Abstract
Purpose
This study aimed at assessing the usefulness of flat detector cone-beam CT virtual navigation-guided transthoracic needle biopsy of small (≤2.0 cm) pulmonary nodules in terms of diagnostic accuracy and complications to evaluate the diagnostic performance and complications of small pulmonary nodules under cone-beam CT (CBCT) guidance.
Materials and methods
A total of 100 patients with 100 solid lung nodules were retrospectively enrolled to undergo transthoracic needle biopsy (TNB) procedures. The mean diameter of lesions was 1.25 cm ± 0.39 (range 0.50–2.00 cm). The needle path was carefully planned and calculated on the CBCT virtual navigation guidance system, which acquired 3D CT-like cross-sectional images. Diagnostic performance, complication rate, and patient radiation exposure were investigated.
Results
The technical success rate of TNB under iGuide CBCT virtual navigation system was 99 % (99/100). The sensitivity, specificity, and accuracy of TNB of small nodules under iGuide CBCT virtual navigation guidance were 98.7 % (77/78), 90.5 % (19/21), and 97.0 % (96/99), respectively. The number of pleural passages with coaxial needle, biopsies, and CBCT acquisitions were 1.09 ± 0.32, 1.20 ± 0.47, and 3.06 ± 1.35, respectively. Complications occurred in 22 (22 %) cases. The mean total procedure time was 12.84 min ± 3.74, resulting in a mean exposure dose of 7.6 mSv ±3.1.
Conclusions
Flat detector Cone-beam CT-guided TNB is a highly accurate and safe diagnostic method for small (≤2.0 cm) lung nodule.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Transthoracic needle biopsy (TNB) is a well-established and commonly used procedure in the work-up of pulmonary nodules, regardless of nodule size [1, 2]. Although TNB can be performed with fluoroscopic or ultrasonographic guidance, CT and CT fluoroscopy-guided TNB have been widely performed in the diagnosis of pulmonary nodules [3]. However, conventional CT guidance has limitations in the lack of real-time monitoring and gantry tilting for a more accessible needle pathway to the target lesion [4–6]. To overcome the disadvantages of conventional CT guidance, CT fluoroscopy has been introduced, and this modality enables real-time monitoring of target lesions and gantry tilting [7]. Hiraki et al. [4] showed the overall accuracy of CT fluoroscopy-guided PTNB was 95.2 %. Nevertheless, limitations include the small gantry bore, radiation exposure to operators, and limited imaging plane orientation [8, 9].
Along with the development of CBCT, a novel technique for TNB guidance recently emerged. It combines advanced needle path planning with 3D CBCT images. The CBCT systems offer real-time visualization of TNB procedures and more flexibility in the orientation of the detector system around the patient compared to traditional CT systems [11, 12]. Therefore, the TNB procedures assisted by advanced 3D needle guidance systems can be performed in a sterile workspace with flexible system angulation capability as well as instantaneous fluoroscopic feedbacks [13]. This technique offers high spatial resolution of less than 1 mm, as well as contrast resolution of 10 HU, which is adequate for lung imaging as lung inherently has a high contrast (soft tissue against air). In August 2009, Artis zeego, the multi-axis system based on robotic technology from Siemens was introduced into our department, where was the first installation unit in China. With its high image quality and its advanced applications such as syngo DynaCT, syngo iPilot and syngo iGuide help to improve the workflow. This paper describes our preliminary experience with TNB for small (≤2.0 cm) pulmonary nodules biopsy under iGuide CBCT guidance system.
Materials and methods
Patients
This retrospective study was approved by the institutional review board of The First Affiliated Hospital of Zhengzhou University, and additional informed consent was obtained from all individual participants for whom identifying information is included in this article. From January 2010 to January 2014, a total of 100 patients with 100 solid pulmonary nodules were retrospectively enrolled to undergo TNB procedures. 100 consecutive patients (69 males and 31 females, mean age 53.08 ± 13.23 years; age range 19–88 years) with CT-confirmed solid pulmonary lesions (≤2.0 cm) were retrospectively enrolled in this study. The lesion size was recorded as the maximum diameter in the image data by one chest radiologist (Han XW, 30 years of experience in image guided TNB).
Image acquisition
As the first step, 3D CBCT images of the patients were acquired with a rotational angiographic system (Artis Zeego, 30 × 40 cm FD detector, Siemens Healthcare, Forchheim Germany). During the image acquisition, the region of interest was positioned in the isocenter and the C-arm rotated 200 degrees in 8 s. As an output, a total number of 397 projection images were generated with an X-ray dose of 0.36 μGy/frame, projection increment 0.5°, 1-k matrix, zoom factor 0, and field of view 480 mm. With the detector used, the imaged volume covered had a cylindrical shape with a height of 185 mm, a diameter of 225 mm, and a voxel size of 0.4 mm. The patients stopped breathing for the entire 8 s 3D CBCT image acquisition.
The resulting raw projection images were then automatically transferred to a workstation (Syngo X Workplace, Siemens Healthcare) for 3D volume reconstruction. As a result, the CBCT images were reconstructed with 5 mm thickness and presented in axial, sagittal and coronal orientations. The time from the end of the data acquisition to the presentation of multiplanar images on the workstation ranged between 43 and 45 s.
Needle path planning and guidance procedure
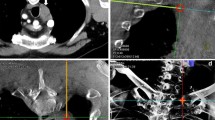
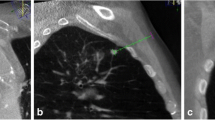
As the second step, the needle path was planned on the same workstation using commercially available software (Syngo iGuide, Siemens Healthcare). Figures 1, 2, 3 and 4 demonstrated this procedure for a 0.5-cm in diameter pulmonary lesion, and Figs. 5, 6, 7, and 8 demonstrated this procedure for a 2.0-cm in diameter pulmonary lesion. The reconstructed 3D volume was first loaded. In the orthogonal multiplanar images, the skin entry point and target lesion positions were manually selected and marked by a cross and a circle, respectively. A virtual path was then generated with its angulations and length calculated and displayed. All three multiplanar images were automatically aligned to the defined path to provide in-plane views (Figs. 2, 6). This procedure could be iteratively performed, modified, and reviewed until a satisfying path was obtained.
CBCT orthogonal multiplanar images with graphics showing planned needle path (yellow line) into target lesion (red circle) (a–c). The cross indicated the skin entry site and the circle indicated the target lesion site. The needle position relative to the anatomical structures was displayed in 3D using volume rendering technique (d)
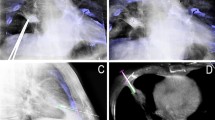
The laser navigation system on the Flat-panel can quickly locate the needle point on skin, Thus the skin entry point could be determined (a). Real-time fluoroscopic images in bull’s eye view (b) and progression views (c). The needle was advanced along the planned needle path (dotted line) from skin entry site (white cross) to target lesion site (white circle). Sufficient tissue sample was obtained (d)
CBCT orthogonal multiplanar images with graphics showing planned needle path (yellow line) into target lesion (red circle) (a–c). The cross indicated the skin entry site and the circle indicated the target lesion site. The needle position relative to the anatomical structures was displayed in 3D using volume rendering technique (d)
In order to use the planned path to align the needle in actual 3D space, the virtual path was then projected and superimposed onto the live fluoroscopic images and displayed on a dedicated live monitor (Figs. 3b, c, 7a, b). The software automatically calculated the C-arm angulations, table motion, image zoom, and then controlled the C-arm moving to reach the desired position. First, the C-arm rotated to the Bull’s Eye View, where the C-arm was angulated in the way that the cross and the circle displayed on the live monitor completely matched and the central X-ray beam was aligned with the planned path (Figs. 3b, 7b). The laser navigation system on the Flat-panel can quickly locate the needle point on skin, Thus the skin entry point could be determined (Fig. 3a). The needle orientation was adjusted until both the tip and hub of the needle in the fluoroscopic image were superimposed and located at the centre of the circle and the cross. Second, after the skin entry point and needle orientation were determined, the needle was advanced under fluoroscopy until the planned target lesion position was reached. The C-arm was rotated back and forth to two different angles subsequently to monitor the needle progression. These two angles provided lateral views (progression view) of the planned needle path and helped to ensure that the needle was advanced along it (Figs. 3c, 7b). Third, a 3D scan was acquired to confirm the final position of the needle (Figs. 4, 8).
PTNB technique
TNBs were performed by or under the supervison of one chest radiologist (Han XW, 30 years of experience in image guided TNB). The patient was placed in either supine or prostrate position and local anaesthesia was given (1 % lidocaine, ≤10 ml). A 16-gauge needle (Quick-Core, Cook Medical Inc., Bloomington, IN, USA) was advanced along the planed path under real-time fluoroscopy. For sampling, the stylet was removed from the guiding needle and replaced by a biopsy needle, and then approximately 0.3–2.0 cm of sample tissue was taken. A cytopathologist was not present, and our criterion was that the total samples; length was more than 0.3 cm to meet the needs of the pathological sections. This procedure could be repeated until sufficient tissue sample was obtained. After sufficient tissue samples were obtained, the coaxial introducer was removed with a position change by placing the patient biopsy side down to reduce the complication rate of pneumothorax [14]. Thereafter, post procedure CT images were acquired to identify procedure-related complications. The patients remained in the hospital for 24 h for observation after the procedure. In case serious symptoms in vital signs or clinical status occurred, repeated imaging and if necessary treatment was performed.
Post-biopsy evaluation
After obtaining sufficient tissue, the coaxial introducer was removed. Post-biopsy complications were investigated with post-procedural CBCT.
Procedural records
We recorded several factors during TNB: the patients’ positions during PTNB, the number of pleural passages, the number of biopsies, the number of CT acquisitions, and total procedure time (defined as the duration from local anaesthesia injection to the end of post-procedure CBCT). Technical success was defined as appropriate location of the coaxial needle within target nodules on procedural CBCT images and adequate tissue sampling on visual inspection. We recorded the total coaxial introducer dwelling time. We also recorded radiation exposure dose during the entire procedure (fluoroscopy dose and CBCT dose), which was given by the SIEMENS zeego angiography series after the interventional procedure.
Pathological results, diagnostic accuracy
The pathological reports of biopsy specimens, surgical specimens, or follow-up images were reviewed to evaluate the final diagnosis of target nodules. Final diagnosis was confirmed in four ways: (1) if the patient underwent surgical resection, the pathological report decided the final diagnosis; (2) if the pathological result of biopsy demonstrated a malignant or a specific benign pathology such as hamartoma or tuberculosis, it was accepted as the final diagnosis; (3) in the cases of non-specific benign pathology (negative for malignancy, chronic inflammation, etc.), follow-up CT helped to decide whether the lesion would be truly or falsely benign, or indeterminate. If the lesion decreased 20 % or more in diameter, we considered the final diagnosis as benign [15]. (4) If a nodule of nonspecific benign pathology did not show sufficient interval decrease in size nor had follow-up images, its final diagnosis was defined as indeterminate. Any PTNB-related complication such as pneumothorax or haemoptysis was also recorded. Pneumothorax was evaluated with post-procedural CBCT and follow-up chest radiographs during the hospitalization.
All data analyses were performed using Excel 2010 (Microsoft, Redmond, WA) and SPSS software (version 13.0; SPSS, Chicago). Numeric data are reported as the mean ± standard deviation.
Results
Nodule characteristics, procedural records and radiation doses
The mean size of nodules was 1.25 cm ± 0.39, ranging from 0.50 to 2.00 cm. Among the 100 nodules, 97 nodules were solid and three ground-glass nodules (GGNs). 65 nodules were located in the right lobe and 35 nodules in the left lobe. The patients underwent TNB in the supine position in 69 cases and in the prone position in 31 cases. The number of pleural passages with coaxial needle, biopsies and CBCT acquisitions was 1.09 ± 0.32 (range 1–3), 1.20 ± 0.47 (range 1–3), and 3.06 ± 1.35 (range 2–8), respectively. Mean total procedure time was 12.84 min ± 3.74 (range 6–20 min). The technical success rate of TNB under iGuide CBCT virtual navigation system was 99.0 % (99/100). One patient with severe chronic obstructive pulmonary disease and a 0.78-cm lesion developed severe pneumothorax with a volume of approximately 50 % during the TNB procedure. This patient was not get adequate tissue sampling on visual inspection and was treated with chest tube drainage due to severe difficulty breathing. We recorded the case as technical failure. The total coaxial introducer dwelling time in our study was 8.2 min ± 3.4. The overall procedural time ranged from 6 to 20 min, resulting in a mean exposure dose of 7.6 mSv ± 3.1.
Pathological results, diagnostic accuracy and complications
Detailed pathological results are listed in Table 1. Among the 99 nodules, 76 nodules were malignant and 21 benign. The 76 malignant lesions consisted of lung adenocarcinomas (n = 48), squamous cell carcinomas (n = 15), small cell lung cancers (n = 10), metastases (n = 2), mesothelioma of pleura (n = 2) and pulmonary blastoma (n = 1). The 21 malignant lesions consisted of lung inflammations (n = 7), tuberculosis (n = 5), hamartoma (n = 2) and carcinoid (n = 2). There were three patients misdiagnosed. One patient’s initial biopsy results were squamous cell carcinoma (nodule size = 1.0 cm), but was later confirmed as an adenosquamous carcinoma by post-operative pathological outcome. The initial biopsy of the other two patients was interpreted as lung inflammation. Both nodules (nodule size = 0.79 cm and 1.66 cm) were enlarged more than 20 % in size on follow-up chest CT after 2 months. TNB procedures were performed again and the both patients were confirmed with lung adenocarcinoma.
Finally, the sensitivity, specificity and accuracy of TNB of very small nodules under the Siemens Artis Zeego iGuide CBCT virtual navigation guidance were 98.7 % (77/78), 90.5 % (19/21) and 97.0 % (96/99), respectively. One patient with severe chronic obstructive pulmonary disease and a 0.78-cm lesion developed severe pneumothorax with a volume of approximately 50 % during the TNB procedure. This patient was not get adequate tissue sampling on visual inspection and was treated with chest tube drainage due to severe difficulty breathing. We recorded the case as technical failure. Another patient with ground-glass nodule developed post-operative pneumothorax with volume about 30 % and hence underwent chest tube drainage. There were other eight patients with pneumothorax volume below 5 %, and no specific treatment was given as the patients were asymptomatic and clinically stable. Post-operative hemoptysis occurred in 12 patients, but the symptom was self-limiting and disappeared within 3 days.
Discussion
With the increasing utilisation of chest CT in clinical practice as well as in lung cancer screenings, early lung cancers manifesting as small nodules have been detected more frequently than ever before. So, the need for accurate diagnosis of these small lung nodules with a high suspicion of malignancy is evident [16, 17].
Initial experiences suggested that CBCT-guided TNB could be a useful, safe and accurate procedure for diagnosis of indeterminate lung nodules, with possible advantages over fluoro-CT-guided biopsy [10, 18]. It is well known that limited access due to a closed CT gantry increases the difficulty of TNB and increases radiation exposure for the operator, whereas the greater working space provided by the C-arm cone-beam system facilitates needle placement [10, 19]. The CBCT system offers advanced needle planning under real-time needle guidance, using a combination of 3D images and fluoroscopy, with good angulation and rotation. Another advantage of CBCT is the improved resolution of images, which have superior contrast, without distortion, compared to fluoro-CT images [20]. Moreover, under CT guidance the needle path can be followed only on a single plane during needle insertion, a limitation in comparison with the 3D images offered by CBCT [10].
Our study shows that the technical success rate of TNB for small nodule (≤2.0 cm) under iGuide Cone-beam CT virtual navigation system was 99.0 %, and diagnostic accuracy rate of 97.0 %. We observed 1 (1 %) case of technical failure in our study, whereas Hiraki et al. [4] observed six (0.6 %). In consideration of the previously mentioned diagnostic performance and technical failure rate of TNBs, we believe that CBCT-guided TNB is a highly accurate diagnostic method for use with pulmonary lesions [4, 21]. Previously, Ohno et al. [1] and Wallace et al. [13] reported the accuracy of CT-guided TNB of small nodules as only 52 and 88 %, respectively. And even in the case of CT-fluoroscopy-guided PTNB, the diagnostic accuracy of these small nodules has been reported to be 92.7 % [22]. In our study diagnostic accuracy was 97.0 % for lesions 2.0 cm or smaller. This compares favourably with the Hiraki et al. [4] and JY Choo et al. studies [17], in which diagnostic accuracy was 92.7 and 98.0 %, respectively. So, flat detector C-arm CT-guided TNB is a highly accurate and safe diagnostic method for small (≤2.0 cm) lung nodule. In fact, Chiara et al. [23] reported that lesion size and safety of the method showed no significant differences between two groups of patients divided by lesion size (≤3 and >3 cm). We believe that the advantages of cone-beam CT guidance, such as real-time imaging guidance and great flexibility in entry site selection, can contribute to high diagnostic accuracy in small lesions. The “bull’s eye view” provided by the real-time fluoroscopy capability of the CBCT virtual navigation system aligns the detector, the skin entry site and the target, contributing to a more accurate and safer biopsy. As an example, this system can help us avoid adjacent structures such as the rib, bronchus or vessels. Furthermore, the total coaxial introducer dwelling time in our study was only 8.2 min ± 3.4, which was shorter than that of TNB under CT-fluoroscopy guidance in another previous study (10.9 ± 8.2 min) [6] and shorter than that under conventional CT guidance (mean 14.4–23.8 min) [24, 25].
The pneumothorax rate 10 % (10/100) was found to be lower than CT or CTF-guided TNB procedure. With respect to TNB-related complications, pneumothorax has been reported to occur in approximately 25–40 % of overall lung biopsy cases [21]. The incidence of TNB-related pneumothorax was low in our study. We believe that this low incidence of pneumothorax was possible as the virtual navigation system enabled us to select a safer and more accurate targeting route in navigating the needle approach to the target. In addition, the coaxial needle technique also played a significant role in the reduction of complications by avoiding repeated pleural punctures or passages. Of the ten patients who had pneumothorax, five patients (50 %) had pulmonary emphysema along the needle pathway, and this may suggest that considering emphysema in the needle pathway would be more likely to lead to the occurrence of pneumothorax. Post-operative hemoptysis occurred in 12 patients, but the symptom was self-limiting and disappeared within 3 days. Interestingly, Among the 100 nodules, There patients with ground-glass nodules all developed post-operative haemoptysis. Maybe ground-glass feature of nodules significantly increased the haemoptysis rate. We think that the relatively loose compactness of these nodules may have negatively affected the natural compression of injured tissue after biopsy and that patent airways and blood vessels within the lesion could also increase the risk of haemoptysis after cutting needle biopsy.
There were three cases (3.0 %) misdiagnosed in the study. One patients’ initial biopsy results were squamous cell carcinoma (nodule size = 1.0 cm), but was later confirmed as an adenosquamous carcinoma by post-operative pathological outcome. The initial biopsy of the other two patients was interpreted as lung inflammation. Both nodules (nodule size = 0.79 and 1.66 cm) were enlarged by more than 20 % in size on follow-up chest CT after 2 months. TNB procedures were performed again and both the patients were confirmed with lung adenocarcinoma. A negative result always poses a diagnostic challenge. A non-specific benign result does not exclude malignancy, and further evaluation is required. The lesion may be malignant, but the sample obtained may lie outside the nodule borders or come from a necrotic area, thus preventing pathologists from establishing a correct diagnosis. Clinical and radiographic follow-up are warranted. If further growth occurs after a non-specific benign diagnosis is obtained with TNB, repeat biopsy or resection is indicated [26].
In terms of radiation dose, a mean exposure dose was 7.2 mSv ± 3.1. In the study by Chiara et al. [23] that dealt with pulmonary nodules with C-arm cone-beam CT, the total dose was equal to 11.62 mSv. Braak et al. [27] performed 92 biopsies in 88 patients with a similar guidance and reported a dose value equal to 9.95 mSv. On the basis of our results and the data from the literature regarding lung biopsies performed with CBCT guidance, despite the limited amount of currently available data and the lack of homogeneity, a slight reduction in dose could be hypothesised with the use of iGuide guidance. It could reduce the total dose delivered because it can reduce the number of CBCT scans. While, in the Hwang et al. study [15] that dealt with cone-beam CT-guided biopsy, the mean effective dose was 4.6 mSv. Less dose (comparing with 7.2mSv in our study) can be explained that we usually performed three CBCT scans per case at least, whereas Hwang et al. [15] performed cone-beam CT scans only twice and did not perform a postprocedural cone-beam CT scan. Radiation dose in our study is similar to that of standard chest CT (7 mSv) [28], the radiation dose of cone-beam CT guided biopsy may not be a substantial problem, although a continuous effort must be made to reduce the radiation dose through use of a small field of view or collimation.
The limitations of this technique should be also mentioned. As patient movements may result in motion artr, facts and affect the registration accuracy of the projected path, the key factor for achieving successful needle guidance is to generate high-quality 3D CBCT images. To this aim, during the imaging procedure, the patient should be well stabilized. However, this requirement may not be fulfilled and limits its applications for some patients who have difficulty sustaining a breath hold for the duration of imaging. Another main limitation of the study is the small sample size (100 patients) that limits the statistical power.
In conclusion, flat detector C-arm CT-guided TNB is a highly accurate and safe diagnostic method for small (≤2.0 cm) lung nodule.
References
Ohno Y, Hatabu H, Takenaka D et al (2003) CT-guided transthoracic needle aspiration biopsy of small (< or =20 mm) solitary pulmonary nodules. Am J Roentgenol 180:1665–1669
de Jiao C, Li TF, Han XW et al (2014) Clinical applications of the C-arm cone-beam CT-based 3D needle guidance system in performing percutaneous transthoracic needle biopsy of pulmonary lesions. Diagn Interv Radiol 20:470–474
Geraghty PR, Kee ST, McFarlane G et al (2003) CT-guided transthoracic needle aspiration biopsy of pulmonarynodules: needle size and pneumothorax rate. Radiology 229:475–481
Hiraki T, Mimura H, Gobara H et al (2009) CT fluoroscopy-guided biopsy of 1,000 pulmonary lesions performed with 20-gauge coaxial cutting needles: diagnostic yield and risk factors for diagnostic failure. Chest 136:1612–1617
Kim GR, Hur J, Lee SM et al (2011) CT fluoroscopy-guided lung biopsy versus conventional CT-guided lung biopsy: a prospective controlled study to assess radiation doses and diagnostic performance. Eur Radiol 21:232–239
Braak SJ, van Strijen MJ, van Leersum M et al (2003) Real-time 3D fluoroscopy guidance during needle interventions: technique, accuracy, and feasibility. Am J Roentgenol 194:445–451
Bissoli E, Bison L, Gioilis E et al (2003) Multislice CT fluoroscopy: technical principles, clinical applications and dosimetry. Radiol Med 106:201–212
Kirchner J, Kickuth R, Laufer U et al (2003) CT fluoroscopy-assisted puncture of thoracic and abdominal masses: a randomized trial. Clin Radiol 57:188–192
Yamagami T, Kato T, Iida S et al (2004) Percutaneous needle biopsy for small lung nodules beneath the rib under CT scan fluoroscopic guidance with gantry tilt. Chest 126:744–747
Jin KN, Park CM, Goo JM et al (2010) Initial experience of percutaneous transthoracic needle biopsy of lung nodules using C-arm cone-beam CT systems. Eur Radiol 20:2108–2115
Sang ML, Chang MP, Kyung HL et al (2013) C-arm cone-Beam CT-guided Percutaneous Transthoracic needle Biopsy of lung nodules: clinical experience in 1108 patients. Radiology 271:291–300
Kalender WA, Kyriakou Y (2007) Flat-detector computed tomography (FD-CT). Eur Radiol 17:2767–2779
Wallace MJ, Kuo MD, Glaiberman C et al (2009) Three-dimensional C-arm cone-beam CT: applications in the interventional suite. J Vasc Interv Radiol 20:S523–S537
O’Neill AC, McCarthy C, Ridge CA et al (2012) Rapid needle-out patient-rollover time after percutaneous CT-guided transthoracic biopsy of lung nodules: effect on pneumothorax rate. Radiology 262:314–319
Hwang HS, Chung MJ, Lee JW et al (2010) C-arm cone-beam CT-guided percutaneous transthoracic lung biopsy: usefulness in evaluation of small pulmonary nodules. Am J Roentgenol 195:400–407
Henschke CI, McCauley DI, Yankelevitz DF et al (1999) Early lung cancer action project: overall design and findings from baseline screening. Lancet 354:99–105
Ji YC, Chang MP, Nyoung KL et al (2013) Percutaneous transthoracic needle biopsy of small (≤1 cm)lung nodules under C-arm cone-beam CT virtual navigation guidance. Eur Radiol 23:712–719
Carrafiello G, Fontana F, Mangini M et al (2012) Initial experience with percutaneous biopsies of bone lesions using XperGuide cone-beam CT (CBCT): technical note. Radiol Med 117:1386–1397
Gupta R, Cheung AC, Bartling SH et al (2008) Flat-panel volume CT: fundamental principles, technology, and applications. Radiographics 28:2009–2022
El-Sheik M, Heverhagen JT, Alfke H et al (2001) Multiplanar reconstructions and three-dimensional imaging (computed rotational osteography) of complex fractures by using a C-arm system: initial results. Radiology 221:843–849
Priola AM, Priola SM, Cataldi A et al (2010) Diagnostic accuracy and complication rate of CT-guided fine needle aspiration biopsy of lung lesions: a study based on the experience of the cytopathologist. Acta Radiol 51:527–533
Wallace MJ, Krishnamurthy S, Broemeling LD et al (2002) CT-guided percutaneous fine-needle aspiration biopsy of small (< or 10-cm) pulmonary lesions. Radiology 225:823–828
Chiara F, Alessandra M, Federico F et al (2014) C-arm cone-beam computed tomography needle path overlay for percutaneous biopsy of pulmonary nodules. Radiol Med 119:820–827
Carlson SK, Felmlee JP, Bender CE et al (2005) CT fluoroscopy-guided biopsy of the lung or upper abdomen with a breath-hold monitoring and feedback system: a prospective randomized controlled clinical trial. Radiology 237:701–708
Schaefer PJ, Schaefer FK, Heller M et al (2007) CT fluoroscopy guided biopsy of small pulmonary and upper abdominal lesions: Efficacy with a modified breathing technique. J Vasc Interv Radiol 18:1241–1248
Poulou LS, Tsagouli P, Ziakas PD et al (2013) Computed tomography-guided needle aspiration and biopsy of pulmonary lesions: a single-center experience in 1000 patients. Acta Radiol 54:640–645
Braak SJ, van Strijen MJ, van Es HW et al (2011) Effective dose during needle interventions: cone-beam CT guidance compared with conventional CT guidance. J Vasc Interv Radiol 22:455–461
Mettler FA Jr, Huda W, Yoshizumi TT et al (2008) Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008(248):254–263
Acknowledgments
This study was funded by the national high tech research and development program (863 Program) (Grant number: 2015AA020301).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Jiao, D., Yuan, H., Zhang, Q. et al. Flat detector C-arm CT-guided transthoracic needle biopsy of small (≤2.0 cm) pulmonary nodules: diagnostic accuracy and complication in 100 patients. Radiol med 121, 268–278 (2016). https://doi.org/10.1007/s11547-015-0604-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-015-0604-3