Abstract
Background
Perioperative blood transfusions have been associated with increased morbidity and poorer oncologic outcomes for numerous surgical procedures. However, this issue is understudied among patients with gastroesophageal malignancies. The objective was to clarify the risk factors and impact of perioperative transfusions on quality of life and surgical and oncologic outcomes among patients undergoing gastric and esophageal cancer surgery.
Methods
Patients undergoing curative-intent resections for gastroesophageal cancers between 2010 and 2018 were included. Perioperative blood transfusion was defined as any transfusion within 24 h pre-operatively, during surgery, or the primary post-operative hospitalization period. Patient and tumor characteristics, surgical and oncological outcomes, and quality of life were compared.
Results
A total of 435 patients were included. Perioperative transfusions occurred in 184 (42%). Anemia, blood loss, female sex, open surgical approach, and operative time emerged as independent risk factors for transfusions. Factors found to be independently associated with overall survival were neoadjuvant therapy, tumor size and stage, major complications, and mortality. Transfusions did not independently impact overall survival, disease-free survival, or quality of life.
Conclusions
Perioperative transfusions did not impact oncologic outcomes or quality of life among patients undergoing curative-intent surgery for gastroesophageal cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastroesophageal cancer patients are at high risk of anemia due to a multitude of cancer- and treatment-related factors 1. Anemia is often treated with red cell (pRBC) transfusions in this population, especially in the perioperative period for those undergoing gastroesophagectomy 2. While necessary to preserve life during or after major surgery, transfusions have been associated with increased morbidity, worse oncologic outcomes, and decreased quality of life (QoL) in studies that evaluated patients with other cancers 3,4,5.
Since transfusions have been associated with increased cancer recurrence and risk of developing new tumors 3,4, 6,7,8,9,10,11,12, some question the value of perioperative transfusions in cancer patients 1,8,13,14,15,16,17. The negative effects of transfusion are thought to be due to transfusion-related immunomodulation; allogenic red blood cell transfusion reduces the activity of natural killer cells and T lymphocytes 4,6,7,9,10. These cells are required to prevent dissemination of circulating and quiescent cancer cells and for providing resistance to infections 6,18. However, the effect of perioperative transfusions among gastroesophageal cancer patients is understudied and not defined clearly.
Therefore, the purpose of this study was to assess the impact of perioperative pRBC transfusions on operative, oncologic, and QoL outcomes among patients with gastroesophageal cancers.
Methodology
Study Subjects
Patients who underwent treatment for gastroesophageal cancer from January 2010 to December 2018 were identified from a prospectively collected database. Patients undergoing curative-intent surgery for gastric and esophageal cancers were included while palliative, prophylactic, and benign resections; rare cancers (non-adenocarcinoma or squamous cell carcinoma) and other synchronous and/or prior cancers were excluded.
Definition of Perioperative Transfusions
Patients were classified as having been transfused in the perioperative period if they received red cell transfusions 24 h pre-operatively, during surgery, or within the post-operative hospitalization period of their cancer resection 9,13,15,19,20,21,22,23.
Data Collection and Classification
The primary outcomes were cumulative overall and disease-free survival. Secondary outcomes included patient and tumor characteristics, operative outcomes, complications, and quality of life determined at baseline and at every follow-up visit. All data were collected prospectively and extracted from paper and electronic medical records.
Age-adjusted Charlson comorbidity index (CCI) was used to categorize comorbidities and age 24. Node-negative and T1–2 stage tumors were classified as early-stage cancer 25 while any node-positive disease and T3–4 were classified as locally advanced cancer 26,27. Anemia was categorized according to the World Health Organization’s (WHO’s) cut-offs: mild (110 g/L to normal), moderate (80 g/L to 110 g/L), and severe anemia (less than 80 g/L) 28. All tumors were classified according to the American Joint Committee on Cancer (AJCC) eighth edition 29,30. Post-operative complications were classified using the Clavien-Dindo score (CDS) 31. Death certificates and Quebec cancer registry data were used to determine mortality. Functional Assessment of Cancer Therapy-Esophageal (FACT-E) questionnaires administered at every outpatient clinic appointment were used to determine QoL scores 32.
Statistical Analysis
Data were analyzed using Mann-Whitney U, Fisher-Exact, Kaplan-Meier, log-rank, and χ2 tests. Multiple logistic regression was used for determining independent risk factors for transfusions with the model being built using statistically significant factors identified on univariate analysis and clinically relevant parameters. Cox proportional hazards regression model was employed to determine variables independently predictive of overall (OS) and disease-free survival (DFS). Prism 8.0 by GraphPad and SAS 9.4 by SAS Institute were utilized for statistical analysis. Data are presented as median interquartile range. A p value of less than 0.05 determined statistical significance.
Results
Of 766 gastroesophageal resections performed between 2010 and 2018, 446 met inclusion criteria. Among those, 11 (2%) were excluded due to lack of transfusion data leaving 435 patients included in the final analysis, of which 184 (42%) received transfusions perioperatively.
Patients were older and had more severe comorbidities in the transfusion group. Adenocarcinoma predominated in both groups and clinical stage was higher in the transfusion group (stage III-IV: pRBC: 106 (57%), no pRBC: 111 (45%); p = 0.002). Coagulation parameters, rates of neoadjuvant therapy (pRBC 124 (67%), no pRBC 160 (64%); p = 0.570), and quantity of transfusions before surgery (pRBC 0 0–2 units, no pRBC 0 0–1 units; p = 0.342) did not vary among groups. Anemia was more prevalent in the transfusion group at all timepoints: hemoglobin at diagnosis (pRBC 124 108–138 g/L, no pRBC 137 119–148 g/L; p < 0.001), pre-operatively (pRBC 109 99–120 g/L, no pRBC 126 115–138 g/L; p < 0.001), and on day of surgery (pRBC 102 89–113 g/L, no pRBC 121 115–132 g/L; p < 0.001). Patient characteristics are presented in Table 1.
Transfusions were more prevalent among those who had surgery using the open approach (Table 2). Duration of surgery was comparable among groups while estimated blood loss (EBL) was higher in the transfusion group (pRBC 500 250–750 mL, no pRBC 250 150–400 mL; p < 0.001). Transfused patients received 1 0–2 pRBC units per patient intra-operatively and 1 0–2 units post-operatively, with 38 (43%) of post-operative transfusions occurring in patients who had also received pRBC transfusions during surgery. The rate of transfusions decreased from 50 to 40% over the study period (Fig. 1). Severe post-operative complications (Clavien-Dindo 3–4) (pRBC 56 (30%), no pRBC 37 (15%); p < 0.001) and 30-day mortality (pRBC 14 (8%), no pRBC 2 (1%); p < 0.001) were higher in the transfusion group.
Tumors were larger in the transfusion group (pRBC 4.3 ± 3.2 cm, no pRBC 3.3 ± 2.4 cm; p = 0.003) and had more lymph node (LN) metastasis (pRBC 2 0–7 nodes, no pRBC 1 0–3 nodes; p = 0.031). Total number of LNs retrieved and lymphovascular and perineural invasion were comparable among groups. Pathological stage, positive margins, and rates of pathologic complete response were similar between groups. However, tumors were more invasive (T4 pRBC 34 (18%), no pRBC 18 (7%); p < 0.001) and had a higher LN status in the transfusion group. Oncological outcomes are presented in Table 3. On multivariate analysis, independent risk factors for receiving perioperative pRBC transfusions (Table 4) were female sex, moderate to severe anemia on day of surgery, EBL above 400 mL, open approach, and prolonged operative time.
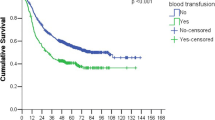
Kaplan-Meier survival curves for DFS and OS are depicted in Fig. 2. Those who did not receive pRBC transfusions had higher DFS and OS on univariate analysis. Figure 3 demonstrates an inverse relationship between survival and quantity of pRBC transfused for both DFS and OS.
Cox proportional hazard analysis for DFS (Table 5) demonstrated the following factors to independently influence DFS: neoadjuvant therapy and pathological stage negatively impacted DFS. Table 6 depicts factors that were independently associated with OS: neoadjuvant therapy, major complications (Clavien-Dindo score 3–5), pathological stage, and tumor size above 3 cm negatively impacted OS. When controlling for other factors, transfusions were not found to independently impact DFS or OS.
Patient-reported QoL scores from FACT-E questionnaires are presented in Fig. 4. Quality of life was similar between groups at all timepoints from diagnosis to follow-up 3 years post-operatively. Trends in overall quality of life show a decrease in the early post-operative period and rebound to higher than pre-operative levels after 3 months post-operatively, but these differences did not reach statistical significance.
Discussion
This study demonstrated that perioperative pRBC transfusions can be administered safely for patients undergoing curative-intent surgery for gastroesophageal cancers without impacting QoL or surgical and oncological outcomes. Independent risk factors for transfusion in this series were anemia, intra-operative blood loss, open approach surgery, prolonged operative time, and female sex. Importantly, transfusions did not negatively impact surgical outcomes and long-term survival. The results of this study are significant as perioperative transfusions have been associated with poorer oncologic and surgical outcomes for other cancers, but this has not been studied in gastroesophageal cancer patients.
The relationship between perioperative blood transfusions and survival is controversial. Many studies have shown a significant deleterious effect on survival related to perioperative transfusions while some demonstrated similarity 6,22. Often, these studies have been limited by small sample sizes and use of univariate analysis alone, making interpretation of the true impact of transfusions challenging 22,23,33,34. Furthermore, large database studies are limited by a lack of granularity of data 15,22,33. Several studies reporting worse long-term oncologic outcomes in transfused patients do not include some significant parameters in their multivariate model such as intra-operative blood loss and pre-operative hemoglobin level. When multivariate modeling has adjusted for such important covariates, a deleterious impact of transfusions has not been demonstrated 18,23,35,36. These articles demonstrate that transfusions, even though they tend to be associated with decreased survival in some studies, do not independently impact survival when confounding variables are incorporated into multivariate analysis, which is in keeping with the findings we report in this analysis.
Disease-free survival was lower in the transfused group in our univariate analysis, which mirrors other studies 13,22. However, this significant difference disappeared on multivariate analysis when covariates were taken into consideration, indicating that transfusions are a confounding factor more than a prognostic indicator 21. Neoadjuvant therapy and pathological tumor stage independently influenced DFS 6,13,15,21, 37,38. Neoadjuvant therapy has a positive correlation with DFS since it is used for locally advanced cancers to improve local tumor control. According to our results and those reported by others, age, sex, comorbidities, complications, tumor size, and approach did not influence DFS independently 6,39,40. Our results demonstrate that perioperative transfusions do not independently impact disease-free survival and that multivariate analysis is necessary to determine whether transfusions impact survival.
Overall survival in this series was significantly longer on univariate analysis among non-transfused patients, implying transfusions may deleteriously impact survival 16,22,34,35. However, we demonstrated that this difference disappears with multivariate analysis where transfusion does not influence OS when confounders are considered 35. Contrary to our findings, transfusions remained an independent prognostic factor for OS in some studies 13,34. This could be attributed to the variables used in the multivariate Cox regression analysis, as these studies did not include some clinically important covariates such as complications and tumor size. The benefit of this single-center review of prospectively followed patients is the depth of data available, allowing for analysis of possible confounding variables related to outcomes. Our results showed that, as expected, stage, tumor size, complications, and mortality impact OS while transfusions alone do not.
Despite the differences in tumor stage, comorbidities approach, and complications observed among transfused and non-transfused patients in this cohort, QoL at all timepoints was similar among groups. We observed a trend towards improved QoL after neoadjuvant therapy and surgery, which can be attributed to dysphagia relief following commencement of treatment. While the differences in QoL at various timepoints did not reach statistical significance in this series, this is likely because gastric and esophageal cancer patients were grouped together in the analysis. Our group has previously demonstrated significant improvements in long-term QoL from baseline in upper gastrointestinal cancer patients 41. Importantly, no differences in QoL were observed at any timepoint between transfused and non-transfused patients in this cohort.
Our results indicate that moderate to severe anemia, intra-operative blood loss, increased operative time, and female sex are independent risk factors for perioperative transfusion on multivariate analysis, as has been reported 14, 22, 36, 42, 43. Two benchmark studies showed that women tend to have a higher transfusion rate and volume, which can be explained by clinicians applying the same absolute transfusion thresholds irrespective of sex even though the WHO’s anemia cut-offs for women are lower 44. In addition, no cut-off values or transfusion guidelines exist specifically for post-menopausal women. Consequently, female surgical patients tend to receive transfusions more often than men 44 and may explain why female sex emerged as an independent risk factor for transfusion in this series.
Limitations of this study include the retrospective nature of the data analysis. Nevertheless, the large patient cohort strengthens the validity of our findings. Additionally, this work was carried out in a single center, limiting the generalizability of our findings. Although neoadjuvant therapy protocols for esophageal and gastric cancer have evolved greatly over the study period, our center has been treating gastric and esophageal adenocarcinomas (the majority of patients in this study) with taxane-based triplet therapy (docetaxel, cisplatin, and 5-fluorouracil) since 2007 45, making this cohort relatively homogenous in this respect despite the lengthy study interval. Administration of adjuvant therapy was not evaluated, which could be a confounder for some variables such as quality of life and long-term survival. In addition, selection bias was present as the decision to transfuse is subjective and some practitioners may have been more liberal with transfusions than others. Although the overall rate of perioperative transfusions in this cohort is high (46%), intra-operative transfusion rates (24%) were in line with globally reported standards. Our data also show transfusions were given more liberally in the earlier years of the study. Indeed, a trend towards decreasing overall transfusion rates over time was observed, consistent with changes in practice globally. All tumors were reclassified using AJCC’s 8th edition while other studies predominantly used the 7th edition. This may cause difficulty in eliciting accurate comparisons between studies, but the eighth edition has been shown to be valid and will be employed in future studies.
Conclusion
Perioperative transfusions are associated with higher cancer stage, comorbidities, and post-operative complications but are not an independent predictor of long-term oncologic or quality of life outcomes after gastroesophagectomy for cancer. Perioperative care physicians should not be biased against transfusion, when required, for fear of worsening long-term outcomes in gastroesophageal cancer patients.
References
Clevenger B, Richards T. Pre-operative anaemia. Anaesthesia 2015;70:20-28.
Birgegard G, Aapro MS, Bokemeyer C, Dicato M, Drings P, Hornedo J, Krzakowski M, Ludwig H, Pecorelli S, Schmoll H, Schneider M, Schrijvers D, Shasha D, Van Belle S. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology 2005;68:3-11.
Marik PE. The hazards of blood transfusion. British Journal Hospital Medicine (London England 2005) 2009;70:12-15.
Velasquez JF, Cata JP. Transfusions of blood products and cancer outcomes. Revista Española de Anestesiología y Reanimación 2015;62:461-467.
Koutsavlis I. Transfusion Thresholds, Quality of Life, and Current Approaches in Myelodysplastic Syndromes. Anemia 2016;https://doi.org/10.1155/2016/8494738.
Reeh M, Ghadban T, Dedow J, Vettorazzi E, Uzunoglu FG, Nentwich M, Kluge S, Izbicki JR, Vashist YK. Allogenic Blood Transfusion is Associated with Poor Perioperative and Long-Term Outcome in Esophageal Cancer. World Journal of Surgery 2017;41:208-215.
Wehry J, Agle S, Philips P, Cannon R, Scoggins CR, Puffer L, McMasters KM, Martin RCG. Restrictive blood transfusion protocol in malignant upper gastrointestinal and pancreatic resections patients reduces blood transfusions with no increase in patient morbidity. American Journal of Surgery 2015;210:1197-1204.
Squires MH, Maithel SK. Transfusion and Gastric Cancer Resection: In Reply to Yang and colleagues. Journal of the American College of Surgeons 2015;221:996.
Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. British Journal of Anaesthesiology 2013;110:690-701.
Chang CM, Quinlan SC, Warren JL, Engels EA. Blood transfusions and the subsequent risk of hematologic malignancies. Transfusion 2010;50:2249-2257.
Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Reviews 2007;21:327-348.
Dellinger EP, Anaya DA. Infectious and immunologic consequences of blood transfusion. Critical Care 2004;2:S18-23.
Squires MH 3rd, Kooby DA, Poultsides GA, Weber SM, Bloomston M, Fields RC, Pawlik TM, Votanopoulos KI, Schmidt CR, Ejaz A, Acher AW, Worhunsky DJ, Saunders N, Levine EA, Jin LX, Cho CS, Winslow ER, Russell MC, Staley CA, Maithel SK. Effect of Perioperative Transfusion on Recurrence and Survival after Gastric Cancer Resection: A 7-Institution Analysis of 765 Patients from the US Gastric Cancer Collaborative. Journal of the American College of Surgeons 2015;221:767-777.
Shen JG, Cheong JH, Hyung WJ, Kim J, Choi SH, Noh SH. Pretreatment anemia is associated with poorer survival in patients with stage I and II gastric cancer. Journal of Surgical Oncology 2005;91:126-130.
Liu XW, Ma MZ, Huang H, Wang YN. Effect of perioperative blood transfusion on prognosis of patients with gastric cancer: a retrospective analysis of a single center database. BMC Cancer 2018;18:649.
Chang CC, Sun JT, Chen JY, Chen YT, Li PY, Lee TC, Su MJ, Wu JM, Yen TH, Chu FY. Impact of Peri-Operative Anemia and Blood Transfusions in Patients with Gastric Cancer Receiving Gastrectomy. Asian Pacific Journal of Cancer Prevention 2016;17:1427-1431.
Craig SR, Adam DJ, Yap PL, Leaver HA, Elton RA, Cameron EW, Sang CT, Walker WS. Effect of blood transfusion on survival after esophagogastrectomy for carcinoma. Annals of Thoracic Surgery 1998;66:356-361.
Sánchez-Bueno F, García-Marcilla J, Pérez-Abad J, Vicente R, Aranda F, Lujan J, Parrilla P. Does perioperative blood transfusion influence long-term prognosis of gastric cancer? Digestive Diseases and Sciences 1997;42:2072-2076.
Zhang Q, Wu H, Zhang J, Qi Q, Zhang W, Xia R. Preoperative Immune Response is Associated with Perioperative Transfusion Requirements in Glioma Surgery. Journal of Cancer 2019;10:3526-3532.
Behrends M, DePalma G, Sands L, Leung J. Association between intraoperative blood transfusions and early postoperative delirium in older adults. Journal of American Geriatric Society 2013;61:365-370.
Rausei S, Ruspi L, Galli F, Tirotta F, Inversini D, Frattini F, Chiappa C, Rovera F, Boni L, Dionigi G, Dionigi R. Peri-operative blood transfusion in gastric cancer surgery: prognostic or confounding factor? International Journal of Surgery 2013;11:S100-103.
Ojima T, Iwahashi M, Nakamori M, Nakamura M, Naka T, Katsuda M, Iida T, Hayata K, Yamaue H. Association of allogeneic blood transfusions and long-term survival of patients with gastric cancer after curative gastrectomy. Journal of Gastrointestinal Surgery 2009;13:1821-1830.
Moriguchi S, Maehara Y, Akazawa K, Sugimachi K, Nose Y. Lack of Relationship between Perioperative Blood-Transfusion and Survival-Time after Curative Resection for Gastric-Cancer. Cancer 1990;66:2331-2335.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases 1987;40:373-383.
Berry MF. Esophageal cancer: staging system and guidelines for staging and treatment. Journal of Thoracic Diseases 2014;6:S289-297.
Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. New England Journal of Medicine 2001;345:725-730.
Roses RE, Datta J, You YN. Defining the optimal treatment of locally advanced gastric cancer. ACS Clinical Research Program. 2019 cited 2020 February 11; Available from: https://bulletin.facs.org/2019/05/defining-the-optimal-treatment-of-locally-advanced-gastric-cancer/.
Khusun H, Yip R, Schultink W, Dillon DH. World Health Organization hemoglobin cut-off points for the detection of anemia are valid for an Indonesian population. Journal of Nutrition 1999;129:1669-1674.
Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Annals of Cardiothoracic Surgery 2017;6:119-130.
Cancer AJC. AJCC Cancer Staging Form Supplement, in AJCC Cancer Staging Manual, Eighth Edition. Chicago, IL. p. 520.
Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, Tsubosa Y, Satoh T, Yokomizo A, Fukuda H, Sasako M. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surgery Today 2016;46:668-685.
Darling G, Eton DT, Sulman J, Casson AG, Cella D. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer 2006; 107:854-863.
Kaneda M, Horimi T, Ninomiya M, Nagae S, Mukai K, Takeda I, Shimoyama H, Chohno S, Okabayashi T, Kagawa S, Orita K. Adverse affect of blood transfusions on survival of patients with gastric cancer. Transfusion 1987;27:375-377.
Lee J, Chin JH, Kim JI, Lee EH, Choi IC. Association between red blood cell transfusion and long-term mortality in patients with cancer of the esophagus after esophagectomy. Diseases of the Esophagus 2018;https://doi.org/10.1093/dote/dox123.
Choi JH, Chung HC, Yoo NC, Lee HR, Lee KH, Kim JH, Roh JK, Min JS, Lee KS, Kim BS, Lim HY. Perioperative blood transfusions and prognosis in patients with curatively resected locally advanced gastric cancer. Oncology 1995;52:170-175.
Bortul M, Calligaris L, Roseano M, Leggeri A. Blood transfusions and results after curative resection for gastric cancer. Tumori Journal 2003;2:S27-30.
Alnimer Y, Hindi Z, Katato K. The Effect of Perioperative Bevacizumab on Disease-Free and Overall Survival in Locally Advanced HER-2 Negative Breast Cancer: A Meta-Analysis. Breast Cancer 2018;https://doi.org/10.1177/1178223418792250.
Greer SE, Pipas JM, Sutton JE, Zaki BI, Tsapakos M, Colacchio TA, Gibson JJ, Wiener DC, Ripple GH, Barth RJ Jr. Effect of neoadjuvant therapy on local recurrence after resection of pancreatic adenocarcinoma. Journal of the American College of Surgeons 2008;206:451-457.
Di Costanzo F, Gasperoni S, Manzione L, Bisagni G, Labianca R, Bravi S, Cortesi E, Carlini P, Bracci R, Tomao S, Messerini L, Arcangeli A, Torri V, Bilancia D, Floriani I, Tonato M. Adjuvant chemotherapy in completely resected gastric cancer: A Randomized phase III trial conducted by GOIRC. Journal of the National Cancer Institute 2008;100:388-398.
Woelfel IA, Fernandez LJ, Idowu MO, Takabe K. A high burden of comorbid conditions leads to decreased survival in breast cancer. Gland Surgery 2018;7:216-227.
Ronellenfitsch U, Najmeh S, Andalib A, Perera RM, Rousseau MC, Mulder DS, Lerri LE. Functional outcomes and quality of life after proximal gastrectomy with esophagogastrostomy using a narrow gastric conduit. Annals of Surgical Oncology 2015;22:772-779.
Osorio J, Jerico C, Miranda C, Garsot E, Luna A, Miro M, Santamaria M, Artigau E, Rodriguez-Santiago J, Castro S, Feliu J, Aldeano A, Olona C, Momblan D, Ruiz D, Galofre G, Pros I, Garcia-Albeniz X, Lozano M, Pera M. Perioperative transfusion management in gastric cancer surgery: Analysis of the Spanish subset of the EURECCA oesophago-gastric cancer registry. Cirugía Española 2018;96:546-554.
Voelter V, Schuhmacher C, Busch R, Peschel C, Siewert JR, Lordick F. Incidence of anemia in patients receiving neoadjuvant chemotherapy for locally advanced esophagogastric cancer. Annals of Thoracic Surgery 2004;78:1037-1041.
Gombotz H, Schreier G, Neubauer S, Kastner P, Hofmann A. Gender disparities in red blood cell transfusion in elective surgery: a post hoc multicentre cohort study. BMJ Open 2016;https://doi.org/10.1136/bmjopen-2016-012210.
Ferri LE, Ades S, Alcindor T, Chasen M, Marcus V, Hickeson M, Artho G, Thirlwell MP. Perioperative docetaxel, cisplatin, and 5-fluorouracil (DCF) for locally advanced esophageal and gastric adenocarcinoma: a multicenter phase II trial. Annals of Oncology 2012;23:1512-1517.
Acknowledgments
We would like to thank Aya Siblini for assisting with securing research ethics review board approval and Ludovic Aubin and Samantha Lancione for assisting with obtaining complications and quality of life data, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval was obtained from the Research Ethics Board of McGill University Health Centre in Montreal, Canada (file # 2019-5085).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Kammili, A., Kaneva, P., Lee, L. et al. Perioperative Transfusions for Gastroesophageal Cancers: Risk Factors and Short- and Long-Term Outcomes. J Gastrointest Surg 25, 48–57 (2021). https://doi.org/10.1007/s11605-020-04845-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-020-04845-7