Abstract
Purpose
Haralick features Texture analysis is a recent oncologic imaging biomarker used to assess quantitatively the heterogeneity within a tumor. The aim of this study is to evaluate which Haralick’s features are the most feasible in predicting tumor response to neoadjuvant chemoradiotherapy (CRT) in colorectal cancer.
Materials and Methods
After MRI and histological assessment, eight patients were enrolled and divided into two groups based on response to neoadjuvant CRT in complete responders (CR) and non-responders (NR). Oblique Axial T2-weighted MRI sequences before CRT were analyzed by two radiologists in consensus drawing a ROI around the tumor. 14 over 192 Haralick’s features were extrapolated from normalized gray-level co-occurrence matrix in four different directions. A dedicated statistical analysis was performed to evaluate distribution of the extracted Haralick’s features computing mean and standard deviation.
Results
Pretreatment MRI examination showed significant value (p < 0.05) of 5 over 14 computed Haralick texture. In particular, the significant features are the following: concerning energy, contrast, correlation, entropy and inverse difference moment.
Conclusions
Five Haralick’s features showed significant relevance in the prediction of response to therapy in colorectal cancer and might be used as additional imaging biomarker in the oncologic management of colorectal patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Early diagnosis and accurate staging of rectal cancer are raising an essential role in the oncologic patients’ management particularly in personalized treatment strategies. Nowadays, magnetic resonance imaging (MRI) is considered the imaging modality of choice for loco-regional staging of rectal cancer [1, 2]. The validation of the technique for this purpose has been based on the ability to distinguish normal rectal wall from pathologic tissues on the basis of the high contrast resolution achievable on T2-weighted sequences [3]. However, its role in the evaluation of response to therapy is challenging due to the difficulty in discriminating fibrotic to viable residual tissue after neoadjuvant chemoradiotherapy (CRT) through morphologic approach with T2-weighted image [4].
Visual assessment has several limitations compared to quantitative measurements, such as inferior inter-reader agreement due to human eye error [5]. To overcome this issue, a multiparametric approach [6, 7] including T2-weighted, diffusion-weighted images (DWI) [8,9,10] and dynamic contrast-enhanced MRI (DCE-MRI) have been proposed [11, 12] with improved results but not already optimal to assure a personalized treatment to patients. Moreover, multiparametric MRI morphological assessment cannot assess the tumor at the cellular level.
Taking into account the weaknesses in visual process, there is the need to find new accurate quantitative semi-automatic/automatic diagnostics techniques [13]. Recently, new MRI biomarkers, such as Texture analysis, were investigated. Texture analysis is a non-invasive method to evaluate tissue heterogeneity of tumor lesions [14]. In literature, Texture parameters derived from T2-weighted images of rectal cancer have the potential role as imaging biomarkers of tumoral response to neoadjuvant CRT [15]. Texture analysis evaluates the spatial variation of gray levels within an image, through mathematical equations that generate several parameters associated with the Texture of an image. It is possible to extract Texture parameters using statistical (first-order, second-order, and high-order), model-based or transform methods [16]. In particular, Haralick’s Texture analysis, known as the spatial gray-level dependence matrix method, let the study of second-order statistics of pixels at different spacings and angles of adjacent or nearest-neighbor pixels [17, 18]; this statistical method reflects the spatial and signal intensity interrelationships between adjacent in-plane voxels (e.g. contrast, homogeneity, second-order entropy and energy), useful for a quantitative analysis of the tissue corresponding to different histology observed for instance in fibrotic tissue instead of viable residual tumor [19].
An early prediction of patients who can respond to therapy or not should be advisable in future target and it will allow a considerable change in patients’ treatment management. Thus, the purpose of this paper was to determine which of the quantitative parameters extrapolated from Haralick’s Texture analysis most suitable in predicting complete tumor response to neoadjuvant therapy and to evaluate the possible correlation among these parameters.
Materials and methods
Population study
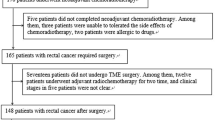
This retrospective study involved a sub-cohort of prospectively enrolled patients involved in the Italian Association for Cancer Research (AIRC) trial study “MR Imaging Biomarkers in Response Evaluation to Neoadjuvant Chemoradiotherapy in Rectal Cancer” I.G. 2013/14129. The investigation was approved by our institutional ethics committee and all patients gave written informed consent. All patients had histologically proved colorectal adenocarcinoma and locally advanced tumor stage from II (cT3-4, N0, M0) to III (cT2-4, N + , M0) following the UICC 2009. Exclusion criteria were considered the following: (a) patients with a histological partial response to therapy; (b) patients who have not completed the neoadjuvant treatment (e.g. hypersensitivity to the study drugs); (c) patients who have not been surgically treated, thus histological results are not available; (d) patients treated with concurrent and experimental drugs or participation in another clinical trial. Patients selected were divided into two groups based on response to treatment as complete responder (CR) and non-responder (NR), as shown in Fig. 1.
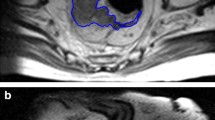
Flow chart of methodology: data were organized into two categories by visual inspection of radiologists (1a and 1b); manual segmentation of complete responder (2a, green line) and Non-Responder (2b, red line) was performed; after manual segmentation, each MRI was elaborated to extrapolate Haralick’s Texture data (from 3 to 6)
Study protocol
All patients underwent 3 MRI examinations, as have been already extensively described in another study [14]. MRI scanning were executed before, during and after neoadjuvant therapy. Between 6 and 8 weeks after the CRT, total mesorectal excision (TME) was performed and an experienced pathologist analyzed the gross specimen. Due to the specific purpose of this study, we have focused our analyses only on pretreatment Oblique Axial T2-weighted MRI examinations for the assessment of imaging biomarkers capable to discriminate from responder and non-responder patients prior to the beginning of neoadjuvant therapy.
MRI examination
All MRI acquisitions were performed using a 3T scanner (Discovery MR750, General Electrics, Milwaukee, Wisconsin, USA). A standard clinical imaging protocol used for rectal cancer study was performed including routinely and dedicated sequences, such as T1 and T2-weighted with fat saturation/suppression, DWI, ADC and dynamic contrast-enhanced sequences as described in another study [15]. For the specific purpose of our study, we analyzed high-resolution T2-weighted fast recovery fast-spin echo (2D FRFSE) sequence (TR, 2086–4172 ms; TE, 11.4–122.3 ms; Nex, 2; slice thickness, 4 mm; matrix, 512 × 512) acquired angled to the axial planes orthogonal to the long axis of the rectum to obtain an Oblique Axial T2-weighted planes [19,20,21]. High-resolution T2-weighted images grant an optimal morphologic evaluation by allowing a precise tumor segmentation; additionally, the texture parameters have a good reproducibility on T2-weighted as described in literature [19, 22].
Texture analysis
Haralick’s texture analysis is a statistical technique, known as the spatial gray-level dependence matrix method. Using it is possible to study second-order statistics of pixels at different spacings and direction of adjacent or nearest-neighbor pixels. As Freeborough and Fox demonstrated [23], a Texture discriminant function derived from MRI brain scans using a spoiled gradient-echo technique on a 1.5 T system gave significantly different values for Alzheimer sufferers compared to normal controls [17].
Two radiologists (XXX and XXX) with 7 and 11 years of experience in rectal cancer MRI evaluation, respectively, performed the segmentation step in consensus. The tumor region has been manually drawn from pre-CRT oblique Axial T2-weighted MRI image, slice by slice for the entire tumor volume by means of a free open-source segmentation platform (ITK-SNAP version 4.11.0; www.itk-snap.org) [24, 25]. After the segmentation process, segmented images were computed with Haralick’s textural analysis method [18] as described by Soomro et al. 2017 [26] for feature extraction (Fig. 1).
Based on previous study, 14 Haralick’s Texture features were selected over 192 total features as shown in Table 1, and every segmented ROI have been computed from normalized gray-level co-occurrence matrix (GLC) in the four main direction (viz: 0°, 45°, 90° and 135°) [18].
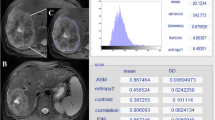
The entire volume of the tumor was computed at each voxel with Gray level co-occurence matrices (GLCM) and the selected features were extracted. GLCM can be mainly described as an histogram in two dimension that assesses the co-occurrence frequency of two pixel intensities at a specified offset compared to each other over the region where the texture is computed. Each texture feature computes a specific relation of pixels with their local neighborhood [27]. In detail, Energy provides knowledge about uniformity of image with a 0–1 range (the highest value 1 expresses low variation in image with respect to intensity). Contrast measures local fluctuation: its high value indicates the higher intensity variation among pixels in the image. Correlation recognizes the parallelism among image gray levels with values between − 1 and + 1 where + 1 indicates a higher linear dependencies of image gray levels. The chaotic distribution of image gray levels was quantified by Entropy. At last, inverse difference moment is referred to as uniformity quantifying the affinity of co-occurrence gray levels [26].
Statistical analysis
To evaluate distribution of the extracted Haralick’s features, statistical analysis was performed computing mean and standard deviation using Statistical analysis with SPSS (21.0; SPSS, Chicago, IL, USA) and MedCalc version 12.7.2 (MedCalc Software, Ostend, Belgium). Dedicated engineers performed a normalization of the GLC matrices by taking the neighbor pixel values and reference pixel values paying attention to probability rather than just counting of co-occurrences as suggested Wibmer and colleagues [27]. Data obtained were matched with clinical data as well as histological evaluation. Linear regression was performed to evaluate the association between texture parameters and histological results. The Wald test from the regression model was performed and p values ≤ 0.05 were considered statistically significant.
Results
From a total of 90 consecutive patients enrolled in the aforementioned trial, 82 patients were excluded due to: (a) histological partial response to therapy (n = 21); (b) non-completion of the neoadjuvant treatment at the time of the present study (n = 46); (c) lack of surgical treatment (n = 2); and (d) lack of histological results at the time of patient selection (n = 13).
Thus, the final population consisted of eight patients (two females, six males, median age 65.5 years, range 58–78 years) with locally advanced colorectal adenocarcinoma at tumor stages II (cT3-4, N0, M0) and III (cT2-4, N + , M0) confirmed by preliminary biopsy. All patients follow a neoadjuvant CRT and after a time-span of 6-8 weeks, they underwent Total Mesorectal Excision surgery (TME) followed by the histological assessment performed by an expert gastrointestinal pathologist.
Preliminary results on Haralick’s Texture analysis has shown that 5 over 14 features could hire an important role as MRI biomarker to differentiate between complete responder and non-responder patients affected by rectal cancer (all p < 0.05): Energy, Contrast, Correlation, Entropy, inverse difference moment. CR patients have significantly higher values of energy, correlation and inverse difference moment features in comparison with NR (all p < 0.05), whereas contrast and entropy show significantly lower values for CR compared to NR (all p < 0.05). Moreover, no significant differences among all the directions were observed within the same group (all p > 0.05). Full data are reported in Table 2 and Fig. 2.
Discussion
This paper represents a preliminary technical study on Haralick’s textural features and the possibly to evaluate the prognostic trend of colorectal cancer with MRI semi-automatic image analysis.
The T2-weighted colorectal MRI of CR patients showed less disorder and randomness than NR patients imaging. Moreover, T2-weighted images of CR patients have higher energy, inverse difference moment and correlation values, indicating uniformity in image, as well as lower entropy and contrast values, revealing lower randomness and dissimilarity in their gray levels in comparison with NR patients. Contrariwise, T2-weighted images of NR patients have higher entropy and contrast, representing higher randomness or disorder and dissimilarity in image gray levels.
Texture Analysis can be included in a wider field of research called Radiomics that has the aim to exploit the full potential of medical imaging [28]. As some studies described, radiomic signature identifies a general prognostic tumor phenotype, for instance in lung and head and neck tumor [29]. Moreover, with the integration of genomics and microarray could built the basis of a wider diagnostic branch called radiogenomic [30], a potential powerful tool for clinical decision able to assess, with higher accuracy than each single diagnostic tool, the response to neoadjuvant therapy or the tailored treatment based on the tumor phenotype leading to an overall improvement of patient management.
As demonstrated by Ng et al. [31], texture analysis allows the evaluation of the heterogeneity within a tumor. Recent studies demonstrated that texture parameters derived from T2-weighted images of rectal cancer potentially might assume a role as imaging biomarkers in detecting tumoral response to neoadjuvant CRT [14]. Their capability on reflecting tumor heterogeneity may be further used in clinical practice, integrated to the other diagnostic tools, to improve the selection of tailored patients’ therapy to avoid under/over treatment that could slow down the care process. Haralick analysis has been preferred in our study for the reasons as follow: first, fractal-based Texture models are computationally intensive as the model is estimated during the Texture extraction process; second, there is a lack in orientation sensitivity and these models are not suitable for describing local image structures [27].
Our study has several limitations. First, due to the technical purpose our results have been achieved in a small population retrospectively analyzed and should be confirmed with a more representative population. Second, we did not analyze with Haralick’s Texture other MRI sequences as DWI, ADC or MRI perfusion. Third, we did not perform a proper 3D volumetric Texture evaluation because the software available allows only a single-slice evaluation contrarily to the Texture Analysis described by Wibmer et al. [27]. More studies are encouraged for a deeper analysis of the topic.
Conclusion
Our preliminary results showed that energy, contrast, correlation, entropy and inverse difference moment, are the Haralick’s features that may have a significant relevance in predicting the response to therapy in patients with colorectal cancer. In particular, the association of such imaging features with additional genomics and microarray data can potentially provide a comprehensive overview of tumor characteristics, allowing for an effective targeted therapy and moving towards a personalized treatment in patients with rectal cancer. Further prospective multicentre trials are advisable to achieve a large-scale validation of our results in clinical practice.
References
Beets-Tan RG, Lambregts DM, Maas M, Bipat S, Barbaro B, Caseiro-Alves F, Curvo-Semedo L, Fenlon HM, Gollub MJ, Gourtsoyianni S, Halligan S, Hoeffel C, Kim SH, Laghi A, Maier A, Rafaelsen SR, Stoker J, Taylor SA, Torkzad MR, Blomqvist L (2013) Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 23(9):2522–2531. https://doi.org/10.1007/s00330-013-2864-4
Laghi A, Ferri M, Catalano C, Baeli I, Iannaccone R, Iafrate F, Ziparo V, Passariello R (2002) Local staging of rectal cancer with MRI using a phased array body coil. Abdom Imaging 27(4):425–431. https://doi.org/10.1107/s00261-001-0123-7
Group MS (2006) Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ 333(7572):779. https://doi.org/10.1136/bmj.38937.646400.55
van den Broek JJ, van der Wolf FS, Lahaye MJ, Heijnen LA, Meischl C, Heitbrink MA, Schreurs WH (2017) Accuracy of MRI in restaging locally advanced rectal cancer after preoperative chemoradiation. Dis Colon Rectum 60(3):274–283. https://doi.org/10.1097/dcr.0000000000000743
Maas M, Lambregts DM, Lahaye MJ, Beets GL, Backes W, Vliegen RF, Osinga-de Jong M, Wildberger JE, Beets-Tan RG (2012) T-staging of rectal cancer: accuracy of 3.0 Tesla MRI compared with 1.5 Tesla. Abdom Imaging 37(3):475–481. https://doi.org/10.1007/s00261-011-9770-5
Attenberger UI, Pilz LR, Morelli JN, Hausmann D, Doyon F, Hofheinz R, Kienle P, Post S, Michaely HJ, Schoenberg SO, Dinter DJ (2014) Multi-parametric MRI of rectal cancer—do quantitative functional MR measurements correlate with radiologic and pathologic tumor stages? Eur J Radiol 83(7):1036–1043. https://doi.org/10.1016/j.ejrad.2014.03.012
Hotker AM, Tarlinton L, Mazaheri Y, Woo KM, Gonen M, Saltz LB, Goodman KA, Garcia-Aguilar J, Gollub MJ (2016) Multiparametric MRI in the assessment of response of rectal cancer to neoadjuvant chemoradiotherapy: a comparison of morphological, volumetric and functional MRI parameters. Eur Radiol 26(12):4303–4312. https://doi.org/10.1007/s00330-016-4283-9
Curvo-Semedo L, Lambregts DM, Maas M, Beets GL, Caseiro-Alves F, Beets-Tan RG (2012) Diffusion-weighted MRI in rectal cancer: apparent diffusion coefficient as a potential noninvasive marker of tumor aggressiveness. J Magn Reson Imaging JMRI 35(6):1365–1371. https://doi.org/10.1002/jmri.23589
Jung SH, Heo SH, Kim JW, Jeong YY, Shin SS, Soung MG, Kim HR, Kang HK (2012) Predicting response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer: diffusion-weighted 3 Tesla MR imaging. J Magn Reson Imaging JMRI 35(1):110–116. https://doi.org/10.1002/jmri.22749
Cai PQ, Wu YP, An X, Qiu X, Kong LH, Liu GC, Xie CM, Pan ZZ, Wu PH, Ding PR (2014) Simple measurements on diffusion-weighted MR imaging for assessment of complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol 24(11):2962–2970. https://doi.org/10.1007/s00330-014-3251-5
Intven M, Reerink O, Philippens ME (2015) Dynamic contrast enhanced MR imaging for rectal cancer response assessment after neo-adjuvant chemoradiation. J Magn Reson Imaging JMRI 41(6):1646–1653. https://doi.org/10.1002/jmri.24718
Gollub MJ, Gultekin DH, Akin O, Do RK, Fuqua JL 3rd, Gonen M, Kuk D, Weiser M, Saltz L, Schrag D, Goodman K, Paty P, Guillem J, Nash GM, Temple L, Shia J, Schwartz LH (2012) Dynamic contrast enhanced-MRI for the detection of pathological complete response to neoadjuvant chemotherapy for locally advanced rectal cancer. Eur Radiol 22(4):821–831. https://doi.org/10.1007/s00330-011-2321-1
Hamilton PW, Bartels PH, Thompson D, Anderson NH, Montironi R, Sloan JM (1997) Automated location of dysplastic fields in colorectal histology using image texture analysis. J Pathol 182(1):68–75. https://doi.org/10.1002/(sici)1096-9896(199705)182:1<68:aid-path811>3.0.co;2-n
De Cecco CN, Ganeshan B, Ciolina M, Rengo M, Meinel FG, Musio D, De Felice F, Raffetto N, Tombolini V, Laghi A (2015) Texture analysis as imaging biomarker of tumoral response to neoadjuvant chemoradiotherapy in rectal cancer patients studied with 3-T magnetic resonance. Invest Radiol 50(4):239–245. https://doi.org/10.1097/RLI.0000000000000116
De Cecco CN, Ciolina M, Caruso D, Rengo M, Ganeshan B, Meinel FG, Musio D, De Felice F, Tombolini V, Laghi A (2016) Performance of diffusion-weighted imaging, perfusion imaging, and texture analysis in predicting tumoral response to neoadjuvant chemoradiotherapy in rectal cancer patients studied with 3T MR: initial experience. Abdom Radiol (NY) 41(9):1728–1735. https://doi.org/10.1007/s00261-016-0733-8
Castellano G, Bonilha L, Li LM, Cendes F (2004) Texture analysis of medical images. Clin Radiol 59(12):1061–1069. https://doi.org/10.1016/j.crad.2004.07.008
Ahmed A, Gibbs P, Pickles M, Turnbull L (2013) Texture analysis in assessment and prediction of chemotherapy response in breast cancer. J Magn Reson Imaging JMRI 38(1):89–101. https://doi.org/10.1002/jmri.23971
Haralick RM Shanmugam K, Dinstein I (2007) Textural features for image classification. In: IEEE Xplore Document. IEEE. http://ieeexplore.ieee.org/document/4309314/
Gourtsoyianni S, Doumou G, Prezzi D, Taylor B, Stirling JJ, Taylor NJ, Siddique M, Cook GJR, Glynne-Jones R, Goh V (2017) Primary rectal cancer: repeatability of global and local-regional MR imaging texture features. Radiology. https://doi.org/10.1148/radiol.2017161375
Jalil O, Afaq A, Ganeshan B, Patel UB, Boone D, Endozo R, Groves A, Sizer B, Arulampalam T (2017) Magnetic resonance based texture parameters as potential imaging biomarkers for predicting long-term survival in locally advanced rectal cancer treated by chemoradiotherapy. Colorectal Dis 19(4):349–362. https://doi.org/10.1111/codi.13496
Nketiah G, Elschot M, Kim E, Teruel JR, Scheenen TW, Bathen TF, Selnaes KM (2017) T2-weighted MRI-derived textural features reflect prostate cancer aggressiveness: preliminary results. Eur Radiol 27(7):3050–3059. https://doi.org/10.1007/s00330-016-4663-1
Kaur H, Choi H, You YN, Rauch GM, Jensen CT, Hou P, Chang GJ, Skibber JM, Ernst RD (2012) MR imaging for preoperative evaluation of primary rectal cancer: practical considerations. Radiographics 32(2):389–409. https://doi.org/10.1148/rg.322115122
Freeborough PA, Fox NC (1998) MR image texture analysis applied to the diagnosis and tracking of Alzheimer’s disease. IEEE Trans Med Imaging 17(3):475–479. https://doi.org/10.1109/42.712137
Yoo TS, Ackerman MJ, Lorensen WE, Schroeder W, Chalana V, Aylward S, Metaxas D, Whitaker R (2002) Engineering and algorithm design for an image processing Api: a technical report on ITK–the insight toolkit. Stud Health Technol Inform 85:586–592
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31(3):1116–1128. https://doi.org/10.1016/j.neuroimage.2006.01.015
Soomro MH, Giunta G, Laghi A, Caruso D, Ciolina M, Marchis CD, Conforto S, Schmid M (2017) Haralick’s texture analysis applied to colorectal T2-weighted MRI: a preliminary study of significance for cancer evolution. In: Biomedical Engineering, 03 Feb 2017. ACTA Press, Alberta https://doi.org/10.2316/P.2016.852-019
Wibmer A, Hricak H, Gondo T, Matsumoto K, Veeraraghavan H, Fehr D, Zheng J, Goldman D, Moskowitz C, Fine SW, Reuter VE, Eastham J, Sala E, Vargas HA (2015) Haralick texture analysis of prostate MRI: utility for differentiating non-cancerous prostate from prostate cancer and differentiating prostate cancers with different Gleason scores. Eur Radiol 25(10):2840–2850. https://doi.org/10.1007/s00330-015-3701-8
Li M, Fu S, Zhu Y, Liu Z, Chen S, Lu L, Liang C (2016) Computed tomography texture analysis to facilitate therapeutic decision making in hepatocellular carcinoma. Oncotarget 7(11):13248–13259. https://doi.org/10.18632/oncotarget.7467
Parmar C, Leijenaar RT, Grossmann P, Rios Velazquez E, Bussink J, Rietveld D, Rietbergen MM, Haibe-Kains B, Lambin P, Aerts HJ (2015) Radiomic feature clusters and prognostic signatures specific for lung and head and neck cancer. Sci Rep 5:11044. https://doi.org/10.1038/srep11044
Incoronato M, Aiello M, Infante T, Cavaliere C, Grimaldi AM, Mirabelli P, Monti S, Salvatore M (2017) Radiogenomic analysis of oncological data: a technical survey. Int J Mol Sci. https://doi.org/10.3390/ijms18040805
Ng F, Ganeshan B, Kozarski R, Miles KA, Goh V (2013) Assessment of primary colorectal cancer heterogeneity by using whole-tumor texture analysis: contrast-enhanced CT texture as a biomarker of 5-year survival. Radiology 266(1):177–184. https://doi.org/10.1148/radiol.12120254
Acknowledgments
This study is funded by AIRC (Associazione Italiana per la Ricerca sul Cancro) Investigator Grant 2013/14129.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Authors declare that they have no conflict of interest.
Ethical standards
All human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
All patients gave their informed consent prior to their inclusion in the study.
Rights and permissions
About this article
Cite this article
Caruso, D., Zerunian, M., Ciolina, M. et al. Haralick’s texture features for the prediction of response to therapy in colorectal cancer: a preliminary study. Radiol med 123, 161–167 (2018). https://doi.org/10.1007/s11547-017-0833-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-017-0833-8