Abstract
Gastrointestinal tract (GI) perforations can occur due to various causes such as trauma, iatrogenic factor, infectious condition, peptic ulcer, inflammatory disease, or a neoplasm. Because GI perforations represent an emergency and life-threatening condition, prompt diagnosis and surgical treatment are required in most cases. However, according to the underlying causes of GI perforations, additional treatment strategies may be needed. Adjuvant chemotherapy or immunotherapy may be required in various GI neoplasms such as adenocarcinoma, lymphoma or gastrointestinal stromal tumor. Inflammatory bowel disease is a chronic disease repeating cycle of intermittent, thus appropriate medical treatment and periodic follow-up are also required. Moreover, vascular intervention may have a role in some cases of mesenteric ischemia associated with mesenteric artery occlusion. Recently, computed tomography (CT) has been the first choice for patients with suspected GI perforations, because CT plays an important role in the accurate assessment of the perforation site, the pathology causing the perforation and the ensuing complications. This review will illustrate characteristic CT findings that differentiate underlying pathologies causing GI perforations to help clinicians decision-making regarding an optimal treatment plan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastrointestinal tract (GI) perforations can occur secondary to various conditions, including traumatic and iatrogenic causes, peptic ulcers, neoplasms, or inflammation [1, 2]. Most perforations are life-threatening and require prompt diagnosis and surgical treatment. Presently, various therapeutic options, including conservative therapy, endoscopic and laparoscopic procedures, as well as conventional laparotomy are available to manage this condition. To treat GI perforations following surgery, additional medical therapy may be required in a few cases to treat the underlying disease that caused the perforation. Accurate determination of the site and cause of GI perforations is important to plan optimal treatment for non-traumatic GI perforations [3]. Computed tomography (CT) plays an important role in the accurate assessment of the perforation site, the pathology causing the perforation and the ensuing complications [4]. This article focuses on characteristic CT findings that differentiate underlying pathologies causing GI perforations (except traumatic and iatrogenic GI perforation) to facilitate decision-making regarding an optimal treatment plan.
CT technique
Our abdominal CT protocol for evaluating acute abdomen should include the entire abdomen from the diaphragm to the pelvic floor. Intravenous contrast material is necessary unless contraindications exist. After precontrat CT scan, 150 ml of contrast material is administered at a flow rate of 3–4 ml/s using a mechanical injector. Contrast-enhanced CT scan is performed at a fixed delay of 75 s. The arterial phase CT is optional for evaluating arterial GI bleeding.
Although extraluminal leakage of the oral contrast is a specific sign of GI perforations, oral contrast material is not administrated due to safety issue such as aspiration and subsequent complication [1, 2]. Contiguous axial images less than 5-mm thickness are obtained, and multi-planar reconstruction may be applied when necessary. In addition to routine abdomen window setting, lung window setting is recommended for detecting extraluminal air.
General CT findings of GI perforation
Direct and indirect CT findings are useful to identify and localize a GI perforation. Direct signs include the following: (a) discontinuity of the GI wall, (b) the presence of extraluminal air and, (c) extraluminal leakage of oral contrast (Fig. 1) [4,5,6]. Direct visualization of a GI wall defect is a pathognomonic finding; however, it is observed in < 50% of patients with GI perforation. Multi-planar reformation images may be helpful in equivocal cases (Fig. 1). The presence of extraluminal air is highly specific for the diagnosis of GI perforations, and CT is highly sensitive in detecting even small quantities of free air. CT performed using lung window settings can detect even a small amount of free air (Fig. 1). The quantity and location of free air help in localizing the site of perforation. Concentrated free air in close proximity to the bowel indicates the site of perforation [3]. Indirect signs include segmental wall thickening, abnormal bowel enhancement, perivisceral fat stranding, inflammatory changes (abscess or an inflammatory mass related to the bowel), or a fluid collection in the surrounding organs [2].
A 57-year-old man with gastric ulcer perforation. a CT scan with lung window setting shows tiny free air bubbles (arrows) around the liver. b Up-right chest x-ray shows no free air under the right hemidiaphragm. C Axial contrast-enhanced CT scan shows a focal ulceration (arrow) with perigastric fat stranding in the anterior wall of the gastric antrum. d Coronal-reformatted CT image shows a focal defect (arrow) in the gastric ulcer
CT findings based on causes of GI perforation
Neoplastic conditions
Adenocarcinoma
Gastric adenocarcinoma is the most common type of primary gastric malignancy. However, perforation is rarely observed with this malignancy with a reported incidence rate of 0.4–6% [7]. Perforation of gastric cancer frequently occurs at the tumor site secondary to tumor necrosis and ischemia and is usually observed in elderly patients with advanced-stage disease. However, it may occur in patients with early gastric cancer with deep ulcerated lesions. CT evaluation can indicate underlying gastric cancer based on the following findings: focal or diffuse gastric wall thickening with inhomogeneous enhancement, perigastric soft tissue extension, or invasion of surrounding organs (Fig. 2). Metastatic lymphadenopathy or distant metastasis provides additional clues to diagnose cancer [8]. However, the preoperative assessment of the exact tumor extent is difficult owing to secondary inflammatory changes and peritonitis in patients with perforated cancer.
A 45-year-old man with perforated gastric adenocarcinoma. a Contrast-enhanced CT scan shows a large amount of pneumoperitoneum (asterisks) around the liver. b Contrast-enhanced CT scan shows a focal wall defect (arrow) and extraluminal air bubbles. Irregular thickening with the enhancement of the gastric wall (arrowheads) suggests underlying gastric cancer. c The resected specimen of the subtotal gastrectomy shows a relatively well-defined ulcerative mass (arrowheads) located in the antrum of the stomach. The perforated mucosa (arrow) is noted in the center of an ulcerative gastric cancer
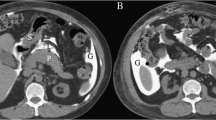
Perforation in patients with colorectal adenocarcinoma is uncommon, with a reported incidence ranging from 1.2–10% [9]. Such a perforation can occur at the tumor site or at some distance from the tumor, the latter being referred to as diastatic perforation. Perforation at the tumor site occurs most frequently in right-sided colon cancers secondary to tumor necrosis [10]. Diastatic perforation of the cecum commonly occurs in left-sided colon cancers, which undergo complete stenosis with consequent upwards colon distension [10]. A competent ileocecal valve plays a role in diastatic perforation (Fig. 3). Colon cancer may cause obstructive colitis which refers to ulcero-inflammatory and necrotizing condition in the colon proximal to stenosing lesions, and the ischemic segment of obstructive colitis can be complicated with bowel perforation [11]. CT findings of underlying colon cancer include focal colonic wall thickening or mass lesion with luminal narrowing (Fig. 3). The clinical manifestations of a perforation are determined by tumor location in that right-sided colonic perforations commonly present as free perforations with peritonitis, whereas left-sided colonic perforations commonly present as localized abscess formation rather than free perforation [12]. Identification of irregular focal wall thickening of the adjacent colonic segments is important to differentiate cancer-induced colonic perforation from that caused by various benign conditions. However, severe pericolic inflammation with a large abscess or peritoneal spillage may impede accurate diagnosis because these situations can overlap various conditions with colonic perforation [13].
A 78 year-old man with diastatic perforation of the cecum due to ascending colon adenocarcinoma. a Axial contract-enhanced CT scan shows irregular focal wall thickening with enhancement (arrow) in the ascending colon. Extraluminal gas bubbles (arrowheads) are visualized in the intraperitoneal cavity. b Coronal-reformatted contrast-enhanced CT scan shows a focal wall defect (arrowhead) in the cecum proximal to the tumor. A competent ileocecal valve (arrow) is visualized
Although perforated GI adenocarcinoma is associated with a high morbidity and mortality rate, the long-term outcome is not worse compared to that associated with non-perforated cancer. Notably, the tumor stage is more important in determining the long-term survival rate [9, 14].
Lymphoma
GI perforation is a potentially life-threatening complication of lymphoma affecting the GI tract. It commonly occurs in patients with T-cell lymphoma, post-transplant lymphoproliferative disease, and patients undergoing chemo and radiotherapy. Non-Hodkin’s lymphoma of B-cell origin is most common type of GI lymphoma, the prevalence of perforation is higher in patients with T-cell lymphoma (approximately 50%) than in patients with B-cell lymphoma (< 30%) [15]. The small intestine is the most common site of perforation (59%) in lymphoma, followed by the large intestine (22%), and the stomach (16%) [16].
A previous study has reported that the incidence of perforation in patients with GI lymphoma was 9% and that in approximately 50% of cases the perforation occurred at the initial presentation of lymphoma, and in the remaining cases it occurred after the initiation of chemotherapy (within the first 4 weeks of treatment) [16]. The mechanism of perforation in GI lymphoma could include the following processes: [1] lymphoma itself injures the intestinal microvasculature resulting in ischemia or [2] treatment-related complications such as neutropenic colits, infectious colitis, radiation enteritis or colonic pseudo-obstruction may cause perforation [16].
Typical CT findings of underlying GI lymphoma include a circumferentially homogeneous thickened segment with aneurysmal dilatation of the lumen, multifocal bowel involvement, lymphadenopathy, and/or hepatosplenomegaly (Fig. 4). However, bowel wall thickening caused by T-cell lymphoma is not as severe as that caused by B-cell lymphoma. Moreover, because the small bowel is a preferential site of involvement, CT signs of perforation are subtle; the detection of focal wall defects may be limited, and only a small amount of extraluminal air or a localized fluid collection without air is detected (Fig. 5) [16].
A 57-year-old woman with perforated diffuse large B-cell lymphoma in the small bowel. a, b Contrast-enhanced CT scans show segmental hypoattenuated wall thickening (arrows) with a focal wall defect (arrowhead) and dilated bowel lumen. Homogeneous enlarged lymphadenopathy is observed in the small bowel mesentery (white asterisks) and left paraaortic area (black asterisk). c The resected specimen of the jejunal segmental resection shows the perforated mucosa (arrow) in the erythematous elevated lesion (lymphoma) of the jejunum
A 63-year-old man with perforated enteropathy-type peripheral T-cell lymphoma in the small bowel. a Upper gastrointestinal series image shows contrast leakage (arrow) at the proximal jejunum. b, c Contrast-enhanced CT scans do not show a wall defect or extralunminal free air. Mild wall thickening (arrows) with luminal dilatation is visualized in the proximal jejunum
Gastrointestinal stromal tumors
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of the GI tract and accounts for 2.5% of all GI tumors. Spontaneous rupture of GISTs can cause a GI perforation, although it is rare [17]. Larger tumors are more vulnerable to rupture because of intratumoral necrosis. Moreover, exophytic morphology, internal cystic necrosis, and a rapid growth rate are other risk factors associated with ruptured GISTs [8]. On CT imaging, ruptured GISTs appear heterogeneous lesions with irregular margins, and internal heterogeneous areas with a lamellated or whorled pattern, reflecting necrosis or hemorrhage (Fig. 6). An air-fluid level may be associated with these lesions secondary to the formation of a fistula between an adjacent bowel loop and the primary tumor (Fig. 6). However, pneumoperitoneum does not commonly occur following GIST rupture because capsular perforation of GISTs usually causes massive bleeding resulting in hemoperitoneum. Ascites is an uncommon presentation of non-ruptured GISTs, even in patients with advanced peritoneal dissemination. Therefore, GIST rupture should be suspected in cases of ascites or hemoperitoneum accompanying these tumors [8, 18]. GIST rupture increases the risk of tumor recurrence secondary to peritoneal seeding; thus, adjuvant treatment with tyrosine kinase inhibitors should be considered in such cases [19].
A 59-year-old man with ruptured ileal GIST. Axial (a) and coronal-reformatted (b) contrast-enhanced CT scans show a heterogeneous necrotic ileal mass (arrows) with exophytic growth. Intratumoral gas (asterisk), disruption of the tumor wall (arrowhead), and peritumoral inflammatory stranding suggest tumor rupture. The mass was confirmed as a high risk of malignant potential GIST
Metastasis
GI metastasis can cause serious complications, such as perforation, obstruction, intussusception, and hemorrhage. Perforation of GI metastasis is an emergency and is associated with a high mortality risk and poor prognosis. The mechanism of perforation of GI metastases is attributed to spontaneous tumor necrosis, chemotherapy-induced necrosis, or increased luminal pressure secondary to bowel obstruction [20].
Common primary tumors that cause perforation of GI metastases include lung, colon, uterine, ovarian, and breast cancers, as well as melanoma [21]. Lung cancer metastases show a greater tendency to undergo necrosis than that observed with other malignant metastases. The small bowel is commonly involved and the jejunum is more commonly affected by the perforation than the ileum. CT findings of GI metastases are nonspecific such as an intraluminal polypoid mass or wall thickening with variable enhancement (Fig. 7). However, most cases of GI perforations show features of wall thickening. Only a small quantity of free air is observed in the vicinity of the perforated bowel because the small bowel is the preferred site of perforation [22].
A 37-year-old man who underwent total gastrectomy for gastric cancer, with perforated metastatic colon cancer. Axial (a, b) and coronal-reformatted (c) contrast-enhanced CT scans show focal wall thickening with enhancement (arrows) and stricture in the transverse colon. Adjacent abscess formation (arrowheads in b, c) with minimal quantity of extraluminal air is visualized, suggesting sealed-off perforation of metastatic colon cancer, although the wall defect is not definitely visualized on CT. Metastaic adenocacinoma of the transverse colon was confirmed by colonosopic biopsy
Appendiceal mucinous neoplasm
An appendiceal mucinous neoplasm is usually larger and ruptures more easily than a non-neoplastic mucocele because it is associated with continued mucin production and neoplastic excretion. The tumor penetrates the appendiceal wall and causes localized sealing-off of the appendix, or it may progress to diffuse intraperitoneal mucious ascites (referred to pseudomyxoma peritonei) over months or even years. Pseudomyxoma peritonei causes intestinal obstruction and is often fatal [23].
Accurate preoperative diagnosis of mucinous cystic neoplasms is important for appropriate surgical treatment. The typical CT findings of an appendiceal mucocele are a thin-walled cystic dilated appendix (> 1.3 cm) occasionally showing mural calcification (Fig. 8). A focal wall defect with localized or diffuse extra-appendiceal low-attenuation mucinous fluid indicates a perforation (Fig. 8). Scalloping of the visceral surface of the liver or spleen is the characteristic feature of pseudomyxoma peritonei [8]. Patients with a mucinous adenocarcinoma show a high risk of perforation and poorer prognosis than patients with low-grade appendiceal mucinous neoplasm because cellular mucin spreading beyond the right lower quadrant occurs more aggressively in the former. CT evidence of enhancing wall nodularity or irregularity raises the suspicion of mucinous adenocarcinoma [24].
A 64-year-old woman with perforated appendiceal mucinous neoplasm. Axial (a) and coronal-reformatted (b) contrast-enhanced CT scans show a dilated appendix (asterisks) with rim calcification. An appendiceal wall defect (arrow) and localized fluid collection (arrowheads) are observed and represent pseudomyxoma peritonei. The mass was confirmed as an appendiceal mucinous adenocarcinoma
Advanced stage and high-grade lesions, spread of mucus beyond the right lower quadrant, and cellular extra-appendiceal mucin are significant prognostic factors in these cases. Perforation and spillage of cellular mucin even when localized to the right lower quadrant is associated with a greater risk of recurrence than that observed with extra-appendiceal acellular mucin. Appendectomy or cecectomy is preferred in patients of perforated low-grade appendiceal mucinous neoplasm with extra-appendiceal acellular mucin, whereas laparotomy may be required to confirm peritoneal dissemination in cases with cellular mucin spread. Patients with peritoneal deposits should undergo additional surgical debulking and intraperitoneal chemotherapy [23, 25].
Non-neoplastic conditions
Peptic ulcer disease
Peptic ulcer disease is a major cause of non-traumatic gastroduodenal perforation. The lifetime prevalence of perforation in patients with peptic ulcer disease is approximately 5%. Perforated peptic ulcer (PPU) often presents with an acute abdomen with a high mortality rate ranging from 13–20%. Risk factors associated with PPU include older age, multiple comorbidities, and the use of nonsteroidal anti-inflammatory drugs (NSAIDs) or steroid [26].
PPU is commonly identified in the gastric antrum and the duodenal bulb, whereas traumatic perforations occur in the 2nd or the 3rd portion of the duodenum. Characteristic CT findings of PPU include ulceration of the gastroduodenal wall with preserved normal gastric wall stratification (Fig. 9). Strong enhancement of the ulcer, marked periulceral wall thickening, and loss normal wall stratification are findings that favor the presence of a malignant ulcer. Pneumoperitoneum is the most sensitive finding, and can be extensive in the intra-abdominal cavity (Fig. 9). Air bubbles in contact with the stomach or duodenum is a specific finding in patients with PPU. Perigastric or periduodenal fluid, and adjacent fat stranding are sensitive, but less specific findings [27].
A 45-year-old man with gastric ulcer perforation. a CT scan with lung window setting shows a large amount of pneumoperitoneum (asterisk) in the perihepatic space and the lesser sac. The falciform ligament is well visualized (the falciform ligament sign, arrow). b Coronal-reformatted contrast-enhanced CT scan shows outpouching focal ulceration (arrow) in the lesser curvature of the gastric antrum, indicating a benign ulcer
Although clinically stable patients with sealed-off perforation are occasionally managed conservatively, PPU is primarily managed surgically. However, outcomes in patients surgically may be unfavorable, regardless of the repair performed [26].
Inflammatory bowel disease
Crohn’s disease (CD) is a common inflammatory condition affecting the small bowel. Free perforation occurs in 1–3% of all cases of CD as an initial manifestation of this condition or during the course of the disease (Fig. 10). Although the mechanism of free perforation is debatable, it can be caused by upstream bowel dilatation complicated by stenosis with increased intraluminal pressure. Exacerbation of toxic colitis also contributes to ischemia-induced bowel perforation, and bowel resection is warranted in such cases [28]. CD is a chronic condition that often follows a relapsing–remitting course; therefore, the perforation is usually sealed-off by the surrounding omentum and other viscera. Thus, sinus tract, fistula, and sealed-off perforations are more common than acute free perforation. This condition is not an emergency and can be treated with elective surgery [27, 29]. Mural thickening with the target sign, comb sign (prominent vasa recta), and fibrofatty proliferation are CT features that suggest underlying CD (Fig. 10). Mesenteric or intra-abdominal abscess or phlegmon, or a fistulous tract or extraluminal air in close to the involved bowel loops raises perforation [27].
A 23-year-old man with Crohn’s disease with free perforation. a Contrast-enhanced CT scan shows a wall defect (arrow) in the small bowel and multiple extraluminal air bubbles (arrowheads). On contrast-enhanced CT scans, wall thickening in the ileocolic valve (arrow in b), diffuse wall thickening with enhancement (arrows in c) in the ileum, and prominent vasa recta (asterisk in c) suggest active inflammatory Crohn’s disease
Free perforation associated with ulcerative colitis (UC) is a rare complication that occurs in approximately 2% of all cases. Unlike CD, UC primarily affects the mucosal layer of the colon and rectum. Thus, free perforation in patients with UC is associated with toxic megacolon during the course of a severe fulminating disease that is caused by extensive inflammation beyond the mucosal layer, with consequently impaired colonic motility and dilatation [29]. The radiological hallmark of active UC is the presence of colonic mural thickening and enhancement. A distended colon with an abnormal haustral pattern and segmental colonic parietal thinning is pathognomonic of toxic megacolon and emergency colectomy is warranted to treat the perforation [30].
Appendicitis
Appendiceal perforation is a complication of acute appendicitis that occurs in 18–35% of patients presenting with this condition [31]. Simple appendectomy can effectively treat non-perforated appendicitis and is associated with a shorter hospital stay than a laparoscopic appendectomy, which is more difficult in cases with perforation. Moreover, conversion to open appendectomy is needed in a few cases. Thus, the treatment strategy usually involves initial nonsurgical management with the administration of antibiotics or percutaneous drainage, followed by interval appendectomy. Thus, prompt identification of appendiceal perforation is important considering the surgical implications and complications. However, differentiation of a nonperforated from perforated appendicitis is not simple, because perforated appendicitis can be a localized process without a typical clinical presentation. Moreover, the amount of extraluminal air is usually small or absent in perforated appendicitis [2].
Recently, CT is being considered as the imaging modality of choice to accurately diagnose acute appendicitis to avoid the complications associated with perforations, as well as to identify the early signs of inflammation and impending perforation that warrant prompt surgical intervention [32]. CT findings including the presence of extraluminal air, extraluminal appendicolith, abscess, phlegmon and a focal defect in the enhancing appendiceal wall are useful to improve the diagnostic accuracy of CT in cases of perforated appendicitis (Fig. 11) [33].
Diverticulitis
Diverticular perforation can complicate diverticulitis. Perforated diverticulitis warrants aggressive therapy and/or operative management. Diverticular perforation ranges from mild phelgmonous change, abscess formation, microperforation with peritonitis, rarely to free perforation with fecal peritonitis. Perforated diverticulitis may occur at any colonic site; however, sigmoid colon is the most common affected [27]. Typical CT features of perforated diverticulitis include a thickened bowel wall, inflamed diverticulum, disproportionate pericolic fat stranding, pericolic abscess or small quantities of extraluminal air (Fig. 12) [34]. Pneumoperitoneum does not commonly occur in patients with perforated diverticulitis [35]. Notably, specific CT criteria are required to differentiate diverticulitis from colon cancer. CT findings that typically suggest diverticulitis are the presence of inflamed diverticula, a stenotic bowel segment measuring > 10 cm in length, sloping transition zones, colonic wall thickness < 1 cm, and the absence of enlarged pericolonic lymph nodes. Nevertheless, this diagnosis remains difficult [36].
A 63-year-old man with perforated diverticulitis in the ascending colon. a, b Contrast-enhanced CT scans show an inflamed diverticulum (arrows) in the ascending colon and extraluinal gas (arrowhead in b) adjacent to the diverticulum. Disproportionate pericolic fat stranding is also noted (asterisks)
Ischemic
Diffuse or localized vascular insufficiency of the GI wall secondary to multiple causes leads to ischemia and infarction, with consequent perforation. This condition may occur secondary to direct vascular occlusion, strangulated bowel obstruction, hypoperfusion associated with nonocclusive vascular disease, and/or various vasculitides. CT findings of GI ischemia vary and are determined by the etiology, the severity of bowel ischemia (superficial mucosal ischemia vs. transmural necrosis/infarction), and the presence and degree of intramural hemorrhage, edema, and/or superinfection [37]. Common CT findings of GI ischemia leading to perforation include a thin-wall bowel, hypoenhancement or lack of bowel enhancement, localized mesenteric edema, gas through the mesenteric vein into portal vein, and/or pneumatosis intestinalis (Fig. 13). Evaluation of arteries and veins from diseased bowel helps in determining the etiology of bowel ischemia (embolic, thrombotic, or dissection-induced) (Fig. 13). In general, usually, intestinal ischemia with perforation is treated surgically, although additional treatment varies based on the cause of ischemia [38].
An 80-year-old man with superior mesenteric artery (SMA) thrombosis and pneumoperitoneum. a CT scan with lung window settings shows subdiaphragmatic air bubbles (arrowheads). b Contrast-enhanced CT scan shows SMA thrombosis (arrow) and a lack of bowel enhancement in the thin-walled small bowel (arrowhead) with accompanying ascites
Sterocoral
Sterocoral perforation is rare and refers to localized mucosal ulceration and ischemic necrosis of the bowel wall caused by a stercoraceous mass. Most (77%) often involves the sigmoid colon or the rectum. Risk factors include conditions leading to longstanding constipation as drugs (narcotics, NSAIDs, analgesia, and methadone), scleroderma and obstructing colonic lesions (neoplasm or stricture), old age, and chronically bedridden state. CT findings of stercoral perforation include pericolic fat stranding in the segment of the bowel showing fecal impaction, focal colonic wall thickening secondary to ischemia- and ulceration-induced edema, extraluminal air bubbles, or abscess formation (Fig. 14). Treatment is resection of the affected bowel, colostomy and creation of Hartmann’s pouch [31, 39].
Mimicker: balanced pnemoperitoneum
Pneumatosis intestinalis is defined as the presence of gas within the GI wall. Pneumatosis intestinalis may be idopathic or might be associated with various disorders. Air dissection from subserosal cysts may cause intraperitoneal free air. Free intraperitoneal air causes tamponade of subserosal cysts, thus maintaining a balance between intacystic air and pneumoperitoneum (therefore referred to as balanced pneumoperitoneum) (Fig. 15). This condition should not be used as the sole indication to perform laparotomy. Approximately 50% of cases of balanced pneumoperitoneum can be successfully managed non-operatively. Emergency surgery may be essential, particularly in patients with signs of strangulated bowel obstruction or ischemia [40].
Conclusion
CT shows high diagnostic performance in detecting and localizing GI perforation by demonstrating direct signs of perforation such as discontinuity of the bowel wall or the presence of extraluminal free air and indirect signs such as the presence of phlegmon, abscess, peritoneal fluid, or extraluminal foreign body. Additionally, CT facilitates accurate assessment of underlying causes of GI perforation by identifying typical imaging features of various GI diseases (Table 1). Clinicians need to be familiar with specific CT findings associated with various conditions causing GI perforation and their correlation with relevant clinical setting to ensure prompt accurate diagnosis and treatment in an attempt to reduce mortality and morbidity rates in these patients.
References
Yeung KW, Chang MS, Hsiao CP, Huang JF. CT evaluation of gastrointestinal tract perforation. Clin Imaging. 2004;28:329–33.
Furukawa A, Sakoda M, Yamasaki M, Kono N, Tanaka T, Nitta N, et al. Gastrointestinal tract perforation: CT diagnosis of presence, site, and cause. Abdom Imaging. 2005;30:524–34.
Kim SH, Shin SS, Jeong YS, Heo SH, Kim JW, Kang HK. Gastrointestinal tract perforation: MDCT findings according to the perforation sites. Korean J Radiol. 2009;10:63–70.
Ghekiere O, Lesnik A, Hoa D, Laffargue G, Uriot C, Taourel P. Value of computed tomography in the diagnosis of the cause of nontraumatic gastrointestinal tract perforation. J Comput Assist Tomogr. 2007;31:169–76.
Hainaux B, Agneessens E, Bertinotti R, De Maertelaer V, Rubesova E, Capelluto E, et al. Accuracy of MDCT in predicting site of gastrointestinal tract perforation. Am J Roentgenol. 2006;187:1179–83.
Imuta M, Awai K, Nakayama Y, Murata Y, Asao C, Matsukawa T, et al. Multidetector CT findings suggesting a perforation site in the gastrointestinal tract: analysis in surgically confirmed 155 patients. Radiat Med. 2007;25:113–8.
Roviello F, Rossi S, Marrelli D, De Manzoni G, Pedrazzani C, Morgagni P, et al. Perforated gastric carcinoma: a report of 10 cases and review of the literature. World J Surg Oncol. 2006;4:19.
Kim SW, Kim HC, Yang DM. Perforated tumours in the gastrointestinal tract: CT findings and clinical implications. Br J Radiol. 2012;85:1307–13.
Abdelrazeq AS, Scott N, Thorn C, Verbeke CS, Ambrose NS, Botterill ID, et al. The impact of spontaneous tumour perforation on outcome following colon cancer surgery. Colorectal Dis. 2008;10:775–80.
Osian G. Emergency surgery for colorectal cancer complications: obstruction, perforation, bleeding. In: Ho Y-H, editor. Contemporary issues in colorectal surgical practice. China: In Tech; 2012. pp. 75–87.
Chang HK, Min BS, Ko YT, Kim NK, Kim H, Kim H, et al. Obstructive colitis proximal to obstructive colorectal carcinoma. Asian J Surg. 2009;32:26–322.
Welch JP, Donaldson GA. Perforative carcinoma of colon and rectum. Ann Surg. 1974;180(5):734–40.
Kim SW, Shin HC, Kim IY, Kim YT, Kim CJ. CT findings of colonic complications associated with colon cancer. Korean J Radiol. 2010;11:211–21.
Shih CH, Yu MC, Chao TC, Huang TL, Jan YY, Chen MF. Outcome of perforated gastric cancer: twenty years experience of one institute. Hepatogastroenterology. 2010;57:1320–4.
Byun JH, Ha HK, Kim AY, Kim TK, Ko EY, Lee JK, et al. CT findings in peripheral T-cell lymphoma involving the gastrointestinal tract. Radiology. 2003;227:59–67.
Vaidya R, Habermann TM, Donohue JH, Ristow KM, Maurer MJ, Macon WR, et al. Bowel perforation in intestinal lymphoma: incidence and clinical features. Ann Oncol. 2013;24:2439–43.
Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38(Suppl 5):S39–51.
Cegarra-Navarro MF, de la Calle MA, Girela-Baena E, Garcia-Santos JM, Lloret-Estan F, de Andres EP. Ruptured gastrointestinal stromal tumors: radiologic findings in six cases. Abdom Imaging. 2005;30:535–42.
Rutkowski P, Nowecki ZI, Michej W, Debiec-Rychter M, Wozniak A, Limon J, et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol. 2007;14:2018–27.
Shiraishi M, Hiroyasu S, Nosato E, Shimoji H, Kusano T, Muto Y. Perforation due to metastatic tumors of the ileocecal region. World J Surg. 1998;22:1065–8.
Romano LFS, Silva M, Granata R, Rugglero G. Imaging of gastrointestinal tract perforation in the oncologic patients. In: Romano LPA, editor. Imaging of alimentary tract perforation. New York: Springer; 2015. p. 115–132.
Kim SY, Ha HK, Park SW, Kang J, Kim KW, Lee SS, et al. Gastrointestinal metastasis from primary lung cancer: CT findings and clinicopathologic features. AJR Am J Roentgenol. 2009;193:W197–201.
Smeenk RM, van Velthuysen ML, Verwaal VJ, Zoetmulder FA. Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surg Oncol. 2008;34:196–201.
Lim HK, Lee WJ, Kim SH, Kim B, Cho JM, Byun JY. Primary mucinous cystadenocarcinoma of the appendix: CT findings. AJR Am J Roentgenol. 1999;173:1071–4.
Yantiss RK, Shia J, Klimstra DS, Hahn HP, Odze RD, Misdraji J. Prognostic significance of localized extra-appendiceal mucin deposition in appendiceal mucinous neoplasms. Am J Surg Pathol. 2009;33:248–55.
Chung KT, Shelat VG. Perforated peptic ulcer—an update. World J Gastrointest Surg. 2017;9:1–12.
Del Gaizo AJ, Lall C, Allen BC, Leyendecker JR. From esophagus to rectum: a comprehensive review of alimentary tract perforations at computed tomography. Abdom Imaging. 2014;39:802–23.
Leal RF, Ward M, Ayrizono Mde L, de Paiva NM, Bellaguarda E, Rossi DH, et al. Free peritoneal perforation in a patient with Crohn's disease—report of a case. Int J Surg Case Rep. 2013;4:322–4.
Berg DF, Bahadursingh AM, Kaminski DL, Longo WE. Acute surgical emergencies in inflammatory bowel disease. Am J Surg. 2002;184:45–51.
Moulin V, Dellon P, Laurent O, Aubry S, Lubrano J, Delabrousse E. Toxic megacolon in patients with severe acute colitis: computed tomographic features. Clin Imaging. 2011;35:431–6.
Zissin R, Hertz M, Osadchy A, Even-Sapir E, Gayer G. Abdominal CT findings in nontraumatic colorectal perforation. Eur J Radiol. 2008;65:125–32.
Iacobellis F, Iadevito I, Romano F, Altiero M, Bhattacharjee B, Scaglione M. Perforated appendicitis: assessment with multidetector computed tomography. Semin Ultrasound CT MR. 2016;37:31–6.
Horrow MM, White DS, Horrow JC. Differentiation of perforated from nonperforated appendicitis at CT. Radiology. 2003;227:46–51.
Destigter KK, Keating DP. Imaging update: acute colonic diverticulitis. Clin Colon Rectal Surg. 2009;22:147–55.
Lohrmann C, Ghanem N, Pache G, Makowiec F, Kotter E, Langer M. CT in acute perforated sigmoid diverticulitis. Eur J Radiol. 2005;56:78–83.
Ben Yaacoub I, Boulay-Coletta I, Julles MC, Zins M. CT findings of misleading features of colonic diverticulitis. Insights Imaging. 2011;2:69–84.
Furukawa A, Kanasaki S, Kono N, Wakamiya M, Tanaka T, Takahashi M, et al. CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol. 2009;192:408–16.
Rha SE, Ha HK, Lee SH, Kim JH, Kim JK, Kim JH, et al. CT and MR imaging findings of bowel ischemia from various primary causes. Radiographics. 2000;20:29–422.
Heffernan C, Pachter HL, Megibow AJ, Macari M. Stercoral colitis leading to fatal peritonitis: CT findings. AJR Am J Roentgenol. 2005;184:1189–93.
Hsueh KC, Tsou SS, Tan KT. Pneumatosis intestinalis and pneumoperitoneum on computed tomography: Beware of non-therapeutic laparotomy. World J Gastrointest Surg. 2011;3:86–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Lee, N.K., Kim, S., Hong, S.B. et al. CT diagnosis of non-traumatic gastrointestinal perforation: an emphasis on the causes. Jpn J Radiol 38, 101–111 (2020). https://doi.org/10.1007/s11604-019-00910-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-019-00910-7