Abstract
The genus Fusariella, typified by F. atrovirens, is characterised by semi- to macronematous, mononematous conidiophores, with cylindrical, subulate or lageniform phialidic conidiogenous cells that produce catenate, septate, curved to straight, subhyaline to brown conidia. During a survey of hyaline-spored hyphomycetes from karst areas in Thailand, we collected a new species of Fusariella with curved conidia and introduce it in this paper as Fusariella curvata sp. nov. In addition, all hitherto described species of Fusariella are reviewed. The result of phylogenetic analyses, based on combined SSU, LSU, TEF and RPB2 sequence data, indicates that the genus belongs in the family Bionectriaceae (Hypocreales, Sordariomycetes).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family Bionectriaceae (Hypocreales, Sordariomycetes) was established by Rossman et al. (1999) and consolidated in recent studies by Maharachchikumbura et al. (2015, 2016), who accepted 38 genera in the family. The sexual morphs of Bionectriaceae are characterised by uniloculate perithecial, rarely cleistothecial ascomata that are white, yellow, orange to tan or brown, do not change colour in KOH or lactic acid, and are generally superficial, lacking a stroma, or immersed in the substratum (Rossman et al. 1999; Maharachchikumbura et al. 2016). The asexual morphs in Bionectriaceae are hyphomycetous, acremonium- or gliocladium-like. The conidiogenous cells are phialidic and produce unicellular to multi-septate, ellipsoidal, fusiform to subfusiform, hyaline to greenish hyaline or bright-coloured conidia (Rossman et al. 1999; Maharachchikumbura et al. 2016).
The genus Fusariella Sacc. was established by Saccardo (1884) to accommodate Fusariella atrovirens (Berk.) Sacc., and is characterised by semi-macronematous, mononematous, unbranched or variously branched conidiophores, with curved, cylindrical, subulate or lageniform, hyaline to pale brown phialides that produce catenate, acrogenous, straight or slightly curved, fusiform, cylindrical, clavate or obclavate, subhyaline to brown conidia (Hughes 1949; Chabelska-Frydman 1964; Roy and Rai 1968; Ellis 1971, 1976; Seifert et al. 2011). Nineteen species epithets for Fusariella are listed in Index Fungorum (2016) and MycoBank (2016; http://www.mycobank.org/). In this study, all species are revisited and a synopsis to the species of Fusariella is provided.
Although the genus Fusariella has been known for more than 130 years since it was established by Saccardo (1884), its natural classification is still uncertain because of the lack of molecular data and a comprehensive taxonomic treatment (Index Fungorum 2016). In this study, we propose taxa at the ranks of family, order and class based on phylogenetic analysis and morphology.
During a survey of hyphomycetes in karst areas of Thailand, one hitherto unknown Fusariella species was found that is described here. The classification of Fusariella is determined taking into account morphology and phylogenetic analyses of ITS, SSU, LSU, RPB2 and TEF1 sequence data.
Materials and methods
Collection and isolation of fungi
Dead materials (stems, wood and leaves) from a variety of plants were randomly collected during May to August 2015 from karst areas at Ang Kep Nam Wat Tham Khao Hin Phayanak (Wat Tham Sao Hin Payanak) (20°19′16.58 - 30.12″N, 99°51′40.72 - 54.50″E), Mae Sai District, Chiang Rai Province in Thailand. Samples were taken to the laboratory in zip-lock plastic bags for examination. The specimens were incubated in sterile moist chambers and examined using a Motic SMZ-168 series microscope. Fungi were removed with a needle and placed in a drop of distilled water on a slide for morphological study. Photographs of fungal structures were captured using a Nikon Eclipse 80i compound microscope with a Canon 450D digital camera. All measurements were made by the Tarosoft® Image FrameWork program. Photo plates were made with Adobe Photoshop CS6 Extended version 13.0.1 (Adobe Systems, USA). Isolation onto potato dextrose agar (PDA) or malt extract agar (MEA) was performed by the single spore isolation method (Chomnunti et al. 2014). Herbarium material is deposited in the herbarium of Mae Fah Luang University (MFLU), Chiang Rai, Thailand and the Herbarium of the Department of Plant Pathology (HGUP), Agricultural College, Guizhou University, Guiyang, China. Living cultures are deposited at the Mae Fah Luang University Culture Collection (MFLUCC) and the Culture Collection at the Department of Plant Pathology, Agriculture College, Guizhou University, China (GUCC). Faces of Fungi and Index Fungorum numbers are registered (Jayasiri et al. 2015; Index Fungorum 2016).
DNA extraction, PCR amplification and sequencing
Genomic DNA was extracted from fungal mycelium grown on PDA or MEA at room temperature with the Fungal gDNA Kit (BioMIGA, USA), according to the manufacturer’s instructions. The internal transcribed spacer region of ribosomal DNA (ITS), small subunit nuclear ribosomal DNA (SSU), large subunit nuclear ribosomal DNA (LSU), RNA polymerase II second largest subunit (rpb2) and the translation elongation factor-1 alpha (tef1) genes were amplified via polymerase chain reaction (PCR) using the following primers: ITS5 and ITS4 (White et al. 1990) for ITS, NS1 and NSS4 (White et al. 1990) for SSU, LROR and LR5 (Vilgalys and Hester 1990) for LSU, fRPB2-5F and fRPB2-7cF (Liu et al. 1999) for rpb2, and EF1-983F and EF1-2218R (Rehner 2001) for tef1. The PCR products were sequenced with the same primers.
Phylogenetic analyses
Original sequences were checked using BioEdit version 7.0.5.3 (Hall 1999), and most reference sequences originated from the publications of Maharachchikumbura et al. (2015, 2016). The remaining homogenous sequences were obtained by BLAST searches (Altschul et al. 1990) from GenBank. All sequences used in this study are listed in Table 1. Alignments for each locus were done in MAFFT v7.212 (Katoh and Standley 2013) and manually verified in MEGA 6.06 (Tamura et al. 2013). Conserved blocks were selected from the initial alignments with Gblocks 0.91b (Castresana 2000). The interleaved NEXUS files were formatted with PAUP* 4.0b10 (Swofford 2002) and manually formatted for Bayesian inference (BI) analyses. BI, maximum parsimony (MP) and maximum likelihood (ML) were used in this study for phylogenetic analyses. For BI analysis, the best model of evolution was determined using MrModeltest v2 (Nylander 2004). BI analysis was done with MrBayes v 3.2.5 (Ronquist et al. 2012). MP analysis was performed in PAUP* 4.0b10 (Swofford 2002). ML analysis was performed in raxmlGUI v 1.3.1 (Silvestro and Michalak 2012). Phylogenetic trees were drawn with TreeView 1.6.6 (Page 1996).
Results
Molecular phylogeny
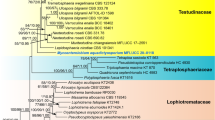
The aligned sequence matrix comprises SSU (1047 bp), LSU (891 bp), EF1-α (1020 bp) and RPB2 (1043 bp) sequence data for 31 taxa and one outgroup taxon for a total of 4001 characters, of which 904 were parsimony informative and 2591 characters were constant. The results of ML analysis based on combined SSU, LSU, EF1-α and RPB2 sequence data are shown in Fig. 1.
Phylogenetic tree generated from maximum likelihood (ML) analysis based on combined SSU, LSU, TEF and RPB2 sequence data for the order Hypocreales. Bootstrap support values for ML greater than 50 % and Bayesian posterior probabilities greater than 0.5 are indicated above or below the nodes as MLBS/PP. The new isolate is in bold and blue. The tree is rooted with Verticillium dahliae ATCC 16535
In the present study, we found that the strain of F. curvata (MFLUCC 15-0844) forms a clade together with the strains of Hydropisphaera peziza (G.J.S. 91-101) with 94 % ML bootstrap support and 100 % Bayesian posterior probabilities within the family Bionectriaceae (Hypocreales, Sordariomycetes).
Taxonomy
Generic description
Fusariella Sacc., Atti Inst. Veneto Sci. lett., ed Arti, Sér. 6 2: 463 (1884)
= Kurssanovia Pidopl., Mykrobiol. Zh. 9(2–3): 57 (1948)
= Tylomyces Cortini, Atti R. Acad. Lincei, Rendiconti Cl. Sci. Fis., sér. 5 30: 63 (1921)
Type species: Fusariella atrovirens (Berk.) Sacc., Fung. 2: 463 (1884)
Description — See Ellis (1971)
Notes: In total, 17 species of the genus Fusariella are accepted in this study, including our new species, Fusariella curvata. We also confirmed that F. cladosporioides, F. polysciadis and F. populi are factually unfaithful. Phylogenetic analysis of SSU, LSU, EF1-α and RPB2 sequence data indicates that this genus belongs in the family Bionectriaceae and this treatment is supported by morphology.
Species descriptions
Fusariella curvata C.G. Lin, Yong Wang bis & K.D. Hyde, sp. nov. (Fig. 2)
Index Fungorum number: IF552354; Facesoffungi number: FoF 02514
Etymology: In reference to the tip of each conidium being curved laterally
Holotype: MFLU 15-3268
Asexual morph Colonies on natural substrate effuse, greyish green. Mycelium partly superficial and partly immersed. Conidiophores macronematous, mononematous, erect, branched, straight or flexuous, smooth, 1–3-septate, hyaline, up to 70 μm long, up to 4.5 μm wide. Conidiogenous cells monophialidic, integrated, terminal or laterally intercalary, straight or flexuous, subulate, smooth, hyaline, 19.5–31.5 μm (\( \overline{x} \) = 23.9 μm, n = 20) long, 2.0–3.0 μm (\( \overline{x} \) = 2.4 μm, n = 20) wide at the base, tapering slightly to the apex to 1.0–2.0 μm (\( \overline{x} \) = 1.6 μm, n = 20) wide. Conidia catenate, acrogenous, usually straight but sometimes curved, fusiform, obclavate, rounded at the base and pointed at the apex, mostly the apical cell of each conidium curved laterally, 1–3-septate (mostly 3-septate), slightly constricted at the septa, smooth, hyaline when young, pale olivaceous when mature, 18–24 μm (\( \overline{x} \) = 20.5 μm, n = 50) long, 3.0–4.5 μm (\( \overline{x} \) = 3.8 μm, n = 50) wide at the widest point. Sexual morph Undetermined.
Culture characteristics: Conidia germinating on PDA within 24 h. Colonies on MEA effuse, in concentric rings with radial wrinkles in the centre, white from above, light yellow from below, reaching a diam. of 2–3 cm in 20 days at 25 °C.
Material examined: THAILAND, Chiang Rai, Mae Sai District, Ang Kep Nam Wat Tham Khao Hin Phayanak (Wat Tham Sao Hin Payanak), 20°19′16.58 - 30.12″N, 99°51′40.72 - 54.50″E, on decaying Quercus sp. leaf, 19 June 2015, C.-G. Lin, WTSP 10-1 (MFLU 15-3268, holotype; HGUP 8001, isotype), living culture MFLUCC 15-0844 = GUCC 8001.
Notes: The shape of conidium (tip mostly curved laterally) clearly distinguishes this species from all other Fusariella species. Conidia of our new species are similar to those of F. sinensis H.M. Liu & T.Y. Zhang, but the conidiogenous cells of our new species are smooth and hyaline, while those of F. sinensis are distinctly verrucose and coloured. Additionally, conidia of our new species are longer but narrower than those of F. sinensis (10–15 × 4.4–5.5 μm, \( \overline{x} \) = 13.5 × 5 μm) (Liu and Zhang 2006). Fusariella concinna (Syd.) S. Hughes is similar to our new species in conidial shape, sometimes the conidia of F. concinna are slightly curved or bent above. However, conidia of F. concinna mostly are curved, fusoid but tapering towards the apex, whereas those of our new species are straight, fusiform, obclavate. Additionally, conidia of F. concinna are hyaline to slightly coloured with a well-defined basal scar, while those of our new species are hyaline when young, pale olivaceous when mature.
Other species accepted in the genus Fusariella
Sixteen known species of Fusariella are re-evaluated and recognised:
-
1.
Fusariella atrovirens (Berk.) Sacc., Fung. 2: 463 (1884) (Fig. 3)
Fig. 3 Fusariella atrovirens (redrawn from Seifert et al. 2011)
≡ Fusisporium atrovirens Berk., in Smith, Engl. Fl., Fungi (Edn 2) (London) 5(2): 351 (1836)
≡ Fusarium atrovirens (Berk.) Mussat, in Saccardo, Syll. fung. (Abellini) 15: 144 (1900)
Description and illustrations — See Hughes (1949)
Notes: Fusariella atrovirens is the type species of the genus Fusariella. The conidia of this species are similar to those of F. bizzozeriana but its conidiophores are minutely verruculose, while those of F. bizzozeriana are smooth (Hughes 1949). Type material of this species is Mycotheca veneta, 1038.
-
2.
Fusariella bizzozeriana (Sacc.) S. Hughes, Mycol. Pap. 28 (6): 6, 1949 (Fig. 4)
Fig. 4 Fusariella bizzozeriana (redrawn from Hughes 1949)
≡ Sporidesmium bizzozerianum Sacc., Mycotheca veneta: no. 365 (1876)
≡ Clasterosporium bizzozerianum (Sacc.) Sacc. [as ‘Closterosporium’], Michelia 2(no. 7): 289 (1881)
Description and illustrations — See Hughes (1949)
Notes: See notes of F. atrovirens. Type material of this species is Mycotheca veneta, 365.
-
3.
Fusariella candida Matsush., Matsush. Mycol. Mem. 7: 51 (1993) (Fig. 5)
Fig. 5 Fusariella candida (redrawn from Matsushima 1993) (scale bar = 10 μm)
Description and illustrations — See Matsushima (1993)
Notes: It is a distinct species. The conidia of all Fusariella species are coloured when mature, except in F. candida and F. formosana. Fusariella candida has very short conidiophores (6.5–14 μm) (Matsushima 1993). Type material of this species is MFC-0P411 (Matsushima Fungus Collection, Kobe).
-
4.
Fusariella concinna (Syd.) S. Hughes, Mycol. Pap. 28: 8 (1949) (Fig. 6)
Fig. 6 Fusariella concinna (redrawn from Hughes 1949)
≡ Clasterosporium concinnum Syd., Annales Mycologici 31 (1–2): 94 (1933)
Description and illustrations — See Hughes (1949)
Notes: Fusariella bizzozeriana and F. viridiatra are similar to F. concinna in conidial shape. However, the latter species develops hyaline to slightly coloured conidia, whereas those of F. bizzozeriana are olivaceous at the base, the upper parts nearly hyaline and those of F. viridiatra are green brown. Type material of this species is Herb. I.M.I. 8669.
-
5.
Fusariella echinulata H.M. Liu & T.Y. Zhang, Mycosystema 25(4): [513] (2006) (Fig. 7)
Fig. 7 Fusariella echinulata (redrawn from Liu and Zhang 2006) (scale bar = 10 μm)
Description and illustrations — See Liu and Zhang (2006)
Notes: Liu and Zhang (2006) described this species with obovoid conidia, which are actually obclavate based on their original description “the conidia are rounded at the base, tapered at apex” and the illustration.
Fusariella obstipa is similar to F. echinulata in conidial shape. However, the latter species develops pale olivaceous conidia, whereas those of F. obstipa are subhyaline. Type material of this species is HSAUP031168.
-
6.
Fusariella egyptiaca Mouch. [as ‘aegyptiacum’], in Mouchacca & Nicot, Revue Mycol., Paris 37(3): 181 (1973) [1972] (Fig. 8)
Fig. 8 Fusariella egyptiaca (redrawn from Ellis 1976) (scale bar = 5 μm)
Description and illustrations — See Ellis (1976)
Notes: Morphologically, F. formosana and F. hughesii are similar to F. egyptiaca in having subcylindrical to obclavate conidia. However, the conidia of F. egyptiaca are dark blackish brown, whereas those of F. formosana are hyaline and F. hughesii are initially hyaline, at maturity greenish blue to grey when in mass.
-
7.
Fusariella formosana Matsush., Matsush. Mycol. Mem. 4: 9 (1985) (Fig. 9)
Fig. 9 Fusariella formosana (redrawn from Matsushima 1985) (scale bar = 10 μm)
Description and illustrations — See Matsushima (1985)
Notes: Fusariella formosana is similar to F. egyptiaca and F. hughesii in conidial shape, see notes of F. egyptiaca. Type material of this species is MFC-10015.
-
8.
Fusariella helanshanensis Y.M. Wu & T.Y. Zhang, Mycosystema 28(5): 653 (2009) (Fig. 10)
Fig. 10 Fusariella helanshanensis (redrawn from Wu et al. 2009) (scale bar = 10 μm)
Description and illustrations — See Wu et al. (2009)
Notes: This species is very similar to F. obstipa in conidial shape and size, but differs from it by the middle cell of conidia being inflated (Wu et al. 2009). Type material of this species is HSAUPII054119 and ex-type is HMAS 196216.
-
9.
Fusariella hughesii Chab.-Frydm., Can. J. Bot. 42(11): 1485 (1964) (Fig. 11)
Fig. 11 Fusariella hughesii (redrawn from Matsushima 1982) (scale bar = 10 μm)
Description and illustrations — See Chabelska-Frydman (1964) and Matsushima (1982)
Notes: It differs from the other Fusariella species in its light green conidia (Chabelska-Frydman 1964). Type material is Herb. I.M.I. 82236.
-
10.
Fusariella indica R.Y. Roy & B. Rai, Trans. Br. Mycol. Soc. 51(2): 333 (1968) (Fig. 12)
Fig. 12 Fusariella indica (redrawn from Roy and Rai 1968) (scale bar = 20 μm)
Description and illustrations — See Roy and Rai (1968)
Notes: This species differs in producing chains of three kinds of conidia, viz. (i) long obclavate, 1–3-septate, 12–15 × 4.5−5 μm; (ii) short obclavate, 1-septate 8–12 × 4.5−6 μm; (iii) fusiform, 1−3-septate, 12–16 × 4.5−5 μm (Roy and Rai 1968). These three kinds of conidia are hyaline when young, becoming greenish black at maturity. Type material of this species is Herb. I.M.I. 127253.
-
11.
Fusariella intermedia Mouch. & Nicot, Revue Mycol., Paris 37(3): 181 (1973) [1972] (Fig. 13)
Fig. 13 Fusariella intermedia (redrawn from Ellis 1976) (scale bar = 5 μm)
Description and illustrations — See Ellis (1976)
Notes: It is similar to F. kansensis in conidial shape, as both species produce curved and fusiform conidia. However, the conidia of F. intermedia are smaller than those of F. kansensis (22–27 × 7–9 μm).
-
12.
Fusariella kansensis (Ellis & Barthol.) M.B. Ellis, More Dematiaceous Hyphomycetes (Kew): 459 (1976) (Fig. 14)
Fig. 14 Fusariella kansensis (redrawn from Ellis 1976) (a scale bar = 5 μm, b without scale bar)
≡ Clasterosporium kansense Ellis & Barthol., Erythea 4: 28 (1896)
Description and illustrations — See Ellis (1976)
Notes: Hughes (1958) firstly proposed Clasterosporium kansense as a synonym of F. bizzozeriana, but the rough-walled phialides and much broader conidia distinguish it from F. bizzozeriana (Ellis 1976). Ellis (1976) re-described this species with the following morphological characters: conidia curved, 3-septate, constricted at the septa, smooth-walled, grey, 22–27 × 7–9 μm, conidiophores hyaline to pale olivaceous brown, 15–30 × 3–4 μm, phialides often verruculose, confirming its position in Fusariella. Fusariella kansensis differs from F. intermedia by producing larger conidia; see the notes of F. intermedia.
-
13.
Fusariella obstipa (Pollack) S. Hughes, Mycol. Pap. 28: 9 (1949) (Fig. 15)
Fig. 15 Fusariella obstipa (redrawn from Hughes 1949)
≡ Dendryphion obstipum Pollack, Mycologia 39(5): 617 (1947)
Description and illustrations — See Pollack (1947) and Hughes (1949)
Notes: Amongst the species that produce more or less obclavate conidia, F. echinulata is most similar to F. obstipa. However, conidia of F. echinulata are pale olivaceous. Type material of this species is BPI 442857, deposited as Dendryphion obstipum.
-
14.
Fusariella sarniensis M.B. Ellis, More Dematiaceous Hyphomycetes (Kew): 460 (1976) (Fig. 16)
Fig. 16 Fusariella sarniensis (redrawn from Ellis 1976) (scale bar = 5 μm)
Description and illustrations — See Ellis (1976)
Notes: The conidia are straight, cylindrical, rounded at the apex, truncate at the base, 3-septate, pale to mid-grey, smooth-walled, 15–18 × 5–7 μm (Ellis 1976). The type material of this species is I.M.I. 35730.
-
15.
Fusariella sinensis H.M. Liu & T.Y. Zhang, Mycosystema 25(4): 514 (2006) (Fig. 17)
Fig. 17 Fusariella sinensis (redrawn from Liu and Zhang 2006) (scale bar = 10 μm)
Description and illustrations — See Liu and Zhang (2006)
Notes: Fusariella sinensis is similar to F. obstipa in conidial shape, as both produce obclavate conidia. However, F. sinensis differs by having distinctly verrucose and coloured phialides (Liu and Zhang 2006). Type material of this species is HSAUP031039.
-
16.
Fusariella viridiatra Sacc., Syll. fung. (Abellini) 4: 395 (1886) (Fig. 18)
Fig. 18 Fusariella viridiatra (redrawn from Lindau 1910)
≡ Fusisporium atrovirens Sacc. Fung. Ital., fig. 45 (1881)
Description and illustrations — See Lindau (1910)
Notes: Fusariella viridiatra is similar to F. intermedia and F. kansensis in conidial shape, as all produce fusiform and curved conidia. However, F. viridiatra differs by producing green brown conidia.
Species excluded from Fusariella
-
1.
Fusariella cladosporioides P. Karst., Hedwigia 30: 248 (1891)
Notes: Karst (1891) briefly described it as “Caespituli effuai, minuti, hypophylli, griseo-olivacei. Hyphae brevissimae, ramosae, articulatae. Conidia bacillaria, apicem versus attenuata, curvata, raro recta, vulgo pauciseptata, fumoso-hyalina vel hyalina (sub lente), 50–100 × 4–6 mmn.”, without any illustration (Hughes 1949).
Crous et al. (2013) proposed F. cladosporioides as a synonym of Pseudocercospora myrticola (Speg.) Deighton, 1976. In this study, we agree with this arrangement.
-
2.
Fusariella polysciadis (Henn.) Wollenw. [as ‘polysciatis’], Fusaria autographica delineate 1: no. 433 (1916)
≡ Pionnotes polysciadis Henn. [as ‘polysciatis’], Bot. Jb. 34: 57 (1904)
≡ Cercosporella polysciadis (Henn.) Hansf. [as ‘polysciatis’], Proc. Linn. Soc. London 155: 43 (1943) [1942–43]
Notes: This species is not a Fusariella because “the conidia are formed in typical Cercospora manner on simple or branched geniculate conidiophores” (Hansford 1943).
-
3.
Fusariella populi Garb., Bull. Soc. Mycol. Fr. 33: 89 (1917)
= ? Stigmina radiosa (Lib.) Goid., Annali Bot.: 11 (1938)
Notes: Garbowski (1917) described a new Fusariella species as follows: “Caespitulis griseo-olivaceis in macula arida brunneola; conidiophoris subnullis; hyphis sporophoris hyalinis, fliformibus, 2 μ circ. latis; conidiis numerosis, fusoideis, curvulis vel rectis 2-septatis, ad septa leviter constrictis, utrinque rotundatis, guttulatis, olivaceis glabris, 30–35 × 5–7 μm.” (Saccardo 1931; Hughes 1949).
Goidànich (1936) regarded Fusariella populi as a synonym of Stigmina radiosa, while Hughes (1949) only stated that this species did not belong in Fusariella. We concur with their conclusions.
Discussion
In this study, all the earlier described species of Fusariella were reviewed and a synopsis of Fusariella species is provided Table 2. Seventeen accepted species of the Fusariella are included; three species, F. cladosporioides, F. polysciadis and F. populi, are to be excluded.
Prior to this study, the natural classification of the genus Fusariella had not been determined. In this study, the phylogeny of the order Hypocreales is inferred from sequence data (SSU, LSU, EF1-α and RPB2) and a phylogenetic tree is provided to infer the phylogenetic position of the genus Fusariella. Based on phylogenetic analysis and morphology, the genus Fusariella belongs to the family Bionectriaceae.
Maharachchikumbura et al. (2015) accepted 38 genera within the family Bionectriaceae (Hypocreales, Sordariomycetes). The asexual morphs of the family Bionectriaceae are acremonium- or gliocladium-like hyphomycetous, e.g. Acremonium Link, Clonostachys Corda, Gliomastix Guég., Kutilakesa Subram., having phialidic conidiogenous cells and hyaline to bright-coloured conidia (Seifert et al. 2011).
In the tree generated from ML analysis based on combined ITS, SSU, LSU, TEF and RPB2 sequence data for the family Bionectriaceae (data not shown), the strain of F. curvata (MFLUCC 15-0844) grouped together with Hydropisphaera arenula (NRRL 13963) with 59 % ML bootstrap support and sister to the Hydropisphaera erubescens (ATCC 36093, ATCC 44545, ATCC 36092, HMAS 91779 and A.R. 2766) clade. The asexual morphs of Hydropisphaera were reported as Acremonium (Rossman et al. 1999), Cephalosporiopsis (Rossman et al. 1999) or Gliomastix (Lechat et al. 2010). Morphologically, these genera are similar to Fusariella in producing unbranched or branched conidiophores, phialidic conidiogenous cells and unicellular to multi-septate, ellipsoidal, fusiform to subfusiform, hyaline to greenish hyaline or bright-coloured conidia (Seifert et al. 2011; Maharachchikumbura et al. 2015).
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410
Bischoff JF, Chaverri P, White JF (2005) Clarification of the host substrate of Ascopolyporus and description of Ascopolyporus philodendrus sp. nov. Mycologia 97(3):710–717. doi:10.3852/mycologia.97.3.710
Castlebury LA, Rossman AY, Sung GH, Hyten AS, Spatafora JW (2004) Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycol Res 108:864–872. doi:10.1017/s0953756204000607
Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17(4):540–552
Chabelska-Frydman C (1964) A new species of Fusariella from Israel. Can J Bot 42(11):1485–1488
Chaverri P, Bischoff JF, Evans HC, Hodge KT (2005) Regiocrella, a new entomopathogenic genus with a pycnidial anamorph and its phylogenetic placement in the Clavicipitaceae. Mycologia 97(6):1225–1237. doi:10.3852/mycologia.97.6.1225
Chomnunti P, Hongsanan S, Aguirre-Hudson B, Tian Q, Peršoh D, Dhami MK, Alias AS, Xu J, Liu X, Stadler M, Hyde KD (2014) The sooty moulds. Fungal Divers 66(1):1–36. doi:10.1007/s13225-014-0278-5
Crous PW, Braun U, Hunter GC, Wingfield MJ, Verkley GJM, Shin HD, Nakashima C, Groenewald JZ (2013) Phylogenetic lineages in Pseudocercospora. Stud Mycol 75:37–114. doi:10.3114/sim0005
Currie CR, Wong B, Stuart AE, Schultz TR, Rehner SA, Mueller UG, Sung GH, Spatafora JW, Straus NA (2003) Ancient tripartite coevolution in the attine ant-microbe symbiosis. Science 299(5605):386–388. doi:10.1126/science.1078155
Ellis MB (1971) Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, England
Ellis MB (1976) More Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, England
Garbowski ML (1917) Les champignons parasites récueillie dans le gouvernement de Podolie (Russie), pendant l'été 1915. Bull Soc mycol Fr 33:73–91
Goidànich G (1936) Morfologia, biologia e sisstematica di un fungo parassita delle foglie di pioppo [Stigmina radiosa (Lib.) G. Goid.]. Ann Bot Rome 21:1–12
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hansford CG (1943) Contributions towards the fungus flora of Uganda. V. Fungi Imperfecti. Proc Linn Soc London 155:34–67
Hughes SJ (1949) Studies on micro-fungi, 1. The genus Fusariella Saccardo. Mycol Pap 28(29):1–11
Index Fungorum (2016) Home page at: http://www.indexfungorum.org/names/Names.asp
Jayasiri SC, Hyde KD, Ariyawansa HA, Bhat J, Buyck B, Cai L, Dai Y-C, Abd-Elsalam KA, Ertz D, Hidayat I, Jeewon R, Jones EBG, Bahkali AH, Karunarathna SC, Liu J-K, Luangsa-ard JJ, Lumbsch HT, Maharachchikumbura SSN, McKenzie EHC, Moncalvo J-M, Ghobad-Nejhad M, Nilsson H, Pang K-L, Pereira OL, Phillips AJL, Raspé O, Rollins AW, Romero AI, Etayo J, Selçuk F, Stephenson SL, Suetrong S, Taylor JE, Tsui CKM, Vizzini A, Abdel-Wahab MA, Wen T-C, Boonmee S, Dai DQ, Daranagama DA, Dissanayake AJ, Ekanayaka AH, Fryar SC, Hongsanan S, Jayawardena RS, Li W-J, Perera RH, Phookamsak R, de Silva NI, Thambugala KM, Tian Q, Wijayawardene NN, Zhao R-L, Zhao Q, Kang J-C, Promputtha I (2015) The Faces of Fungi database: fungal names linked with morphology, phylogeny and human impacts. Fungal Divers 74(1):3–18. doi:10.1007/s13225-015-0351-8
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30(4):772–780
Lechat C, Farr DF, Hirooka Y, Minnis AM, Rossman AY (2010) A new species of Hydropisphaera, H. bambusicola, is the sexual state of Gliomastix fusigera. Mycotaxon 111(1):95–102
Lindau G (1910) Dr. L. Rabenhorst’s Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz. 2nd edn. Verlag von Eduard Kummer, Leipzig, Germany
Liu H-M, Zhang T-Y (2006) Two new species of Fusariella from soil in China. Mycosystema 25(4):513–515. doi:10.13346/j.mycosystema.2006.04.001
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Mol Biol Evol 16(12):1799–1808
Lutzoni F, Kauff F, Cox CJ, McLaughlin D, Celio G, Dentinger B, Padamsee M, Hibbett D, James TY, Baloch E, Grube M, Reeb V, Hofstetter V, Schoch C, Arnold AE, Miadlikowska J, Spatafora J, Johnson D, Hambleton S, Crockett M, Shoemaker R, Sung G-H, Lücking R, Lumbsch T, O’Donnell K, Binder M, Diederich P, Ertz D, Gueidan C, Hansen K, Harris RC, Hosaka K, Lim Y-W, Matheny B, Nishida H, Pfister D, Rogers J, Rossman A, Schmitt I, Sipman H, Stone J, Sugiyama J, Yahr R, Vilgalys R (2004) Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. Am J Bot 91(10):1446–1480. doi:10.3732/ajb.91.10.1446
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Huang S-K, Abdel-Wahab MA, Daranagama DA, Dayarathne M, D’Souza MJ, Goonasekara ID, Hongsanan S, Jayawardena RS, Kirk PM, Konta S, Liu J-K, Liu Z-Y, Norphanphoun C, Pang K-L, Perera RH, Senanayake IC, Shang QJ, Shenoy BD, Xiao YP, Bahkali AH, Kang JC, Somrothipol S, Suetrong S, Wen TC, Xu JC (2015) Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers 72(1):199–301. doi:10.1007/s13225-015-0331-z
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Bhat JD, Dayarathne MC, Huang S-K, Norphanphoun C, Senanayake IC, Perera RH, Shang Q-J, Xiao Y, D’souza MJ, Hongsanan S, Jayawardena RS, Daranagama DA, Konta S, Goonasekara ID, Zhuang W-Y, Jeewon R, Phillips AJL, Abdel-Wahab MA, Al-Sadi AM, Bahkali AH, Boonmee S, Boonyuen N, Cheewangkoon R, Dissanayake AJ, Kang J, Li Q-R, Liu JK, Liu XZ, Liu Z-Y, Luangsa-ard JJ, Pang K-L, Phookamsak R, Promputtha I, Suetrong S, Stadler M, Wen T, Wijayawardene NN (2016) Families of Sordariomycetes. Fungal Divers 79(1):1–317. doi:10.1007/s13225-016-0369-6
Matsushima T (1982) Matsushima Mycological Memoirs, No. 02. Published by the author, Kobe, Japan
Matsushima T (1985) Matsushima Mycological Memoirs No. 04. Published by the author, Kobe, Japan
Matsushima T (1993) Matsushima Mycological Memoirs, No. 07. Published by the author, Kobe, Japan
Mel’nik V, Lee S, Groenewald JE, Crous PW (2004) New hyphomycetes from Restionaceae in fynbos: Parasarcopodium ceratocaryi gen. et sp. nov., and Rhexodenticula elegiae sp. nov. Mycol Prog 3(1):19–28. doi:10.1007/s11557-006-0072-1
Nylander JAA (2004) MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University
Page RD (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12(4):357–358
Pollack FG (1947) Two additions to the fungi imperfecti. Mycologia 39(5):617–621
Rehner S (2001) Primers for elongation factor 1-α (EF1-α). Available online at: http://www.aftol.org/pdfs/EF1primer.pdf
Rehner SA, Samuels GJ (1994) Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol Res 98(6):625–634. doi:10.1016/S0953-7562(09)80409-7
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61(3):539–542
Rossman AY, Samuels GJ, Rogerson CT, Lowen R (1999) Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Stud Mycol 42:1–248
Roy RY, Rai B (1968) Fusariella indica sp. nov. Trans Br Mycol Soc 51(2):333–335
Saccardo PA (1884) Sylloge fungorum omnium hucusque cognitorum, vol 4. Sumptibus Auctoris, Patavii
Saccardo PA (1931) Sylloge fungorum omnium hucusque cognitorum, vol 25. Sumptibus Auctoris, Patavii
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Bolchacova E, Voigt K, Crous PW, Miller AN, Wingfield MJ, Aime MC, An KD, Bai FY, Barreto RW, Begerow D, Bergeron MJ, Blackwell M, Boekhout T, Bogale M, Boonyuen N, Burgaz AR, Buyck B, Cai L, Cai Q, Cardinali G, Chaverri P, Coppins BJ, Crespo A, Cubas P, Cummings C, Damm U, de Beer ZW, de Hoog GS, Del-Prado R, Dentinger B, Dieguez-Uribeondo J, Divakar PK, Douglas B, Duenas M, Duong TA, Eberhardt U, Edwards JE, Elshahed MS, Fliegerova K, Furtado M, Garcia MA, Ge ZW, Griffith GW, Griffiths K, Groenewald JZ, Groenewald M, Grube M, Gryzenhout M, Guo LD, Hagen F, Hambleton S, Hamelin RC, Hansen K, Harrold P, Heller G, Herrera G, Hirayama K, Hirooka Y, Ho HM, Hoffmann K, Hofstetter V, Hognabba F, Hollingsworth PM, Hong SB, Hosaka K, Houbraken J, Hughes K, Huhtinen S, Hyde KD, James T, Johnson EM, Johnson JE, Johnston PR, Jones EB, Kelly LJ, Kirk PM, Knapp DG, Koljalg U, Kovacs GM, Kurtzman CP, Landvik S, Leavitt SD, Liggenstoffer AS, Liimatainen K, Lombard L, Luangsa-Ard JJ, Lumbsch HT, Maganti H, Maharachchikumbura SS, Martin MP, May TW, McTaggart AR, Methven AS, Meyer W, Moncalvo JM, Mongkolsamrit S, Nagy LG, Nilsson RH, Niskanen T, Nyilasi I, Okada G, Okane I, Olariaga I, Otte J, Papp T, Park D, Petkovits T, Pino-Bodas R, Quaedvlieg W, Raja HA, Redecker D, Rintoul T, Ruibal C, Sarmiento-Ramirez JM, Schmitt I, Schussler A, Shearer C, Sotome K, Stefani FO, Stenroos S, Stielow B, Stockinger H, Suetrong S, Suh SO, Sung GH, Suzuki M, Tanaka K, Tedersoo L, Telleria MT, Tretter E, Untereiner WA, Urbina H, Vagvolgyi C, Vialle A, Vu TD, Walther G, Wang QM, Wang Y, Weir BS, Weiss M, White MM, Xu J, Yahr R, Yang ZL, Yurkov A, Zamora JC, Zhang N, Zhuang WY, Schindel D, Consortium FB (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci U S A 109(16):6241–6246. doi:10.1073/pnas.1117018109
Seifert KA, Morgan-Jones G, Gams W, Kendrick B (2011) The genera of hyphomycetes. CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands
Silvestro D, Michalak I (2012) raxmlGUI: a graphical front-end for RAxML. Org Divers Evol 12(4):335–337
Spatafora JW, Sung GH, Sung JM, Hywel-Jones NL, White JF (2007) Phylogenetic evidence for an animal pathogen origin of ergot and the grass endophytes. Mol Ecol 16(8):1701–1711. doi:10.1111/j.1365-294X.2007.03225.x
Sung GH, Spatafora JW (2004) Cordyceps cardinalis sp. nov., a new species of Cordyceps with an east Asian-eastern North American distribution. Mycologia 96(3):658–666. doi:10.2307/3762183
Sung G-H, Hywel-Jones NL, Sung J-M, Luangsa-ard JJ, Shrestha B, Spatafora JW (2007) Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol 57:5–59. doi:10.3114/sim.2007.57.01
Swofford DL (2002) PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods). Version 4.0b10. Sinauer Associates, Sunderland, MA
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729. doi:10.1093/molbev/mst197
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172(8):4238–4246
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, California, pp 315–322
Wu Y-M, Geng Y-H, Zhang T-Y (2009) Soil dematiaceous hyphomycetes from the Inner Mongolia Plateau, China. Mycosystema 28(6):652–655
Zhang N, Blackwell M (2002) Molecular phylogeny of Melanospora and similar pyrenomycetous fungi. Mycol Res 106:148–155. doi:10.1017/s0953756201005354
Zhang N, Castlebury LA, Miller AN, Huhndorf SM, Schoch CL, Seifert KA, Rossman AY, Rogers JD, Kohlmeyer J, Volkmann-Kohlmeyer B, Sung G-H (2006) An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia 98(6):1076–1087. doi:10.3852/mycologia.98.6.1076
Zhang S, Zhang Y-J, Liu X-Z, Zhang H, Liu D-S (2013) On the reliability of DNA sequences of Ophiocordyceps sinensis in public databases. J Ind Microbiol Biotechnol 40:365–378. doi:10.1007/s10295-012-1228-4
Zhao P, Luo J, Zhuang W-Y (2011) Practice towards DNA barcoding of the nectriaceous fungi. Fungal Divers 46:183–191. doi:10.1007/s13225-010-0064-y
Acknowledgements
We would like to thank Prof. Shaun Pennycook (Landcare Research Manaaki Whenua, New Zealand) for advising on the fungal name. The research is supported by the National Natural Science Foundation of China (no. NSFC 31560489), the grant [JD2014018] from Education Department of Guizhou Province and Fundamental Research on Science and Technology, Ministry of Science and Technology of China (2014FY120100).
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Roland Kirschner and Pedro W. Crous
Rights and permissions
About this article
Cite this article
Lin, CG., Chen, Y., McKenzie, E.H.C. et al. The genus Fusariella . Mycol Progress 15, 1313–1326 (2016). https://doi.org/10.1007/s11557-016-1246-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-016-1246-0