Abstract
Blyttia spiralis (Forssk.) D.V.Field & J.R.I.Wood, a threatened and medicinally important plant, belongs to the family Apocynaceae. Widespread habitat destruction has adversely affected natural populations of this species. This paper reports the first efficient plant propagation system for B. spiralis using nodal segments as explants. The greatest number of shoots was obtained on Murashige and Skoog (MS) medium containing 6-benzylaminopurine (BAP; 4.0 mg L−1) through bud breaking. Shoots were further multiplied through repetitive transfer of original explants and by subculturing of in vitro-raised shoots. Key physiochemical factors affecting various stages of micropropagation were systematically tested. The maximum number of shoots (35.45 ± 2.64), with a mean length of 6.69 ± 1.03 cm, was achieved on 0.8% agar-gelled shoot proliferation medium (SPM: MS + 0.5 mg L−1 each of BAP and kinetin [Kin] + 0.1 mg L−1 α-naphthaleneacetic acid [NAA]), with a combination of 50 mg L−1 ascorbic acid; 25 mg L−1 each of citric acid, l-arginine, and adenine sulfate; and 150 mg L−1 ammonium sulfate and 100 mg L−1 activated charcoal (AC) in culture bottles. In tests of rooting conditions, 61.5% of the shoots rooted in vitro on ¼-strength MS medium containing 2.0 mg L−1 indole-3-butyric acid (IBA) and 200 mg L−1 AC; 89.4% of the shoots rooted ex vitro in Soilrite®, if the shoot bases were pretreated with 250 mg L−1 IBA for 5 min. Plantlets rooted by both procedures were gradually acclimatized in a greenhouse. About 85% of ex vitro-rooted and 55% of in vitro-rooted plantlets were successfully established in soil. The described method can be employed for the large-scale propagation and conservation of Blyttia spiralis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Blyttia spiralis (Forssk.) D.V.Field & J.R.I.Wood [synonym = Pentatropis spiralis (Forssk.) Decne.]Footnote 1 (Field and Wood 1983), family Apocynaceae, is a medicinal perennial climber and is commonly known as Aakari Bel (Bhandari 1995) (in Hindi, Bel means “a climber”). B. spiralis is reported as one of the “life-supporting plants” of Little Rann of Kachchh, Gujarat (a unique ecosystem and natural heritage place in India), as this plant is an important source of food for both humans and livestock (Pawar and Patil 2008; Ishnava et al. 2011). This species is located in tropical parts of Africa, Australia, and Asia (Rasool et al. 1991a).
Traditionally, B. spiralis is used as a tonic in the treatment of gonorrhea and as a purgative (Rasool et al. 1991a; Ali 2013). Extracts from this species contain a number of biologically active compounds such as acyclic diterpene ester (cis-phytyl-1-palmitate), triterpene (cycloart-22-ene-3α, 25-diol), taraxasterol, ψ-taraxasterol, and squalene, which contribute to its alterative, astringent, cooling, and emetic properties (Rasool et al. 1991a, b). Recently, an important friedelane triterpenoid, pentatronol, has been reported from B. spiralis (Shan et al. 2013). In addition, bergenin (equivalent to phenylbutazone) is present in pods, which have antiinflammatory effects on carrageenan-induced rat paw edema (Indian Medicinal Plants 2009). Indigenous tribal communities use root powder of this plant to cure fever, indigestion, and dysentery (Joshi et al. 2013). Bark extracts are used as lotions to treat muscular troubles and sores and also as a constituent of toothpowders and gargles (Indian Medicinal Plants 2009). Also, the plant is known for its antimicrobial and antifungal activities (Khanum et al. 2013).
In nature, B. spiralis propagates through seeds. Owing to their edible nature, the young seeds are collected by local people, which reduce the supply of seeds available for propagation (Pawar and Patil 2008). Further, germination of seeds is reduced in the absence of suitable moisture/precipitation, and newly germinated seedlings are also sensitive to drought stresses and might not live to maturity (Khanum et al. 2013). In addition, established plants are used as fodder for goats. B. spiralis generally grows on the boundaries/hedges of agricultural fields (Bhandari 1995). Increased mechanization in agriculture and substitution of conventional boundaries (which are supposed to be natural conservatories/repositories of bio-resources/native plants) with artificial ones made of stone also adversely affect the natural populations of this plant species (Pawar and Patil 2008). Currently, this plant is categorized as a threatened (vulnerable) species in the Karachi region of Pakistan (Hussain et al. 2010). Furthermore, ecological niche modeling (ENM; an important conservational tool to determine the present and future distribution of a species) and maximum entropy (MAXENT) modeling for future scenarios forecasted a high risk of habitat loss for B. spiralis by 2050 in the southeastern Sindh region of Pakistan (Khanum et al. 2013).
Ex situ conservation strategies may be employed as an alternative to natural cultivation, especially for species facing high risk of habitat loss. Medicinal plants should also be protected by a “conservation through utilization” strategy (Sanchez et al. 2011); however, this requires large-scale plant production. Plant tissue culture provides a number of techniques for mass propagation and germplasm conservation of threatened species, especially those for which vegetative propagation methods are not available or feasible. Moreover, the rate of multiplication under in vitro conditions is often better than under traditional/vegetative conditions (Bhojwani and Dantu 2013). Until now, there have been no reports available on methods for either vegetative propagation or tissue culture of B. spiralis, so the experiments described here were conducted to develop a tissue culture protocol for mass propagation of this plant. In order to establish a sustainable regeneration protocol, various physical factors (type of explant, season of collection, repetitive transfer of the original explants, gelling agent, culture vessel, and rooting substrate) and chemical factors (plant growth regulators [PGRs], additives, activated charcoal [AC], and sucrose concentration) were evaluated during different stages of micropropagation. To improve the rooting efficiency of regenerated shoots, two rooting methods were compared.
Materials and Methods
Plant material selection and decontamination
Field surveys were conducted in the peripheral areas of Jodhpur (a district in northwestern India) during the months of July to September (Monsoon season) for selection of morphologically/phenotypically healthy plants. After selecting the mother/source plant, three types of explant (having at least one node) were used for culture establishment: apical shoot tip (2–3 × 0.1–0.2 cm), fresh nodal segment (4–5 × 0.3–0.4 cm), and old nodal segment (4–5 × 0.4–0.5 cm). To examine the effect of different seasons on culture establishment, explants were taken four times a year (January–March, April–June, July–September, and October–December) from the mother/source plant. The shoot segments were directly decontaminated using 0.5% (v/v) sodium hypochlorite solution (NaClO) for 15–16 min and thereafter washed thoroughly with autoclaved water five to six times under a laminar air hood.
Nutrient media and environmental conditions in growth chamber
The basal nutrient medium used for the present study was Murashige and Skoog (MS) (Murashige and Skoog 1962), supplemented with 3% (w/v) sucrose and 0.02% (w/v) inositol (HiMedia®, Mumbai, India) and gelled with 0.8% (w/v) agar–agar bacteriological grade (Qualigens Fine Chemicals, Mumbai, India). The pH of the medium was adjusted to 5.8 ± 0.02 using 1 N KOH or HCl before autoclaving at 1.1 kg cm−2, 121°C for 15 min. The cultures were incubated in a growth room maintained at a temperature of 26 ± 2°C, a light intensity of 40–50 μmol m−2 s−1photon flux density (PFD; provided by cool and white fluorescent tubes [Philips, Mumbai, India]) for 14–16-h photoperiod, and 55–60% relative humidity (RH).
Effect of plant growth regulators on culture establishment, shoot regeneration, and multiplication
For shoot bud induction, surface-sterilized explants were vertically placed on autoclaved MS medium (pH 5.8 ± 0.02) containing various (6-benzylaminopurine [BAP] or kinetin [Kin]) at concentrations of 1.0, 2.0. 3.0, 4.0, or 5.0 mg L−1 (all PGRs were from HiMedia® and added prior to autoclaving). MS basal medium with no cytokinins served as the control. The cultures showing shoot induction were further amplified using two methods: (i) reculturing of the original explants (after harvesting the first batch of sprouts) onto fresh MS medium containing BAP (0.5, 1.0, 1.5, or 2.0 mg L −1) alone or in combination with α-naphthaleneacetic acid (NAA; 0.05, 0.1, 0.25, or 0.5 mg L−1), for four passages at an interval of 4 wk each; and (ii) subculturing of in vitro-raised sprouts (after a cluster of four to five shoots had formed) on MS medium supplemented with BAP, Kin, or N6-[2-isopentenyl] adenine (2-iP; 0.5, 1.0, or 2.0 mg L−1) alone or in combination with NAA (0.1 mg L−1). The cultures produced by this second method were maintained for 3 yr by regular subculturing at intervals of 5–6 wk, without any loss of growth and vigor.
Evaluation of physiochemical factors affecting shoot multiplication
Effect of additives. After optimizing the shoot proliferation medium (SPM) in terms of PGRs, a combination of additives (50 mg L−1 ascorbic acid; 25 mg L−1 each of citric acid, adenine sulfate, and l-arginine; and 150 mg L−1 ammonium sulfate [(NH4)2SO4]; all additives were from HiMedia® and added prior to autoclaving) was incorporated into the SPM, as suggested by Rathore et al. (2013), for evaluating their effect on growth of shoot cultures.
Effect of gelling agent. After optimizing PGRs and additives, two gelling agents (agar–agar bacteriological grade (0.8%, w/v; Qualigens Fine Chemicals), and gellan gum (0.14%, w/v; Sigma-Aldrich®, St. Louis, MO) and liquid medium were evaluated to compare their effects on the shoot multiplication rate.
Effect of activated charcoal. Once PGRs, additives, and gelling agent were optimized for shoot multiplication, the effect of AC (100 mg L−1) was also evaluated in the SPM, as suggested by Rathore et al. (2013).
Effect of culture vessel type. Screw-cap culture bottles (420 mL; 70 mm diameter × 130 mm height; Siddhivinayak Glass Concepts, Firozabad, India), Borosil® flasks (250 mL; Borosil®, Mumbai, India), Magenta™ boxes (Kasablanka, Mumbai, India), and Phyta Jars (HiMedia®) were evaluated to compare and select the best culture vessel for shoot multiplication on SPM.
Rooting and acclimatization
In vitro rooting and microtuberization. For rooting under aseptic conditions, the multiplied shoots were excised individually from shoot clumps and placed on different strengths of MS media (MS full, MS ½, or MS ¼) supplemented with various concentrations (0.5, 1.0, 2.0, or 3.0 mg L−1) of root-inducing PGRs (naphthoxyacetic acid [NOA] or indole-3-butyric acid [IBA]) and AC (200 mg L−1). The rooting media were gelled using 0.8% (w/v) agar–agar bacteriological grade (Qualigens Fine Chemicals) and contained sucrose (30 g L−1; PGRs were added preautoclaving). After optimizing the medium strength and PGR for rooting, the sucrose concentration was increased to 40, 50, 60, or 70 g L−1 in order to study its effect on in vitro tuberization of B. spiralis.
Ex vitro rooting and effect of rooting substrate. For rooting under ex vitro conditions, in vitro-raised shoots were harvested individually and their bases (the bottom 3–5 mm of the shoot) were dipped in NOA or IBA at concentrations of 50, 100, 250, or 500 mg L−1 alone and in combination, for 1, 3, 5, or 7 min. The auxin-treated shoots were transplanted to bottles containing either sterilized Soilrite® [a mix of expanded perlite (horticulture grade), Irish peat moss, and exfoliated vermiculite (1:1:1 [v/v/v]), Keltech Energies Limited, Bengaluru, India] or sterilized soil. Sterilization for both substrates was done by autoclaving at 1.1 kg cm−2 pressure and 121°C temperature for 45 min. Both substrates were irrigated with MS ¼ liquid solution.
Hardening and soil transfer
The glass bottles (420 mL; 70 mm diameter × 130 mm height; Siddhivinayak Glass Concepts) containing in vitro-rooted plantlets and shoots transplanted for ex vitro rooting were covered with polycarbonate caps and placed in an area of a greenhouse having high RH (80–90%) and low temperature (28 ± 2°C) conditions. For the next 2–3 wk, the caps were gradually released to acclimatize the plantlets and removed at the end of that period. The bottles were kept in the greenhouse (with open caps) for the next 2–3 wk but were moved to the axle fan section having low RH (45–55%) and high temperature (36 ± 2°C) for gradual hardening. Successfully hardened plantlets were transplanted to black polybags (16 cm height × 10.5 cm width; Lodha Plastic Industries, Jodhpur, India) containing field soil, kept in a greenhouse for the next 2–3 wk, and finally transferred to the nursery.
Experimental layout and statistical analysis
All the experiments were set up in a randomized block design (RBD) with a minimum of 20 replicates per treatment, and experiments were performed three times. Observations such as shoot number and shoot length and root number and root length were scored over a period of 2–5 wk depending on the experiment. The data were statistically analyzed using analysis of variance (ANOVA), and differences among the mean values were compared with Duncan’s multiple range test (P < 0.05) using SPSS ver. 17 (SPSS Inc., Chicago, IL). All the results were represented as mean ± SD of three experiments.
Results and Discussion
Effect of explant type and collection season on culture establishment
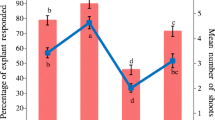
Out of the three types of explant evaluated for culture initiation, freshly sprouted nodal segments were found the best in terms of percentage response and shoot number. The other two types of explant, apical shoot tip and old nodal segment, responded comparatively poorly (Fig. 1a ) and therefore were not used for further studies. Similar findings regarding the effects of explant type on culture establishment and regeneration were also reported in Lawsonia inermis (Ram and Shekhawat 2011), Leptadenia reticulata (Rathore et al. 2013), and Agastache foeniculum (Moharami et al. 2014). In addition, the responses of explants in culture were also significantly affected by the season of collection, as explants harvested during the rainy season in Rajasthan, India (i.e., July to September), gave the best culture initiation responses (Fig. 1b ). According to Phulwaria et al. (2011), some bud/growth-arresting factors are diluted or degraded during the rainy season, and this in turn results in enhanced bud breaking in culture.
(a) Effect of different explant types on percentage response and number of shoots. (b) Effect of different collection seasons on culture establishment in terms of percentage response and number of shoots. Error bars indicate SD. Mean values sharing the same letter do not differ significantly (P < 0.05) according to Duncan’s multiple range test.
Effect of plant growth regulators (PGRs) on culture establishment, shoot regeneration, and multiplication
Fresh nodal explants (collected from the mother plant; Fig. 2a ) cultured on MS medium containing various concentrations of cytokinin (BAP or Kin) responded within 6–8 d of incubation and showed bud breaking/sprouting (Fig. 2b ). Explants cultured on MS medium devoid of any cytokinin failed to exhibit bud breaking. Among the treatments containing cytokinins, 4.0 mg L−1 BAP produced the greatest number of shoots (5.10 ± 0.87) and shoot mean length (2.95 ± 0.53 cm), after 4 wk (Table 1). BAP is one of the most important commercial cytokinins. It is used for bud breaking and reinvigoration in tissue cultures (Zhang et al. 2010), and the efficacy of BAP over other cytokinins has been well documented in a number of plant species (Rathore et al. 2013; Patel et al. 2014b).
Shoot bud induction, multiple shoot production, and rooting protocols for Blyttia spiralis. (a) B. spiralis growing in the field. (b) Bud breaking from nodal segments on MS medium containing BAP (4.0 mg L−1), after 2 wk. (c) Reculture of the original explants on MS medium containing BAP (1.0 mg L−1) and NAA (0.1 mg L−1). (d) Shoot clump grown on 0.8% (w/v) agar-gelled SPM + additives + AC (100 mg L−1), in screw-cap culture bottle, after 5 wk. (e) In vitro-rooted shoots on MS ¼ medium containing IBA (2.0 mg L−1) and AC (200 mg L−1). (f) Ex vitro-rooted shoots in Soilrite®, pretreated with IBA (250 mg L−1) for 5 min. (g) Acclimatization of rooted plantlets under greenhouse conditions. (h) Successfully hardened plants of B. spiralis.
In the first approach to shoot multiplication, the original explants were recultured on different concentrations and combinations of BAP and NAA. Of these, MS medium supplemented with BAP (1.0 mg L−1) and NAA (0.1 mg L−1) produced the maximum number of shoots (8.1 ± 0.91 per explant) after the second passage of repeated transfer (Fig. 2c ). During the third and fourth passages, however, fewer shoots were formed, and the number decreased with each passage (Fig. 3a ). This approach for shoot multiplication has also been employed in a few other plant species such as Leptadenia reticulata (Rathore et al. 2013) and Caralluma edulis (Patel et al. 2014b).
(a) Effect of reculturing passages of the original explants on shoot number and shoot length. Medium: MS + BAP (1.0 mg L−1) and NAA (0.1 mg L−1). (b) Effect of different types of culture vessels on shoot multiplication of B. spiralis. Medium: 0.8% (w/v) agar-gelled SPM + additives + AC (100 mg L−1). Error bars indicate SD. Mean values sharing the same letter do not differ significantly (P < 0.05) according to Duncan’s multiple range test.
In another approach to shoot multiplication, the first batch of shoot sprouts (after a cluster of four to five shoots had formed) was transferred to MS medium supplemented with various types and concentrations of PGRs. Among these, MS medium having a combination of 0.5 mg L−1 each of BAP and Kin, and 0.1 mg L−1 NAA (SPM) was found the best for shoot proliferation and produced a maximum number of shoots (≈20–22) after 5 wk (data not shown). This result shows that an appropriate combination of cytokinin(s) and auxin(s) is a prerequisite for better shoot proliferation and growth (Moharami et al. 2014; Phulwaria et al. 2014; Masondo et al. 2015). According to Su and Zhang (2014), plant regeneration is an outcome of a complex network of interconnecting hormonal signaling pathways rather than being regulated by either auxins or cytokinins alone. The cause for this interaction may be that cytokinins promote stem cell proliferation in the central region of the meristem, whereas auxin stimulates organ primordium initiation from the surrounding region, and that this cross-talk mechanism in turn regulates developmental processes such as shoot meristem development and shoot branching (Shimizu-Sato et al. 2009; Su et al. 2011). The cultures produced by this second approach were maintained for 3 yr by regular subculturing at intervals of 5–6 wk, without any loss of growth and vigor.
Evaluation of physiochemical factors affecting shoot multiplication
Effect of additives. Incorporation of additives (a combination of 50 mg L−1 ascorbic acid; 25 mg L−1 each of citric acid, l-arginine, and adenine sulfate; and 150 mg L−1 ammonium sulfate) to the PGR-optimized SPM significantly (P < 0.05) improved the multiplication rate and produced green and healthy shoots (Table 2). Ascorbic acid and citric acid act as a source of antioxidants, whereas l-arginine and adenine sulfate are known for their nitrogen-reducing and cytokinin-like properties, respectively. Recently, Sivanandhan et al. (2015) also reported that inclusion of adenine sulfate (15 mg L−1) yielded a higher frequency of multiple shoots in Withania somnifera. Further, ammonium sulfate is reported to promote nitrate absorption and buffer the nutrient medium (Ivanova and Van Staden 2008). The beneficial role of these additives on in vitro growth has been acknowledged in several reports (Rathore et al. 2013; Patel et al. 2014b).
Effect of gelling agent. Of the two gelling agents tested, 0.8% (w/v) agar-solidified medium was found better than 0.14% (w/v) gellan-gum-gelled medium in terms of both shoot multiplication rate and shoot quality. In the present study, liquid medium was also found less effective than agar-solidified medium (Table 2). Similar to these results, agar-gelled medium was also found better than gellan-gum-gelled medium in a majority of Indian potato cultivars (Sharma et al. 2011). Due to its stability, high gel clarity, and resistance to metabolism during culture, agar is the most commonly used gelling agent in plant tissue culture (Jain and Babbar 2002), although gellan gum has been found better than agar in some studies (Masondo et al. 2015). According to Ziv (1991), the gelling agent is not an inert component of the medium but may influence the nutrient and water availabilities to the cultured tissues and hence influence shoot quality and multiplication rate.
Effect of activated charcoal. In comparison to AC-lacking medium, AC-containing medium produced sturdier shoots with well-developed foliage and a slightly (but not significantly) higher multiplication rate (Table 2). These effects of AC during successive subculturing may be attributable to its irreversible adsorption of inhibitory compounds such as phenolic exudates, oxidized phenolics, and toxic metabolites, all of which are presumed to be deleterious for the health of cultures (Rathore et al. 2013). In addition, AC is involved in a number of stimulatory activities such as releasing its own natural substances as well as releasing the adsorbed nutrients and PGRs, which eventually become available to the plant tissues in cultures (Thomas 2008).
Effect of culture vessel type. Among the four culture vessels tested, screw-cap culture bottles were the best for in vitro shoot multiplication of B. spiralis (Fig. 3b ). Although the shoot multiplication rate was fair in Magenta™ boxes, it was significantly lower (P < 0.05) than that in culture bottles. In Phyta Jars, considerable hyperhydration was recorded with comparatively fewer shoots than either culture bottles or Magenta™ boxes, and in Borosil® flasks (250 mL), the lowest multiplication rate was observed. The headspace of tissue culture vessels affects the gaseous composition (e.g., ethylene, methane, and carbon dioxide), light penetration, RH, ventilation rates, photosynthesis, dark respiration, and leaf pigmentation. These factors individually and together affect shoot elongation and proliferation, and probably the hyperhydric degradation processes, in culture (Islam et al. 2005; Kacar et al. 2010).
The maximum number of shoots (35.45 ± 2.64) and maximum mean shoot length (6.69 ± 1.03 cm) were observed when cultured on 0.8% (w/v) agar-gelled SPM (MS + 0.5 mg L−1 each of BAP and Kin + 0.1 mg L−1 NAA) containing additives and AC (100 mg L−1) in screw-cap culture bottles, after 5 wk (Fig. 2d and Table 3).
Rooting and acclimatization
In vitro rooting. Among the medium strengths tested, MS at ¼ strength was found the best for in vitro rooting in terms of both root number and root length (Fig. 4a ). MS ¼ medium containing AC (200 mg L−1) and IBA (2.0 mg L−1) formed the greatest number of roots per shoot (4.05 ± 0.81) with the greatest mean root length (2.55 ± 0.60 cm; Table 4). On a higher concentration of IBA (>3.0 mg L−1), excessive callusing at the shoot base and leaf yellowing were observed. However, a small mass of callus was also observed at the base of most shoots up to a concentration of 2.0 mg L−1 IBA (Fig. 2e ). The root-inducing effects of IBA may be credited to its preferred uptake and transport, greater stability than other auxins, and effects on gene activation (Ludwig-Muller 2000), and its effects on root induction are in accordance with many previous reports in different plant species (Rathore et al. 2013; Moharami et al. 2014; Patel et al. 2014a, b).
(a) Effect of strength of MS salts on root number and root length of B. spiralis. (b) Effect of different sucrose concentrations on in vitro tuber formation of B. spiralis. Medium in (a) and (b): IBA 2.0 mg L−1 + AC (200 mg L−1). Error bars indicate SD. Mean values sharing the same letter do not differ significantly (P < 0.05) according to Duncan’s multiple range test.
Effect of sucrose concentrations on in vitro tuber formation. When the concentration of sucrose in optimized rooting medium (MS ¼ + IBA–2.0 mg L−1 + AC–200 mg L−1) was increased, tuber formation was observed. Of the concentrations tested, 60 g L−1 sucrose was found the best in terms of percentage tuberization (Fig. 4b ). Similar to these results, formation of tubers/storage organs under in vitro conditions on higher sucrose concentrations has been reported in a number of tuberous plant species (Kamarainen-Karppinen et al. 2010; Fan et al. 2011). In the present study, the absence of AC in the rooting medium negatively influenced tuber formation irrespective of the sucrose concentration.
Ex vitro rooting. In vitro-raised shoots could be successfully rooted under ex vitro conditions when treated with various concentrations of auxins for different time durations. In comparison to NOA, IBA was found better in terms of root development, and a comparatively greater number of shoots rooted (89.4% under the best treatment conditions). Of the treatments tested, shoot bases treated with IBA (250 mg L−1) for 5 min formed the greatest number of roots per shoot (8.11 ± 1.37), with a mean length of 7.25 ± 1.03 cm, after 5 wk (Fig. 2f and Table 5). Either short or long treatment duration than 5 min resulted in reduced root production. The role of IBA in ex vitro rooting is documented in various plant species (Lodha et al. 2014; Patel et al. 2014b). In terms of tuber formation, the ex vitro rooting technique was found unsuitable, as no tuber formation was achieved by this method. This result again confirms the role of increased sucrose concentration(s) in tuber/storage organ formation in culture.
Effect of rooting substrates on ex vitro rooting. Of the two rooting substrates tested, Soilrite® was better: About 90% of shoots rooted in Soilrite®, in comparison to 55% in soil (data not shown). Owing to its porosity, Soilrite® is easy for roots to penetrate and holds more water than soil, which may collectively promote the growth of young roots of in vitro-raised shoots. Similar results have also been observed in Tecomella undulata (Varshney and Anis 2012), where Soilrite® appeared to be a better rooting substrate than vermiculite or garden soil. Rooting under ex vitro conditions is an attractive alternative to in vitro rooting, because ex vitro rooting can lower the cost of a micropropagation protocol up to 30–75% (Lodha et al. 2014; Patel et al. 2014b). It also reduces the time required for further hardening as plantlets developed through ex vitro rooting do not need any added acclimatization prior to field transfer (Chandra et al. 2010). Additionally, ex vitro-rooted plantlets have more vigor to tolerate environmental pressures during the hardening procedure (Phulwaria et al. 2011). As noted above, the one case in which ex vitro rooting is less satisfactory is when tuber formation is desired.
Hardening and soil transfer
The different conditions of temperature and relative humidity (RH) in different parts of the greenhouse coupled with gradual opening of the polycarbonate caps (of bottles; Fig. 2g ) assisted in acclimatization of plantlets to ex vitro conditions. About 85% of ex vitro-rooted and 55% of in vitro-rooted plantlets were successfully hardened and survived under field conditions (data not shown). In the present study, the percentage of hardened plantlets developed through ex vitro rooting was significantly higher (P < 0.05) than that developed from in vitro rooting. Similar findings have been observed in Lawsonia inermis (Ram and Shekhawat 2011) and C. edulis (Patel et al. 2014b). The reason for this may be that in ex vitro rooting, callus formation does not occur at the root–shoot junction; thus, it is more similar to a natural root system. Although in vitro-developed roots are thick, they lack root hairs and are easily broken (another hurdle during transplantation and handling), which eventually lowers the transplant survival rate in the field (Yan et al. 2010). The successfully hardened plants were transplanted into black polybags (Fig. 2h ) and kept in the greenhouse until transferred to the nursery.
Conclusions
This is the first report of an efficient in vitro propagation system for B. spiralis, a vulnerable, medicinally important, and life-supporting tuberous species. The present study demonstrates that a micropropagation protocol is influenced not only by PGRs but also by various physical factors (type of explant, season of collection, repetitive transfer of the original explants, gelling agent, culture vessel, and rooting substrate) and chemical factors (a combination of ascorbic acid, citric acid, l-arginine, and adenine sulfate; ammonium sulfate, AC, and sucrose concentration). Furthermore, the in vitro rooting technique was suitable for micro-tuberization, whereas ex vitro rooting was better in terms of increasing the transplant survival rate. The protocol described here will enable the ex situ conservation of B. spiralis, which may promote the “conservation through utilization” strategy. Conservation of this species ex situ is important not only because of its medicinal value but also as a means of strengthening arid and semiarid ecosystems.
Notes
* Refer to http://www.theplantlist.org/tpl1.1/record/tro-2602043 for correct naming convention of this species.
References
Ali N (2013) Brine shrimp cytotoxicity of crude methanol extract and antispasmodic activity of α-amyrin acetate from Tylophora hirsuta Wall. BMC Complement Altern Med 13:135–142
Bhandari MM (1995) Flora of the Indian Desert. In: MPS reports, Jodhpur, India, p 201
Bhojwani SS, Dantu PK (2013) Plant tissue culture: an introductory text. Springer, New York, pp 245–274
Chandra S, Bandopadhyay R, Kumar V, Chandra R (2010) Acclimatization of tissue cultured plantlets: from laboratory to land. Biotechnol Lett 32:1199–1205
Fan M, Liu Z, Zhou L, Lin T, Liu Y, Luo L (2011) Effects of plant growth regulators and saccharide on in vitro plant and tuberous root regeneration of cassava (Manihot esculenta Crantz). J Plant Growth Regul 30:11–19
Field DV, Wood JRI (1983) A new name for Pentatropis spiralis auctt., and the resurrection of the genus Blyttia (Asclepiadaceae). Kew Bull 38:215–220
Hussain SS, Ahmed M, Siddiqui MF, Wahab M (2010) Threatened and endangered native plants of Karachi. Int J Biol Biotechnol 7:259–266
Indian Medicinal Plants (2009) http://indian-medicinal-plants.blogspot.in/2009/06/p-paederia-foetida-linn.html. Cited 3 Jan 2015
Ishnava K, Ramarao V, Mohan JSS, Kothari IL (2011) Ecologically important and life supporting plants of little Rann of Kachchh, Gujarat. J Ecol Nat Environ 3:33–38
Islam MT, Dembele DP, Keller ERJ (2005) Influence of explant, temperature and different culture vessels on in vitro culture for germplasm maintenance of four mint accessions. Plant Cell Tissue Organ Cult 81:123–130
Ivanova M, Van Staden J (2008) Effect of ammonium ions and cytokinins on hyperhydricity and multiplication rate of in vitro regenerated shoot of Aloe polyphylla. Plant Cell Tissue Organ Cult 92:227–231
Jain N, Babbar SH (2002) Gum katira—a cheap gelling agent for plant tissue culture media. Plant Cell Tissue Organ Cult 71:223–229
Joshi EB, Jain BK, Joshi PN, Soni HB (2013) Prevalence of traditional medications through native floral elements among tribal communities of Kachchh arid ecosystem, Gujarat, India. Int J Environ 2:184–201
Kacar YA, Bicen B, Varol I, Mendi YY, Serce S, Cetiner S (2010) Gelling agents and culture vessels affect in vitro multiplication of banana plantlets. Genet Mol Res 9:416–424
Kamarainen-Karppinen T, Virtanen E, Rokka VM, Pirttila AM (2010) Novel bioreactor technology for mass propagation of potato microtubers. Plant Cell Tissue Organ Cult 101:245–249
Khanum R, Mumtaz AS, Kumar S (2013) Predicting impacts of climate change on medicinal asclepiads of Pakistan using Maxent modeling. Acta Oecol 49:23–31
Lodha D, Patel AK, Rai MK, Shekhawat NS (2014) In vitro plantlet regeneration and assessment of alkaloid contents from callus cultures of Ephedra foliata (Unth phog), a source of anti-asthmatic drugs. Acta Physiol Plant 36:3071–3079
Ludwig-Muller J (2000) Indole-3-butyric acid in plant growth and development. Plant Growth Regul 32:219–230
Masondo NA, Aremu AO, Finnie JF, Van Staden J (2015) Growth and phytochemical levels in micropropagated Eucomis autumnalis subspecies autumnalis using different gelling agents, explant source, and plant growth regulators. In Vitro Cell Dev Biol Plant 51:102–110
Moharami L, Hosseini B, Ravandi EG, Jafari M (2014) Effects of plant growth regulators and explant types on in vitro direct plant regeneration of Agastache foeniculum, an important medicinal plant. In Vitro Cell Dev Biol Plant 50:707–711
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Patel AK, Agarwal T, Phulwaria M, Kataria V, Shekhawat NS (2014a) An efficient in vitro plant regeneration system from leaf of mature plant of Leptadenia reticulata (Jeewanti): a life giving endangered woody climber. Ind Crop Prod 52:499–505
Patel AK, Phulwaria M, Rai MK, Gupta AK, Shekhawat S, Shekhawat NS (2014b) In vitro propagation and ex vitro rooting of Caralluma edulis (Edgew.) Benth. & Hook. f.: an endemic and endangered edible plant species of the Thar Desert. Sci Hortic 165:175–180
Pawar S, Patil DA (2008) Ethnobotany of Jalgaon District, Maharashtra. Daya Books, Delhi, p 239
Phulwaria M, Patel AK, Rathore JS, Ram K, Shekhawat NS (2014) An improved micropropagation and assessment of genetic stability of micropropagated Salvadora oleoides using RAPD and ISSR markers. Acta Physiol Plant 36:1115–1122
Phulwaria M, Ram K, Gahlot P, Shekhawat NS (2011) Micropropagation of Salvadora persica—a tree of arid horticulture and forestry. New For 42:317–327
Ram K, Shekhawat NS (2011) Micropropagation of commercially cultivated Henna (Lawsonia inermis) using nodal explants. Physiol Mol Biol Plant 17:281–289
Rasool N, Ahmad VU, Malik A (1991a) Terpenoids from Pentatropis spiralis. Phytochemistry 30:1331–1332
Rasool N, Khan AQ, Ahmad VU, Malik A (1991b) A new cycloartane-type triterpene from Pentatropis spiralis. J Nat Prod 54:889–892
Rathore MS, Rathore MS, Shekhawat NS (2013) Ex vivo implications of phytohormones on various in vitro responses in Leptadenia reticulata (Retz.) Wight. & Arn.—an endangered plant. Environ Exp Bot 86:86–93
Sanchez AC, Osborne PE, Haq N (2011) Climate change and the African baobab (Adansonia digitata L.): the need for better conservation strategies. Afr J Ecol 49:234–245
Shan WG, Zhang LW, Xiang JG, Zhan ZJ (2013) Natural friedelanes. Chem Biodivers 10:1392–1434
Sharma S, Venkatasalam EP, Patial R, Latawa J, Singh S (2011) Influence of gelling agents and nodes on the growth of potato microplant. Potato J 38:41–46
Shimizu-Sato S, Tanaka M, Mori H (2009) Auxin-cytokinin interactions in the control of shoot branching. Plant Mol Biol 69:429–435
Sivanandhan G, Selvaraj N, Ganapathi A, Manickavasagam M (2015) Effect of nitrogen and carbon sources on in vitro shoot multiplication, root induction and withanolides content in Withania somnifera (L.) Dunal. Acta Physiol Plant 37:12. doi:10.1007/s11738-014-1758-7
Su YH, Liu YB, Zhang XS (2011) Auxin–cytokinin interaction regulates meristem development. Mol Plant 4:616–625
Su YH, Zhang XS (2014) The hormonal control of regeneration in plants. Curr Top Dev Biol 108:35–69
Thomas TD (2008) The role of activated charcoal in plant tissue culture. Biotechnol Adv 26:618–631
Varshney A, Anis M (2012) Improvement of shoot morphogenesis in vitro and assessment of changes of the activity of antioxidant enzymes during acclimation of micropropagated plants of Desert Teak. Acta Physiol Plant 34:859–867
Yan H, Liang C, Yang L, Li Y (2010) In vitro and ex vitro rooting of Siratia grosvenorii, a traditional medicinal plant. Acta Physiol Plant 32:115–120
Zhang H, Horgan KJ, Reynolds PH, Jameson PE (2010) 6-Benzyladenine metabolism during reinvigoration of mature Pinus radiata buds in vitro. Tree Physiol 30:514–526
Ziv M (1991) Vitrification: morphological and physiological disorders of in vitro plants. In: Debergh PC, Zimmerman RH (eds) Micropropagation: technology and application. Kluwer, Dordrecht, pp 45–69
Acknowledgments
AKP and KR are thankful to the University Grant Commission (UGC), New Delhi, for providing Special Assistance Program (SAP) in the form of Centre of Advanced Study (CAS) to the Department of Botany, Jai Narain Vyas University, Jodhpur. DL gratefully acknowledges the financial support from the Council of Scientific and Industrial Research (CSIR), New Delhi, in the form of Junior and Senior Research Fellowship (JRF-SRF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Jorge Canhoto
Rights and permissions
About this article
Cite this article
Patel, A.K., Lodha, D., Ram, K. et al. Evaluation of physiochemical factors affecting high-frequency plant regeneration of Blyttia spiralis (synonym: Pentatropis spiralis), a threatened climber of medicinal value. In Vitro Cell.Dev.Biol.-Plant 52, 10–19 (2016). https://doi.org/10.1007/s11627-015-9738-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-015-9738-1