Abstract
Purpose
To compare morphological and functional MRI metrics and determine which ones perform best in assessing response to neoadjuvant chemoradiotherapy (CRT) in rectal cancer.
Materials and methods
This retrospective study included 24 uniformly-treated patients with biopsy-proven rectal adenocarcinoma who underwent MRI, including diffusion-weighted (DW) and dynamic contrast-enhanced (DCE) sequences, before and after completion of CRT. On all MRI exams, two experienced readers independently measured longest and perpendicular tumour diameters, tumour volume, tumour regression grade (TRG) and tumour signal intensity ratio on T2-weighted imaging, as well as tumour volume and apparent diffusion coefficient on DW-MRI and tumour volume and transfer constant Ktrans on DCE-MRI. These metrics were correlated with histopathological percent tumour regression in the resected specimen (%TR). Inter-reader agreement was assessed using the concordance correlation coefficient (CCC).
Results
For both readers, post-treatment DW-MRI and DCE-MRI volumetric tumour assessments were significantly associated with %TR; DCE-MRI volumetry showed better inter-reader agreement (CCC=0.700) than DW-MRI volumetry (CCC=0.292). For one reader, mrTRG, post-treatment T2 tumour volumetry and assessments of volume change made with T2, DW-MRI and DCE-MRI were also significantly associated with %TR.
Conclusion
Tumour volumetry on post-treatment DCE-MRI and DW-MRI correlated well with %TR, with DCE-MRI volumetry demonstrating better inter-reader agreement.
Key Points
• Volumetry on post-treatment DCE-/DW-MRI sequences correlated well with histopathological tumour regression.
• DCE-MRI volumetry demonstrated good inter-reader agreement.
• Inter-reader agreement was higher for DCE-MRI volumetry than for DW-MRI volumetry.
• DCE-MRI volumetry merits further investigation as a metric for evaluating treatment response.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In patients with rectal cancer, accurate assessment of response to chemoradiotherapy has increased in importance as the variety of treatment options has grown and a controversial non-operative approach may even be considered for some patients in whom complete tumour regression is believed to have occurred [1]. In addition, tumour shrinkage after neoadjuvant therapy may allow for a less invasive organ-sparing surgical approach and a higher rate of sphincter-preservation. However, while routine magnetic resonance imaging (MRI) has a well established role in pre-therapeutic staging of patients with rectal cancer [2], its role in response assessment after chemoradiotherapy is less well established, largely because of the difficulty encountered in distinguishing between therapy-induced scarring and residual viable tumour on T2-weighted sequences [3, 4].
To overcome these limitations [5, 6], other MRI approaches have been proposed in recent years, including the assessment of changes in T2 signal intensity [7] or of changes in tumour volume, and the non-invasive interrogation of biological tumour properties using diffusion-weighted (DW)-MRI [8–17] and dynamic contrast-enhanced (DCE)-MRI [18–24]. In addition, some researchers have attempted volumetric rather than single region-of-interest-based tumour assessment on T2-weighted or DW-MRI sequences, and reported promising results [11, 25–27].
While investigators have studied many different metrics of interest to rapidly move the field along and inform the debate on the utility of post-treatment MRI, the various metrics have not been applied in parallel in a single, homogeneous cohort of patients so that their relative value could be directly compared. As such, it is still unclear which of these metrics performs best in assessing response after neoadjuvant treatment. The purpose of this study was therefore to determine which of numerous morphological, volumetric and biological MRI metrics performs best in a head-to-head comparison using a cohort of patients with locally advanced rectal cancer from a single institution who were managed similarly with respect to neoadjuvant chemoradiation dose and imaging schedule.
Materials and methods
Patient selection
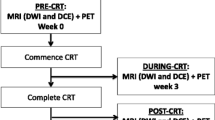
The institutional review board approved this retrospective, Health Insurance Portability and Accountability Act (HIPAA)-compliant study and issued a waiver of informed consent. Our institution’s Picture Archiving and Communication Systems (PACS) and electronic medical records were searched for the years 2007 to 2013 to identify patients who (1) had biopsy-proven primary rectal adenocarcinoma; (2) underwent long-course neoadjuvant chemoradiotherapy; (3) underwent MRI examinations before the start and after the completion of neoadjuvant treatment featuring DW and/or DCE sequences; and (4) had surgical tumour resection performed at our institution.
This initial search for patients with at least a pre-therapeutic MRI and primary rectal adenocarcinoma yielded a total of 812 patients (1/1/2007 to 1/27/2013). Given that post-operative re-staging was not performed uniformly or regularly at our institution until 2010 as well as the increasing use of induction chemotherapy or definitive chemotherapy at our institution, most patients did not meet the inclusion criteria above, resulting in a total of 26 homogeneous patients with available post-therapeutic MRI examination. Of these, one patient had to be excluded due to the placement of a rectal stent before post-treatment MRI interfering with imaging, and another patient was excluded due to partial excision of the tumour before MRI. The final cohort therefore consisted of 24 patients (see Fig. 1). Three patients were excluded from analysis of DW imaging, because no DW-MRI sequence was available (n=2) or because of imaging artefacts (n=1). The median interval between the two MRI examinations was 96 (range: 71–131) days, and the median interval between a second MRI and surgery was 20 (range: 3–54) days. All patients underwent standard long-course chemoradiation with 50–50.4 Gy in 25–28 fractions and application of 5- fluorouracil between the first and the second MRI, according to National Comprehensive Cancer Network (NCCN) guidelines [28].
Magnetic resonance imaging (MRI) and image analysis
MRI examinations were performed on different MRI scanners manufactured by GE Healthcare (Waukesha, WI, USA) at a field strength of 1.5 Tesla (n=40) or 3 Tesla (n=8) using a standardized MRI protocol that included standard high-resolution T2-weighted imaging in axial, sagittal, coronal and oblique orientation (TR: 4400-5000; TE: 90-110; echo train length: 12–24; slice thickness: 3–4 mm; interslice gap: 1 mm; FOV: 20 cm; matrix: 320 x 160; NEX: 2), an axial DW sequence (single-shot spin-echo EPI sequence, b-values: 0 and 750–1,000 s/mm2; TR: 1,800–5,550ms; TE: 60–112ms; slice thickness: 3–5 mm; interslice gap: 1 mm; FOV: 18–40 cm; matrix: 96–256 x 96–128; NEX: 3–6; mean acquisition time: 2.4 min) and a sagittal DCE-MRI sequence (TR: 3.1–7.9 ms; TE: 0.9–4.2 ms; slice thickness: 4–10 mm; no interslice gap; FOV: 20–34 cm; matrix: 256–320 x 128-192; mean temporal resolution: 8.3 (5–11.5) s; 30–40 phases; mean acquisition time: 5.2 min). A bolus of Gd- DTPA (Magnevist, Bayer Schering, Berlin-Wedding, Germany) at a constant dose of 0.1 mmol/kg was power injected at a rate of 2 ml/s followed by a saline flush for all patients.
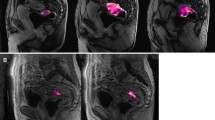
Two readers with experience reading rectal MRI (Reader 1: AMH with 5 years of experience; Reader 2: MJG with 10 years of experience) independently assessed each pre- and post-treatment MRI, blinded to clinical and histopathological information. They each assessed the following metrics, which were chosen either because they were already frequently used for tumour assessment or because they had been reported to provide significant value in response assessment on rectal MRI after therapy (see Fig. 2 a–d for examples):
(a) T2- weighted image (sagittal): Measurement of longest tumour diameter. (b) T2- weighted image (axial-oblique): Measurement of product of largest bidirectional measurements (excluding the lumen) and T2 volumetry (region of interest (ROI) was drawn on every slice with visible tumour). (c) diffusion-weighted image (axial, b=1000 s/mm2): tumour volumetry on diffusion-weighted imaging (DWI; ROI was drawn on every slice with visible tumour) and calculation of apparent diffusion coefficient (ADC). (d) T1- weighted dynamic contrast-enhanced (DCE) image (sagittal): tumour volumetry on DCE. Ktrans was derived from a ROI in maximal tumour extent
-
(1)
Longest diameter of the tumour on any plane (measured on T2-weighted sequences, currently part of many response assessment approaches for other tumours, including RECIST [29]).
-
(2)
Product of largest bidirectional tumour measurements (largest diameter and perpendicular tumour diameter) of the tumour on the axial oblique plane, excluding the lumen (measured on T2-weighted sequences, a measurement similar to that proposed by the World Health Organization [30]).
-
(3)
Relative T2 ratio (SIrel = SItumor/SImuscle) based on a single region of interest (ROI) in a representative region of the tumour and an ROI in the centre of the obturator internus muscle, carefully avoiding any intramuscular fat (a metric introduced by Kluza et al. [7]).
-
(4)
Tumour volume on T2-weighted high-resolution sequences, with plane selection being determined based on best visibility of the tumour (e.g. coronal for low rectal cancers) and kept constant for pre- and post-therapeutic measurements [26].
-
(5)
Tumour volume on DW imaging (measured on the DW sequence) and voxel-wise calculation of apparent diffusion coefficient (ADC) values using a monoexponential model, based on the volumetric assessment [9, 25, 31].
-
(6)
Tumour volume on DCE sequences and the transfer constant Ktrans of the generalized Tofts model [32], measured by drawing a region of interest on a slice in maximal tumour extent, including all visible tumour on this slice [23]. A population-derived arterial input function and T1 reference times (1,317 ms at 1.5T and 1,597 ms at 3T) were used.
-
(7)
MR-based tumour regression grade (mrTRG), see Patel et al. [5, 6] for examples.
All morphological tumour assessments (longest tumour diameter, product of bidirectional tumour measurements, T2 ratio) were performed with commercial PACS software (Centricity, GE Healthcare, Waukesha, WI, USA). For volumetric tumour assessments (T2-weighted, DW- or DCE-MRI sequences), each radiologist drew an ROI encircling the entire tumour on every slice where it appeared, using the software Image J (version 1.47m, National Institutes of Health, Bethesda, MD, USA [33]). The data from these ROIs were then analyzed by in-house software written in Matlab (Matlab R2014b, The MathWorks, Inc, Natick, MA, USA) to calculate the volume, apparent diffusion coefficients (mean value for the whole tumour volume) and transfer constant Ktrans values, respectively.
Histopathological workup of the specimens
Each patient underwent surgical resection of the tumour by our colorectal surgeons, each with colorectal cancer surgery specialty training and certified in the performance of total mesorectal excision (TME). Either a low anterior resection (LAR) or abdominoperineal resection (APR) was performed and the specimen was examined with histopathology according to institutional standards. Apart from the routine histopathological assessment including the assessment for surgical margins and other histological features (tumour type and differentiation, involvement of the perineural (PN) or lymphovascular (PV) space by tumour, T stage and N stage; AJCC 7th edition) the percent tumour response was estimated based on the amount of fibrosis and inflammatory tissue versus the amount of residual viable carcinoma in the lesion [34, 35].
Statistical analysis
Summary statistics were calculated for pre- and post-treatment MRI parameters, including percent change. Wilcoxon signed rank tests were used to confirm whether the median difference for each parameter was significantly different from zero. To analyze agreement between the two readers, we used the concordance correlation coefficient [36], including 95 % confidence intervals (CIs). This coefficient combines measures of both precision and accuracy to determine how far the observed data deviate from the line of perfect concordance (i.e. the line at 45° on a square scatterplot). The closer the coefficient is to 1, the better the agreement between the two readers for that imaging parameter. Cohen’s kappa for pairwise agreement analysis was used for categorical parameters, including 95 % CIs. Spearman’s rank correlation was used to analyze the correlation of the imaging parameters with percent tumour response, including 95 % CIs. A Bland Altman assessment for agreement was used to compare the two readers using the absolute difference as a percentage of the average of the two readers, including 95 % limits of agreement. The type-one error was set to 5 % two-sided. R version 3.1.1 was used for all analyses, including the epiR, psych and mada packages.
Results
Patients
The 24 patients included in the study had a mean age of 57 (range: 37–86) years; 19 of them were male and five were female. The pre-treatment clinical stage was cT2 in three patients (all of them also cN+), T3 in 17 patients and T4 in four patients. Histopathological tumour stage after resection was pT0 in four (percentage tumour regression %TR: 100 %), pT1 in two (%TR: 80–90 %), pT2 in seven (all N0, %TR: 30–95 %), pT3N0 in four (%TR: 60–95 %) and pT3N1 in seven (%TR: 25–99 %) patients.
Median values for each MRI parameter on pre- and post-treatment MRI are given in Table 1, as are the median percentage changes in the parameters from pre- to post-treatment MRI. The data indicate that the median difference between pre- and post-treatment parameters is significantly different from zero, except for ADC and Ktrans.
Inter-reader agreement
Inter-reader agreement was found to differ greatly among the MRI parameters measured, with agreement on pre-treatment values generally being superior to agreement on post-treatment values (Table 2, Fig. 3). In the context of response assessment after therapy, the best agreement was found for ADC (CCC: 0.766, 95 % CI 0.510–0.898), DCE volumetry (CCC: 0.700, 95 % CI 0.527–0.817) and T2 volumetry (CCC: 0.609, 95 % CI 0.401–0.758), when assessing absolute post-treatment values. Bland Altman plots on the percent change scale indicate that the bias is minimal, and the limits of agreement are sufficiently close for us to conclude there is good agreement (Fig. 3).
Bland-Altman plots for the measured MRI parameters at pre- and post-therapeutic examinations that were significantly associated with tumour response (units: mm3). The Bland-Altman plot shows the percent difference of the measurement between the two readers versus the average of the two readers’ measurements. The middle dotted line is drawn at the mean difference, and the upper and lower lines are the 95 % limits of agreement (calculated using the standard deviation)
Correlation between MRI parameters and histopathological tumour regression
The only two parameters that were significantly associated with histopathological tumour regression for both readers were post-treatment values for DW imaging (DWI) volumetry (rho = −0.574, 95 % CI −0.794 to −0.222 for reader 1; rho = −0.453, 95 % CI −0.724 to −0.061 for reader 2) and post-treatment DCE volumetry (rho = −.547, 95 % CI −0.779 to −0.184 for reader 1; rho = −0.522, 95% CI −0.764 to −0.150 for reader 2).
For reader 1 alone, both the percentage change in T2 volume (rho = −0.636, 95 % CI −0.827 to −0.313) and post-treatment absolute T2 volume (rho = −0.565, 95 % CI −0.789 to −0.209) were significantly associated with histopathological tumour regression. For this reader, percentage changes in DWI and DCE-MRI tumour volumes (rho = −0.579, 95 % CI −0.796 to −0.229 and rho = −0.745, 95 % CI −0.883 to −0.488, respectively) and morphological assessment of therapeutic response (mrTRG) were also found to be highly significant (rho = −0.751, 95 % CI −0.886 to −0.499).
None of the other ‘morphological’ parameters (longest tumour diameter, product of bidirectional tumour measurements, T2 ratio) or functional parameters (ADC, Ktrans) were found to be associated with tumour regression for either reader (Table 3).
Discussion
In our study, volumetric tumour measurements on post-therapeutic DW-MRI and DCE-MRI sequences were the only metrics significantly associated with percentage tumour regression on histopathology for both readers, and inter-reader agreement was higher for DCE-MRI volumetry than for DW-MRI volumetry.
Neoadjuvant chemoradiation is known to cause tumour shrinkage and to lower the rate of local recurrence in patients with advanced rectal cancer [37]. However, the exact degree of tumour regression induced varies widely among individual patients, ranging from complete pathological response (pCR) to (rarely) only minor tumour shrinkage. Accurate assessment of therapeutic response would be of great clinical value, as it could help in selecting the best therapeutic approach (potentially a less-invasive organ-sparing surgical approach) and in determining the feasibility of non-operative management, which is generally reserved for those with a complete clinical response to neoadjuvant therapy [1]. Response quantitation remains difficult on routine T2-weighted rectal MRI, in part due to scarring, which may contain tumour [3, 4]. In our study, T2 volumetry was useful, whereas uni- and bi-dimensional measurements were not. This makes intuitive sense, since measuring anything less than the entire tumour volume presupposes spherical tumour geometry that can be approximated closely by using known equations. Dimension-based response assessment seems to work fairly well for solid organ lesions with a roughly spherical growth pattern, and thus RECIST and WHO methods have predominated. However, in the luminal GI tract, it is much more difficult to derive an accurate estimation of initial tumour burden and treatment response at imaging.
The success of DWI and DCE volumetry in predicting tumour regression in our study can be interpreted to mean that in contrast to dimension-based measurements from T2-weighted imaging, and even T2 volumetry, measurements of the volumes of protons with restricted mobility (DWI) and of tissue with abnormally permeable microvasculature (DCE) more accurately approximate true tumour volume because of the biological differences between tumours and normal cellular systems.
Our finding that DWI volumetry after neoadjuvant treatment correlates with histopathological tumour regression aligns well with prior investigations, in particular with those of Ha et al. [11] and Curvo-Semedo et al. [25], who also found that DWI volumetry performed better in the detection of pCR than did volumetry on T2-weighted sequences or ADC values. These results were recently verified in a bi-institutional study [38]. To our knowledge, the use of DCE-MRI tumour volumetry for assessment of rectal cancer response to chemoradiation has not previously been studied, and represents new information regarding rectal cancer response assessment. Since DCE-MRI volumetry performed well in this study and demonstrated reproducibility across both readers, the authors believe this parameter deserves to be included in future investigations and validated in a prospective trial.
As noted above, the parameters ADC and Ktrans, which can be calculated from the volumetric measurements on DW and DCE sequences, respectively, did not perform well in our study. Prior results achieved with these parameters have been heterogeneous and somewhat contradictory [39]. Some authors have reported correlations between low pre-treatment ADC values and/or the change of ADC values through treatment and good histopathological response [8, 15, 31], while others have reported associations between low pre-treatment ADC values and more aggressive tumours [9, 14] or could not find any associations between ADC values and tumour response or pCR status at all [25]. Similarly, some authors have reported an association between post-treatment values of Ktrans and pCR status [23], while others were unable to identify the same association [24]. The reasons for these contradictory results may lie in differences in methodological aspects of the studies, such as the selection of different b-values and models to calculate ADC from DW-MRI and the use of different temporal resolutions, different pharmacokinetic models and different methods for calculating the arterial input function (based on individual measurements or a population-based prediction) for DCE-MRI.
The fact that volumetric tumour assessment on T2-weighted imaging and mrTRG assessment correlated well with tumour regression for one reader but not the other highlights the possibility for discrepancies in tumour assessment between readers – even amongst experienced readers as in this study. The reproducibility of measured MRI parameters is of great importance, in particular, if these parameters are to be used in a clinical setting, for example, to distinguish patients with histopathological complete response from those without. Therefore, our results should be regarded as a preliminary assessment to identify a group of parameters for which a more detailed investigation, possibly in a prospective trial with multiple readers of differing levels of experience, is warranted. The addition of DCE-MRI volumetry to previously investigated parameters might be of particular value, as this new parameter not only correlated well with tumour regression, but also demonstrated superior inter-reader agreement. The issue of reproducibility extends to the different histopathological grading systems that are in use – in particular, since recent investigations have questioned both their reproducibility and their clinical use [40, 41]. The grading system used in this study [34] allows for the pathologist to specify a percentage tumour regression, which resulted in a higher inter-reader reproducibility [35] compared to other (e.g. Dworak or Mandard) scoring systems. However, the known difficulties in the histopathological tumour assessment after chemoradiation warrant further investigations into the reproducibility of this parameter.
Our study has limitations: First, we applied strict inclusion criteria to assemble a patient cohort as homogenous as possible. However, the fact that only 24 patients were included in this study limits the generalizability of our results. In addition, the total of only four patients with complete pathological response was too small to allow for more detailed statistical sub-analysis of results for this clinically important group. Secondly, due to our retrospective study design, minor deviations in MRI protocols (i.e. b-values in DW imaging) could not be accounted for and might have influenced our results. Finally, though we found significant associations between post-therapeutic tumour volume on DCE-MRI and regression on histopathology, and good inter-reader agreement for this parameter, a cut-off value is needed for this parameter, so it can be used in clinical practice (e.g. to identify patients with pCR). However, the establishment of such a threshold requires a trial with a higher number of patients and was not possible in our study.
In conclusion, our study demonstrated that DCE-MRI tumour volumetry on post-treatment examinations correlated well with histopathological tumour regression and showed better inter-observer agreement than tumour volumetry on DW imaging. Therefore, this parameter should be incorporated into the assessment of tumour response to neoadjuvant treatment of rectal cancer in future prospective trials.
References
Habr-Gama A (2006) Assessment and management of the complete clinical response of rectal cancer to chemoradiotherapy. Colorectal Dis 8(Suppl 3):21–24
Beets-Tan RG, Beets GL (2011) Local staging of rectal cancer: a review of imaging. J Magn Reson Imaging 33(5):1012–1019
Barbaro B, Fiorucci C, Tebala C, Valentini V, Gambacorta MA, Vecchio FM et al (2009) Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology 250(3):730–739
Kuo LJ, Chern MC, Tsou MH, Liu MC, Jian JJ, Chen CM et al (2005) Interpretation of magnetic resonance imaging for locally advanced rectal carcinoma after preoperative chemoradiation therapy. Dis Colon Rectum 48(1):23–28
Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P et al (2011) Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 29(28):3753–3760
Patel UB, Brown G, Rutten H, West N, Sebag-Montefiore D, Glynne-Jones R et al (2012) Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer. Ann Surg Oncol 19(9):2842–2852
Kluza E, Rozeboom ED, Maas M, Martens M, Lambregts DM, Slenter J et al (2013) T2 weighted signal intensity evolution may predict pathological complete response after treatment for rectal cancer. Eur Radiol 23(1):253–261
Barbaro B, Vitale R, Valentini V, Illuminati S, Vecchio FM, Rizzo G et al (2012) Diffusion-weighted magnetic resonance imaging in monitoring rectal cancer response to neoadjuvant chemoradiotherapy. Int J Radiat Oncol Biol Phys 83(2):594–599
Curvo-Semedo L, Lambregts DM, Maas M, Beets GL, Caseiro-Alves F, Beets-Tan RG (2012) Diffusion-weighted MRI in rectal cancer: apparent diffusion coefficient as a potential noninvasive marker of tumor aggressiveness. J Magn Reson Imaging 35(6):1365–1371
Elmi A, Hedgire SS, Covarrubias D, Abtahi SM, Hahn PF, Harisinghani M (2013) Apparent diffusion coefficient as a non-invasive predictor of treatment response and recurrence in locally advanced rectal cancer. Clin Radiol 68(10):e524–e531
Ha HI, Kim AY, Yu CS, Park SH, Ha HK (2013) Locally advanced rectal cancer: diffusion-weighted MR tumour volumetry and the apparent diffusion coefficient for evaluating complete remission after preoperative chemoradiation therapy. Eur Radiol 23(12):3345–3353
Jung SH, Heo SH, Kim JW, Jeong YY, Shin SS, Soung MG et al (2012) Predicting response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer: diffusion-weighted 3 Tesla MR imaging. J Magn Reson Imaging 35(1):110–116
Kim SH, Lee JM, Hong SH, Kim GH, Lee JY, Han JK et al (2009) Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology 253(1):116–125
Lambrecht M, Vandecaveye V, de Keyzer F, Roels S, Penninckx F, van Cutsem E et al (2012) Value of diffusion-weighted magnetic resonance imaging for prediction and early assessment of response to neoadjuvant radiochemotherapy in rectal cancer: preliminary results. Int J Radiat Oncol Biol Phys 82(2):863–870
Sun YS, Zhang XP, Tang L, Ji JF, Gu J, Cai Y et al (2010) Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion-weighted MR imaging for early detection of tumor histopathologic downstaging. Radiology 254(1):170–178
van der Paardt MP, Zagers MB, Beets-Tan RGH, Stoker J, Bipat S (2013) Patients Who Undergo Preoperative Chemoradiotherapy for Locally Advanced Rectal Cancer Restaged by Using Diagnostic MR Imaging: A Systematic Review and Meta-Analysis. Radiology 269(1):101–112
Cai P, Wu Y, An X, Qiu X, Kong L, Liu G et al (2014) Simple measurements on diffusion-weighted MR imaging for assessment of complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol 24(11):2962–2970
de Lussanet QG, Backes WH, Griffioen AW, Padhani AR, Baeten CI, van Baardwijk A et al (2005) Dynamic contrast-enhanced magnetic resonance imaging of radiation therapy-induced microcirculation changes in rectal cancer. Int J Radiat Oncol Biol Phys 63(5):1309–1315
Devries AF, Griebel J, Kremser C, Judmaier W, Gneiting T, Kreczy A et al (2001) Tumor microcirculation evaluated by dynamic magnetic resonance imaging predicts therapy outcome for primary rectal carcinoma. Cancer Res 61(6):2513–2516
Devries AF, Kremser C, Hein PA, Griebel J, Krezcy A, Ofner D et al (2003) Tumor microcirculation and diffusion predict therapy outcome for primary rectal carcinoma. Int J Radiat Oncol Biol Phys 56(4):958–965
Dinter DJ, Horisberger K, Zechmann C, Wenz F, Brade J, Willeke F et al (2009) Can dynamic MR imaging predict response in patients with rectal cancer undergoing cetuximab-based neoadjuvant chemoradiation? Onkologie 32(3):86–93
Oberholzer K, Menig M, Pohlmann A, Junginger T, Heintz A, Kreft A et al (2012) Rectal cancer: Assessment of response to neoadjuvant chemoradiation by dynamic contrast-enhanced MRI. J Magnet Reson Imaging 38(1):119–126
Gollub MJ, Gultekin DH, Akin O, Do RK, Fuqua JL, Gonen M et al (2012) Dynamic contrast enhanced-MRI for the detection of pathological complete response to neoadjuvant chemotherapy for locally advanced rectal cancer. Eur Radiol 22(4):821–831
Lim JS, Kim D, Baek SE, Myoung S, Choi J, Shin SJ et al (2012) Perfusion MRI for the prediction of treatment response after preoperative chemoradiotherapy in locally advanced rectal cancer. Eur Radiol 22(8):1693–1700
Curvo-Semedo L, Lambregts DM, Maas M, Thywissen T, Mehsen RT, Lammering G et al (2011) Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy--conventional MR volumetry versus diffusion-weighted MR imaging. Radiology 260(3):734–743
Nougaret S, Rouanet P, Molinari N, Pierredon MA, Bibeau F, Azria D et al (2012) MR volumetric measurement of low rectal cancer helps predict tumor response and outcome after combined chemotherapy and radiation therapy. Radiology 263(2):409–418
Nougaret S, Fujii S, Addley HC, Bibeau F, Pandey H, Mikhael H et al (2013) Neoadjuvant chemotherapy evaluation by MRI volumetry in rectal cancer followed by chemoradiation and total mesorectal excision: Initial experience. J Magn Reson Imaging 38(3):726–732
Benson AB, Bekaii-Saab T, Chan E, Chen Y, Choti MA, Cooper HS et al (2012) Rectal cancer. J Natl Compr Cancer Netw 10(12):1528–1564
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47(1):207–214
Kim SH, Lee JY, Lee JM, Han JK, Choi BI (2011) Apparent diffusion coefficient for evaluating tumour response to neoadjuvant chemoradiation therapy for locally advanced rectal cancer. Eur Radiol 21(5):987–995
Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10(3):223–232
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675
Shia J, Guillem JG, Moore HG, Tickoo SK, Qin J, Ruo L et al (2004) Patterns of morphologic alteration in residual rectal carcinoma following preoperative chemoradiation and their association with long-term outcome. Am J Surg Pathol 28(2):215–223
Trakarnsanga A, Gönen M, Shia J, Nash GM, Temple LK, Guillem JG et al (2014) Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst 106(10)
Lin LI (1989) A concordance correlation coefficient to evaluate reproducibility. Biometrics 45(1):255–268
Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C et al (2012) Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol Off J Am Soc Clin Oncol 30(16):1926–1933
Lambregts, Doenja MJ, Rao S, Sassen S, Martens MH, Heijnen LA, Buijsen J et al (2014) MRI and Diffusion-Weighted MRI Volumetry for Identification of Complete Tumor Responders After Preoperative Chemoradiotherapy in Patients With Rectal Cancer: A Bi-institutional Validation Study. Ann Surg. doi:10.1097/SLA.0000000000000909
Hötker AM, Garcia-Aguilar J, Gollub MJ (2014) Multiparametric MRI of rectal cancer in the assessment of response to therapy: a systematic review. Dis Colon Rectum 57(6):790–799
Chetty R, Gill P, Govender D, Bateman A, Chang HJ, Deshpande V et al (2012) International study group on rectal cancer regression grading: interobserver variability with commonly used regression grading systems. Hum Pathol 43(11):1917–1923
Vallbohmer D, Bollschweiler E, Brabender J, Wedemeyer I, Grimminger PP, Metzger R et al (2012) Evaluation of histological regression grading systems in the neoadjuvant therapy of rectal cancer: do they have prognostic impact? Int J Color Dis 27(10):1295–1301
Acknowledgments
The authors thank Ada Muellner, MS for editing the manuscript. The research of Kaitlin M. Woo and Mithat Gönen was partly supported by NIH/NCI Cancer Center Support Grant P30 CA008748. The scientific guarantor of this publication is Dr. Marc J. Gollub. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. Mithat Gönen PhD and Kaitlin M. Woo MS kindly provided statistical advice for this manuscript. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. No study subjects or cohorts have been previously reported. Methodology: retrospective, experimental, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hötker, A.M., Tarlinton, L., Mazaheri, Y. et al. Multiparametric MRI in the assessment of response of rectal cancer to neoadjuvant chemoradiotherapy: A comparison of morphological, volumetric and functional MRI parameters. Eur Radiol 26, 4303–4312 (2016). https://doi.org/10.1007/s00330-016-4283-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4283-9