectively) stimulated formation of shoots (20.7, 47.3, and 57.7%, respectively, for each IAA concentration) and roots (86.6, 71.0, and 100%, respectively, for each IAA concentration). However, when the net ZnSO4 concentration was increased to 17.2 mg L−1 and tested in combination with IAA (0.1, 0.5, and 1.0 mg L−1), only the 0.1 mg L−1 IAA treatment (MS13) induced shoots (38.5%) and roots (71.0%). The other two IAA concentrations (0.5 and 1.0 mg L−1) in combination with 17.2 mg L−1 ZnSO4, as well as all three IAA concentrations (0.1, 0.5, and 1.0 mg L−1) in combination with 8.6 mg L−1 ZnSO4 (the ZnSO4 concentration in the regular MS medium), induced only roots. It is noteworthy that 15 shoots per hypocotyl explant were induced by the best treatment (MS11), which contained 12.9 mg L−1 ZnSO4 plus 1.0 mg L−1 IAA (Table 2). The explants continued to simultaneously form shoots and roots on the same (MS11) medium in a Petri dish (Fig. 1a ). This effect was more pronounced in screw-capped bottles, which are larger and have more headspace volume (Fig. 1b, c ). For each monthly subculture, 15 shoots could be recovered; so up to the fourth subculture, 60 shoots could be collected from a single hypocotyl segment during a 4-mo period (Fig. 1e ). These well-developed plantlets were later transplanted ex vitro inside the greenhouse.
O. basilicum has been shown to induce good shoot regeneration on MS medium supplemented with 6-benzylaminopurine (BAP) plus auxins (indole-3-butyric acid [IBA] or IAA), as reported by various authors (Sahoo et al. 1997; Phippen and Simon 2000; Begum et al. 2002). In the present study, the use of ZnSO4 (12.9 mg L−1) plus IAA (1.0 mg L−1) was sufficient to produce the maximum number of shoots (15 shoots) from the hypocotyl explants. Ekmekci and Aasim (2014) reported that hypocotyl explants of O. basilicum produced the maximum number of shoots per explant (5.17) on MS medium containing 2 mg L−1 thidiazuron (TDZ). Similarly, TDZ (4 mg L−1) was also found to be the best cytokinin for maximum callus and shoot induction in basil (Phippen and Simon 2000). Nevertheless, the results presented in this article suggest that the presence of ZnSO4 plus auxin, without added cytokinin, was sufficient and efficient for shoot regeneration in O. basilicum. Based on this finding, it can be hypothesized that ZnSO4 is involved either directly or indirectly in regulating cytokinin and subsequent shoot regeneration in O. basilicum. The results here confirm that the presence of ZnSO4 plus IAA in the culture medium is an essential requirement for shoot induction and/or plant regeneration in O. basilicum.
Total phenolic content in O. basilicum
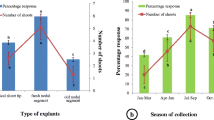
The total phenolic content in tissues varied widely: from 23 to 71 mg GAE g−1 DW (in vitro), from 54 to 127 mg GAE g−1 DW (ex vitro), and from 88 to 132 mg GAE g−1 DW (flowering time) (Fig. 2a ). Thus, the lowest total phenolic content (23 mg GAE g−1 DW) was found in the in vitro-grown stem, while old leaf tissue collected during flowering time contained the highest phenolic content (132 mg GAE g−1 DW). A similar observation was made in buckwheat (Fagopyrum esculentum), in which the identified phenolic content levels varied significantly among plant organs and vegetative growth stages (Sytar et al. 2014). Moreover, variations in the concentration of phenolic contents in plants during their phenological cycle were also reported by Çirak et al. (2007). These authors found that among different tissues samples, the fully opened flower had higher phenolic content than stem, leaf, and other reproductive parts. Data published in the literature and the experimental findings here confirm that it would be appropriate to use tissues at flowering time for screening of secondary metabolite levels in O. basilicum.
Total phenolic content (a) and total rosmarinic acid content (b) in stem, shoot tip, and old leaf of O. basilicum L. grown on MS medium with a final concentration of 12.90 mg L−1 ZnSO4 plus 1.0 mg L−1 IAA (medium MS11). Material was sampled in vitro (8 wk), ex vitro (16 wk), and at flowering time (21 wk).
Rosmarinic acid accumulation
The HPLC-measured rosmarinic acid content in O. basilicum ranged from 0.5 to 13.0 mg g−1 DW. Rosmarinic acid levels in old leaf tissue collected during flowering time were remarkably higher than other tissues (Fig. 2b ). These observations are in agreement with those of De-Eknamkul and Ellis (1985), who reported that the yield of both biomass and rosmarinic acid responded synchronously to changes in macronutrient and phytohormone concentrations in Anchusa officinalis. Moreover, different auxins produced quantitatively different responses. The auxin 2,4-dichlorophenoxyacetic acid (2,4-D) was found to delay the initiation and reduce the subsequent rate of rosmarinic acid synthesis in Anchusa officinalis (De-Eknamkul and Ellis 1985), whereas the same auxin had the opposite effect in Coleus blumei cultures (De-Eknamkul and Ellis 1988). Also, it was reported that α-naphthaleneacetic (NAA) yielded the highest rosmarinic acid accumulation in cultures of Anchusa officinalis (De-Eknamkul and Ellis 1988) and Solenostemon scutellarioides (Sahu et al. 2013). Thus, it may be concluded that the effect of auxins on rosmarinic acid accumulation is species dependent.
The present study shows that the accumulation of rosmarinic acid is fivefold higher in old leaf tissue at flowering time than in old leaf tissue from in vitro-grown samples (Fig. 2b ). The results reported here are in accordance with those of Kintzios et al. (2003), who reported that rosmarinic acid increased at the flowering stage in leaves of acclimatized plants, with levels reaching up to 20 mg g−1 DW. A similar observation was also reported in O. basilicum (Kiferle et al. 2011). According to Juliani et al. (2008), the concentration of rosmarinic acid in basil leaves increased during flowering relative to that at the vegetative stage. The present study revealed that the manipulation of the culture medium composition was a feasible option for improving plantlet production of O. basilicum, providing a source for isolation of rosmarinic acid.
Conclusions
The major problem with the use of Lamiaceae species for pharmaceutical purposes is the wide plant-to-plant variability due to genetic and biochemical heterogeneity. This article describes a protocol for rapid plant regeneration through direct shoot regeneration in O. basilicum, which was developed by testing variable concentrations of ZnSO4·7H2O and IAA on MS medium. This method can be used for the production of elite/quality plant materials at a much faster rate than the conventional indirect regeneration protocols of the species. Plant propagation via direct organogenesis is a valuable method for the clonal propagation and ex vitro conservation of O. basilicum genetic resources. The present investigation elucidates that cultures of O. basilicum can serve as a potential source of secondary metabolites under suitable conditions.
References
Archana R, Namasivayam A (2002) A comparative study of different crude extracts of Ocimum sanctum on noise stress. Phytother Res 16:579–580
Begum F, Amin M, Azad M (2002) In vitro rapid clonal propagation of Ocimum basilicum L. Plant Tissue Cult 12:27–35
Çirak C, Radušienė J, Ivanauskas L, Janulis V (2007) Variation of bioactive secondary metabolites in Hypericum origanifolium during its phenological cycle. Acta Physiol Plant 29:197–203
Dahleen LS, Bregitzer P (2002) An improved media system for high regeneration rates from barley immature embryo-derived callus cultures of commercial cultivars. Crop Sci 42:934–938
De-Eknamkul W, Ellis B (1985) Effects of auxins and cytokinins on growth and rosmarinic acid formation in cell suspension cultures of Anchusa officinalis. Plant Cell Rep 4:50–53
De-Eknamkul W, Ellis BE (1988) Rosmarinic acid: production in plant cell cultures. In: Bajaj YPS (ed) Medicinal and aromatic plants I. Biotechnology in agriculture and forestry, vol 4. Springer, Berlin, Heidelberg, pp 310–329
Dube S, Upadhyay P, Tripathi S (1989) Antifungal, physicochemical, and insect-repelling activity of the essential oil of Ocimum basilicum. Can J Bot 67:2085–2087
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1–42
Ekmekci H, Aasim M (2014) In vitro plant regeneration of Turkish sweet basil (Ocimum basilicum L.). J Anim Plant Sci 24:1758–1765
Gopi C, Ponmurugan P (2006) Somatic embryogenesis and plant regeneration from leaf callus of Ocimum basilicum L. J Biotechnol 126:260–264
Graham RD, Welch RM, Bouis HE (2001) Addressing micronutrient malnutrition through enhancing the nutritional quality of staple foods: principles, perspectives and knowledge gaps. Adv Agron 70:77–142
Hakkim FL, Shankar CG, Girija S (2007) Chemical composition and antioxidant property of holy basil (Ocimum sanctum L.) leaves, stems, and inflorescence and their in vitro callus cultures. J Agric Food Chem 55:9109–9117
Heywood VH (1993) Flowering plants of the world. BT Batsford Ltd, London, p 263
Hossain B, Hirata N, Nagatomo Y, Akashi R, Takaki H (1997) Internal zinc accumulation is correlated with increased growth in rice suspension culture. J Plant Growth Regul 16:239–243
Juliani HR, Koroch AR, Simon JE (2008) Basil: a new source of rosmarinic acid. In: Ho CT, Simon JE, Shahidi F, Shao Y (eds) Dietary supplements. American Chemical Society Symposium Series, vol 987. ACS, Washington, DC, pp 129–143
Kiferle C, Lucchesini M, Mensuali-Sodi A, Maggini R, Raffaelli A, Pardossi A (2011) Rosmarinic acid content in basil plants grown in vitro and in hydroponics. Cent Eur J Biol 6:946–957
Kintzios S, Makri O, Panagiotopoulos E, Scapeti M (2003) In vitro rosmarinic acid accumulation in sweet basil (Ocimum basilicum L.). Biotechnol Lett 25:405–408
Kirtikar KR, Basu BD (2003) Indian medicinal plants with illustrations, 2nd edn. Vol VIII. Oriental Enterprises, Uttaranchal, India, pp 2701–2705
Kobayashi A, Sakamoto A, Kubo K, Rybka Z, Kanno Y, Takatsuji H (1998) Seven zinc‐finger transcription factors are expressed sequentially during the development of anthers in petunia. Plant J 13:571–576
Lepp NW (1981) Effect of heavy metal pollution on plants. Vol. 1. Effects of trace metals on plant function. Applied Science, London and New Jersey
Marigo G, Boudet AM (1979) Effects of an increase in levels of phenolic compounds on the auxin content and growth of Lycopersicum esculentum. Z Pflanzenphysiol 92:33–38
Maxwell SE, Delaney HD (2004) Designing experiments and analyzing data: a model comparison perspective (2nd ed.). Erlbaum, Mahwah, NJ
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol 15:473–497
Oguchi K, Tanaka N, Komatsu S, Akao S (2004a) Characterization of NADPH‐dependent oxidoreductase induced by auxin in rice. Physiol Plant 121:124–131
Oguchi K, Tanaka N, Komatsu S, Akao S (2004b) Methylmalonate-semialdehyde dehydrogenase is induced in auxin-stimulated and zinc-stimulated root formation in rice. Plant Cell Rep 22:848–858
Pande D, Iqbal M, Srivastava P (2000) Effect of ZnSO4 and CuSO4 on regeneration and lepidine content in Lepidium sativum L. Biol Plant 43:253–256
Phippen WB, Simon JE (2000) Shoot regeneration of young leaf explants from basil (Ocimum basilicum L.). In Vitro Cell Dev Biol Plant 36:250–254
Rady MR, Nazif NM (2005) Rosmarinic acid content and RAPD analysis of in vitro regenerated basil (Ocimum americanum) plants. Fitoterapia 76:525–533
Ramesh SA, Choimes S, Schachtman DP (2004) Over-expression of an Arabidopsis zinc transporter in Hordeum vulgare increases short-term zinc uptake after zinc deprivation and seed zinc content. Plant J Mol Biol 54:373–385
Saeki Y, Yasukouchi A, Nagatomo Y, Takaki H (2000) Distinctive expression of a zinc-binding protein in rice callus grown in medium with high zinc concentration. Soil Sci Plant Nutr 46:209–216
Sahoo Y, Pattnaik S, Chand P (1997) In vitro clonal propagation of an aromatic medicinal herb Ocimum basilicum L. (sweet basil) by axillary shoot proliferation. In Vitro Cell Dev Biol Plant 33:293–296
Sahu R, Dewanjee S, Gangopadhyay M (2013) Bioproduction and optimization of rosmarinic acid production in Solenostemon scutellarioides through media manipulation and conservation of high yielding clone via encapsulation. Nat Prod Commun 8:1275–1278
Shier WT (1994) Metals as toxins in plants. Toxin Rev 13:205–216
Siddique I, Anis M (2007) Rapid micropropagation of Ocimum basilicum using shoot tip explants pre-cultured in thidiazuron supplemented liquid medium. Biol Plant 51:787–790
Sytar O, Borankulova A, Hemmerich I, Rauh C, Smetanska I (2014) Effect of chlorocholine chlorid on phenolic acids accumulation and polyphenols formation of buckwheat plants. Biol Res 47:19
Welch RM, Shuman L (1995) Micronutrient nutrition of plants. Crit Rev Plant Sci 14:49–82
Yanagisawa S (2004) Dof domain proteins: plant-specific transcription factors associated with diverse phenomena unique to plants. Plant Cell Physiol 45:386–391
Zhao Y (2010) Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol 61:49–64
Acknowledgments
The authors thank TUBITAK for partial financial support. We are grateful to the Department of Biology, Abant Izzet Baysal University, for providing lab facilities. We also thank N. Sahbaz for providing the O. basilicum seeds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Neftali Ochoa-Alejo
Rights and permissions
About this article
Cite this article
Verma, S.K., Sahin, G., Das, A.K. et al. In vitro plant regeneration of Ocimum basilicum L. is accelerated by zinc sulfate. In Vitro Cell.Dev.Biol.-Plant 52, 20–27 (2016). https://doi.org/10.1007/s11627-015-9739-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-015-9739-0