Abstract
Gastrointestinal (GI) tract perforation is a life-threatening condition that can occur at any site along the alimentary tract. Early perforation detection and intervention significantly improves patient outcome. With a high sensitivity for pneumoperitoneum, computed tomography (CT) is widely accepted as the diagnostic modality of choice when a perforated hollow viscus is suspected. While confirming the presence of a perforation is critical, clinical management and surgical technique also depend on localizing the perforation site. CT is accurate in detecting the site of perforation, with segmental bowel wall thickening, focal bowel wall defect, or bubbles of extraluminal gas concentrated in close proximity to the bowel wall shown to be the most specific findings. In this article, we will present the causes for perforation at each site throughout the GI tract and review the patterns that can lead to prospective diagnosis and perforation site localization utilizing CT images of surgically proven cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastrointestinal (GI) tract perforation is a life-threatening condition that can occur at any site along the alimentary tract. The standard management is prompt surgical intervention, with patient morbidity and mortality rising significantly when diagnosis and treatment are delayed [1]. With delayed management, sepsis and multi-organ failure result in nearly 75% of perforation cases and the mortality rate approaches 30% [1–3]. In contrast, early perforation detection and intervention significantly improves patient outcome. With a high sensitivity for pneumoperitoneum, computed tomography (CT) is widely accepted as the diagnostic modality of choice when a perforated hollow viscus is suspected [4–6].
Direct findings on CT which confirm the presence of a perforation include focal bowel wall discontinuity, extraluminal gas, and extraluminal enteric contrast (when administered). Indirect signs of bowel perforation on CT include segmental bowel wall thickening, abnormal bowel wall enhancement, perivisceral fat stranding or fluid, and abscess [4, 7–12].
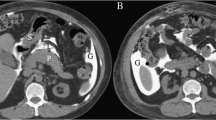
While confirming the presence of a perforation is critical, clinical management and surgical technique also depend on localizing the perforation site. CT is accurate in detecting the site of perforation in about 85% of cases [4, 7, 13]. If free intraperitoneal gas is found only in the upper abdomen, a proximal GI perforation is more likely. In contrast, when free intraperitoneal gas is only identified within the pelvis, the perforation site is likely to be colon or, less frequently, small bowel [7, 14]. If the gas is isolated to the extraperitoneum, a retroperitoneal perforation is more likely, including the second or third segments of the duodenum, ascending or descending colon, or distal third of the rectum. To further pinpoint the site of perforation, the presence of any of the following three CT findings has been shown to be the most reliable; segmental bowel wall thickening, focal bowel wall defect, or bubbles of extraluminal gas concentrated in close proximity to the bowel wall (Fig. 1) [4]. In this article, we will present the causes for perforation at each site throughout the GI tract and review the patterns that can lead to prospective diagnosis and perforation site localization utilizing CT images of surgically proven cases (Table 1).
A The three CT findings proven reliable in localizing a perforation site: (1) Segmental bowel wall thickening (arrowhead), (2) Focal bowel wall defect (long arrow), and (3) Concentrated extraluminal gas (short arrow). B The same image demonstrates CT findings contributory in diagnosing a bowel perforation, however, not shown to be consistently accurate in localizing a perforation site: (1) Subdiaphragmatic gas (arrowhead), (2) Perivisceral fat stranding (long arrow), and (3) Free intraperitoneal fluid (short arrow).
CT technique
At our institution, when an abnormality of the alimentary tract is suspected clinically, we obtain contiguous axial images from the thoracic inlet through the upper abdomen to evaluate the esophagus or from the lung bases through the ischial tuberosities to evaluate the subdiaphragmatic GI tract. Intravenous contrast injection with a low osmolarity contrast medium is performed unless a contraindication exists. 5 mm maximum axial slice thickness images are acquired in the portal venous phase at a 60 s delay. Coronal and sagittal multiplanar reformatted images are routinely obtained. CT image interrogation with wide window settings (bone and lung windows) is complementary to conventional abdominal windows for detecting extraluminal gas [15, 16].
Oral contrast
When administering oral contrast in a case of suspected bowel perforation, dilute water-soluble iodinated contrast (not barium-based contrast) should be administered. Extravasated ingested contrast material is a direct sign of bowel perforation, and when present, has a high specificity for localizing the perforation site. However, patients with peritoneal symptoms tend to poorly tolerate enteric contrast and waiting for intraluminal contrast opacification can delay emergent management. The sensitivity of extravasation of enteric contrast material varies from 19% to 42% [4, 17–19]. The relatively low observation is believed to be due to the rapid sealing of a large percentage of perforation sites and the supine positioning used for CT, which is unlikely to show extravasation of an anterior perforation. Therefore, enteric contrast can be helpful in identifying a site of perforation; however, it is most often noncontributory, and the absence of visible extravasation does not exclude a perforation.
Esophagus
Esophageal perforation often presents acutely with nonspecific thoracic manifestations including, but not limited to, chest pain, odynophagia, vomiting, and shock. Therefore, CT is the most common modality utilized in detecting esophageal perforation in the emergent setting, and has been shown to be complimentary to conventional esophagography and direct visualization in delineating the perforation location and underlying etiology [20–22]. As with elsewhere in the GI tract, delay in diagnosis accounts for the highest morbidity and mortality. Therefore, prompt detection and management is critical. The more common etiologies include spontaneous rupture (Boerhaave syndrome), foreign body ingestion, tumor, trauma, and iatrogenic causes.
Boerhaave syndrome
With Boerhaave syndrome, spontaneous emetogenic perforation results from the rapid increase in intraluminal pressure encountered with vomiting in the setting of incomplete cricopharyngeal relaxation [20]. The most common rupture location is the distal left posterior wall, which classically presents with pneumomediastinum and left pleural effusion [20]. At CT, the esophageal wall defect may be detectable. Left-sided mediastinal gas and fluid and a left pleural effusion are often visualized (Fig. 2).
Spontaneous: Boerhaave syndrome. A–D Axial and coronal soft tissue and bone algorithm images demonstrate a focal wall defect along the left side of the esophagus (arrows) and regional pneumomediastinum (arrowheads). E Subsequent esophagram confirms the perforation with extravasation of enteric contrast (arrowhead).
Foreign body
Foreign bodies can result in perforation through penetration injury in the setting of sharp object ingestion or via pressure necrosis when a foreign body becomes lodged against the thin esophageal wall. Food bolus (usually meat) is the most commonly encountered esophageal impaction (Fig. 3) [20, 23, 24]. An underlying abnormality such as stricture is often discovered at the time of retrieval [20, 25]. Bones from fish or chicken account for the second most common esophageal foreign body (Fig. 4) [20, 26]. When the history suggests this type of foreign body, barium studies are discouraged as they may obscure visualization at the time of endoscopic examination and retrieval attempt [20, 24, 27].
Foreign body: food impaction. A, B Axial and coronal CT images demonstrate debris distending the esophagus (long arrows), with extraluminal paraesophageal fluid (arrowheads) and gas (short arrows). C Contrast ingested during esophagram fails to pass the debris/obstruction (arrows). D Post endoscopy and disimpaction; contrast readily flows into the stomach.
Foreign body: fish bone. A Axial lung algorithm image shows extensive pneumomediastinum (blue arrowheads). B A loculated retrocardiac collection (blue arrowhead) containing a linear radiopaque density (arrow), confirmed to be a fish bone. C, D Soft tissue and bone algorithm images show an esophageal wall defect (arrows) in communication with the collection. E Esophagram confirms retrocardiac extravasation (arrows).
Tumor
Perforation of esophageal carcinoma is a rare complication that most often results from palliative measures such as radiation therapy, instrumentation at the time of stent placement, or via pressure necrosis from a previously placed stent [28, 29]. Esophagorespiratory fistulas are a known complication that can result from transmural disruption from an esophageal carcinoma or lung primary. The diagnosis may first be suggested in the setting of lung abscesses or recurrent pneumonias developing in this patient population [29]. At CT, a bronchoesophageal fistula may be visualized as a direct air-filled communication between the structures (Fig. 5). To maximize survival, the diagnosis of esophagopulmonary fistula must be established before the onset of resultant lung infections [29].
Tumor: lung carcinoma with erosion into the esophagus; esophagobronchial fistula. A, B Axial and coronal CT images demonstrate left lower lobe bronchial narrowing (arrows) and lobar collapse, along with fistulous communication to the esophagus (arrowheads) in a patient with recurrent lung carcinoma. C The esophageal wall disruption led to a contained extraluminal gas collection within the posterior mediastinum (arrow).
Trauma
Esophageal perforation from external blunt trauma or a penetrating injury is rare, likely due to the small size and protected posterior position of the esophagus. Esophagography with water-soluble contrast is an effective imaging study in diagnosing an esophageal tear. However, the presenting symptoms of a traumatic esophageal injury are nonspecific and frequently attributed to more commonly encountered injuries [30]. Therefore, CT findings are often the first indication of a traumatic esophageal injury. An esophageal mural defect with posterior pneumomediastinum is diagnostic (Fig. 6). When not attributable to another cause, subcutaneous cervical gas or posterior pneumomediastinum should raise suspicion for esophageal perforation and guide further work up to include esophagography and/or direct inspection.
Iatrogenic
Traumatic esophageal perforation is much more likely to result from iatrogenic causes [31]. Specifically, intraluminal trauma from therapeutic endoscopic procedures such as stricture dilatation and stent placement has the highest association. Effective management depends on an early clinical suspicion and accurate interpretation of diagnostic imaging [31]. The CT features will be similar to those described with trauma above, however, with the additional history of a recent esophageal procedure (Fig. 7).
Iatrogenic: EGD balloon dilatation attempt of an esophageal stricture. A–C Superior to inferior axial images show esophageal dilatation, stricture, and posterior wall disruption, respectively (white arrows) with extravasation of oral contrast (blue arrowhead). D, E Coronal image and subsequent esophagram demonstrate the stricture (white arrows) and ingested contrast pooling in the posterior mediastinum (blue arrowheads).
Stomach/duodenum
The primary etiologies leading to gastroduodenal perforation include ulcerative disease, blunt or penetrating trauma, malignancy, and iatrogenic causes [7, 9]. Gastric and duodenal perforations are discussed together in this section due to the similar causative pathology, clinical presentation, and management options. The imaging features can also overlap; however, there are some key differences which will be discussed below.
Ulcerative disease
Pharmaceutical advancements (mainly the introduction proton pump inhibitors) along with an increased recognition of the importance of treating Helicobacter pylori infection have led to a considerable decrease in the incidence of peptic ulcer disease over the past three decades [1, 32]. Nevertheless, peptic ulcer disease remains the most common cause for gastroduodenal perforation [1]. Peptic ulcer perforation is most commonly found in the gastric antrum or duodenal bulb, with the duodenal ulcer perforation risk estimated at 5%–10% [1, 7]. At CT imaging, an ulceration or focal wall defect may be visible (Figs. 8, 9). Pneumoperitoneum is the most sensitive finding and can be extensive. However, the absence of extraluminal gas does not exclude gastroduodenal ulcer perforation, particularly at the onset of symptoms [7, 33]. Localized extraluminal gas in contact with the stomach or the duodenum is specific for localizing the perforation site to the adjacent viscus. Gastric and proximal duodenal perforations rarely result in air trapped within the mesenteric root or sigmoid recess, which would therefore strongly favor a perforation of the colon or distal small bowel [7, 14]. As the duodenum distal to the bulb is retroperitoneal, extraluminal gas in the right anterior pararenal space is a reliable CT finding for diagnosing a distal duodenal perforation. Sensitive, though less specific findings overlap with perforations elsewhere and include focal bowel wall thickening, perigastric or periduodenal fluid, and adjacent mesenteric fat stranding [7, 10]. Most patients with a perforated ulcer are managed surgically by suturing the perforation and preoperative confirmation of the ulcer location can impact surgical technique [1, 32, 34, 35]. While not common at our institution, a spontaneously sealed duodenal ulcer may occasionally be managed nonoperatively [7, 36].
Ulcerative disease: duodenal ulcer (same patient as Fig. 1). A, B Sagittal and coronal zoomed in images show a focal defect along the superior wall of the duodenal bulb (long arrows). Note the subdiaphragmatic intraperitoneal gas (arrowhead) and fluid (short arrow). C There is a concentrated gas bubble adjacent to the defect (long arrow) as well as perivisceral fat stranding (arrowhead).
Marginal ulceration occurring after Roux-en-Y gastrojejunostomy is a known and relatively common late complication of the operation. As with peptic ulcer disease, medical management is often sufficient. However, when perforation of a marginal ulcer occurs, the impact can be devastating. The reported incidence is ~1% of the patients undergoing laparoscopic gastric bypass, and the perforation occurs on average 1.5 years after the operation [37]. On CT imaging, the salient findings are similar to peptic ulcer disease described above, however, will be located at the gastrojejunostomy anastomotic site (Fig. 10) [37].
Ulcerative disease: gastrojejunostomy with marginal ulceration. A Axial and B, C coronal images demonstrate a focal defect with adjacent extraluminal collection at the gastrojejunostomy anastomosis (arrows) in a patient 1 year post-gastric bypass. This history and imaging appearance was suspicious for, and confirmed at surgery to be, a contained perforation of a marginal ulcer.
Trauma
With a thick muscular wall and protected location, the stomach is relatively resistant to external trauma. Penetrating injuries are more likely than blunt trauma to lead to gastric perforation, and the presence of a distended stomach increases the risk of rupture from either mechanism [1, 38]. CT features may show disruption of the gastric wall with perigastric fluid (Fig. 11). A large amount of gas may be released into the peritoneal space. With penetrating injuries, a wound track extending to the stomach may be evident (Fig. 12). Gastric trauma is often associated with concomitant left hemidiaphragmatic injury. When present, contamination of the chest cavity from spillage of gastric contents can place the patient at increased risk for subsequent empyema.
Trauma: gastric stab wound with an unknown object. A There is a focal defect along the lateral wall of the stomach (arrow), with associated extravasation of ingested contrast and gas (arrowhead). B Axial image more inferiorly demonstrates pooling of extravasated contrast along the inferior margin of the spleen (arrow) and a tiny subcutaneous foreign body along the trajectory of the stab wound (arrowhead).
The retroperitoneal position of the duodenum provides protection from most penetrating injuries. However, with fixed attachments and proximity to the vertebral column, the descending and horizontal segments are at a relatively high risk for perforation in the setting of blunt trauma (Fig. 13) [7].
Trauma: motor vehicle accident with duodenal perforation. A Axial image showing thickening of the descending segment of the duodenum (long arrows), with retroperitoneal gas extending along the anterior margin of the pancreas (short arrow). B, C Coronal images showing the second part of the duodenum to be thickened (long arrows), with adjacent extraluminal gas (short arrow).
Tumor
GI tract tumors are more likely to perforate as a consequence of trauma or iatrogenic injuries [39–45]. When spontaneous perforation occurs, the instigating factor tends to be underlying ischemia and necrosis (Fig. 14) [39–45]. Importantly, bowel perforation occasionally occurs proximal to the tumor secondary to the increased intraluminal pressure resulting from bowel obstruction or in the setting of a mucin-producing neoplasm [43]. Perforation of gastric tumors is rare, with a reported incidence of 0.4%–6.0%, and predominately occurs in malignant tumors at advanced stages (T3 or higher) [43, 44]. Therefore, when presenting as a perforation, CT imaging features to suggest an underlying malignancy are also often evident and include irregular thickening and enhancement of the gastric wall, perivisceral soft-tissue extension, peritoneal spread of disease, and lymphadenopathy [43]. When gastric carcinoma perforation occurs at an earlier stage, the sensitivity for CT identifying the malignancy is limited. However, in an elderly patient with a perforated deep ulcer on imaging, malignancy should be suspected [43, 46, 47].
Tumor: periampullary duodenal mass. A Enteric contrast spills into the hepatorenal space and extends posterior to the third portion of the duodenum (arrowheads). B The periampullary mass is suggested on this image (arrowhead) and subsequently confirmed endoscopically. A feeding tube extends past the mass, to the ligament of Trietz (arrow).
Iatrogenic
Any object placed into the GI tract has the potential to result in perforation [48–50]. Diagnostic and therapeutic techniques such as esophagogastroduodenoscopy (EGD) and endoscopic retrograde cholangiopancreatography (ERCP) are safe in the majority of patients, with reported perforation rates of 0.03%–0.3% [49, 50]. With EGD, the esophagus is the most common perforation site (51%), followed by the duodenum (32%), jejunum (6%), and stomach (3%) [50]. The thick, muscular gastric wall is felt to be protective. With ERCP, the retroperitoneal duodenum is by far the most frequent bowel perforation site, and is more common when a sphincterotomy is performed (Fig. 15) [49]. Unfortunately, as with other etiologies, the morbidity and mortality rates for iatrogenic gastroduodenal perforation are high. CT imaging plays a crucial role in patients with suspected iatrogenic perforation. In a Mayo Clinic study of 72 EGD perforations, the only factors determined to be predictive of which patients were more likely to fail nonoperative management were the post-procedure CT findings of free fluid or contrast extravasation [50].
Iatrogenic. Duodenal perforation during ERCP sphincterotomy for extraction of a common bile duct stone. A ERCP fluoroscopic image of the right upper quadrant in the AP projection post-sphincterotomy demonstrates an irregular collection of contrast (arrowheads). The lack of outlined mucosal folds and peculiar configuration favors an extraluminal location. In addition, there are adjacent vertically oriented collections of air that fail to conform to bowel, in keeping with extraluminal air (short arrows). Cholecystectomy clips are present (long arrow). Subsequent CT with axial (B) and coronal (C, D) images confirming the perforation by documenting extraluminal gas (arrows) and contrast (arrowheads).
Small bowel
Perforation of the jejunum or ileum tends to present with nonspecific clinical symptoms and therefore the diagnosis is most often made at the time of CT imaging. Etiologies for small bowel perforation include inflammatory, infectious, and ischemic conditions, small bowel diverticulitis, mechanical obstruction, trauma, malignancy, iatrogenic causes, and foreign bodies [51].
Inflammatory
Crohn disease is a common inflammatory condition to affect small bowel and can rarely lead to free perforation from the colon (1.6%) or small bowel (0.7%) [52]. While the ileum is the most commonly inflamed segment of bowel in Crohn patients, the most frequent site for small bowel perforation is less clear, with some studies claiming jejunum and others claiming ileum as the most common culprit [52, 53]. More common than free perforation, Crohn disease leads to sinus tracts, fistulas, and contained perforations sealed off by inter-loop adhesions, leading to localized phlegmonous changes and abscess formation. Rarely, perforation can be the presenting finding in a patient otherwise undiagnosed as having Crohn disease [54]. CT plays a critical role in this population, identifying the signs of small bowel perforation as well as the imaging features suggestive of Crohn disease as the underlying etiology (Fig. 16) [55].
Inflammatory: Crohn disease. A Coronal image showing mucosal hyperenhancement of a segment of small bowel, in keeping with active inflammation (long arrows). A large amount of sub-diaphragmatic gas is noted (blue arrowheads) as is a complex loculated collection/abscess in the left lower quadrant (short arrows). B There is a chronically distended loop of bowel in the left lower quadrant containing enterolith (long arrows). The distention resulted from chronic delayed transit through the stenotic, and now actively inflamed, distal segment of bowel shown in A. The superimposed active inflammation likely led to a complete obstruction and subsequent proximal perforation as confirmed by the focal defect along the medial wall in communication with the abscess (short arrows). Free air is noted anteriorly (blue arrowhead).
Ischemic
Small bowel perforation can result from the inflammation and subsequent necrosis that develops in the setting of mesenteric ischemia. Underlying causes include bowel strangulation, obstruction, large vessel occlusion, or vasculitis. With vasculitides, small systemic visceral vessels may be involved and not directly demonstrable by CT [16, 56]. However, mural and perivisceral changes at CT can be suggestive of underlying ischemia, including bowel wall thickening, mural hypoperfusion, and localized mesenteric fluid (Fig. 17) [16, 56, 57]. Specific, though often late, findings of bowel infarction include a lack of bowel wall enhancement, gas within the mesenteric venous system, and bowel wall pneumatosis [16, 56, 57].
Ischemic: vasculitis. Mucosal hyperenhancement and wall thickening of a segment of small bowel (long arrows), confirmed to be ischemic at surgery. Note the adjacent extraluminal gas and fluid (short arrow). Segmental bowel wall thickening and concentrated extraluminal gas is specific for this to be the perforation site.
Trauma
Encompassing more surface area within the peritoneal cavity than any other organ, small bowel has a high propensity for injury in the setting of penetrating abdominal trauma. 80% of gunshot wounds and 30% of stab wounds that disrupt the peritoneum lead to small bowel injury [1, 58]. Delaying surgical management or wound exploration to obtain a CT in these patients is controversial. When a CT is obtained, free intraperitoneal gas alone is not diagnostic of bowel injury as air can be introduced into the peritoneal cavity by the mechanism of injury. A wound track extending to an injured segment of bowel has been shown to be the most sensitive CT finding (Fig. 18) [16, 59].
Trauma: bullet. A, B Inferior to superior axial images showing locules of gas along a bullet track (long arrows) that traveled anterior to posterior, crossing small bowel and mesentery before becoming lodged within the right presacral musculature (arrowhead). A hematoma/complex fluid collection surrounds a small bowel loop with irregular appearing walls (short arrows). C Axial image through the level of the liver showing free intraperitoneal fluid and air (arrow).
In contrast, small bowel perforation from blunt abdominal trauma is an infrequent complication. When perforation results from blunt trauma, abdominal CT has a sensitivity of 64% and accuracy of 82% in detecting the site of perforation [16, 60]. Even in the absence of the direct CT signs of GI tract perforation discussed earlier, infiltration of the mesentery and/or a moderate to large volume of intraperitoneal fluid (in the absence of a solid organ injury) should raise concern for an occult bowel injury [16, 60, 61].
Tumor
Small bowel perforation from an underlying malignancy most often results from GI lymphoma, and while lymphoma can arise anywhere within the alimentary tact, the vast majority of GI lymphoma perforations occur within the small bowel [16, 39, 41, 43, 62]. Bowel perforation from GI lymphoma is more common in the setting of T-cell lymphoma, post-transplant lymphoproliferative disorder, and after chemotherapy or radiation treatment [63–67].
At CT, the signs of perforation can be subtle, with only a small amount of extraluminal gas or fluid. The characteristic bowel appearance of a circumferentially thickened segment with aneurysmal dilatation of the lumen can suggest the underlying etiology [66]. The presence of multifocal bowel involvement, lymphadenopathy, and hepatosplenomegaly can be additional clues in suggesting GI lymphoma [43].
Gastrointestinal stromal tumors (GISTs) are most commonly located in the stomach or small bowel and rarely spontaneously rupture [40–43]. At CT, ruptured GISTs tend to appear heterogeneous in attenuation, with a lamellated pattern thought to reflect areas of hemorrhage or necrotic degeneration [43]. Ascites is uncommon for GISTs. Therefore, when otherwise unexplained, a patient with a GIST who develops ascites should raise suspicion for tumor rupture (Fig. 19) [40, 43]. Risk factors for GIST rupture include a large size, exophytic configuration, large internal cystic or necrotic component, and a rapid growth rate [42, 43].
Tumor: gastrointestinal stromal tumor (GIST). A Axial images showing a heterogeneous attenuation mass in the proximal jejunum (short arrows), confirmed surgically to represent a GIST. Note the adjacent extraluminal fluid collection (arrowheads). B Just superiorly, there are locules of intraperitoneal gas within the left hemiabdomen (long arrows).
Perforation of a small bowel metastasis most often results from lung carcinoma [43]. The CT appearance is often nonspecific, though the presence of an intraluminal polypoid mass or wall thickening with variable patterns of contrast enhancement in the setting of a known primary malignancy should raise suspicion [43, 68].
Iatrogenic
Bowel injury at the time of laparoscopic abdominal surgery most commonly affects the small bowel and is usually identified and corrected at the time of injury [16]. When iatrogenic bowel injury is unrecognized intraoperatively, there is a high post-operative morbidity rate. The CT appearance can be challenging, as intraperitoneal gas is not unexpected in the recently post-laparoscopy state. Oral contrast can be useful, as extraluminal ingested contrast in the setting of an intact anastomotic site suggests the diagnosis of accidental bowel injury [16].
Foreign body
Intuitively, ingested foreign bodies are more likely to lead to small bowel perforation when sharp or shaped in a manner that predisposes to failed passage (long, nonflexible, >3 cm in diameter). Fish bones and chicken bones are the most common inadvertently ingested foreign bodies to cause perforation (Fig. 20). Foreign body perforations rarely result in a large amount of free intraperitoneal gas, as the bowel insult tends to be gradual, and the injured site concurrently covers with a fibrinous exudate [16]. Concentrated extraluminal gas locules within the mesentery and infiltration of fat near a thickened bowel segment are the most common CT findings (Fig. 21) [16]. Identifying the foreign body confirms the diagnosis, and is more common with foreign bodies of radiodense material such as metal or calcium [16, 69, 70]. In cases of metal foreign bodies, a bone window setting may be useful in identifying the object [16].
Foreign body: fish bone. A–D Sequential axial images showing a thickened loop of small bowel in the left lower quadrant with locules of extraluminal gas along the mesenteric margin (white arrows). In B, there is a linear radiopaque density within the inflamed small bowel loop (blue arrowhead), confirmed at surgery to be a fish bone.
Foreign body: corner from medication packaging. A Axial imaging showing a few locules of mesenteric gas (arrows). B Just inferiorly, there is a mildly thickened segment of small bowel, with an intraluminal linear opacity extending into the anterior left wall (arrowhead). C At surgery, the perforation was confirmed to be secondary to the corner of a medication package, inadvertently ingested while taking the medication.
Appendix
Appendicitis
CT has a high sensitivity and specificity for detecting and diagnosing acute appendicitis [71–77]. Unfortunately, the data are inconsistent as to the effectiveness for CT to prospectively detect appendiceal perforation, particularly in an early stage [71, 78–82]. The presence of an abscess, extraluminal gas, or ileus strongly correlates with appendiceal perforation (Fig. 22) [71]. However, with early or micro-perforation, these definitive findings are uncommon, and tend to be subtle if present. Periappendiceal stranding and fluid can be encountered with the perforated or nonperforated appendix and an enhancement defect in the appendiceal wall has been shown to have low sensitivity and specificity for appendiceal perforation [71]. Despite these limitations, determining whether or not the appendix has ruptured has important prognostic and management implications. In the setting of perforation, morbidity and mortality are higher, and conservative preoperative management, including intravenous antibiotics or abscess drainage, is often indicated to reduce the extent of subsequent surgery [71, 83, 84].
Tumor
Appendiceal mucinous neoplasms (neoplastic mucoceles) can spread to the peritoneum as pseudomyxoma peritonei through extraluminal invasive carcinomas or ruptured adenomas [85]. At CT, cystic dilatation of the appendix with a luminal diameter greater than 1.3 cm, along with the presence of mural calcification, suggests a mucocele (Fig. 23) [43]. There is overlap in distinguishing between benign and low-grade malignant processes at both imaging and pathology [85]. At CT, enhancing appendiceal wall nodularity favors the presence of an invasive mucinous cystadenocarcinoma [43, 86].
Tumor: appendiceal mucinous neoplasm. A Coronal image showing a normal base of the appendix (arrowhead), with abrupt dilatation of the mid and distal appendix to over 4 cm (arrows). B–D Axial images showing extensive intraperitoneal gelatinous ascites, in keeping with pseudomyxoma peritonei. The mucocele is outlined by mural calcification (long arrows), with a focal defect corresponding to the rupture site (short arrow).
Colon
Tumor
Colon adenocarcinoma can lead to perforation via two mechanisms: (1) necrosis at the site of the mass and (2) a functional closed-loop obstruction between the mass and a competent ileocecal valve, resulting in colonic perforation proximal to the mass [43, 87, 88]. The most commonly involved segments to perforate include the sigmoid colon and cecum [43, 87, 89]. At CT, extraluminal gas can be extensive. Identifying signs of perforation in the setting of irregular colonic wall thickening and infiltrative pericolonic soft tissue can favor the diagnosis (Fig. 24) [7, 43].
Tumor: colon adenocarcinoma. A Thickened segment of sigmoid colon (arrows) with nodular soft tissue attenuation spread to the pericolonic fat (arrowheads). B Thick-walled extraluminal collection (long arrow) extends antero-inferior from the mass and abuts the bladder. Gas is noted within the bladder nondependently (short arrow). This was confirmed at surgery to be a sigmoid adenocarcinoma with contained perforation and fistualization to the bladder.
Self-expanding metal stents are increasingly being used in the setting of malignant colorectal obstruction as a bridge to scheduled surgery or as palliative option in patients with advanced stage disease [90]. Perforation is a potential complication that can occur days to months after stent placement, and is more common in patients previously treated with chemotherapy [90]. CT imaging tends to be straightforward, with visualization of the stent extending through the site of colonic wall disruption (Fig. 25).
Diverticulitis
Perforated diverticulitis represents the most serious complication of diverticular disease and can occur at any site along the colon [91]. In Western countries, diverticular disease, as well as diverticular perforation, most commonly arises along the sigmoid colon [92]. At CT, diverticulitis appears as segmental wall thickening and pericolonic fat stranding in the setting of underlying diverticular disease [92]. CT has a high sensitivity for detecting complications secondary to diverticulitis including abscess formation and focal-contained perforations. Contained perforations present as small extraluminal pockets of gas. Less often, diffuse pneumoperitoneum and even retroperitoneal and mediastinal gas can occur via subperitoneal communications (Fig. 26) [93].
Diverticulitis: sigmoid colon. A Gas tracks to the level of the thoracic inlet (arrowheads). B Gas tracks along the left retroperitoneum (arrowheads). C The air originated at the level of the sigmoid colon, where a thickened segment contains diverticula and is surrounded by extraluminal gas (arrowheads). The sigmoid colon is intraperitoneal, however, the subperitoneal space serves as a common pathway for extraperitoneal communication. The mediastinal gas dissects superiorly from retroperitoneal communications.
Iatrogenic
Colonoscopic perforation is a rare procedural complication; however, as with most other etiologies for bowel perforation, has a high rate of morbidity and mortality [94]. Colonoscopy being performed in patients with multiple comorbidities or for therapeutic purposes is at increased risk for perforation [94–96]. Plain radiography or CT may be ordered when colonoscopic perforation is suspected. The presence of extraluminal gas can be confirmatory (Fig. 27). However, patients are often managed based on the presence or absence of generalized peritonitis even without radiologic evidence of perforation [94]. At colonoscopy, barotrauma from pneumatic distention is the attributed etiology for most right-sided colonic tears. In contrast, most left-sided perforations result from direct mechanical trauma from the endoscope, in which the perforation site is typically along the anti-mesenteric colonic wall [94, 97, 98].
Iatrogenic: perforation after colonoscopy. A Pneumatosis coli (arrowheads) and pericolonic gas surround the ascending colon (arrows). This presumably corresponds to the perforation level. B, C Axial and coronal images showing air tracking along the right pararenal spaces (white arrows) and a small amount of intraperitoneal gas collects anterior to the liver (arrowhead). Barotrauma from pneumatic distention was the attributed etiology. As the patient lacked symptoms of peritonitis, conservative management was elected.
Note that post-polypectomy syndrome, a transmural injury at the site of an excised polyp, mimics perforation by presenting with similar clinical signs and symptoms of peritonitis [94, 99]. CT scan may reveal focal mural thickening with pericolonic fluid and stranding at the polypectomy site, however, no pneumoperitoneum will be evident [94, 97].
Foreign body
Colorectal foreign bodies can result from antegrade passage of ingested objects or retrograde insertion during acts of sexual stimulation or assault. The foreign body can usually be diagnosed with plain radiography and removed transanally via manual manipulation [100]. When peritoneal symptoms are present or if the foreign body is not readily reducible, CT can be complimentary in diagnosing the exact position of the foreign body and evaluating for perforation. At CT, perforation can be confirmed by bowel wall disruption and the extraluminal position of the object (Fig. 28). Perforations are often treated by surgical repair and may require proximal loop colostomy [101].
Foreign body: intraperitoneal zucchini. A Coronal image showing a thickened segment of the rectosigmoid colon (short arrow), with an adjacent collection of fluid containing locules of gas (long arrow). B, C Coronal and axial images show a large, predominately air density, foreign body freely positioned within the peritoneal cavity (arrowheads) with adjacent locules of gas (long arrows). This corresponded to a rectally inserted zucchini that had perforated through the bowel wall. Reproduced with permission, courtesy of Dr. Frank Gaillard, Radiopaedia.org.
Conclusion
CT is an excellent resource in detecting and localizing a GI tract perforation site at any location from the esophagus to the rectum (Table 2). Early detection of a perforation has critical implications for patient morbidity and mortality. Preoperatively diagnosing the site and cause for the perforation is important for surgical planning. A focal bowel wall defect is a highly specific CT finding, though has a limited sensitivity. In challenging cases, localize the gas as predominately intraperitoneal or extraperitoneal. Then, search for a focal defect, concentrated extraluminal gas, and/or segmental bowel wall thickening to further localize the perforation origin.
References
Espinoza R, Rodriguez A (1997) Traumatic and nontraumatic perforation of hollow viscera. Surg Clin N Am 77(6):1291–1304
Bohnen JM, Mustard RA, Oxholm SE, Schouten BD (1988) APACHE II score and abdominal sepsis. A prospective study. Arch Surg 123(2):225–229
Christou NV, Barie PS, Dellinger EP, Waymack JP, Stone HH (1993) Surgical Infection Society intra-abdominal infection study. Prospective evaluation of management techniques and outcome. Arch Surg 128(2):193–198 (discussion 198–199)
Hainaux B, Agneessens E, Bertinotti R, et al. (2006) Accuracy of MDCT in predicting site of gastrointestinal tract perforation. AJR Am J Roentgenol 187(5):1179–1183
Stapakis JC, Thickman D (1992) Diagnosis of pneumoperitoneum: abdominal CT vs. upright chest film. J Comput Assist Tomogr 16(5):713–716
Earls JP, Dachman AH, Colon E, Garrett MG, Molloy M (1993) Prevalence and duration of postoperative pneumoperitoneum: sensitivity of CT vs left lateral decubitus radiography. AJR Am J Roentgenol 161(4):781–785
Kim SH, Shin SS, Jeong YY, et al. (2009) Gastrointestinal tract perforation: MDCT findings according to the perforation sites. Korean J Radiol 10(1):63–70
Yeung KW, Chang MS, Hsiao CP, Huang JF (2004) CT evaluation of gastrointestinal tract perforation. Clin Imaging 28(5):329–333
Furukawa A, Sakoda M, Yamasaki M, et al. (2005) Gastrointestinal tract perforation: CT diagnosis of presence, site, and cause. Abdom Imaging 30(5):524–534
Ghekiere O, Lesnik A, Hoa D, et al. (2007) Value of computed tomography in the diagnosis of the cause of nontraumatic gastrointestinal tract perforation. J Comput Assist Tomogr 31(2):169–176
Brofman N, Atri M, Hanson JM, et al. (2006) Evaluation of bowel and mesenteric blunt trauma with multidetector CT. Radiographics 26(4):1119–1131
Miki T, Ogata S, Uto M, et al. (2004) Multidetector-row CT findings of colonic perforation: direct visualization of ruptured colonic wall. Abdom Imaging 29(6):658–662
Imuta M, Awai K, Nakayama Y, et al. (2007) Multidetector CT findings suggesting a perforation site in the gastrointestinal tract: analysis in surgically confirmed 155 patients. Radiat Med 25(3):113–118
Maniatis V, Chryssikopoulos H, Roussakis A, et al. (2000) Perforation of the alimentary tract: evaluation with computed tomography. Abdom Imaging 25(4):373–379
Zissin R, Konikoff F, Gayer G (2006) CT findings of latrogenic complications following gastrointestinal endoluminal procedures. Semin Ultrasound CT MR 27(2):126–138
Zissin R, Osadchy A, Gayer G (2009) Abdominal CT findings in small bowel perforation. Br J Radiol 82(974):162–171
Becker CD, Mentha G, Schmidlin F, Terrier F (1998) Blunt abdominal trauma in adults: role of CT in the diagnosis and management of visceral injuries. Part 2: gastrointestinal tract and retroperitoneal organs. Eur Radiol 8(5):772–780
Chen CH, Huang HS, Yang CC, Yeh YH (2001) The features of perforated peptic ulcers in conventional computed tomography. Hepatogastroenterology 48(41):1393–1396
Fultz PJ, Skucas J, Weiss SL (1992) CT in upper gastrointestinal tract perforations secondary to peptic ulcer disease. Gastrointest Radiol 17(1):5–8
Young CA, Menias CO, Bhalla S, Prasad SR (2008) CT features of esophageal emergencies. Radiographics 28(6):1541–1553
Gimenez A, Franquet T, Erasmus JJ, Martinez S, Estrada P (2002) Thoracic complications of esophageal disorders. Radiographics 22(Spec No):S247–S258
Katabathina VS, Restrepo CS, Martinez-Jimenez S, Riascos RF (2011) Nonvascular, nontraumatic mediastinal emergencies in adults: a comprehensive review of imaging findings. Radiographics 31(4):1141–1160
Li ZS, Sun ZX, Zou DW, et al. (2006) Endoscopic management of foreign bodies in the upper-GI tract: experience with 1088 cases in China. Gastrointest Endosc 64(4):485–492
Mosca S, Manes G, Martino R, et al. (2001) Endoscopic management of foreign bodies in the upper gastrointestinal tract: report on a series of 414 adult patients. Endoscopy 33(8):692–696
Longstreth GF, Longstreth KJ, Yao JF (2001) Esophageal food impaction: epidemiology and therapy. A retrospective, observational study. Gastrointest Endosc 53(2):193–198
Webb WA (1995) Management of foreign bodies of the upper gastrointestinal tract: update. Gastrointest Endosc 41(1):39–51
Eisen GM, Baron TH, Dominitz JA, et al. (2002) Guideline for the management of ingested foreign bodies. Gastrointest Endosc 55(7):802–806
Ferguson MK (1997) Esophageal perforation and caustic injury: management of perforated esophageal cancer. Dis Esophagus 10(2):90–94
Kim KR, Shin JH, Song HY, et al. (2009) Palliative treatment of malignant esophagopulmonary fistulas with covered expandable metallic stents. AJR Am J Roentgenol 193(4):W278–W282
Beal SL, Pottmeyer EW, Spisso JM (1988) Esophageal perforation following external blunt trauma. J Trauma 28(10):1425–1432
Brinster CJ, Singhal S, Lee L, et al. (2004) Evolving options in the management of esophageal perforation. Ann Thorac Surg 77(4):1475–1483
Stabile BE (1992) Current surgical management of duodenal ulcers. Surg Clin of N Am 72(2):335–356
Grassi R, Romano S, Pinto A, Romano L (2004) Gastro-duodenal perforations: conventional plain film, US and CT findings in 166 consecutive patients. Eur J Radiol 50(1):30–36
Feliciano DV (1992) Do perforated duodenal ulcers need an acid-decreasing surgical procedure now that omeprazole is available? Surg Clin N Am 72(2):369–380
Jordan PH Jr (1988) Morrow C: perforated peptic ulcer. Surg Clin N Am 68(2):315–329
Donovan AJ, Berne TV, Donovan JA (1998) Perforated duodenal ulcer: an alternative therapeutic plan. Arch Surg 133(11):1166–1171
Felix EL, Kettelle J, Mobley E, Swartz D (2008) Perforated marginal ulcers after laparoscopic gastric bypass. Surg Endosc 22(10):2128–2132
Brunsting LA, Morton JH (1987) Gastric rupture from blunt abdominal trauma. J Trauma 27(8):887–891
Byun JH, Ha HK, Kim AY, et al. (2003) CT findings in peripheral T-cell lymphoma involving the gastrointestinal tract. Radiology 227(1):59–67
Cegarra-Navarro MF, de la Calle MA, Girela-Baena E, et al. (2005) Ruptured gastrointestinal stromal tumors: radiologic findings in six cases. Abdom Imaging 30(5):535–542
Chao TC, Chao HH, Jan YY, Chen MF (2005) Perforation through small bowel malignant tumors. J Gastrointest Surg 9(3):430–435
Hohenberger P, Ronellenfitsch U, Oladeji O, et al. (2010) Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg 97(12):1854–1859
Kim SW, Kim HC, Yang DM (1017) Perforated tumours in the gastrointestinal tract: CT findings and clinical implications. Br J Radiol 2012(85):1307–1313
Roviello F, Rossi S, Marrelli D, et al. (2006) Perforated gastric carcinoma: a report of 10 cases and review of the literature. World J Surg Oncol 4:19
Yang CJ, Hwang JJ, Kang WY, et al. (2006) Gastro-intestinal metastasis of primary lung carcinoma: clinical presentations and outcome. Lung Cancer 54(3):319–323
Jwo SC, Chien RN, Chao TC, Chen HY, Lin CY (2005) Clinicopathological features, surgical management, and disease outcome of perforated gastric cancer. J Surg Oncol 91(4):219–225
Shih CH, Yu MC, Chao TC, et al. (2010) Outcome of perforated gastric cancer: twenty years experience of one institute. Hepatogastroenterology 57(102–103):1320–1324
Daliya P, White TJ, Makhdoomi KR (2012) Gastric perforation in an adult male following nasogastric intubation. Ann R Coll Surg Engl 94(7):e210–e212
Enns R, Eloubeidi MA, Mergener K, et al. (2002) ERCP-related perforations: risk factors and management. Endoscopy 34(4):293–298
Merchea A, Cullinane DC, Sawyer MD, et al. (2010) Esophagogastroduodenoscopy-associated gastrointestinal perforations: a single-center experience. Surgery 148(4):876–880 (discussion 881–872)
Hines J, Rosenblat J, Duncan DR, Friedman B, Katz DS (2013) Perforation of the mesenteric small bowel: etiologies and CT findings. Emerg Radiol 20(2):155–161
Greenstein AJ, Mann D, Sachar DB, Aufses AH Jr (1985) Free perforation in Crohn’s disease: I. A survey of 99 cases. Am J Gastroenterol 80(9):682–689
Berg DF, Bahadursingh AM, Kaminski DL, Longo WE (2002) Acute surgical emergencies in inflammatory bowel disease. Am J Surg 184(1):45–51
Freeman HJ (2002) Spontaneous free perforation of the small intestine in Crohn’s disease. Can J Gastroenterol 16(1):23–27
Furukawa A, Saotome T, Yamasaki M, et al. (2004) Cross-sectional imaging in Crohn disease. Radiographics 24(3):689–702
Rha SE, Ha HK, Lee SH, et al. (2000) CT and MR imaging findings of bowel ischemia from various primary causes. Radiographics 20(1):29–42
Furukawa A, Yamasaki M, Takahashi M, et al. (2003) CT diagnosis of small bowel obstruction: scanning technique, interpretation and role in the diagnosis. Semin Ultrasound CT MR 24(5):336–352
Lowe RJ, Boyd DR, Folk FA, Baker RJ (1972) The negative laparotomy for abdominal trauma. J Trauma 12(10):853–861
Shanmuganathan K, Mirvis SE, Chiu WC, et al. (2004) Penetrating torso trauma: triple-contrast helical CT in peritoneal violation and organ injury: a prospective study in 200 patients. Radiology 231(3):775–784
Butela ST, Federle MP, Chang PJ, et al. (2001) Performance of CT in detection of bowel injury. AJR Am J Roentgenol 176(1):129–135
Breen DJ, Janzen DL, Zwirewich CV, Nagy AG (1997) Blunt bowel and mesenteric injury: diagnostic performance of CT signs. J Comput Assist Tomogr 21(5):706–712
Kako S, Oshima K, Sato M, Terasako K, et al. (2009) Clinical outcome in patients with small-intestinal non-Hodgkin lymphoma. Leuk Lymphoma 50(10):1618–1624
Balthazar EJ, Noordhoorn M, Megibow AJ, Gordon RB (1997) CT of small-bowel lymphoma in immunocompetent patients and patients with AIDS: comparison of findings. AJR Am J Roentgenol 168(3):675–680
Dodd GD 3rd, Greenler DP, Confer SR (1992) Thoracic and abdominal manifestations of lymphoma occurring in the immunocompromised patient. Radiol Clin N Am 30(3):597–610
Ghai S, Pattison J, Ghai S, et al. (2007) Primary gastrointestinal lymphoma: spectrum of imaging findings with pathologic correlation. Radiographics 27(5):1371–1388
Lee WK, Lau EW, Duddalwar VA, Stanley AJ, Ho YY (2008) Abdominal manifestations of extranodal lymphoma: spectrum of imaging findings. AJR Am J Roentgenol 191(1):198–206
Levine MS, Rubesin SE, Pantongrag-Brown L, Buck JL, Herlinger H (1997) Non-Hodgkin’s lymphoma of the gastrointestinal tract: radiographic findings. AJR Am J Roentgenol 168(1):165–172
Kim SY, Ha HK, Park SW, et al. (2009) Gastrointestinal metastasis from primary lung cancer: CT findings and clinicopathologic features. AJR Am J Roentgenol 193(3):W197–W201
Goh BK, Tan YM, Lin SE, et al. (2006) CT in the preoperative diagnosis of fish bone perforation of the gastrointestinal tract. AJR Am J Roentgenol 187(3):710–714
Rathaus V, Erez I, Zissin R (2006) Ileal perforation due to an ingested fragment of a skewer: preoperative ultrasonographic diagnosis. J Ultrasound Med 25(3):389–391
Bixby SD, Lucey BC, Soto JA, et al. (2006) Perforated versus nonperforated acute appendicitis: accuracy of multidetector CT detection. Radiology 241(3):780–786
Keyzer C, Zalcman M, De Maertelaer V, et al. (2005) Comparison of US and unenhanced multi-detector row CT in patients suspected of having acute appendicitis. Radiology 236(2):527–534
Lane MJ, Katz DS, Ross BA, et al. (1997) Unenhanced helical CT for suspected acute appendicitis. AJR Am J Roentgenol 168(2):405–409
Lane MJ, Liu DM, Huynh MD, et al. (1999) Suspected acute appendicitis: nonenhanced helical CT in 300 consecutive patients. Radiology 213(2):341–346
Rao PM, Rhea JT, Novelline RA, et al. (1997) Helical CT technique for the diagnosis of appendicitis: prospective evaluation of a focused appendix CT examination. Radiology 202(1):139–144
Rao PM, Rhea JT, Novelline RA, Mostafavi AA, McCabe CJ (1998) Effect of computed tomography of the appendix on treatment of patients and use of hospital resources. New Engl J Med 338(3):141–146
Rhea JT, Halpern EF, Ptak T, et al. (2005) The status of appendiceal CT in an urban medical center 5 years after its introduction: experience with 753 patients. AJR Am J Roentgenol 184(6):1802–1808
Foley TA, Earnest Ft, Nathan MA, et al. (2005) Differentiation of nonperforated from perforated appendicitis: accuracy of CT diagnosis and relationship of CT findings to length of hospital stay. Radiology 235(1):89–96
Horrow MM, White DS, Horrow JC (2003) Differentiation of perforated from nonperforated appendicitis at CT. Radiology 227(1):46–51
Lin CJ, Chen JD, Tiu CM, et al. (2005) Can ruptured appendicitis be detected preoperatively in the ED? Am J Emerg Med 23(1):60–66
Oliak D, Sinow R, French S, Udani VM, Stamos MJ (1999) Computed tomography scanning for the diagnosis of perforated appendicitis. Am Surg 65(10):959–964
Yeung KW, Chang MS, Hsiao CP (2004) Evaluation of perforated and nonperforated appendicitis with CT. Clin Imaging 28(6):422–427
Brown CV, Abrishami M, Muller M, Velmahos GC (2003) Appendiceal abscess: immediate operation or percutaneous drainage? Am Surg 69(10):829–832
Yamini D, Vargas H, Bongard F, Klein S, Stamos MJ (1998) Perforated appendicitis: is it truly a surgical urgency? Am Surg 64(10):970–975
Misdraji J (2010) Appendiceal mucinous neoplasms: controversial issues. Arch Pathol Lab Med 134(6):864–870
Lim HK, Lee WJ, Kim SH, et al. (1999) Primary mucinous cystadenocarcinoma of the appendix: CT findings. AJR Am J Roentgenol 173(4):1071–1074
Abdelrazeq AS, Scott N, Thorn C, et al. (2008) The impact of spontaneous tumour perforation on outcome following colon cancer surgery. Colorectal Dis 10(8):775–780
Kim SW, Shin HC, Kim IY, Kim YT, Kim CJ (2010) CT findings of colonic complications associated with colon cancer. Korean J Radiol 11(2):211–221
Tan KK, Hong CC, Zhang J, Liu JZ, Sim R (2010) Surgery for perforated colorectal malignancy in an Asian population: an institution’s experience over 5 years. Int J Colorectal Dis 25(8):989–995
Fernandez-Esparrach G, Bordas JM, Giraldez MD, et al. (2010) Severe complications limit long-term clinical success of self-expanding metal stents in patients with obstructive colorectal cancer. Am J Gastroenterol 105(5):1087–1093
Humes DJ, Solaymani-Dodaran M, Fleming KM, et al. (2009) A population-based study of perforated diverticular disease incidence and associated mortality. Gastroenterology 136(4):1198–1205
Horton KM, Corl FM, Fishman EK (2000) CT evaluation of the colon: inflammatory disease. Radiographics 20(2):399–418
Oliphant M, Berne AS, Meyers MA (1996) The subperitoneal space of the abdomen and pelvis: planes of continuity. AJR Am J Roentgenol 167(6):1433–1439
Lohsiriwat V (2010) Colonoscopic perforation: incidence, risk factors, management and outcome. World J Gastroenterol 16(4):425–430
Arora G, Mannalithara A, Singh G, Gerson LB, Triadafilopoulos G (2009) Risk of perforation from a colonoscopy in adults: a large population-based study. Gastrointest Endosc 69(3 Pt 2):654–664
Rabeneck L, Paszat LF, Hilsden RJ et al. (2008) Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology 135(6):1899–1906, 1906, e1891
Kim DH, Pickhardt PJ, Taylor AJ, Menias CO (2008) Imaging evaluation of complications at optical colonoscopy. Curr Prob Diagn Radiol 37(4):165–177
Pickhardt PJ, Kim DH, Taylor AJ (2008) Asymptomatic pneumatosis at CT colonography: a benign self-limited imaging finding distinct from perforation. AJR Am J Roentgenol 190(2):W112–W117
Putcha RV, Burdick JS (2003) Management of iatrogenic perforation. Gastroenterol Clin N Am 32(4):1289–1309
Kurer MA, Davey C, Khan S, Chintapatla S (2010) Colorectal foreign bodies: a systematic review. Colorectal Dis 12(9):851–861
Barone JE, Yee J, Nealon TF Jr (1983) Management of foreign bodies and trauma of the rectum. Surg Gynecol Obstet 156(4):453–457
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Del Gaizo, A.J., Lall, C., Allen, B.C. et al. From esophagus to rectum: a comprehensive review of alimentary tract perforations at computed tomography. Abdom Imaging 39, 802–823 (2014). https://doi.org/10.1007/s00261-014-0110-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-014-0110-4