Abstract
Background
Esophageal resection for cancer (EC) is still associated with considerable mortality and morbidity rates. Allogenic blood transfusion (aBT) is associated with poor short-term and long-term outcome in surgical oncology. We aimed to evaluate the effect of aBT in a homogeneous population of EC patients undergoing esophagectomy without perioperative treatment.
Methods
We analyzed 565 esophagectomies performed due to EC. Allogenic blood transfusion was correlated to clinicopathological parameters, perioperative mortality and morbidity as well as the long-term outcome. Results are presented as adjusted odds ratio (OR) or hazard ratio (HR) with 95 % confidence interval (95 % CI).
Results
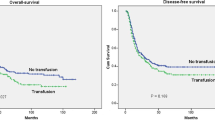
Patients receiving aBT (aBT(+)) had no higher tumor stages or higher rates of lymph node metastasis (P = 0.65 and 0.17, respectively) compared to patients without aBT (aBT(−)). Allogenic blood transfusion was strongly associated with perioperative morbidity (OR 1.9, 95 % CI 1.1–3.5, P = 0.02) and mortality (OR 2.9, 95 % CI 1.0–8.6, P = 0.04). Tumor recurrence rate was significantly higher in aBT(+) patients (P = 0.001). The disease-free and overall survival were significantly longer in aBT(−) compared to aBT(+) patients (P = 0.016 and <0.001, respectively). Patients receiving aBT had almost doubled risk for tumor recurrence (HR 1.8, 95 % CI 1.2–2.5, P = 0.001) and death (HR 2.2, 95 % CI 1.5–3.2, P < 0.001).
Conclusion
Allogenic blood transfusion has a significant impact on the natural course of EC after complete resection. The poor short-term and long-term outcome warrants further evaluation of the underlying molecular mechanisms induced by allogenic blood transfusion in cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Incidence of esophageal cancer (EC) is steadily rising worldwide [1]. Despite significant improvement in long-term survival, the perioperative mortality and morbidity associated with esophagectomy for EC, even in high volume centers, remains high [2]. The reported morbidity and mortality rates associated with esophageal resection reach up to 50 and 20 %, respectively [3, 4]. Esophageal resection represents a major operation associated with potentially high blood loss and long intensive care unit stay. Allogenic blood transfusion (aBT) is frequently performed in EC patients even in non-anemic patients [5]. Besides the potential risk of infectious disease transmission and mismatch incompatibility, aBT has recently been identified as an independent risk factor for perioperative outcome in terms of mortality and morbidity [5]. Furthermore, aBT has been associated with higher rates of tumor recurrence and poor survival in various types of cancer. In addition, in several entities, including EC, autologous blood transfusion (autoBT) or transfusion of white blood cell (WBC)-depleted blood has been associated with a more favorable perioperative and long-term outcome compared to patients with aBT [6–11]. Previously, few studies attempted to address the role of aBT on the clinical outcome in EC. These studies were biased by heterogeneity of the study population, especially in terms of applied treatment regimens [7, 12]. To evaluate the effect of aBT on perioperative and long-term outcome, it is of importance to define a homogeneous study population since any type of systemic therapy or radiation will result in immune modulation that is impossible to detach from the effects initiated by aBT. Furthermore, previous studies only focused on either short- or long-term outcome. The aim of this study was to evaluate the effect of aBT on perioperative mortality and morbidity as well as long-term outcome in patients undergoing complete resection for EC without perioperative treatment.
Patients and methods
The study was approved by the medical ethics committee of the Chamber of Physicians of Hamburg. A total of 714 patients with esophageal cancer underwent esophageal resection at our institution between 1992 and 2010. Only patients with histopathologically proven EC and tumor-free resection margins were included into the study. In total, 565 patients were included in this study. Only patients with local R0 and distant R0 status were included. 24 patients had lung metastases, which were not detected by the preoperative staging. These lung metastases were detected during the thoracic part after completing the abdominal part of the esophagectomy. Thus, these patients underwent R0 lung metastasectomy during esophagectomy. None of the patients received perioperative or postoperative treatment. Informed consent was obtained from all patients before including them in a prospective database. All patients had a detailed preoperative assessment of the general health condition and organ function evaluation. Routine tumor staging included esophago-gastro-duodenoscopy, computed tomography and blood tests. The perioperative mortality was defined as 30-day post-hospital discharge mortality. The perioperative morbidity included only major complications in which further medical or surgical intervention was necessary (Clavien Dindo III–IV) within 30 days after discharge [13]. Clinical follow-up data were obtained by studying the patient clinical charts or by contacting them on an outpatient basis. To evaluate the true impact of aBT on the clinical outcome in EC, we did not define any cutoff points but only compared patients who received (aBT(+)) and who did not receive blood transfusion (aBT(−)) perioperatively. aBT was defined as any aBT in the perioperative in-hospital course. Indication for aBT was primarily based upon patient’s condition. The anemia threshold for transfusion in patients with coronary heart disease was 10 g/dl and in patients without heart disease 7 g/dl. In case of acute life-threatening bleeding, the decision for aBT was made by the doctor in charge. In a hemodynamic stable patient, the indication was primarily based upon surgeon’s discretion. None of the patients received autoBT. Application of leukocyte-depleted blood due to presence of comorbidities resulted in exclusion of the patient from this study.
Statistical analysis
For statistical analysis, SPSS for Windows (IBM SPSS Statistics for Windows, Version 20.0. Released 2011. Armonk, NY: IBM Corp.) was used. For correlation of clinicopathological parameters and aBT, the Chi-square test was used. For variables with a continuous scale, the Mann–Whitney U test was applied. To evaluate the effect of aBT on perioperative morbidity and mortality, univariate and multivariable logistic regression analyses were performed and adjusted odds ratio (OR) with 95 % confidence interval (95 % CI) calculated. Disease-free (DFS) and overall survival (OS) curves of the patients were plotted using the Kaplan–Meier method and analyzed using the log-rank test. The OS was computed as the time period from the date of surgery to either the date of death or last follow-up, whichever occurred first. The DFS was defined as the time period from the date of surgery to the date of recurrence, last follow-up or date of death, whichever occurred first. Univariate and multivariate Cox regression analyses were performed to determine the adjusted hazard ratio (HR) for tumor recurrence and overall survival. Significant statements refer to P values of two-tailed tests that were <0.05.
Results
Characterization of the study population
Out of 714 patients with esophageal resection at our institution between 1992 and 2010, 103 patients had positive resection margins, 13 patients were resected for diseases different from EC, and 33 patients were lost to follow-up or had incomplete data and were therefore excluded from this study. Complete data and follow-up were available from 565 patients. All 565 patients had histopathologically proven EC. None of the patients received neoadjuvant therapy. Distribution according to tumor type (SCC or AC) and operating technique (TH or TA) was balanced among the study population. Median age of the study population was 63.2 years (range 34.5–85.2). Table 1 depicts patient characteristics of the entire study population. Out of 565 patients, 93 (16.5 %) did not receive aBT. Overall perioperative mortality accounted for 14.6 % (N = 81) and perioperative morbidity for 34.4 % (N = 194) in the entire study population.
Correlation of aBT with clinicopathological parameters and perioperative outcome
Table 1 depicts the result of the correlation of aBT with clinicopathological parameters. Patients requiring aBT had lower median preoperative hemoglobin level and displayed higher intraoperative median blood loss (P = 0.003 and <0.001). The median operating time in aBT(+) patients was significantly longer (P = 0.01). Interestingly, aBT(+) had not significantly more advanced disease as reflected by tumor size, presence of lymph node metastasis and tumor grading compared to aBT(−) patients (P = 0.65, P = 0.17 and P = 0.46, respectively). However, patients with aBT(+) had significant higher rates of tumor recurrence (P = 0.001).
The transfusion of allogenic blood was associated with perioperative outcome in terms of morbidity and mortality. Table 2 depicts the results of the multivariable analysis for perioperative morbidity and mortality. Patients with aBT had an adjusted OR of 1.9 (95 % CI 1.1–3.5, P = 0.02) for perioperative morbidity and 2.9 and 95 % CI 1.0–8.6, P = 0.04 for perioperative mortality.
aBT and clinical outcome
To verify that our study group was representative for patients with EC, we calculated the OS according to the seventh edition of the Union International Contre le Cancer (UICC). The OS was found to be dependent upon AJCC stage and comparable to the published data by other groups. The median OS was 21.6 months (95 % CI 18.1–25.1). The stage-specific OS for stage I to IV was 47.7, 39.3, 29.1, 20.5 and 7.4 months, respectively. Patients, who died perioperatively, were excluded from the survival analysis. During the observation period, 212 (51.7 %) patients experienced a relapse of the disease and 258 (62.9 %) patients died. Kaplan–Meier curves plotted for DFS and OS showed a marked decrease in survival between aBT(−) and aBT(+) patients (Fig. 1; Table 3).
A stratified sub-analysis for tumor type, nodal status, operating technique and aBT was performed. Table 3 depicts the results of the stratified sub-analyses. Interestingly, throughout all sub-analyses aBT(−) patients displayed a significant better DFS and OS compared to aBT(+) patients.
aBT a prognostic factor for recurrence and survival
Multivariable analysis according to the Cox regression hazard model using age, sex, tumor size, presence of lymph node and distant metastasis, tumor grading, tumor type, operating technique and aBT for tumor recurrence and overall survival were performed. Allogenic blood transfusion was strongly associated with recurrence (adjusted HR 1.8, 95 % CI 1.2–2.5, P = 0.001) and death (HR 2.2, 95 % CI 1.5–3.2, P < 0.001) (Table 4).
Discussion
This study, consisting of 565 homogeneously, surgically, treated patients revealed that aBT is strongly associated with perioperative and long-term outcome in EC patients.
Over the last two decades, aBT has moved into the limelight of being an independent risk factor for morbidity and mortality in non-oncological and oncological patients [5, 14]. Although the attitude toward transfusion has changed, it remains an individual decision. The landmark transfusion requirements in critical care trial (TRICC trial) is the only prospective randomized study reporting on adverse outcome associated with aBT in the liberal group (hemoglobin <10 g/dl) compared to the restrictive group (hemoglobin <7 g/dl) [15]. Importantly, in patients under 55 years a significant association between increased mortality and liberal transfusion attitude was reported. In addition, a recent study demonstrated an association between aBT at young age and increased risk of developing non-Hodgkin lymphoma [16]. These findings not only pinpoint toward the severity of the impact of aBT on short-term outcome but also imply to a long-lasting immune modulatory effect of aBT. These long-lasting effects result in poor clinical outcomes as it has been shown for cardiac and oncological patients [17–20].
Majority of our patients did not suffer of anemia which has been linked to increased morbidity and mortality [21, 22]. Interestingly, Corwin et al. [23] analyzing anemia and blood transfusion in the critically ill reported that anemia only predicted the probability of transfusion but did not correlate with the outcome. Kulier et al. [24] identified preoperative hemoglobin and aBT as independent risk factors for adverse clinical outcome. At the same hemoglobin level, however, risk of adverse outcome increased out of proportion with the number of blood units. In particular, increased risk of infection, prolonged ventilation, cardiovascular events and renal failure have been linked to aBT(+) [25]. These findings support our data, especially as aBT was besides the tumor size the only significant prognostic factor for prediction of perioperative morbidity.
Several studies suggest association between aBT and tumor recurrence and survival in various tumor types [26–29]. The impact of the immune modulation initiated by aBT has first been shown in transplantation where patients with aBT-induced immunosuppression presented prolonged graft-survival [30]. Allogenic blood transfusion is known to reduce natural killer cell activities and T lymphocyte blastogenesis and increase suppressor T lymphocyte activities [31]. These cells do not only prevent dissemination of circulating and quiescent cancer cells but are also important for resistance toward infection [5]. The aBT-related immune modulation is a sum of preexistent patient characteristics and transfused factors [5]. Interestingly, studies comparing aBT and autoBT underline the association between aBT and poor clinical outcome since patients receiving autoBT have been reported to present significantly better perioperative and long-term outcome in EC compared to patients receiving aBT [8, 12]. The reported role of aBT on perioperative outcome and long-term survival is heterogeneous in EC. Melis et al. [32] correlated the clinical outcome with regard to neoadjuvant treatment, anemia and perioperative complications in patients undergoing esophagectomy for cancer. This study excluded anemia as an independent factor and verified the significance of aBT for prediction of postoperative complications. Infectious complications are more likely in the postoperative course in patients with aBT in contrast to patients receiving autoBT [33]. Furthermore, autoBT is superior over aBT comparing immune response by quantification of circulating immune-competent cells in neoadjuvantly treated patients undergoing resection for EC [12]. Motoyama et al. [8] reported on prolonged DFS in autoBT group in recurrent EC. In contrast to these studies, Nozoe et al. did not identify aBT as an independent prognosticator of survival in EC patients and Ling et al. demonstrated that aBT resulted in poorer survival but white blood cell-depleted blood transfusion did not improve the outcome [7, 34]. Furthermore, Kader et al. [35] were able to demonstrate that aBT had a survival benefit in EC patients treated by radio-chemotherapy only.
In our study, aBT(+) patients demonstrated throughout a poorer outcome compared to aBT(−) patients. This effect remained apparent even after stratification of the study population to the underlying tumor type, disease stage and operating technique. Taken together, these findings indicate an early aBT initiated immune modulatory effect that lasts long. Furthermore, we have been able to demonstrate a prognostic significance of aBT as a marker for perioperative morbidity, mortality and oncological outcome in one large homogenous EC population. Limitation of our study is the retrospective nature and lack of determination of immuno modulatory mediators like CD4, CD8 and natural killer cells or interleukin levels and incorporation of these factors in our comparative analysis.
In conclusion, we were able to demonstrate an association between aBT and morbidity, mortality, tumor recurrence and overall survival in a homogeneously, only surgically, treated EC population. The findings warrant an urgent re-evaluation of the current attitude toward aBT to improve the unsatisfying perioperative and long-term outcome in EC patients.
Abbreviations
- aBT:
-
Allogenic blood transfusion
- AC:
-
Adenocarcinoma
- AJCC:
-
American Joint Committee on Cancer
- autoBT:
-
Autologous blood transfusion
- CD:
-
Cluster of differentiation
- CI:
-
Confidence interval
- DFS:
-
Disease-free survival
- EC:
-
Esophageal cancer
- HR:
-
Hazard ratio
- OR:
-
Odds ratio
- OS:
-
Overall survival
- SCC:
-
Squamous cell carcinoma
- TA:
-
Thoracoabdominal
- TH:
-
Transhiatal
- TRICC:
-
Transfusion requirements in critical care
- WBC:
-
White blood cell
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Makowiec F, Baier P, Kulemann B et al (2013) Improved long-term survival after esophagectomy for esophageal cancer: influence of epidemiologic shift and neoadjuvant therapy. J Gastrointest Surg 17:1193–1201
Wouters MW, Krijnen P, Le Cessie S et al (2009) Volume- or outcome-based referral to improve quality of care for esophageal cancer surgery in The Netherlands. J Surg Oncol 99:481–487
Pennathur A, Luketich JD (2008) Resection for esophageal cancer: strategies for optimal management. Ann Thorac Surg 85:S751–S756
Dixon E, Datta I, Sutherland FR, Vauthey JN (2009) Blood loss in surgical oncology: neglected quality indicator? J Surg Oncol 99:508–512
Rovera F, Dionigi G, Boni L et al (2006) Postoperative infections after oesophageal resections: the role of blood transfusions. World J Surg Oncol 4:80
Nozoe T, Miyazaki M, Saeki H, Ohga T, Sugimachi K (2001) Significance of allogenic blood transfusion on decreased survival in patients with esophageal carcinoma. Cancer 92:1913–1918
Motoyama S, Okuyama M, Kitamura M et al (2004) Use of autologous instead of allogeneic blood transfusion during esophagectomy prolongs disease-free survival among patients with recurrent esophageal cancer. J Surg Oncol 87:26–31
Hajjar LA, Vincent JL, Galas FR et al (2010) Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 304:1559–1567
Lange MM, van Hilten JA, van de Watering LM et al (2009) Leucocyte depletion of perioperative blood transfusion does not affect long-term survival and recurrence in patients with gastrointestinal cancer. Br J Surg 96:734–740
Komatsu Y, Orita H, Sakurada M, Maekawa H, Hoppo T, Sato K (2012) Intraoperative blood transfusion contributes to decreased long-term survival of patients with esophageal cancer. World J Surg 36:844–850. doi:10.1007/s00268-012-1433-3
Takemura M, Osugi H, Higashino M, Takada N, Lee S, Kinoshita H (2005) Effect of substituting allogenic blood transfusion with autologous blood transfusion on outcomes after radical oesophagectomy for cancer. Ann Thorac Cardiovasc Surg 11:293–300
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Goodnough LT, Shander A, Spivak JL et al (2005) Detection, evaluation, and management of anemia in the elective surgical patient. Anesth Analg 101:1858–1861
Hebert PC, Wells G, Blajchman MA et al (1999) A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 340:409–417
Cerhan JR, Engels EA, Cozen W et al (2008) Blood transfusion, anesthesia, surgery and risk of non-Hodgkin lymphoma in a population-based case–control study. Int J Cancer 123:888–894
Doyle BJ, Rihal CS, Gastineau DA, Holmes DR Jr (2009) Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol 53:2019–2027
Cornes P, Coiffier B, Zambrowski JJ (2007) Erythropoietic therapy for the treatment of anemia in patients with cancer: a valuable clinical and economic option. Curr Med Res Opin 23:357–368
Wu WC, Smith TS, Henderson WG et al (2010) Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery. Ann Surg 252:11–17
Bernard AC, Davenport DL, Chang PK, Vaughan TB, Zwischenberger JB (2009) Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg 208:931–937 7 e1–e2; discussion 8–9
Patel MS, Carson JL (2009) Anemia in the preoperative patient. Med Clin N Am 93:1095–1104
Karkouti K, Wijeysundera DN, Beattie WS (2008) Reducing bleeding in cardiac surgery I. Risk associated with preoperative anemia in cardiac surgery: a multicenter cohort study. Circulation 117:478–484
Corwin HL, Gettinger A, Pearl RG et al (2004) The CRIT study: anemia and blood transfusion in the critically ill–current clinical practice in the United States. Crit Care Med 32:39–52
Kulier A, Levin J, Moser R et al (2007) Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation 116:471–479
Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD (2007) Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 116:2544–2552
Weber RS, Jabbour N, Martin RC II (2008) Anemia and transfusions in patients undergoing surgery for cancer. Ann Surg Oncol 15:34–45
Sugita S, Sasaki A, Iwaki K et al (2008) Prognosis and postoperative lymphocyte count in patients with hepatocellular carcinoma who received intraoperative allogenic blood transfusion: a retrospective study. Eur J Surg Oncol 34:339–345
Kneuertz PJ, Patel SH, Chu CK et al (2011) Effects of perioperative red blood cell transfusion on disease recurrence and survival after pancreaticoduodenectomy for ductal adenocarcinoma. Ann Surg Oncol 18:1327–1334
Wang T, Luo L, Huang H et al (2014) Perioperative blood transfusion is associated with worse clinical outcomes in resected lung cancer. Ann Thorac Surg 97:1827–1837
Opelz G, Terasaki PI (1978) Improvement of kidney–graft survival with increased numbers of blood transfusions. N Engl J Med 299:799–803
Baumgartner JM, Silliman CC, Moore EE, Banerjee A, McCarter MD (2009) Stored red blood cell transfusion induces regulatory T cells. J Am Coll Surg 208:110–119
Melis M, McLoughlin JM, Dean EM et al (2009) Correlations between neoadjuvant treatment, anemia, and perioperative complications in patients undergoing esophagectomy for cancer. J Surg Res 153:114–120
Kinoshita Y, Udagawa H, Tsutsumi K et al (2000) Usefulness of autologous blood transfusion for avoiding allogenic transfusion and infectious complications after esophageal cancer resection. Surgery 127:185–192
Ling FC, Hoelscher AH, Vallbohmer D et al (2009) Leukocyte depletion in allogeneic blood transfusion does not change the negative influence on survival following transthoracic resection for esophageal cancer. J Gastrointest Surg 13:581–586
Kader AS, Lim JT, Berthelet E, Petersen R, Ludgate D, Truong PT (2007) Prognostic significance of blood transfusions in patients with esophageal cancer treated with combined chemoradiotherapy. Am J Clin Oncol 30:492–497
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Matthias Reeh, Tarik Ghadban and Josephine Dedow have contributed equally to this work and therefore share first authorship.
Rights and permissions
About this article
Cite this article
Reeh, M., Ghadban, T., Dedow, J. et al. Allogenic Blood Transfusion is Associated with Poor Perioperative and Long-Term Outcome in Esophageal Cancer. World J Surg 41, 208–215 (2017). https://doi.org/10.1007/s00268-016-3730-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-016-3730-8