Abstract
Large bowel perforation is an abdominal emergency that results from a wide range of etiologies. Computed tomography is the most reliable modality in detecting the site of large bowel perforation. The diagnosis is made by identifying direct CT findings such as extraluminal gas or contrast and discontinuity along the bowel wall. Indirect CT findings can help support the diagnosis, and include bowel wall thickening, pericolic fat stranding, abnormal bowel wall enhancement, abscess, and a feculent collection adjacent to the bowel. Common etiologies that cause large bowel perforation are colon cancer, foreign body aspiration, stercoral colitis, diverticulitis, ischemia, inflammatory and infectious colitides, and various iatrogenic causes. Recognizing a large bowel perforation on CT can be difficult at times, and there are various entities that may be misinterpreted as a colonic perforation. The purpose of this article is to outline the MDCT technique used for evaluation of suspected colorectal perforation, discuss relevant imaging findings, review common etiologies, and point out potential pitfalls in making the diagnosis of large bowel perforation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Acute large bowel perforation is an abdominal emergency that requires urgent clinical attention and diagnosis. A wide range of etiologies can result in large bowel perforation and localizing the site of perforation can sometimes be challenging. The overall mortality from large bowel perforation has been reported between 16.9% and 19.6%, emphasizing the importance of making an accurate and timely diagnosis [1, 2]. Plain radiography is commonly the initial imaging examination performed in the diagnostic workup for the acute abdomen. Sensitivity for detection of extraluminal air on plain radiography is only 50%–70%, and the site of perforation is almost never elucidated [3, 4]. Multidetector CT (MDCT) predicts the site of perforation with an accuracy of between 82% and 90% making it the most reliable imaging modality for diagnosing large bowel perforation [5].
This review will outline the MDCT technique used for evaluation of suspected colorectal perforation, discuss relevant imaging findings, review common etiologies, and point out potential pitfalls in making the diagnosis of large bowel perforation.
MDCT technique
MDCT is a rapid imaging modality that is commonly used in the setting of suspected acute abdominal pathology. Colonic perforation is often an unexpected finding in patients presenting with unexplained abdominal pain. At our institution, the protocol for explained abdominal pain is intravenous contrast in the portal venous phase and a forty-five minute prep time with water-soluble oral contrast, if no contraindications exist. A longer oral contrast prep time of 3 h may be used in cases where there is suspected colonic pathology, such as in patients postoperative from bowel surgery. This enables adequate time for contrast to opacify the colon in most patients. In cases where high-grade bowel obstruction is suspected, oral contrast is not utilized. In addition, rectally administered contrast may be used in cases in which there is suspicion for anastomotic leak.
The utility of oral contrast has recently been questioned, given the lack of evidence of any added benefit. Some of the arguments against its use are that it increases length of stay in the emergency department, carries the risk of aspiration and leads to additional cost [6, 7]. A meta-analysis studying the utilization of oral contrast in cases of blunt abdominal trauma found no difference in accuracy of diagnosis with or without oral contrast [7]. In cases of alimentary perforation, either traumatic or nontraumatic, extraluminal contrast has not been reported to be a frequent CT finding [8]. However, extraluminal contrast is a direct and specific sign of perforation and can help elucidate the site of perforation. Therefore, its utilization should be at the discretion of the institution, until further research has been performed that compares the risk versus benefit for the nontraumatic acute abdomen in diagnosis of perforation.
MDCT direct and indirect findings
Direct findings of bowel perforation include extraluminal gas, discontinuity of the bowel wall, and extraluminal contrast. Extraluminal gas has a high specificity for gastrointestinal perforation and the location of extraluminal gas can elucidate the site of perforation; specifically free intraperitoneal gas located only in the supramesocolic and inframesocolic compartments defines 100% of large bowel perforations [9]. Additionally, extraluminal gas exclusively in the pelvis is most often related to colonic and less frequently to distal small bowel perforation. In contrast, extraluminal gas found exclusively around the liver and stomach was found to be specific for an upper GI (stomach or duodenum) perforation [9]. The amount of pneumoperitoneum varies depending on the cause and site of obstruction, as well as the acuity of the pathology. In a chronic process, the perforation is often walled off and localized, and the amount of extraluminal gas and/or fluid may be relatively small and difficult to detect. Other findings that can help localize the site of perforation, listed in order of most to least sensitive, include concentrated bubbles of extraluminal gas in proximity to the perforation site, bowel wall thickening and a mural defect [10].
Colonic perforation may result in pneumoretroperitoneum if the site of perforation is in a retroperitoneal segment of colon, including the ascending and descending colon, and rectum. Additionally, most sigmoid diverticula arise from the extraperitoneal surface of the colon; therefore retroperitoneal or extraperitoneal gas may be a manifestation of sigmoid diverticulitis (Fig. 1).
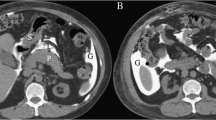
A “cleft sign” or defect in the bowel wall is another direct and specific sign of large bowel perforation. This finding is observed less frequently than extraluminal gas on CT, as it is seen in less than 50% of patients with perforation (Fig. 2) [11]. The detection of free extraluminal contrast can also be a direct sign of bowel perforation, though the sensitivity of extravasation of oral contrast material is low, varying between 19% and 42% [9].
Indirect findings of large bowel perforation include bowel wall thickening, pericolic fat stranding, abnormal bowel wall enhancement, abscess, and an inflammatory mass adjacent to bowel [12]. Indirect findings are extremely useful as they may help in detecting a large bowel perforation site. CT identification of the perforation site increases from 34% to 97% for ascending-to-sigmoid colonic perforations and from 40% to 80% for rectal perforations, when direct CT findings are combined with indirect CT findings [13].
In contrast to a free perforation, diagnosing a contained or localized perforation can be more challenging. In such cases, pneumoperitoneum is often absent and the diagnosis must be made by recognizing extraluminal bowel contents. The finding of a “dirty mass” secondary to fecal spillage has been described as a specific finding of large bowel perforation [14], when located in close proximity to a loop of colon or rectum (Fig. 3A–B). In one study, the sizes of these masses varied from 1 to 6 cm [14].
Perforated sigmoid colon secondary to colon cancer with adjacent “dirty masses.” A Axial CT image demonstrates marked mural thickening of the sigmoid colon (white arrow), consistent with colon cancer. B Coronal CT image showing two large extraluminal gas and fluid collections (black arrows) in close proximity to the sigmoid, secondary to perforation
It can be difficult differentiating extraluminal stool from a normal loop of colon. If a large bowel perforation is not promptly diagnosed, there can be dire consequences for the patient, with rapid development of peritonitis and sepsis (Fig. 4). The usage of wide windows and reformats in the sagittal and coronal planes may help. In addition, the radiologist should trace the bowel from the rectum to the cecum, making note of any gas containing structure that cannot be connected with adjacent bowel. In cases of nonopacified large bowel, repeat CT imaging with oral contrast may be helpful in excluding a perforation. Water-soluble contrast enema or CT with rectal contrast administration can be used as problem solving tools in confirming colonic perforation in equivocal cases.
Feculent peritonitis secondary to perforated diverticulitis. Axial CT image through the pelvis demonstrates a mottled collection of extraluminal gas and stool (“dirty mass”), which was mistaken for a loop of sigmoid colon. Note the collection (white arrow) and the adjacent sigmoid colon (white arrowhead)
There are various mechanisms and pathways for gas to be introduced into the peritoneal cavity other than a breach of the gastrointestinal tract which can lead to false positive cases of large bowel perforation. Examples include recent abdominal surgery and other intraperitoneal instrumentation, barotrauma, bladder perforation, penetrating trauma, and via the female genital tract. In addition, gas in the retroperitoneal or extraperitoneal spaces could also be mistaken for pneumoperitoneum due to the close proximity of these spaces to each other. In particular, gas in the anterior prevesical space (space of Retzius) can be mistaken for pneumoperitoneum, as it is located immediately anterior to the anterior peritoneal lining. Therefore, the radiologist should be aware of any recent procedure or pathologic process involving the abdominal wall, retroperitoneum, extraperitoneal pelvis or groins, which could potentially introduce gas, so as not to mistakenly diagnose a perforated viscus. Some examples include extraperitoneal bladder perforation after cystoscopy, inguinal hernia repair, and Fournier’s gangrene.
Etiologies of large bowel perforation
Neoplasm
Perforation secondary to colonic adenocarcinoma is the cause of 1.2%–10% of large bowel perforations, and the cecum and sigmoid colon are the most common sites of perforating colon carcinoma [12]. There are two mechanisms by which a colon cancer can perforate. The first mechanism is by direct necrosis at the site of the tumor. In cases of tumor necrosis leading to perforation, the amount of extraluminal air is usually small (Fig. 5A–C) [3]. The second mechanism is by “blowout” proximal to the tumor. “Blowout” is defined as a closed-loop obstruction in which the colon cancer causes increased colonic pressure between a competent ileocecal valve and the cancer, leading to a perforation [12]. In cases of blowout, the perforation can be at the site of the tumor or proximal to it (Fig. 6A–B). Frequently the site of perforation is the cecum because of its larger diameter and resultant higher intraluminal pressure as it becomes distended. It is important for the radiologist to ascertain that no signs of perforation exist more proximal to an obstructing colon cancer. Colonic wall thickening proximal to an obstructing colon cancer has been reported to be present in 10% of cases and is associated with ischemia or edema in a viable colon (Fig. 7A–B) [15].
Perforated ascending colon cancer secondary to necrosis. A Coronal CT image demonstrates focal, short segment colonic bowel wall thickening at the hepatic flexure (black arrowheads), consistent with colon cancer. B Coronal CT image shows a small amount of extraluminal gas (white arrowhead) immediately posterior to the colon cancer. C Axial CT image redemonstrates adjacent extraluminal gas (white arrowhead)
Perforated ascending colon secondary to a distal colon cancer. A Coronal CT image demonstrates a stenosing annular colon cancer in the distal ascending colon (white arrow). B Axial CT image demonstrates perforation with extraluminal stool and gas (white arrowhead) in the ascending colon proximal to this mass secondary to “blowout”
Perforated sigmoid colon secondary to an obstructing colon cancer. A Coronal CT image demonstrates mass like thickening of the sigmoid colon (white arrow) with dilatation of the colon proximally and extraluminal gas (white arrowheads) indicating perforation. B Axial CT image depicts marked dilatation of the colon with extraluminal gas (white arrowhead) and circumferential bowel wall thickening involving the right colon secondary to ischemia (white arrow)
While the large majority of neoplastic large bowel obstruction is due to primary colonic adenocarcinoma, serosal metastases and extrinsic compression or invasion from an extracolonic neoplasm can also cause large bowel obstruction, and potentially lead to perforation.
Foreign body ingestion
The most common foreign bodies that lead to perforation are toothpicks and dietary foreign bodies such as fish bones or bone fragments [16]. Sites of perforation are found in intestinal segments with acute angulation such as the ileocecal and rectosigmoid regions [17]. The passage of large amounts of extraluminal gas is limited by the fact that a foreign body produces a gradual erosion of the intestinal wall [18, 19], allowing time for the inflammatory reaction to be walled off (Fig. 8A–B). Therefore, diffuse pneumoperitoneum is rare in the setting of perforation secondary to foreign body ingestion. The most direct assessment for foreign body perforation on CT is identification of the foreign object in close proximity to extraluminal gas. Other findings include localized wall thickening, fat stranding and abscess. With acquisition of sufficiently thin axial sections and coronal and sagittal reformatted images, most foreign objects should be visible on CT. Oral contrast can hinder detection of a foreign object, and if there is prospective suspicion for an ingested foreign object, CT should be performed without the use of positive oral contrast. Unfortunately, in the case of an accidental dietary ingestion, the patient will often not remember the incident and there can often be lag time of weeks to months between ingestion and development of symptoms [19].
Stercoral colitis
In stercoral colitis, fecal impaction causes increased intraluminal and colonic wall pressure which can lead to ischemic pressure necrosis, ulcer formation, and potentially perforation [20]. The most common locations for stercoral ulceration include the anterior rectum proximal to the peritoneal reflection, the antimesenteric border of the rectosigmoid junction and the apex of the sigmoid colon [20]. Ulcers resulting from stercoral colitis are typically located on the antimesenteric border of the intestine, which is less vascularized than the mesenteric border and more sensitive to mechanical constraint [21]. Infrequent sites of involvement include the transverse colon and cecum. Only a minority of patients with fecal impaction will develop stercoral colitis. In uncomplicated fecal impaction the colon should remain thin-walled. Typical findings of stercoral colitis on CT include wall thickening and pericolic fat stranding, with extraluminal gas or an abscess present in the setting of perforation (Fig. 9 ) [20]. Stercoral perforation can lead to the rapid development of peritonitis, sepsis, and potentially death, due to intraperitoneal fecal spillage.
Perforated stercoral colitis. Axial CT image shows a fecaloma (white arrow), bowel wall thickening, surrounding fat stranding and extraluminal gas (white arrowhead) consistent with stercoral perforation. Stercoral colitis with multiple perforation sites (other perforation sites seen on CT are not shown) was at found at surgery
Ischemic colitis
Ischemic colitis is almost always due to nonocclusive disease (low flow state), rather than vascular occlusion. The segments commonly affected are the left colon, and the anastomotic plexus between the inferior mesenteric artery and the hypogastric vascular supply at the rectosigmoid junction [22]. While it is a commonly held belief that the splenic flexure is the most frequent site of colonic ischemia, a recent study found no significant increase in ischemia at the splenic flexure relative to other colonic segments, and there is no data in the literature showing the splenic flexure to be at greater risk [23]. The ischemic process initially involves the mucosal layer of the bowel wall. In most cases, ischemic injury will remain isolated to the mucosa and is a reversible, self-limiting process. However in a minority of cases, the ischemia will be transmural, causing necrosis of the muscular layer, with the potential for perforation and severe sepsis [22].
The most common CT findings of ischemic colitis are bowel wall thickening and pericolic fat stranding. Pneumatosis intestinalis and portomesenteric gas were present less than 10% of the time in one series, with pneumatosis intestinalis associated with more severe cases (Fig. 10A–B) [23]. It is important to recognize that colonic pneumatosis intestinalis can represent a benign finding. Benign pneumatosis intestinalis has been reported in a variety of conditions, including pulmonary disease, systemic diseases, certain medications and organ transplantation [24]. In order to help differentiate between benign and clinically worrisome pneumatosis intestinalis, the radiologist can survey for additional findings to help support a diagnosis. CT findings visualized in conjunction with pneumatosis intestinalis including bowel wall thickening, mesenteric stranding, ascites, bowel dilatation and portomesenteric gas correlate significantly with clinically worrisome cases. Additionally correlation with clinical history, physical examination and laboratory test results are necessary in making a diagnosis. [25].
Perforated ischemic colitis. A Axial CT image demonstrates wall thickening of the distal transverse and proximal descending colon (white arrows), and a small amount of adjacent free fluid (black arrowhead). B Axial CT image shows pneumatosis of involved bowel loops and localized extraluminal gas (white arrowhead)
Pneumatosis cystoides intestinalis is a benign etiology characterized by the presence of gaseous cysts containing nitrogen, hydrogen and carbon dioxide in the intestinal wall, which are submucosal and subserosal in location [26]. Patients are almost always asymptomatic. Occasionally the cysts can rupture and result in pneumoperitoneum, mimicking a frank bowel perforation (Fig. 11 ). Recognition of the cystic shape of these gas collections, in contrast to the linear intramural gas collections characteristic of ischemic bowel, can help make the diagnosis.
Pneumatosis cystoides intestinalis. Axial CT image of the abdomen and pelvis demonstrate numerous intramural air-filled cysts in the transverse colon (white arrow) and adjacent extraluminal air secondary to cyst rupture. Note the lack of wall thickening, ascites or fat stranding. The patient was asymptomatic
Diverticulitis
Diverticulitis is suspected clinically when there is a triad of left lower abdominal pain, fever and leukocytosis. The manifestations of acute diverticulitis on CT can vary widely, ranging from pericolic fat stranding and wall thickening to abscess, fistula formation, free perforation and feculent peritonitis [27]. Cases of perforated diverticulitis can occur at any site in the colon, including the proximal colon, although it most commonly involves the sigmoid colon. Extraluminal gas usually concentrates in close proximity to the involved colon [5]. Peritonitis and free perforation have been shown to occur more often in patients with no prior history of diverticulitis (Fig. 12 ), while patients with a history of diverticulitis tend to present with pericolic abscess or phlegmon [28]. In a large study, patients with a first episode of sigmoid diverticulitis had a greater incidence of free perforation than those with recurrent sigmoid diverticulitis (25.3% vs. 7.9%). Additionally, patients with a first episode required emergency colectomy more often, whereas initial conservative therapy was more common in those with recurrent sigmoid diverticulitis [29].
Due to the proximity of the small bowel mesentery to the sigmoid colon, CT findings of severe sigmoid diverticulitis may occasionally show predominant involvement of the small bowel, with small bowel wall thickening, mesenteric fat stranding and interloop fluid and gas collections. The appendix and mesoappendix can be involved in a similar fashion if the sigmoid colon loops into the right abdomen. Such findings can be mistaken for a small bowel or appendiceal perforation. Awareness of this association, and the presence of diverticular disease with wall thickening of the sigmoid, should alert the radiologist to the correct diagnosis.
Inflammatory bowel disease (IBD)
Large bowel perforation in cases of IBD are rare. Free perforation in Crohn’s disease occurs in about 3% of patients, and can occur during a bout of toxic colitis, or an acute exacerbation of chronic disease, especially when there is a distal obstruction. Sealed off perforations are more common, with CT findings of phlegmon and abscess near the involved colonic segment [10]. Characteristic CT findings of Crohn’s disease, including skip lesions, mesenteric/mesocolic fat proliferation, engorged vasa recta, intramural fat, and fistula formation, may help in making the diagnosis of Crohn’s colitis. Free perforation in ulcerative colitis occurs in about 2% of patients and is often associated with toxic megacolon [30]. Toxic megacolon involves total or segmental nonobstructive colonic distension of at least 6 cm with inflammation of the colonic wall and associated systemic toxicity [31]. The condition is primarily associated with inflammatory bowel disease, however toxic megacolon can also be a complication of infectious and ischemic colitis, especially C. difficile colitis. Plain radiography has traditionally been used to assess for colonic dilatation in patients with suspected toxic megacolon. Dilatation is usually most severe in the ascending and transverse colon. CT can provide more detailed findings regarding the distribution of colonic involvement and degree of wall thickening, including abnormal haustral pattern, segmental colonic wall thinning and nodular pseudopolyps (Fig. 13) [31]. Colonic diameter of greater than 7.7 cm on CT is indicative of the development of toxic megacolon [32]. CT can also be used to detect complications such as perforation, abscess formation and thrombophlebitis.
Infection
Infectious colitis has varied clinical manifestations, depending on the afflicting pathogen. The differential diagnosis for infectious colitis primarily involving the right colon includes salmonella, Yersinia, tuberculosis and amebiasis. Diseases with a propensity for the left colon include schistosomiasis, shigellosis, herpes, gonorrhea, syphilis and lymphogranuloma venereum. Diffuse colonic involvement can be caused by cytomegalovirus and E. coli [33]. Characteristic CT features of infectious colitis include bowel wall thickening and pericolic fat stranding.
C. difficile is the leading enteric infection found in hospitalized patients in the United States with an estimated 3 million new cases of diarrhea and colitis annually [34]. Pseudomembranous colitis is the most severe form of C. difficile colitis, affecting between 0.1% and 10.1% of patients receiving penicillin or cephalosporins. Complications can include fulminant colitis and colonic perforation. If the pathogen is not controlled by the appropriate antibiotic therapy, transmural necrosis and perforation can occur. One study found fifty perfect of scanned hospitalized patients with C. difficile colitis to have positive CT findings. Most patients had segmental involvement, with the rectum and sigmoid colon most affected. In addition, positive scans were associated with leukocytosis, abdominal pain and diarrhea [35].
Neutropenic colitis occurs in the immunocompromised population, commonly in cancer patients receiving chemotherapy. Neutropenic colitis may be multifactorial in etiology with infection, chemotherapy, intramural hemorrhage, ischemia and altered immune function all having putative roles. Although it most commonly occurs in the right colon, it can manifest anywhere in the small or large bowel with CT findings of nonspecific wall thickening, edema and pericolic fat stranding. Transmural necrosis and perforation may occur in severe cases (Fig. 2) [36].
Abscesses can cause pneumoperitoneum and mimic a perforation when ruptured. Clostridium septicum is an anaerobic gram-positive bacillus that superinfects hepatic colon adenocarcinoma metastases which can rupture and be confused with a perforated colon cancer (Fig. 14A–C). C. septicum sepsis is often fulminant with reported mortality rates of approximately 60% [37] and may present as gas gangrene, myonecrosis, sepsis or liver abscesses [38]. C. septicum liver abscesses are rare in the absence of underlying liver disease [39]. In cases of metastatic superinfection, the metastases outgrow its blood supply and provide an anaerobic environment ideal for bacterial growth [40]. Liver abscesses from C. septicum can contain gas and rupture can occur.
Clostridium septicums superinfection of a colonic neoplasm with liver abscesses and free air. A Axial CT image demonstrates an enlarged liver with several collections of intrahepatic gas (black arrowheads), B Axial CT image demonstrates subtle liver masses (white arrow) which are more conspicuous at different window/level settings (not shown), intrahepatic (black arrowheads) and extraluminal gas (black arrow) that has broken through the hepatic capsule, C Axial CT image demonstrates a distal colonic mass (white arrowhead)
Nonneoplastic obstructive causes
Nonneoplastic etiologies of large bowel obstruction leading to perforation are less common. The two most common nonneoplastic etiologies of large bowel obstruction include volvulus (Fig. 15A–B) and diverticulitis, which are estimated to be the cause of large bowel obstruction in an estimated 10%–15% and 10% of cases, respectively [41]. Uncommon etiologies include endometriosis, hernia, fecal impaction, intussusception and inflammatory bowel disease. Regardless of the cause of obstruction, persistent dilatation of the large bowel can lead to ischemia, necrosis and perforation. The role of CT in the setting of large bowel obstruction is to define the etiology and location of the obstruction and to assess the colon for signs of ischemia including wall thickening, pneumatosis, decreased or absent mural enhancement and perforation.
Ogilvie’s syndrome
Acute colonic pseudo-obstruction, also known as Ogilvie’s syndrome, is a rare cause of perforation and occurs mainly in patients with severe illness or that are postoperative. The condition is characterized by massive colonic distension without mechanical obstruction, and perforation occurs in 1%–3% of patients [42, 43].The risk of perforation increases up to 23% when the cecal diameter is greater than 14 cm. [43, 44]. No cases of perforation were reported in a study including four hundred patients when the cecal diameter was less than 12 cm. [43]. In cases of perforation, the mortality rate is high and has been reported to be between 50% and 71% [45]. Typical CT findings include marked diffuse dilatation of the colon and rectum, without a transition point to collapsed bowel. If prior abdominal radiographs or CT scans are available, they may show similar findings, as this is often a chronic condition, however careful comparison must be made, as the dilatation can acutely worsen, putting the patient at risk for perforation.
Iatrogenic causes
Bevacizumab
Over the past decade, vascular endothelial growth factor (VEGF) receptor monoclonal antibodies have become routine in the treatment of various cancers. Bevacizumab is a widely used agent which has been linked to gastrointestinal perforation with a black-box warning issued by the US Food and Drug Administration, recommending that the drug be permanently discontinued in patients with gastrointestinal perforation. In a meta-analysis of 17 randomized-controlled trials, patients that were treated with bevacizumab had a significantly increased risk of gastrointestinal perforation compared with patients treated with control medication, with a relative risk of 2.14. This relative risk jumped to 3.10 when only patients with colorectal cancer were included in the analysis. Overall, the incidence of gastrointestinal perforation with bevacizumab was 0.9% with a mortality of 21.7% [46]. Perforation may occur at a surgical anastomosis, at sites of tumor in/on the bowel wall (Fig. 16) or in otherwise normal segments. Perforation may also occur in association with enteritis/colitis [47]. Additionally, there have been reported association of gastrointestinal perforation with vascular endothelial growth factor tyrosine-kinase inhibitors (VEGFTKI), however a meta-analysis did not show an increased risk of gastrointestinal perforation with VEGFTKIs compared to control medications [48].
Colonoscopy
The most serious complication of colonoscopy is perforation, which has a morbidity rate as high as 43% and mortality rates as high as 25% [49]. In the setting of a diagnostic colonoscopy, the rectosigmoid is the most common site of perforation followed by the cecum, whereas in therapeutic colonoscopy it commonly occurs at the site of the excised polyp (Fig. 17) [50]. The overall incidence of perforation secondary to colonoscopy is very low, with one study reporting a 0.08% incidence of perforation in nearly 80,000 colonoscopies [49].
In cases of perforation secondary to colonoscopy, the radiologist can expect to find retroperitoneal gas in the anterior pararenal space when the perforation involves the posterior wall of the sigmoid, ascending or descending colon [50]. In the case of an anterior wall sigmoid perforation, free extraluminal air near the lesion and in the intraperitoneal spaces may be present [50]. Rectal perforation results in gas in the extraperitoneal pelvic spaces, possibly extending higher and producing bilateral pneumoretroperitoneum if the amount of extraluminal gas is large [3]. Perforations contained within the extraperitoneal or retroperitoneal spaces usually result in minimal symptoms.
CT colonography
Perforation can also be a consequence, albeit rare, from CT colonography. Patients with active bowel inflammation, colon-containing hernia, history of recent colonic biopsy or at an advanced age may be at increased risk of perforation from CT colonography [51, 52]. A large meta-analysis delineated a higher perforation rate in symptomatic versus asymptomatic patients (0.08% vs. 0.02%) with a pooled perforation rate of 0.04%. In terms of location of the perforation, the sigmoid colon was the most common site, involved in 40.7% of perforations, followed by the rectum in 22.2% (Fig. 18 ) and the cecum in 14.8% perforations [53].
Anastomotic leak
Anastomotic leak is a common complication in the immediate postoperative period, and can have devastating consequences, leading to peritonitis, sepsis, and death in as many as 50% of patients if not diagnosed in a timely manner and treated properly. Symptoms most commonly manifest in post-op days 5–7. Low anterior resections carry the highest risk for anastomotic leak [54].
Extravasation of enteric contrast is specific for leak (Fig. 19A–B). At our institution, oral contrast with extended prep time is used to assess integrity of a proximal anastomosis, for example after right hemicolectomy or ileocolic resection. Rectally administered contrast is used to evaluate a left colonic or colorectal anastomosis. A small bore flexible catheter is used to intubate the rectum. In cases of a low colorectal or coloanal anastomosis, the balloon is not inflated, to avoid possible disruption of the anastomosis. In addition to extraluminal contrast extravasation, findings indicative of anastomotic leak include loculated gas and fluid collections and a disproportionate amount of extraluminal gas in the surgical bed [54].
Postoperative anastomotic leak. A Noncontrast CT image demonstrates the rectosigmoid anastomosis (white arrow) with extraluminal fluid collections (white arrowheads). B Rectal contrast-enhanced CT image demonstrates enhancement of the extraluminal collections (white arrowheads) signifying anastomotic leak
There are numerous other causes of colorectal perforation due to iatrogenic injury, including instruments used during laparoscopic or robotic surgery, electrocautery, or other unintentional mural penetration. Percutaneous and endoscopic procedures such as feeding tube insertion, abscess drainages, paracentesis, and colonoscopic stent placement all carry a risk of bowel injury [10].
Conclusion
Large bowel perforation is an acute abdominal emergency, requiring rapid diagnosis for proper treatment. CT findings can often, but not always, localize the perforation to the involved segment of the gastrointestinal tract. Familiarity with the various etiologies of colorectal perforation and their associated CT findings can help the radiologist formulate an accurate differential diagnosis. Providing a specific diagnosis for the cause of large bowel perforation may not always be possible on CT. Many of the above described etiologies may show nonspecific findings of wall thickening and fat stranding. In particular, ischemic, inflammatory and infectious etiologies can all frequently show findings of nonspecific colitis. For example, diverticulitis and ischemic colitis frequently involve the left colon and may look similar. In most cases, they can be distinguished by length of involvement, presence of diverticulosis, and degree of fat stranding, but there can be overlap in these findings. In cases without specific findings, the distribution of colonic involvement and comparison with prior studies, if available, can sometimes help in narrowing the diagnosis. Additionally, correlation with medical history, physical exam findings, comorbidities, and laboratory values is essential for accurate diagnosis.
References
Bielecki K, Kamiński P, Klukowski M (2002) Large bowel perforation: morbidity and mortality. Tech Coloproctol 6(3):177–182
Kriwanek S, Armbruster C, Beckerhinn P, Ditrich K (1994) Prognostic factors for survival in colonic perforation. Int J Colorectal Dis 9(3):158–162
Furukawa A, Sakoda M, Yamasaki M, et al. (2005) Gastrointestinal tract perforation: CT diagnosis of presence, site, and cause. Abdom Imaging 30(5):524–534
Singh JP, Steward MJ, Booth TC, Mukhtar H, Murray D (2010) Evolution of imaging for abdominal perforation. Ann R Coll Surg Engl 92(3):182–188
Kim SH, Shin SS, Jeong YY, et al. (2009) Gastrointestinal tract perforation: MDCT findings according to the perforation sites. Korean J Radiol 10(1):63–70
Levenson RB, Camacho MA, Horn E, et al. (2012) Eliminating routine oral contrast use for CT in the emergency department: impact on patient throughput and diagnosis. Emerg Radiol 19(6):513–517
Lee CH, Haaland B, Earnest A, Tan CH (2013) Use of positive oral contrast agents in abdominopelvic computed tomography for blunt abdominal injury: meta-analysis and systematic review. Eur Radiol 23(9):2513–2521
Maniatis V, Chryssikopoulos H, Roussakis A, et al. (2000) Perforation of the alimentary tract: evaluation with computed tomography. Abdom Imaging 25(4):373–379
Hainaux B, Agneessens E, Bertinotti R, et al. (2006) Accuracy of MDCT in predicting site of gastrointestinal tract perforation. Am J Roentgenol 187(5):1179–1183
Zissin R, Hertz M, Osadchy A, Even-Sapir E, Gayer G (2008) Abdominal CT findings in nontraumatic colorectal perforation. Eur J Radiol 65(1):125–132
Iacobellis F, Berritto D, Grassi R (2015) Diagnostic approach to alimentary tract perforations. Imaging of alimentary tract perforation. Switzerland: Springer International Publishing, p 4
Kim SW, Kim HC, Yang DM (1017) Perforated tumours in the gastrointestinal tract: CT findings and clinical implications. Br J Radiol 2012(85):1307–1313
Imuta M, Awai K, Nakayama Y, et al. (2007) Multidetector CT findings suggesting a perforation site in the gastrointestinal tract: analysis in surgically confirmed 155 patients. Radiat Med 25(3):113–118
Saeki M, Hoshikawa Y, Miyazaki O, et al. (1998) Computed tomographic analysis of colonic perforation:“dirty mass”, a new computed tomographic finding. Emerg Radiol 5(3):140–145
Xiong L, Chintapalli KN, Dodd GD III, et al. (2004) Frequency and CT patterns of bowel wall thickening proximal to cancer of the colon. AJR 182:905–909
Goh BK, Chow PK, Quah HM, et al. (2006) Perforation of the gastrointestinal tract secondary to ingestion of foreign bodies. World J Surg 30(3):372–377
Gayer Gabriela, Petrovitch I, Brooke Jeffrey R (2011) Foreign objects encountered in the abdominal cavity at CT. Radiographics 31(2):409–428
Cianci R, Bianco V, Esposito G, Pizzi AD, Fillippone A. MDCT Imaging of Gastrointestinal Tract Perforation Due to Foreign Body Ingestion. In: Imaging of Alimentary Tract Perforation. Switzerland: Springer International Publishing, 2015; 79–84.
Goh BK, Tan YM, Lin SE, et al. (2006) CT in the preoperative diagnosis of fish bone perforation of the gastrointestinal tract. Am J Roentgenol 187(3):710–714
Heffernan C, Pachter HL, Megibow AJ, Macari M (2005) Stercoral colitis leading to fatal peritonitis: CT findings. Am J Roentgenol 184(4):1189–1193
Facy O, Radais F, Chalumeau C, et al. (2007) Stercoral perforation of the colon. Physiopathology and treatment strategy. Gastroenterol Clin Biol 31(12):1069–1070
Balthazar EJ, Yen BC, Gordon RB (1999) Ischemic colitis: CT evaluation of 54 cases 1. Radiology 211(2):381–388
Cruz C, Abujudeh HH, Nazarian RM, Thrall JH (2015) Ischemic colitis: spectrum of CT findings, sites of involvement and severity. Emerg Radiol 22(4):357–365
Ho LM, Paulson EK, Thompson WM (2007) Pneumatosis intestinalis in the adult: benign to life-threatening causes. Am J Roentgenol 188(6):1604–1613
Lee KS, Hwang S, Rúa SM, et al. (2013) Distinguishing benign and life-threatening pneumatosis intestinalis in patients with cancer by CT imaging features. Am J Roentgenol 200(5):1042–1047
Azzaroli F, Turco L, Ceroni L, et al. (2011) Pneumatosis cystoides intestinalis. World J Gastroenterol 17(44):4932–4936
Kaiser AM, Jiang JK, Lake JP, et al. (2005) The management of complicated diverticulitis and the role of computed tomography. Am J Gastroenterol 100(4):910–917
Chapman J, Davies M, Wolff B, et al. (2005) Complicated diverticulitis: is it time to rethink the rules? Ann Surg 242(4):576–583
Ritz JP, et al. (2011) Outcome of patients with acute sigmoid diverticulitis: multivariate analysis of risk factors for free perforation. Surgery 149(5):606–613
Berg DF, Bahadursingh AM, Kaminski DL, Longo WE (2002) Acute surgical emergencies in inflammatory bowel disease. Am J Surg 184(1):45–51
Moulin V, Dellon P, Laurent O, et al. (2011) Toxic megacolon in patients with severe acute colitis: computed tomographic features. Clin Imaging 35(6):431–436
Imbriaco M, Balthazar EJ (2001) Toxic megacolon: role of CT in evaluation and detection of complications. Clin Imaging 25(5):349–354
Thoeni RF, Cello JP (2006) CT imaging of colitis. Radiology 240(3):623–638
Levy AD, Mortele KJ, Yeh BM (2015) Infectious colitis. Gastrointestinal imaging. USA: Oxford University Press, p 236
Ash L, Baker ME, O’Malley CM Jr, et al. (2006) Colonic abnormalities on CT in adult hospitalized patients with Clostridium difficile colitis: prevalence and significance of findings. Am J Roentgenol 186(5):1393–1400
Kirkpatrick ID, Greenberg HM (2003) Gastrointestinal complications in the neutropenic patient: characterization and differentiation with abdominal CT 1. Radiology 226(3):668–674
Kennedy CL, Krejany EO, Young LF, et al. (2005) The α-toxin of Clostridium septicum is essential for virulence. Mol Microbiol 57(5):1357–1366
Khan AA, Davenport K (2006) A reminder of the association between Clostridium septicum and colonic adenocarcinoma. Int Semin Surg Oncol 1(3):12
Kolbeinsson ME, Holder WD, Aziz S (1991) Recognition, management, and prevention of Clostridium septicum abscess in immunosuppressed patients. Arch Surg 126(5):642–645
Urban BA, McCormick R, Fishman EK, Lillemoe KD, Petty BG (2000) Fulminant Clostridium septicum infection of hepatic metastases presenting as pneumoperitoneum. Am J Roentgenol 174(4):962–964
Cappell MS, Batke M (2008) Mechanical obstruction of the small bowel and colon. Med Clin North Am 92(3):575–597
Ponec RJ, Saunders MD, Kimmey MB (1999) Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med 341:137–141
Vanek VW, Al-Salti M (1986) Acute pseudo-obstruction of the colon (Ogilvie’s syndrome): an analysis of 400 cases. Dis Colon Rectum 29:203–210
Geller A, Petersen BT, Gostout CJ (1996) Endoscopic decompression for acute colonic pseudo-obstruction. Gastrointest Endosc 44(2):144–150
Maloney N, Vargas HD (2005) Acute intestinal pseudo-obstruction (Ogilvie’s syndrome). Clin Colon Rectal Surg 18(02):96–101
Hapani S, Chu D, Wu S (2009) Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol 10(6):559–568
Thornton E, Howard SA, Jagannathan J, et al. (1018) Imaging features of bowel toxicities in the setting of molecular targeted therapies in cancer patients. Br J Radiol 2012(85):1420–1426
Qi WX, Sun YJ, Tang LN, Shen Z, Yao Y (2014) Risk of gastrointestinal perforation in cancer patients treated with vascular endothelial growth factor receptor tyrosine kinase inhibitors: a systematic review and meta-analysis. Crit Rev Oncol/Hematol 89(3):394–403
Iqbal CW, Cullinane DC, Schiller HJ, et al. (2008) Surgical management and outcomes of 165 colonoscopic perforations from a single institution. Arch Surg 143(7):701–707
Ponticiello G, Di Nuzzo L, Saturnino PP. Colorectal perforation: assessment with MDCT. In: Imaging of alimentary tract perforation. Switzerland: Springer International Publishing, 2015; 59–60.
Sosna J, Blachar A, Amitai M, et al. (2006) Colonic perforation at CT colonography: assessment of risk in a multicenter large cohort. Radiology 239(2):457–463
Dachman AH (2006) Advice for optimizing colonic distention and minimizing risk of perforation during CT colonography. Radiology 239(2):317–321
Bellini D, Rengo M, De Cecco CN, et al. (2014) Perforation rate in CT colonography: a systematic review of the literature and meta-analysis. Eur Radiol 24(7):1487–1496
Weinstein S, Osei-Bonsu S, Aslam R, Yee J (2013) Multidetector CT of the postoperative colon: review of normal appearances and common complications. Radiographics 33(2):515–532
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Statement of informed consent was not applicable since the manuscript does not contain any patient data.
Rights and permissions
About this article
Cite this article
Kothari, K., Friedman, B., Grimaldi, G.M. et al. Nontraumatic large bowel perforation: spectrum of etiologies and CT findings. Abdom Radiol 42, 2597–2608 (2017). https://doi.org/10.1007/s00261-017-1180-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1180-x