Abstract
An efficient and simple procedure was systematically developed for inducing direct somatic embryogenesis and plantlet regeneration from leaf sheath explants of Curcuma amada Roxb. A two-step culture system was used to induce somatic embryogenesis. The optimized procedure resulted in direct somatic embryogenesis from 93.3% explants after 3-wk culture. Leaf sheath explants were incubated for 2 wk on medium containing 2.24 μM 2,4-dichlorophenoxyacetic acid and 1.11 μM 6-benzyladenine to initiate direct somatic embryogenesis. Thereafter, these explants were transferred to a medium containing 9.10 μM thidiazuron and 1.33 μM α-naphthaleneacetic acid. Elongated somatic embryos obtained from these cultures germinated readily, and the optimal frequency of plantlet development (86.7%) was achieved when embryos were cultured in darkness on 1/2 strength Murashige and Skoog medium containing 1.44 μM gibberellic acid. Histological and scanning electron microscopic studies showed that the initial cell divisions that led to embryo formation occurred in epidermal and subepidermal cells, followed by the development of globular and elongated structures that appeared to be somatic embryos. The presence of a clear protoderm in the globular structures and procambial strands in the elongated structures confirmed that these structures were true somatic embryos. Plantlets derived from somatic embryos were acclimatized successfully to ex vitro conditions at a survival rate of 87.43% and developed with normal phenotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Curcuma amada Roxb. is an aromatic spice crop of the family Zingiberaceae, a group of important tropical monocots which includes turmeric, ginger, and cardamom. It is commonly known as mango ginger and possesses a raw mango-like flavor blended with that of ginger (Banerjee et al. 2012). The aromatic smell raised from C. amada is mainly attributed to presence of car-3-ene and cis-ocimene compounds (Gholap and Bandyopadhyay 1984; Rao et al. 1989; Choudhury et al. 1996). Its rhizomes contain fibers, starch, and more than 68 volatile, aromatic essential oils which are used in food, beverages, cosmetics, and medicines (Srivastava et al. 2001; Mustafa et al. 2005; Policegoudra and Aradhya 2008).
C. amada possesses several medicinal properties, such as stomachic, carminative, aphrodisiac, antipyretic, and laxative properties, and is a potential source of compounds with cholesterol-lowering activities (Kirtikar and Basu 1984; Warrier et al. 1994; Srinivasan et al. 2008). The major bioactive compounds of C. amada include curcuminoids, curcumin, demethoxy curcumin, and bis-demethoxy curcumin. These compounds have been used for their antioxidant, anti-inflammatory, antidepresent, and platelet-aggregation inhibition activities (Policegoudra et al. 2011). It also contain a labdane-type diterpenoid (labda-8(17), 12-diene-15 and 16-dial), which exhibit activity against tuberculosis (Singh et al. 2010).
C. amada is mainly propagated by division of rhizomes which are slow to multiply. Genetic improvement by conventional breeding is also difficult in C. amada because of a lack of sexual reproduction (Balachandran et al. 1990; Prakash et al. 2004). Furthermore, genetic engineering and molecular studies of its resistance to rhizome rot (caused by Pythium sp.) and bacterial wilt (caused by Ralstonia solanacearum) requires a standardized, simple morphogenic protocol (Prasath et al. 2011). Therefore, a reliable protocol for large scale propagation of this rhizomatous spice is necessary. Somatic embryogenesis represents a promising tool for mass propagation as well as for genetic transformation (Nhut et al. 2000; Manrique-Trujillo et al. 2013). Somatic embryos are preferred as a tissue for production of alginate-encapsulated synthetic seeds (Ganapathi et al. 2001; Remakanthan et al. 2013). Tissue culture studies in C. amada have shown only adventitious plantlet formation from rhizome and leaf sheath explants (Prakash et al. 2004; Das et al. 2010; Banerjee et al. 2012), and indirect somatic embryogenesis via callus induction (Soundar Raju et al. 2013). In this study, we report direct somatic embryogenesis and plant regeneration from C. amada leaf sheath explants, and a method that can be used for large scale production of elite C. amada in less time than with previous procedures.
Material and Methods
Plant material, preparation of explants, and culture condition
C. amada plants, procured from Malappuram, Kerala, were established in vitro and used as a source of explants. The culture procedures and media were similar to those described by Soundar Raju et al. (2013). Leaf sheath explants were obtained from 3-mo-old plants. The innermost, tender leaf tips were avoided and only peripheral leaf sheath segments were considered. Leaf sheath segments (1.5 cm long) were inoculated on medium with the abaxial surface facing the medium.
The pH of media used for all experiments was adjusted to 5.7 ± 0.1 and autoclaved at 121°C and 104 kPa for 15 min. All experiments were performed with solid media gelled with 0.8% agar powder (Himedia®, Mumbai, India). Cultures were maintained at 25 ± 2°C, 16 h photoperiod (except as noted below) under 40 μmol m−2 s−1 light intensity provided by white fluorescent tubes and a relative humidity at 55–65%.
Induction of somatic embryogenesis
All media used for somatic embryo induction included the Murashige and Skoog (MS) (Murashige and Skoog 1962) mineral formulation supplemented with B5 vitamins (Gamborg et al. 1968) and 3% sucrose. Preliminary experiments to assess the potential of somatic embryo induction form leaf sheath explants were performed in somatic embryo induction medium 1 (SIM 1) supplemented with 2,4-dichlorophenoxy acetic acid (2,4-D; 2.24, 4.49 μM) alone or with 1.11 μM 6-benzyl adenine (BA). Based on preliminary results obtained using SIM 1, the explants were transferred to somatic embryo induction media 2 (SIM 2) supplemented with BA (4.44, 8.88 μM), kinetin (Kn; 4.64, 9.29 μM), or thidiazuron (TDZ; 4.55, 9.10 μM) alone or in combination with α-naphthalene acetic acid (NAA; 1.33 μM) for 3 wk to determine the optimal levels of growth regulators for somatic embryogenesis.
Germination and hardening
Somatic embryos cultured on SIM 2 medium for 3 wk were individually transferred to germination medium [1/2 strength MS medium supplemented with gibberellic acid (GA3; 0.0, 0.72, 1.44, 2.16, 2.88 μM) and 2% sucrose] and cultured in either dark or light (16 h photoperiod) to induce germination. After 3 wk, tiny plantlets were transferred to growth medium, which consisted of 1/2 strength MS basal medium supplemented with 2% sucrose, and cultured in light with a 16 h photoperiod. Plantlets with 3–4 leaves and 4–5 roots were transferred to plastic cups containing an autoclaved mixture of sand, soil, and vermiculate (1:2:1). Cups were covered with perforated polythene bags to maintain high humidity. The bags were removed when the plants produced new leaves. Some of these primary-hardened plants were selected at random and were transferred to earthen pots containing sand, soil, and cattle manure (1:2:1) for secondary hardening under shade house condition. The survival rate of plantlets was calculated after 1 mo of primary hardening.
Histological and microscopic analysis
Explants before and after differentiation of somatic embryos were selected for histological studies. The explants were fixed in formalin-acetic acid (FAA-95% ethanol; 2:1:17, v/v/v) for 24 h, dehydrated using serial grades of ethanol, and embedded in paraffin wax using the methods of Dam et al. (2010). Thin sections (∼10 μm) were cut using a rotary microtome, mounted on glass slides, and allowed to dry for at least 10 min before staining. Finally, sections were stained with 0.025% toluidine blue O or safranin O and mounted in Di-n-butyl phthalate in xylene (DPX); BDH®, Mumbai, India). The prepared slides were examined through a light microscope (Leica®, Switzerland) and photographed. For scanning electron micrography, samples were frozen in liquid nitrogen and scanned in a low vacuum liquid scanning electron microscope (SEM; Hitachi® S 3400, Tokyo, Japan) with chamber pressure of 30 Pa and an accelerated voltage of 15 kV.
Statistical analysis
All experiments were carried out three times, each time with at least 15 explants. All data were subjected to one-way ANOVA. Data are presented as mean, or mean ± standard error (SE). Mean separations were determined by Duncan’s multiple rage test at a significance level of P < 0.05 (IBM® SPSS statistics 19).
Results
Development of somatic embryos
After 2 wk of culture on SIM 1 containing 2.24 μM 2,4-D and 1.11 μM BA, the leaf sheath explants became swollen and soft (Fig. 1A ). The explants eventually became crimped, and white clusters of small translucent spherical structures were formed on SIM 2 medium containing cytokinin (BA, Kn, TDZ) alone or in combination with NAA (Fig. 1B ). Later, these structures differentiated into globular and elongated stages (Fig. 1C, D ). In contrast, culture of leaf sheath explants on SIM 1 containing 4.49 μM 2,4-D and 1.11 μM BA for more than 3 wk led to a reduction in the percentage of explants forming somatic embryos and an increase in callus formation.
Morphological stages of direct somatic embryogenesis: (A) General view of leaf sheath explant after 2 wk of culture on SIM 1 medium containing 2.24 μM 2,4-D in combination with 1.11 μM BA; (B) Development of translucent spheres (arrow) on SIM 2 medium containing 9.10 μM TDZ in combination with 1.33 μM NAA; (C) Early stages of globular embryo development (arrows); (D) Elongated stages of somatic embryos (arrows); (E) Germination of somatic embryos on 1/2 strength MS medium containing 1.44 μM GA3 under dark condition; (F, G) Germinated somatic embryo; (H) Plantlets regenerated on 1/2 strength MS basal medium under light condition; (I) Plantlets showing shoot and roots just before transfer to mixture of sand, soil, and vermiculite (1:2:1); (J, K) Plantlets during secondary hardening to mixture of sand, soil, and cattle manure. Bars: (A) 100 μm; (B, C) 500 μm; (D) 700 μm; (E) 1.0 mm; (F, G) 1.5 mm; (H, I) 1.0 cm; (J) 1.2 cm.
The one-way ANOVA revealed that the SIM 2 media had significant effects on the percentage of explants with somatic embryo induction (P < 0.05). Somatic embryo induction was observed after 3 wk of culture. In general, Kn was least effective for direct somatic embryogenesis. The best responses occurred using SIM 2 media supplemented with TDZ, with up to 73.3% of explants showing induction of somatic embryos (Table 1). Higher concentrations of BA or TDZ (8.88; 9.10 μM) reduced the induction of somatic embryos (Table 1). Further experimentation confirmed this, and showed also that BA or TDZ more effectively induced somatic embryos when used in combination with NAA (Table 2). The highest percentage (93.3% of explants responding) of somatic embryo induction was obtained on medium supplemented with 9.10 μM TDZ in combination with 1.33 μM NAA.
Somatic embryos did not germinate on the embryo induction medium. After transfer to 1/2 strength MS medium containing GA3, somatic embryos germinated within 10 d (Fig. 1E ). GA3 concentration had a significant effect on somatic embryo germination (Fig. 1F, G ). Germination was significantly better from embryos cultured in the dark (Fig. 2). The highest rate of germination (86.7%) was obtained from somatic embryos cultured in the dark on medium containing 1.44 μM GA3 (Fig. 2).
The small plantlets obtained on the germination medium were further transferred to culture bottle containing 1/2 strength MS basal medium under light (Fig. 1H ). After 3 wk, the plantlets had formed a vigorous root system and 3–4 leaves. Plantlets were than transplanted to plastic cups containing the potting mixture (Fig. 1I, J ) and, after 1 mo of primary hardening, 87.43% of plants survived. Randomly selected primary-hardened plants were transferred for secondary hardening (Fig. 1K ).
Histological and microscopic analysis
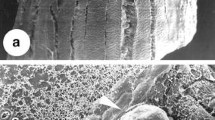
Histological and microscopic analyses gave insight into the cellular origin of somatic embryos and morphological changes that occurred during the induction of somatic embryogenesis. Somatic embryos seemed to form from either a single cell or small group of cells in the leaf sheath explant (Fig. 3A ). Cells in both epidermal and subepidermal regions of the explant that appeared competent for embryogenesis contained dense cytoplasm with small vacuoles and evident nucleus (Fig. 3B ), and were obtained within 14 d of culture on SIM 1. These embryogenic cells divided rapidly, and the embryos ultimately protruded from the surface of the explant after 7 d of culture on SIM 2 (Fig. 3C, D ). Further differentiation of these embryos resulted in the formation of globular and elongated embryos after 15–21 d of culture (Fig. 3E–G ). The clear presence of the protoderm (the outer most layer of a developing embryo) seen in the globular-stage embryos and the procambial strand was seen in the elongated-stage embryo confirms these to be somatic embryos directly formed from leaf sheath explants (Fig. 3H , I ). The SEM analysis further confirmed the direct origin of the embryo from the leaf sheath explant (Fig. 4A, B ) and development of globular embryos with no callus intermediate (Fig. 4C, D ).
Histological evidence of direct somatic embryogenesis from leaf sheath explant of C. amada: (A) Transverse section of 3-mo-old in vitro-grown leaf sheath explant; (B) Embryogenic cells with prominent nucleus, small vacuole, and dense cytoplasm on 14 d of SIM 1 culture (arrows); (C) Active cell division and formation of embryo observed after 7 d of SIM 2 culture (arrows); (D) Histology of explant showing direct appearance of embryos (arrow); (E, F) Direct appearance of globular somatic embryos (arrows) from epidermal and subepidermal regions on 15 d of culture; (G, H) Globular-shaped somatic embryos with protoderm (arrow); (I) Elongated stage somatic embryo with procambial strand observed after 21 d of culture (arrow). Bars: (A, B) 100 μm; (C, D) 300 μm, (E–H) 500 μm, (I) 700 μm.
SEM showing direct somatic embryogenesis from leaf sheath explant of C. amada. (A, B) SEM images showing cell clusters on the surface of the explant observed after 7 d of SIM 2 culture (arrows), (C) Surface of the explant with globular somatic embryos during 15 d of culture (arrows), (D) Enlarged view of globular somatic embryo (arrow).
Discussion
We previously reported indirect regeneration system of somatic embryogenesis from callus-derived cell suspension cultures of C. amada (Soundar Raju et al. 2013). However, direct somatic embryogenesis is a more desirable approach to obtain regenerated plants, similar to the parent plants, since callus formation may cause somaclonal variation (Mizukami et al. 2008). Direct embryogenesis has been also reported to be useful for the regeneration of transgenic plants (Manoharan et al. 1998; Tokuji and Fukuda 1999). In this study, a high percentage of somatic embryo induction directly from leaf sheath explants was observed, without formation of an intermediate callus. In addition, this method is easier and requires less time than the previously described method of indirect regeneration. Therefore, direct somatic embryogenesis from leaf sheath explants could prove to be another effective regeneration system for more rapid propagation of mango ginger.
2,4-D is highly effective for initiation of somatic embryos in many plant species (Ammirato 1983; Fitch and Manshardt 1990; Haider et al. 1993; Anandan et al. 2012). Prolonged culture in high concentration of 2,4-D lead to rapid cell division, which can subsequently result in genetic variation among the in vitro propagated plants (Venkov et al. 2000). Our results showed that the primary treatment in SIM 1 containing a low concentration of 2,4-D and BA for 2 wk is enough to trigger direct somatic embryogenesis. Nhut et al. (2000) reported successful direct somatic embryogenesis in rice using explants pretreated with a low concentration of 2,4-D and BA, as their combination seemed to trigger the morphogenetic competency of the explant, leading to the reception of the signals for embryo development in several species (Franklin et al. 2006; Dam et al. 2010).
Among the three cytokinins, TDZ was better at inducing somatic embryos in SIM 2 than was BA and Kn. TDZ has been used for regeneration studies in many monocots, including banana (Srangsam and Kanchanapoom 2003), bamboo (Lin et al. 2004), and Dendrobium orchid (Chung et al. 2005). TDZ stimulated the conversion of cytokinin nucleotide to more biologically active nucleotides (Laloue and Pethe 1982) and to purine cytokinins. It also promoted the conversion of adenine to adenosine (Capelle et al. 1983).
In this study, the combination of 9.10 μM TDZ and 1.33 μM NAA was the most effective at inducing direct somatic embryogenesis than was TDZ alone. This result is consistent with other reports where TDZ in combination with auxins such as 2,4-D and indole-3-acetic acid produced significantly more somatic embryogenesis in plants like orchid sp. Phalaenopsis (Kuo et al. 2005) and Digitalis trojana (Verma et al. 2012).
In somatic embryos of some species, particularly of plants that undergo dormancy in natural seeds, the germination and growth of embryos into plants can be stimulated by the application of GA3 in the culture medium (Gaj 2004; Manrique-Trujillo et al. 2013). The germination of somatic embryo of some monocots was enhanced by dark conditions (Nhut et al. 2000). In the present study, somatic embryos cultured on 1/2 strength MS medium containing GA3 under dark condition showed enhanced germination. Similar results on stimulatory effects of GA3 were reported for Sesamum indicum (Xu et al. 1997), Eleutherococcus senticosus (Choi et al. 1999), and Panax notoginseng (You et al. 2012).
The histological study showed that epidermal and subepidermal cells were the source of somatic embryos in C. amada. The initial cell divisions occurred in the epidermal and subepidermal cells of the explant. This regeneration pathway is common in several monocots including river lily (Slabbert et al. 1995) and açaí palm (Scherwinski-Pereira et al. 2012). Further, the formation of embryogenic cells with prominent nuclei, small vacuoles, and dense cytoplasm was common for several plant species (Paul et al. 2011). Globular and elongated embryo stages were regarded as key stages in the identification of somatic embryos (Godbole et al. 2002). The presence of protoderm and procambial strands in the developing embryos are the additional key features, which could be used as indicators for somatic embryo formation (Quiroz-Figueroa et al. 2002; Sharma and Millam 2004; Jalil et al. 2008). Histological study of globular and elongated structure in the present study showed clear evidence of protoderm and procambial strands in the developing somatic embryos of C. amada.
Conclusion
This is the first report of direct somatic embryogenesis from leaf sheath explants in mango ginger. This protocol can be used for the multiplication of valuable germplasm on a large scale at a much faster rate, and is easier, compared to the previous method of indirect somatic embryogenesis. Furthermore, these tissues should also be useful for the introduction of genes conferring resistance to pathogens using genetic engineering. Although mango ginger somatic embryo-derived plantlets could be acclimatized and grown under ex vitro condition, the degree to which they display a normal appearance and genetic fidelity needs to be confirmed.
References
Ammirato P (1983) The regulation of somatic embryo development in plant cell cultures: suspension culture techniques and hormone requirements. Biotechnology 1:68–74
Anandan R, Sudhakar D, Balasubramanian P, Gutieǐrrez-Mora A (2012) In vitro somatic embryogenesis from suspension cultures of Carica papaya L. Sci Hortic 136:43–49
Balachandran SM, Bhat SR, Chandel KPS (1990) In vitro clonal multiplication of turmeric (Curcuma spp.) and ginger (Zingiber officinale Rosc.). Plant Cell Rep 8:521–524
Banerjee S, Singh S, Pandey H, Pandey P, Rahman L (2012) Conservation and storage of Curcuma amada Roxb. synseeds on Luffa sponge matrix and RAPD analysis of the converted plantlets. Ind Crop Prod 36:383–388
Capelle SC, Mok DWS, Kirscher SC, Mok MC (1983) Effects of thidiazuron on cytokinin autonomy and the metabolism of N 6-(−∆2isopentenyl)[8-14C]adenosine in callus tissues of Phaseolus lunatus L. Plant Physiol 73:796–802
Choi YE, Kim JW, Yoon ES (1999) High frequency of plant production via somatic embryogenesis from callus or cell suspension cultures in Eleutherococcus senticosus. Ann Bot 83:309–314
Choudhury SN, Rabha LC, Kanjilal PB, Ghosh AC, Leclercq PA (1996) Essential oil of Curcuma amada Roxb. from Northeastern India. J Essent Oil Res 8:79–80
Chung HH, Chen JT, Chang WC (2005) Cytokinins induce direct somatic embryogenesis of Dendrobium Chiengmai pink and subsequent plant regeneration. In Vitro Cell Dev Biol Plant 41:765–769
Dam A, Paul S, Bandyopadhyay TK (2010) Direct somatic embryogenesis and plant regeneration from leaf explants of Limonium sinensis (Girard) Kuntze. Sci Hortic 126:253–260
Das A, Kesari V, Rangan L (2010) Plant regeneration in Curcuma species and assessment of genetic stability of regenerated plants. Biol Plant 54(3):423–429
Fitch MMM, Manshardt RM (1990) Somatic embryogenesis and plant regeneration from immature zygotic embryos of papaya (Carica papaya L.). Plant Cell Rep 9:320–324
Franklin G, Arvinth S, Sheeba CJ, Kanchana M, Subramonian N (2006) Auxin pretreatment promotes regeneration of sugarcane (Saccharum spp. hybrids) midrib segment explants. Plant Growth Regul 50:111–119
Gaj MD (2004) Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regul 43:27–47
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension culture of soybean root cells. Exp Cell Res 50:151–158
Ganapathi TR, Srinivas L, Suprasanna P, Bapat VA (2001) Regeneration of plants from alginate-encapsulated somatic embryos of banana cv. Rasthali (Musa SPP. AAB Group). In Vitro Cell Dev Biol Plant 37:178–181
Gholap AS, Bandyopadhyay C (1984) Characterization of mango-like aroma in Curcuma amada Roxb. J Agric Food Chem 32:57–59
Godbole S, Sood A, Thakur R, Sharma M, Ahuja PS (2002) Somatic embryogenesis and its conversion into plantlets in a multipurpose bamboo, Dendrocalamus hamiltonii Nees et Arn. Ex Munro. Curr Sci 83:885–889
Haider SA, Islam R, Kamal AHM, Rahman SM, Joarder OI (1993) Direct and indirect organogenesis in cultured hypocotyls explants of Abelmoshus esculentus (L.) Moench. Plant Tissue Cult 3:85–89
Jalil M, Chee WW, Othman RY, Khalid N (2008) Morphohistological examination on somatic embryogenesis of Musa acuminata cv. Mas (AA). Sci Hortic 117:335–340
Kirtikar KR, Basu BD (1984) Indian medicinal plants, vol 4. Bishen Singh Mahendra Pal Singh, Dehra Dun
Kuo HL, Chen JT, Chang WC (2005) Efficient plant regeneration through direct somatic embryogenesis from leaf explants of Phalaenopsis ‘Little Steve’. In Vitro Cell Dev Biol Plant 41:453–456
Laloue M, Pethe C (1982) Dynamics of cytokinin metabolism in tobacco cells. In: Wareing PE (ed) Plant growth substances. Academic publishers, New York, pp 185–195
Lin CS, Lin CC, Chang WC (2004) Effect of thidiazuron on vegetative tissue–derived somatic embryogenesis and flowering of bamboo Bambusa edulis. Plant Cell Tissue Org Cult 76:75–82
Manoharan M, Vidya CSS, Sita GL (1998) Agrobacterium-mediated genetic transformation in hot chili (Capsicum annum L. var. Pusa jwala). Plant Sci 131:77–83
Manrique-Trujillo S, Díaz D, Reaño R, Ghislain M, Kreuze J (2013) Sweetpotato plant regeneration via an improved somatic embryogenesis protocol. Sci Hortic 161:95–100
Mizukami M, Takeda T, Satonaka H, Matsuota H (2008) Improvement of propagation frequency with two-step direct somatic embryogenesis from carrot hypocotyls. Biochem Eng J 38:55–60
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Mustafa A, Ali M, Khan NZ (2005) Volatile oil constituents of the fresh rhizome of Curcuma amada Roxb. J Essent Oil Res 17:490–491
Nhut DT, Le BV, Van KTT (2000) Somatic embryogenesis and direct shoot regeneration of rice (Oryza sativa L.) using thin cell layer culture of apical meristematic tissue. J Plant Physiol 157:559–565
Paul S, Dam A, Bhattacharyyarya A, Bandyopadhyay TK (2011) An efficient regeneration system via direct and indirect somatic embryogenesis for the medicinal tree Murraya koenigii. Plant Cell Tissue Org Cult 105:271–283
Policegoudra RS, Aradhya SM (2008) Structure and biochemical properties of starch from an unconventional source-mango ginger (Curcuma amada Roxb.) rhizome. Food Hydrocoll 22:513–519
Policegoudra RS, Aradhya SM, Singh L (2011) Mango ginger (Curcuma amada Roxb.)—a promising spice for phytochemicals and biological activities. J Biosci 36(4):739–748
Prakash S, Elangomathavan R, Seshadri S, Kathiravan K, Ignacimuthu S (2004) Efficient regeneration of Curcuma amada Roxb. plantlets from rhizome and leaf sheath explants. Plant Cell Tissue Org Cult 78:159–165
Prasath D, El-Sharkawy I, Sherif S, Tiwary KS, Jayasankar S (2011) Cloning and characterization of PR5 gene from Curcuma amada and Zingiber officinale in response to Ralstonia solanacearum infection. Plant Cell Rep 30:1799–1809
Quiroz-Figueroa FR, Fuentes-Cerda CFJ, Rojas-Herrera R, Loyola-Vargas VM (2002) Histological studies on the developmental stages and differentiation of two different somatic embryogenesis systems of Coffea arabica. Plant Cell Rep 20:1141–1149
Rao AS, Rajanikanth B, Seshadri R (1989) Volatile aroma components of Curcuma amada Roxb. J Agric Food Chem 37:740–743
Remakanthan A, Menon TG, Soniya EV (2013) Somatic embryogenesis in banana (Musa acuminata AAA cv. Grand Naine): effect of explant and culture conditions. In Vitro Cell Dev Biol Plant 50:127–136
Scherwinski-Pereira SJE, da Silva GS, da Silva RA, Fermino PCP Jr, Luis ZG, de Oliveira FE (2012) Somatic embryogenesis and plant regeneration in açaí palm (Euterpe oleracea). Plant Cell Tissue Org Cult 109:501–508
Sharma SK, Millam S (2004) Somatic embryogenesis in Solanum tuberosum L.: a histological examination of key developmental stages. Plant Cell Rep 23:115–119
Singh S, Kumar JK, Saikia D, Shanker K, Thakur JP, Negi AS, Banerjee S (2010) A bioactive labdane diterpenoid from Curcuma amada and its semisynthetic analogues as antitubercular agents. Eur J Med Chem 45:4379–4382
Slabbert MM, Bruyn MN, Ferreira DI, Pretorius J (1995) Adventitious in vitro plantlet formation from immature floral stems of Crinum macowanii. Plant Cell Tissue Org Cult 43:51–57
Soundar Raju C, Kathiravan K, Aslam A, Shajahan A (2013) An efficient regeneration system via somatic embryogenesis in mango ginger (Curcuma amada Roxb.). Plant Cell Tissue Org Cult 112:387–393
Srangsam A, Kanchanapoom K (2003) Thidiazuron induced plant regeneration in callus culture of triploid banana (Musa sp.) ‘Gros Michel’. AAA group. Songklanakarin J Sci Technol 25:689–696
Srinivasan MR, Chandrasekhara N, Srinivasan K (2008) Cholesterol lowering activity of mango ginger (Curcuma amada Roxb.) in induced hypercholesterolemic rats. Eur Food Res Technol 227:1159–1163
Srivastava AK, Srivastava SK, Shah NC (2001) Constituents of rhizome essential oil of Curcuma amada Roxb. Indian J Essent Oil Res 13:63–64
Tokuji Y, Fukuda H (1999) A rapid method for transformation of carrot (Daucus carota L.) by using direct somatic embryogenesis. Biosci Biotechnol Biochem 63:519–523
Venkov P, Topashka-Ancheva M, Georgieva M, Alexieva V, Karanow E (2000) Genotoxic effect of substituted phenoxyacetic acids. Arch Toxicol 74:560–566
Verma SK, Sahin G, Yucesan B, Eker I, Sahbaz N, Gurel S, Gurel E (2012) Direct somatic embryogenesis from hypocotyls segments of Digitalis trojana Ivan and subsequent plant regeneration. Ind Crop Prot 40:76–80
Warrier PK, Nambiar VPK, Ramankutty C (1994) Indian medicinal plants-a compendium of 500 species, vol. 1. Orient Longman Pvt. Ltd., Chennai, p 106
Xu ZQ, Jia JF, Hu ZD (1997) Somatic embryogenesis in Sesamum indicum L cv. nigrum. J Plant Physiol 150:755–758
You XL, Tan X, Dai JL, Li YH, Choi YE (2012) Large-scale somatic embryogenesis and regeneration of Panax notoginseng. Plant Cell Tissue Org Cult 108:333–338
Acknowledgments
This project was supported by UGC Major Research Project grant F. No. 42-946/2013 (SR) to Dr. A. Shajahan from the University Grant Commission, Govt. of India, New Delhi. The authors thank the Centre for Advanced Studies in Botany, University of Madras for their help in scanning electron microscopic observations. We also thank DST, Govt. of India for providing facilities through the DST-FIST program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Jayasankar Subramanian
Rights and permissions
About this article
Cite this article
Soundar Raju, C., Aslam, A., Kathiravan, K. et al. Direct somatic embryogenesis and plant regeneration from leaf sheath explants of mango ginger (Curcuma amada Roxb.). In Vitro Cell.Dev.Biol.-Plant 50, 752–759 (2014). https://doi.org/10.1007/s11627-014-9653-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-014-9653-x