Abstract
Purpose
The correlation between perioperative blood transfusions and the prognosis after major cancer surgery remains controversial. We investigated the association between perioperative blood transfusion and survival outcomes following major cancer surgeries and analyzed trends in perioperative blood transfusions.
Methods
Data for this population-based cohort study were obtained from the National Health Insurance Service of South Korea. Adult patients who underwent major cancer surgery between January 1, 2016, and December 31, 2020, were included. The primary endpoint was 90-day mortality.
Results
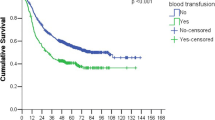
The final analysis included 253,016 patients, of which 55,094 (21.8%) received perioperative blood transfusions. In the multivariable logistic regression model, select factors, including neoadjuvant/adjuvant chemotherapy, an increased preoperative Charlson Comorbidity Index, moderate or severe liver disease, liver cancer surgery, and small bowel cancer surgery, were associated with an increased likelihood of blood transfusion. In the multivariable Cox regression model, patients who received blood transfusion had a significantly higher risk of 90-day mortality (hazard ratio: 5.68; 95% confidence interval: 5.37, 6.00; P < 0.001) than those who did not.
Conclusion
We identified potential risk factors for perioperative blood transfusions. Blood transfusion is associated with an increased 90-day mortality risk after major cancer surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of cancer is increasing rapidly with population growth and aging worldwide [1]. It is projected to become the leading cause of death in every country in the twenty-first century [2]. Cancer treatment often involves major surgery and chemo/radiation therapy, resulting in significant blood loss, bone marrow suppression, and nutritional deficiencies [3], all of which can necessitate blood transfusion [4]. Therefore, examining the impact of perioperative blood transfusions on the prognosis of cancer patients is essential for optimizing their management.
The effect of blood transfusion on the prognosis of cancer patients is controversial. Anemia can negatively affect physical and psychosocial functions and the quality of life and lead to comorbid conditions [3]; therefore, blood transfusions may be unavoidable. Despite significant improvements in blood transfusion safety in recent years, the risk of transmission of emerging pathogens has not been completely eliminated [5]. Excessive transfusion of red blood cells and platelets has been linked to an increased risk of venous and arterial thrombotic events [6]. Some studies suggest that excessive blood transfusions in patients with cancer can exert immunomodulatory effects [7], resulting in cancer recurrence patterns [8], whereas others argue that the opposite is true [9, 10]. Therefore, the correlation between perioperative blood transfusions and the medical prognosis after major cancer surgery remains controversial and requires further investigation.
Therefore, present investigation examined the association between perioperative blood transfusion and survival outcomes following major cancer surgeries and analyzed trends in perioperative blood transfusion between 2016 and 2020. We hypothesized that blood transfusions would be associated with increased mortality rates after cancer surgery.
Methods
Study design and ethical statements
This research was conducted in accordance with the relevant ethical guidelines and approved by the Institutional Review Board (approval number: X-2105-686-904). Data were obtained from the National Health Insurance Service (NHIS) after approval of the study protocol (NHIS-2022-1-336). Informed consent was not required, as the study involved a retrospective analysis of anonymized data from the South Korean NHIS database.
Data source
This study used data from the South Korean NHIS database, which contains comprehensive information on all diagnoses and prescriptions. Diagnoses were recorded using International Classification of Diseases, 10th Revision (ICD-10) codes to enable patients to receive government financial support. The NHIS database also includes the demographic and socioeconomic data of all patients in South Korea.
Inclusion of patients
This study commenced with an initial screening of adult patients who underwent major cancer surgeries under general anesthesia between January 1, 2016, and December 31, 2020. Major cancers included lung, gastric, colorectal, esophageal, small bowel, liver, pancreatic, bile duct, and gallbladder cancer. Table S1 lists the specific types of major cancer surgeries. Four exclusion criteria were applied: the study included only the first major cancer surgery for patients who underwent multiple surgeries, and subsequent cases were excluded. Patients with metastatic cancer (ICD-10 codes C77-C80) and those with missing age data were excluded. Pediatric patients (< 18 years old) were not included. In South Korea, the NHIS provides coverage for 95% of all medical expenditures incurred by patients with cancer, provided that they are registered with the system. Consequently, all individuals avail themselves of this opportunity and enroll in the program.
Blood transfusion
Patients were categorized into two groups based on whether or not they received blood transfusions during the perioperative period for major cancer surgeries. The NHIS database accurately records transfusions of packed red blood cells (pRBCs), platelets (PLTs), and fresh-frozen plasma (FFP) using the corresponding transfusion codes.
Endpoints
The primary endpoint was mortality within 90 days after major cancer surgery. Mortality within this timeframe was further delineated as cancer- or non-cancer-related using data from Statistics Korea, which documents the primary etiology of all fatalities in South Korea using ICD-10 codes. Fatalities due to primary cancer etiology (including progression, recurrence, metastasis, or complications) were classified as cancer-related mortality. All other fatalities were designated as non-cancer related. The secondary endpoint was the trend in blood transfusions between 2016 and 2020.
Covariates
Demographic and socioeconomic data were collected for a comprehensive analysis. We used the NHIS database to gather information on patients’ employment status during surgery. Residences were categorized as urban or rural, based on their location in metropolitan cities or other areas. The NHIS also provides data on household income levels to determine the eligibility for medical aid programs. Patients were divided into five groups based on quartile ratios and the Medical Aid Program group. Data on minimally invasive surgical techniques such as video-assisted thoracic surgery and laparoscopy were also collected. Information on intraoperative remifentanil infusion and administration of neoadjuvant and adjuvant therapies was collected to indirectly reflect the advanced stages of each cancer.
Data on hospital types and annual case volumes of major cancer surgeries were extracted for statistical analyses to account for the varying capacities of the hospitals where cancer surgeries were performed. Hospitals were classified as tertiary or general hospitals. We calculated the annual case volumes of major cancer surgeries for each hospital by dividing the total number of major cancer surgery cases from 2016 to 2020 by five. Patients were stratified into four groups based on the quartile ratios of annual case volumes. To benefit from South Korea’s social welfare system, individuals with disabilities must be registered in the NHIS database. Within the database, patients with disabilities were classified into six groups based on the severity of their disability. For this study, the patients were divided into two groups: those with severe and those with mild-to-moderate disabilities (grades 1–3 and 4–6, respectively). Comorbidities were assessed using the Charlson Comorbidity Index (CCI), which was calculated using the ICD-10 codes, as presented in Table S2.
Statistical analyses
Clinicopathological characteristics were compared between the patients who received blood transfusions (BT group) and those who did not (non-BT group). Categorical variables are presented as numbers and percentages, whereas continuous variables are presented as mean values with standard deviations. Multivariable logistic regression was used to identify the factors associated with perioperative blood transfusion among patients undergoing major cancer surgery. All covariates were included in the adjustment model, and the results are presented as odds ratios (ORs) with 95% confidence intervals (CIs). A multivariable Cox regression analysis was also performed to examine the hazard ratio (HR) for 90-day mortality, with all covariates included in the model, except for CCI, to avoid multicollinearity. We also performed propensity score (PS) matching between the BT and non-BT groups to determine whether or not the HR for 90-day mortality varied between the PS-matched and entire cohorts. PSs were calculated using logistic regression and 1:1 matching was conducted using the nearest neighbor method with a caliper of 0.25. An absolute standardized difference (ASD) < 0.1 was used as a criterion to determine whether or not the two groups were adequately balanced by PS matching.
Finally, subgroup analyses were conducted to determine the significance of the association between perioperative blood transfusions and 90-day mortality for each type of cancer surgery. Log–log plots were used to confirm the assumptions of the Cox proportional hazard model.
All statistical analyses were performed using the R software program (version 4.0.3, R packages, R Project for Statistical Computing, Vienna, Austria), and P < 0.05 indicated significance.
Results
Study population
Figure 1 illustrates the patient selection process used in this study. In total, 296,019 cases of major cancer surgery were recorded in South Korea between January 1, 2016, and December 31, 2020. We excluded 38,560 cases of multiple surgeries performed in a single patient and 3921 cases of metastatic cancer to focus on the initial episodes of major cancer surgery. In addition, 385 patients with missing age data and 137 pediatric patients < 18 years old were excluded. Our final analysis included 253,016 patients who had undergone major cancer surgery. Of these patients, 55,094 (21.8%) received perioperative blood transfusions (BT group) and 197,922 (78.2%) did not (non-BT group). In the BT group, 20.2%, 6.5%, and 1.8% of patients received pRBC, FFP, and platelet transfusions, respectively. Figure 2 illustrates the overall downward trend in the blood transfusion rates for all three types of blood products between 2016 and 2020. Table 1 shows a comparison of the characteristics of the BT and non-BT groups. More patients died within 90 days in the BT group than in the non-BT group (9.3% vs. 1.0%).
Multivariable logistic regression model
Table 2 presents the results of the multivariate logistic regression analysis examining the association between various factors and the likelihood of perioperative blood transfusion. Compared to lung cancer surgery, all other surgery categories were associated with a higher probability of blood transfusion, with small bowel (OR: 4.18; 95% CI 3.94, 4.43; P < 0.001) and liver (OR: 3.25; 95% CI 3.12, 3.39; P < 0.001) cancer surgeries having the highest ORs. Major cancer surgeries performed via video-assisted thoracoscopic surgery (VATS) or laparoscopy were associated with a lower likelihood of blood transfusion than either the open thoracotomy or laparotomy group (OR: 0.38; 95% CI 0.37, 0.39; P < 0.001). Neoadjuvant/adjuvant chemotherapy ([OR: 2.25; 95% CI 2.15, 2.35; P < 0.001]/[OR: 1.29; 95% CI 1.26, 1.32; P < 0.001]) and adjuvant radiotherapy (OR: 2.17; 95% CI 1.66, 1.84; P < 0.001) were associated with an increased risk of blood transfusion. Among the preoperative comorbidities measured using the CCI, patients with moderate or severe liver disease had a significantly higher likelihood of blood transfusion than patients without moderate or severe liver disease (OR: 2.26; 95% CI 2.08, 2.45; P < 0.001).
Survival analyses
Table 3 presents the results of survival analyses examining the association between blood transfusion and 90-day mortality. Patients who received blood transfusions had a significantly higher risk of 90-day mortality than the no blood transfusion group (HR: 5.68; 95% CI 5.37, 6.00; P < 0.001). Among the three types of blood products, pRBC transfusion was associated with the greatest increase in 90-day mortality risk (HR, 3.12; 95% CI 2.93, 3.31; P < 0.001). Furthermore, each additional pint of pRBCs was associated with a 4% increase in the 90-day mortality risk (HR: 1.04; 95% CI 1.04, 1.04; P < 0.001). These results are consistent with the 90-day cancer and non-cancer mortality rates. The HRs with 95% CIs for other covariates are presented in Table S3.
Table S4 shows the characteristics of the BT and non-BT groups before and after PS-matching. All ASDs between the two groups were < 1.0, suggesting adequate balance by PS matching. Table 4 shows the results of the survival analyses in a PS-matched cohort. In the PS-matched cohort, patients who received blood transfusions had a significantly higher risk of 90-day mortality (HR, 4.48; 95% CI 4.20, 4.77; P < 0.001) than those who did not. These results were consistent with the 90-day cancer and non-cancer mortality rates.
Subgroup analyses
Table 5 presents the results of the subgroup analyses for 90-day mortality based on the cancer surgery type. The BT group had a higher 90-day mortality rate than the non-BT group for all cancer surgery types, and it was the highest in gastric cancer surgery (HR: 7.70; 95% CI 6.66, 8.91; P < 0.001).
Discussion
The overall trend of perioperative blood transfusions among patients with major cancers decreased between 2016 and 2020. Platelet transfusion remained almost constant; however, FFP and pRBC transfusions decreased slightly over the years. Cancer surgery, particularly in the small bowel and liver, is closely associated with blood transfusions. Patients who received neoadjuvant chemotherapy or adjuvant radiotherapy were also strongly associated with blood transfusion. Based on the preoperative CCI, moderate or severe liver disease and congestive heart failure were strongly associated with blood transfusion. Patients who underwent VATS or laparoscopy were less likely to receive blood transfusions than the open thoracotomy or laparotomy group. The 90-day cancer-related mortality rate after major cancer surgery was higher in the BT group than in the non-BT group, especially in those who received pRBC. Transfusion with PLT was most frequently associated with 90-day non-cancer mortality. Gastric and liver cancer surgeries were also highly associated with 90-day mortalities.
Recent studies also revealed a trend toward decreasing perioperative pRBC and plasma transfusion rates due to improvements in medical management, such as erythropoiesis-stimulating agents, tranexamic acid, intraoperative cell salvage, acute normovolemic hemodilution, and novel surgical techniques using laparoscopy and robot assistance [11,12,13,14]. Increasing concerns regarding the possible deleterious effects of blood transfusion have also triggered a commitment to more restrictive transfusion thresholds [12, 13].
Liver cancer surgery is highly associated with blood transfusion; however, laparoscopy and VATS are associated with lower blood transfusion rates than the open thoracotomy or laparotomy group. Owing to the invasive nature of the operation, liver transplantation for hepatocellular carcinoma often results in excessive blood loss and hemostatic disorders. Therefore, aggressive supplementation with blood products [15]. Approximately 80% of patients with hepatocellular carcinoma have liver cirrhosis [16], which hinders the ability to synthesize coagulation factors and plasma proteins and causes thrombocytopenia [17]. Laparoscopic surgery uses energy-dividing devices, such as ultrasonic coagulating shears and vessel-sealing devices, along with a magnified surgical view, which reduces the incidence of bleeding, resulting in less intraoperative blood loss [18]. This may explain the reduced association of laparoscopic surgery with perioperative blood transfusion [19]. VATS is a less invasive surgical procedure that results in less intraoperative blood loss [20] and a lower need for blood transfusion [21] than open thoracotomy.
Severe disability and a high CCI reflect a compromised physical status, corresponding to an increased amount of intraoperative blood transfusion during cancer surgery [22]. Liver diseases, such as cirrhosis, are associated with increased bleeding complications and the need for intraoperative blood transfusion [23]. Patients with congestive heart failure are likely to be malnourished or have cachexia or pre-existing anemia, which can be worsened by cancer and subsequent major surgeries, consequently requiring blood transfusions [24].
Chemotherapy drugs are strongly associated with hepatic injury due to hepatic oxidative stress from chemotherapy-generated reactive oxygen species, which increase the operative risk of liver resection and blood loss [25]. Chemotherapy and radiotherapy can have side effects, such as bone marrow depression and gut mucosal sloughing [24]. This leads to anemia, which can cause increased tumor hypoxia. This condition is associated with angiogenesis, apoptosis resistance, and resistance to cytotoxic therapies [26]. Depending on the chemotherapy regimen and type of cancer, thrombocytopenia and its severity vary; thus, platelets are transfused to prevent bleeding caused by reduced platelet counts after radiotherapy and chemotherapy [27].
Platelets are multifunctional immune cells that promote tumor growth and metastasis. Tumor cells form platelet-tumor cell aggregates, which prevent death induced by NK cells and TNF-α [28]. Platelets attracted to tumor sites release their load of vascular endothelial and platelet-derived growth factors and transforming growth factor beta, which contribute to tumor growth and proliferation [29]. However, platelet transfusion has side effects, such as a fever, allergy, bacterial contamination, transfusion-related acute lung injury, and thrombus formation.
RBC transfusions promote venous thromboembolism owing to increased hematocrit and viscosity [30]. With prolonged storage, fresh plasma tends to contain tumor-promoting cytokines. Transfusion-induced immunomodulation contributes to compromised immune surveillance and tumor cell growth, proliferation, and metastasis [29].
Studies have argued that poor outcomes in cancer patients receiving perioperative blood transfusions are due to clinical conditions requiring transfusions rather than blood transfusions [31]. Chronic anemia is frequently associated with sarcopenia, a decreased body mass index, and comorbidities [32]. It results from an advanced oncologic stage often associated with cachexia, consequent malnutrition, and exacerbation of anemia [33].
Patients with gastric cancer experience anemia due to malnourishment from large or obstructing tumors, which causes reduced oral feeding and tumor bleeding. Pernicious anemia is a late clinical presentation of well-known precancerous conditions such as multifocal atrophic gastritis [34, 35]. Neoadjuvant chemotherapy exacerbates anemia in patients with gastric cancer by negatively affecting RBC production, appetite, and the gastrointestinal function [36]. It is also a frequent side effect of imatinib, which is the standard treatment for gastrointestinal stromal tumors.
In liver cancer, hemoglobin levels decrease with increasing severity of liver cirrhosis [37]. Portal hypertension and cirrhosis result in gastrointestinal hemorrhaging, leading to iron deficiency. It can also be caused by reduced blood coagulation owing to impaired hepatocytes and a reduced thrombocyte count. Combination therapies for chronic hepatitis C virus infections, such as peginterferon, ribavirin, and direct-acting antivirals, also increase anemia [35].
Several limitations associated with the present study warrant mention. First, our multivariable model for 90-day mortality did not strongly indicate a dose–response relationship. Second, we could not evaluate the relationship between transfusion and tumor stage progression. Patients with locally advanced cancer may require extended surgical procedures associated with perioperative blood transfusion. While comprehensive data on tumor stage were not collected, our study used confounders, such as VATS or laparoscopy, neoadjuvant and adjuvant chemotherapy, and adjuvant radiotherapy, to indirectly account for tumor stage and surgical extent. Third, we included only major cancer surgeries, and our results might be identical for other cancer types, such as breast, thyroid, and esophageal cancers. Finally, we could not distinguish between patients who underwent curative surgery and those who underwent palliative surgery, which might be another confounding factor in this study.
In conclusion, the overall trend of perioperative blood transfusions among patients with major cancers in South Korea has decreased between 2016 and 2020. We identified the potential risk factors for perioperative blood transfusions. In addition, blood transfusions were found to be associated with an increased 90-day mortality risk after major cancer surgery.
References
Lin L, Yan L, Liu Y, Yuan F, Li H, Ni J. Incidence and death in 29 cancer groups in 2017 and trend analysis from 1990 to 2017 from the Global Burden of Disease Study. J Hematol Oncol. 2019;12:1–21.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018;68:394–424.
Schwartz RN. Anemia in patients with cancer: Incidence, causes, impact, management, and use of treatment guidelines and protocols. Am J Health Syst Pharm. 2007;64:S5–13.
Ludwig H, Van Belle S, Barrett-Lee P, Birgegård G, Bokemeyer C, Gascón P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer. 2004;40:2293–306.
Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–52.
Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168:2377–81.
Connor JP, O’Shea A, McCool K, Sampene E, Barroilhet LM. Peri-operative allogeneic blood transfusion is associated with poor overall survival in advanced epithelial ovarian Cancer; potential impact of patient blood management on Cancer outcomes. Gynecol Oncol. 2018;151:294–8.
Kamei T, Kitayama J, Yamashita H, Nagawa H. Intraoperative blood loss is a critical risk factor for peritoneal recurrence after curative resection of advanced gastric cancer. World J Surg. 2009;33:1240–6.
Rausei S, Ruspi L, Galli F, Tirotta F, Inversini D, Frattini F, et al. Peri-operative blood transfusion in gastric cancer surgery: prognostic or confounding factor? Int J Surg. 2013;11:S100–3.
Pacelli F, Rosa F, Marrelli D, Pedrazzani C, Bossola M, Zoccali M, et al. Do Perioperative Blood Transfusions Influence Prognosis of Gastric Cancer Patients? Analysis of 927 Patients and Interactions with Splenectomy. Ann Surg Oncol. 2011;18:1615–23.
Auvinen M-K, Zhao J, Lassén E, Lubenow N, Seger Mollén A, Watz E, et al. Patterns of blood use in Sweden from 2008 to 2017: a nationwide cohort study. Transfusion. 2020;60:2529–36.
Ecker BL, Simmons KD, Zaheer S, Poe S-LC, Bartlett EK, Drebin JA, et al. Blood transfusion in major abdominal surgery for malignant tumors: a trend analysis using the national surgical quality improvement program. JAMA Surg. 2016;151:518–25.
Roubinian NH, Escobar GJ, Liu V, Swain BE, Gardner MN, Kipnis P, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion. 2014;54:2678–86.
Sano T, Shimada K, Sakamoto Y, Esaki M, Kosuge T. Changing trends in surgical outcomes after major hepatobiliary resection for hilar cholangiocarcinoma: a single-center experience over 25 years. J Hepatobiliary Pancreat Surg. 2007;14:455–62.
de Boer MT, Molenaar IQ, Hendriks HG, Slooff MJ, Porte RJ. Minimizing blood loss in liver transplantation: progress through research and evolution of techniques. Dig Surg. 2005;22:265–75.
Hobeika C, Fuks D, Cauchy F, Goumard C, Soubrane O, Gayet B, et al. Impact of cirrhosis in patients undergoing laparoscopic liver resection in a nationwide multicentre survey. J British Surg. 2020;107:268–77.
Maritti M, Tritapepe L. Anesthesiologic management during surgery for hepatocellular carcinoma. In: Hepatocellular Carcinoma. Cham: Springer; 2022. p. 209–17.
Chen X, Feng X, Wang M, Yao X. Laparoscopic versus open distal gastrectomy for advanced gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized comparative studies. Eur J Surg Oncol. 2020;46:1998–2010.
Zhou S, Wang X, Zhao C, Liu Q, Zhou H, Zheng Z, et al. Laparoscopic vs open colorectal cancer surgery in elderly patients: short- and long-term outcomes and predictors for overall and disease-free survival. BMC Surg. 2019;19:137.
Li Z, Liu C, Liu Y, Yao S, Xu B, Dong G. Comparisons between minimally invasive and open esophagectomy for esophageal cancer with cervical anastomosis: a retrospective study. J Cardiothorac Surg. 2020;15:128.
Kent MS, Hartwig MG, Vallières E, Abbas AE, Cerfolio RJ, Dylewski MR, et al. Pulmonary open, robotic and thoracoscopic lobectomy (PORTaL) study: an analysis of 5721 cases. Ann Surg. 2022.
Al-Refaie WB, Parsons HM, Markin A, Abrams J, Habermann EB. Blood transfusion and cancer surgery outcomes: a continued reason for concern. Surgery. 2012;152:344–54.
de Goede B, Klitsie P, Lange J, Metselaar H, Kazemier G. Morbidity and mortality related to non-hepatic surgery in patients with liver cirrhosis; a systematic review. Best Pract Res Clin Gastroenterol. 2012;26:47–59.
Fearon KC, Jenkins JT, Carli F, Lassen K. Patient optimization for gastrointestinal cancer surgery. Br J Surg. 2012;100:15–27.
Anderson CD, Chari RS. Chemotherapy liver injury. Surgery. 2010;147:195–6.
Deschner M, Vasanthamohan L, Zayed S, Lazo-Langner A, Palma D, D’Souza D, et al. The impact of red blood cell transfusion on mortality and treatment efficacy in patients treated with radiation: a systematic review. Clin Transl Radiat Oncol. 2022;33:23–9.
Wu Y, Aravind S, Ranganathan G, Martin A, Nalysnyk L. Anemia and thrombocytopenia in patients undergoing chemotherapy for solid tumors: a descriptive study of a large outpatient oncology practice database, 2000–2007. Clin Ther. 2009;31(Pt 2):2416–32.
Wang J, Zhou P, Han Y, Zhang H. Platelet transfusion for cancer secondary thrombocytopenia: platelet and cancer cell interaction. Transl Oncol. 2021;14: 101022.
Goubran HA, Elemary M, Radosevich M, Seghatchian J, El-Ekiaby M, Burnouf T. Impact of transfusion on cancer growth and outcome. Cancer Growth Metastasis. 2016;9:32797.
Ramsey G, Lindholm PF. Thrombosis risk in cancer patients receiving red blood cell transfusions. Semin Thromb Hemost. 2019;45(6):648–56.
Tarantino I, Ukegjini K, Warschkow R, Schmied BM, Steffen T, Ulrich A, et al. Blood transfusion does not adversely affect survival after elective colon cancer resection: a propensity score analysis. Langenbecks Arch Surg. 2013;398:841–9.
Kwon HY, Kim BR, Kim YW. Association of preoperative anemia and perioperative allogenic red blood cell transfusion with oncologic outcomes in patients with nonmetastatic colorectal cancer. Curr Oncol. 2019;26:357–66.
Petrelli F, Ghidini M, Ghidini A, Sgroi G, Vavassori I, Petrò D, et al. Red blood cell transfusions and the survival in patients with cancer undergoing curative surgery: a systematic review and meta-analysis. Surg Today. 2021;51:1535–57.
Vannella L, Lahner E, Osborn J, Annibale B. Systematic review: gastric cancer incidence in pernicious anaemia. Aliment Pharmacol Ther. 2013;37:375–82.
Stein J, Connor S, Virgin G, Ong DEH, Pereyra L. Anemia and iron deficiency in gastrointestinal and liver conditions. World J Gastroenterol. 2016;22:7908.
Kouyoumdjian A, Trepanier M, Al Shehhi R, Cools-Lartigue J, Ferri LE, Lee L, et al. The effect of preoperative anemia and perioperative transfusion on surgical outcomes after gastrectomy for gastric cancer. J Surg Res. 2021;259:523–31.
Singh S, Manrai M, Parvathi V, Kumar D, Srivastava S, Pathak B. Association of liver cirrhosis severity with anemia: does it matter? Ann Gastroenterol. 2020;33:272.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, S., Song, IA. & Oh, T.K. The association of perioperative blood transfusion with survival outcomes after major cancer surgery: a population-based cohort study in South Korea. Surg Today 54, 712–721 (2024). https://doi.org/10.1007/s00595-023-02783-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-023-02783-w