Abstract
Epidermal growth factor receptors (EGFR, HER2, HER3) activate signal transduction pathways involved in cancer proliferation, apoptosis, differentiation, metastasis, and angiogenesis. Their overexpression and activation are associated with unfavorable prognosis of cancer patients. Therefore, they are attractive targets for cancer therapy. Due to the development of drug resistance, therapeutic monoclonal antibodies and synthetic small molecule tyrosine kinase inhibitors directed against EGFR family members may fail with fatal consequences for cancer patients. Medicinal plants raised considerable interest during the past years as valuable resources to develop novel treatment therapies targeting epidermal growth factor receptors and their downstream signal transduction pathways. The present review gives an overview of isolated phytochemicals that inhibit these signaling routes. Inhibitors have been described that down-regulate the mRNA or protein expression of EGFR, HER2, or HER3 or inhibit the phosphorylation of these receptors and/or their downstream signaling kinases. Remarkably, a wealth of in vivo experiments complemented in vitro data, indicating that natural products are also active in living animals bringing this research concept closer to clinical applicability. The combination of receptor-inhibiting natural product with standard anticancer drugs frequently caused increased or even synergistic tumor inhibition in vitro and in vivo. It deserves further evaluation, if and how epidermal growth factor receptor-targeting natural products can be integrated into clinical oncology as well as to define their role for more tumor-specific and individualized tumor therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy regimens derived from results of clinical trials are valuable for determining optimal treatment options for large populations of patients. However, an individual patient’s response to chemotherapy can be very different from the predicted response of the average population, and the reasons for this variation are largely unknown. Several clinical and pathological factors have been identified as having prognostic value of treatment outcome and survival of cancer patients, e.g., tumor size, lymph node and distant metastasis, tumor grade, and, more recently, specific molecular biomarkers. These prognostic factors help to classify the standard risk of subpopulations of patients with the same tumor entity, but are still unable to predict the response of specific individuals to therapy. Therefore, there is an urgent need for reliable molecular tests to predict the individual patient’s risk of death from the disease irrespective of the treatment (prognostic markers) and sensitivity or resistance to chemotherapy (predictive markers). Such tests are necessary to develop individualized treatment schedules in the future. The field of chemotherapy is currently undergoing a paradigm shift from classical cytotoxic chemotherapy towards targeted therapy with the aim to eradicate tumor cells more efficiently with fewer side effects on normal tissue. Proteins encoded by genes carrying tumor-specific mutations serve as preferential targets for the development of novel drugs in cancer therapy.
It is now well accepted that mutations in three main types of genes contribute to carcinogenesis: oncogenes, tumor suppressor genes, and stability genes. Oncogene activation (gain-of-function mutation) results from point mutations, chromosomal translocation, or gene amplification. Without such mutations, tumors cannot grow (tumor addiction). On the other hand, mutations in tumor suppressor genes are loss-of-function mutations, e.g., missense mutations, chromosomal deletions or insertions, or epigenetic silencing, that allow the tumor to grow unchecked by normal cellular control mechanisms. Stability genes or caretakers including DNA repair genes controlling genomic stability and genes responsible for organizing mitotic recombination and chromosomal segregation. Inactivating mutations in these genes are dangerous because they increase the mutational rate in other genes. Out of the large number of potential targets for targeted chemotherapy, we focus on the epidermal growth factor receptor family in the present overview. Growth factor receptors have a tremendous relevance in cancer biology. Therefore, the therapeutic intervention to silence the function of epidermal growth factors and their related signaling pathways represents a highly attractive approach to improve treatment success of solid tumors.
Epidermal growth factor receptors in cancer biology
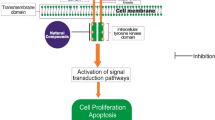
There are four human epidermal growth factor receptors (EGFR/ERBB1/HER1, HER2/ERBB2/c-neu, HER3, and HER4). After ligand binding, they activate downstream signaling routes, which regulate proliferation, differentiation, apoptosis, metastasis, and angiogenesis. Their 3D structures are represented in Fig. 1. EGFR and HER2 are over-expressed in many solid tumors, which is associated with unresponsiveness to chemo- and radiotherapy as well as short survival times of patients (see below) [1]. The heterodimer structure of EGFR/HER2 is depicted in Fig. 2. Thus far, 10 ligands have been identified, i.e., the epidermal growth factor family (EGF, transforming growth factor-α, β-cellulin, epiregulin, HB-EGF, AR) and the neuregulin family (heregulin, neuregulins) [2, 3]. Upon binding of a ligand to an EGFR monomer, homo-dimerization takes place with a second EGFR molecule or with another HER member. Similarly, HER2 can dimerize with HER3 or HER4 and HER3 with HER4. Ligand binding and dimerization leads to intracellular phosphorylation of HER receptors and thereby activation of the downstream signaling pathways. The existence of 10 ligands of different homo- and heterodimers consisting of four receptors create a considerable flexibility and complexity for signal transduction [2–4]. This complexity is even further increased by varying the duration and strength of receptor signaling, receptor internalization, and recycling as well as rates of phosphorylation and dephosphorylation [5].
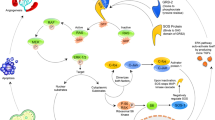
Dimerization stimulates intrinsic tyrosine kinase activity of EGFR, which regulates specific signal transduction cascades, e.g., Raf/Mek/Erk, PI3K/PDK1/Akt, PLCγ/PKC, MAPK, and JNK signaling routes. Constitutive EGFR activation as consequence of point mutations or gene amplification causes deregulated cellular processes such as proliferation, invasion, angiogenesis, cell motility, cell adhesion, inhibition of apoptosis, and DNA synthesis. The kinase activity is also associated with autophosphorylation of five tyrosine residues in the C-terminal EGFR domain. Mutations affecting EGFR expression foster carcinogenesis.
The extraordinary relevance of EGFR in tumor biology makes it an exquisite molecular target for tumor therapy. Apart from therapeutic antibodies, several small molecules have been developed as EGFR inhibitors [6]. For example, gefitinib (Iressa®; Astra Zeneca, DE, USA) and erlotinib (Tarceva®; OSI-774, Genentech Inc., CA, USA) are first-generation inhibitors used for the treatment of non-small cell lung cancer and other tumor types [7]. Both quinazolinamines exhibit their inhibitory activity by competing with ATP for the ATP binding pocket of EGFR.
Despite considerable successes with these EGFR tyrosine kinase inhibitors in cancer therapy, resistance against these chemical compounds develop due to the selection of point-mutated EGFR variants [8]. Therefore, there is an urgent need for the identification of novel EGFR tyrosine kinase inhibitors. In recent years, medicinal plants came into the center of interest as resources for novel treatment strategies to target EGFR family members.
Role of epidermal growth factor receptors for drug resistance and patient prognosis
EGFR-expressing cell lines
The connection between EGFR and classical cytotoxic drug resistance has been known for more than two decades. Murine sarcoma 180 (S180) cells selected for resistance towards doxorubicin overexpress EGFR compared to drug-sensitive wild-type S180 cells [9, 10]. As subsequently shown, EGFR expression also plays a role for drug resistance of tumor cells not previously selected treated with cytostatic drugs. Since kidney carcinomas are frequently unresponsive to chemotherapy, they represent a suitable model to study inherent drug resistance. EGFR expression of human primary cell cultures of renal cell carcinomas of 18 patients subjected to hierarchical cluster analyses showed that the expression of c-ErbB1 and c-ErbB2 was higher in resistant cell cultures compared to sensitive cell cultures [11]. EGFR is involved in drug resistance by affection of apoptosis, DNA repair, or the induction of resistance gene expression [12]. These in vitro results were translated to clinical tumors. Tumors with EGFR expression were significantly more frequent resistant to doxorubicin than EGFR negative or weakly expressing cancers [13, 14].
Glioblastoma multiforme (GBM) is the most aggressive form of adult human brain tumor [15]. Malignant gliomas often show resistance to adjuvant radio- and chemotherapy due to the accumulation of genetic alterations that cause oncogene activation, e.g., EGFR [16]. In most GBMs, amplification and rearrangement of the EGFR gene resulted in mutant receptors, called ΔEGFR (kinase-deficient mutant EGFR) that enhanced tumorigenicity in vivo, and caused cisplatin resistance [17].
In addition to cisplatin, EGFR also reduced the activity of microtubule poisons, i.e., vincristine and paclitaxel [18]. Combination treatment of human ΔEGFR-expressing GBM cells with EGFR-directed tyrosine kinase inhibitor and cisplatin synergistically induced apoptosis in vitro and in vivo [17, 18].
Furthermore, the combination treatment of a c-Met kinase inhibitor and either an EGFR kinase inhibitor or cisplatin enhanced cytotoxicity of mutant EGFR-expressing GBM cells [19]. Taken together, EGFR expression is associated with drug resistance in vitro making it an exquisite target for novel drugs inhibiting EGFR function. EGFR-mediated resistance is also known for cytotoxic natural products. Two interesting bioactive compounds derived from the Chinese coniferous tree Cephalotaxus hainanensis are cephalotaxine (CET) and its ester homoharringtonine (HHT) [20]. Although HHT possessed the highest growth inhibitory activity towards human leukemic cells [21], human ΔEGFR-expressing GBM cells were 14-fold more resistant to HHT than control cells [22]. These findings indicated a causative role of mutation-activated EGFR for cellular resistance towards CET and HHT. Similar results have been obtained for artesunate (ART), which is a semisynthetic derivative of artemisinin, the active principle of Artemisia annua L. This herb was traditionally used in Chinese medicine for the treatment of fever and chills. Nowadays, artemisinin is used as anti-malarial drug [23]. Artemisinin and its derivative ART also reveal profound anti-cancer activity [24–26]. In sum, drug resistance mediated by EGFR is not restricted to established anticancer drugs but also occurs towards other cytotoxic compounds of natural origin. Hence, EGFR-mediated resistance may represent a general type of cellular defense mechanisms towards a broad range of toxic xenobiotics.
EGFR in clinical tumors
EGFR is expressed in different human tumors, e.g., in cancers of the lung, head and neck, colon, pancreas, breast, ovary, bladder and kidney, and gliomas. EGFR expression correlated is of prognostic significance for cancer patients. Patients with EGFR-overexpressing tumors reveal worse prognosis [27]. To illustrate this, we exemplarily focus on lung cancer in more detail.
Lung cancer is the leading cause of cancer mortality worldwide, and cure rates are less than 15 % [28]. Lung cancers are classified into two histological types: small cell carcinoma and non-small cell carcinoma. The majority of bronchogenic carcinomas can be classified into four histological types: small cell carcinoma, adenocarcinomas, squamous cell lung carcinomas, and large cell carcinomas. The histological features, clinical course, and response to therapy indicate that small cell lung carcinoma (SCLC) is a separate entity. The behavior of the other three histological subtypes is similar and therefore is referred to as non-small cell lung carcinoma (NSCLC) [29]. NSCLC represent the majority of lung cancers and are usually associated with poor prognosis. While SCLC is drug sensitive, NSCLC are the opposite. Clinical oncology still regards resistance to chemotherapy NSCLC patients as a major problem. As numerous mechanisms are operative in drug-resistant tumors [11, 30, 31], the relative quantitative contributions of each of these resistance mechanisms have to be determined. Hence, understanding the complex genetic network in clinically relevant drug resistance needs more holistic approaches to the entire battery of genes conferring drug resistance.
In 81 human primary squamous cell lung carcinomas, EGFR expression was described as prognostic factor [13]. EGFR expression level was reduced in NSCLC patients with long-term survival [32]. In addition, down-regulation of HER2 expression played an important role in resistant NSCLC [33]. Interestingly, carcinomas of smokers expressed EGFR more frequently than carcinomas of nonsmokers do [34]. The importance of EGFR signaling in lung cancer and its beneficial effects on patient survival led to clinical usage of the EGFR inhibitor erlotinib for the treatment of NSCLC [33].
Taken together, these data clearly speak for an important role of EGFR in drug resistance in vitro as well as in the clinical setting. This is why it appears an exquisite target for novel drugs specifically inhibiting EGFR function and signaling.
HER2, HER3, and HER4 in clinical tumors
HER2 overexpression plays a major role in breast cancer, but it can be also found in other tumor types. HER2 positivity in breast cancers varies from 10 to 40 % [35–38]. The overexpression of HER2 mRNA and protein is a poor prognostic factor [39, 40] and correlated with poor responsiveness to chemotherapy [36]. While EGFR and HER2 have been intensively studied during the past years, less data are available for HER3 and HER4. The prognostic significance of HER3 has been discussed in a contradictory manner. Some authors reported associations of HER3 expression to poor prognosis in breast cancer patients, while others described HER3 as a favorable prognostic factor [41]. HER4 mediates anti-proliferative and differentiation effects [35]. Hence, it is plausible that this receptor represents a favorable prognostic marker in breast cancer patients [41–43].
Since the development of monoclonal antibody C225 to treat EGFR-positive cancers [44], many other therapeutic antibodies and small molecule tyrosine kinase inhibitors against EGFR and HER2 have been developed [45]. In contrast, HER4 does not serve as target for drug development because of its positive prognostic significance. While EGFR- or HER2-overexpressing cancers are adverse prognostic factors if standard cytotoxic chemotherapy is applied, the contrary occurs upon application of EGFR or HER2 inhibitors. Tumors with high EGFR or HER expression are preferentially killed by such targeted antibodies and small molecule inhibitors [37]. This is an instructive example how the specific therapeutic targeting of proteins with worse prognosis can be exploited to improve treatment success rates. Unfortunately, tumors can also develop resistance against EGFR- or HER2-directed antibodies and small molecules, and the search for novel drugs to fight cancer continues.
In this context, the tremendous chemodiversity of phytochemicals comes into play. Novel compounds from natural sources may serve as lead compounds for a new generation of drugs eradicating resistant tumors.
Inhibition of epidermal growth factor signaling by phytochemicals
Natural products as resource for cancer treatment
As pointed out by a survey of the National Cancer Institute, USA, the majority of established cancer drugs are natural products, derivatives of natural products, or drugs mimicking the mode of action of natural products [46]. Searching in nature for novel scaffolds is a promising way to find new chemical tools to bypass and overcome such drug resistance. Novel natural product inhibitors may serve as lead compounds for drug development. A plethora of data in the literature shows that natural products can serve as inhibitors for EGFR-associated signaling molecules such as the RAS/RAF/MEK/ERK and PI3K/AKT/mTOR pathways. This indicates that the identification of novel inhibitors from natural resources is not beyond the scope of expectations.
Inhibitors of EGFR signaling
Phytochemicals from different chemical classes such as flavonoids, terpenoids, and alkaloids have been shown to exert their cytotoxic activity towards cancer cells by affecting EGFR signaling (Table 1). Some specific compounds were intensively investigated such as genistein, curcumin, quercetin, resveratrol, and (−) epigallocatechin-3-gallate. The frequent observation that natural products act in a multifactorial manner [47] applies here as well. As shown in Table 1, phytochemicals inhibited both phosphorylation and expression of EGFR (by ubiquitination and degradation). Furthermore, natural compounds inhibited the phosphorylation of downstream kinases either as consequence of EGFR inhibition or by binding of compounds to corresponding kinase domains of signal transducers. In addition, translocation of kinases (e.g., ERK, MAPK) from the cytosol to the nucleus can be blocked by some compounds. As consequence of silencing EGFR signaling routes, various effects were observed in cancer cells, e.g., induction of cell cycle arrest and apoptosis, inhibition of cell mobility, and inhibition of invasion of metastasis.
It is important to note that several compounds have been shown to exert their effects not only in vitro but also in vivo, e.g., curcumin, (−) epigallocatechin-3-gallate, 11,11′-dideoxy-verticillin, quercetin, deguelin, proanthocyanidins, luteolin, artesunate, platycodin D, berberine, capsaicin, and delfinidin. Further evaluation of the compounds mentioned in Table 1 in terms of EGFR inhibition was performed with in silico molecular docking analyses on human EGFR tyrosine kinase domain. Molecular docking analyses in silico on human EGFR tyrosine kinase domain revealed silibinin to interact with comparable binding energies as the known inhibitor, lapatinib with similar docking poses (Fig. 3). Anti-tumor activity in vivo represents a precondition to consider compounds for clinical application. It is also interesting to study the interaction of natural products with anticancer drugs. Phytochemicals caused increased or even synergistic inhibition of tumor growth in combination with established drugs. This has been shown for the combinations of curcumin plus gefitinib/erlotinib, honokiol plus cetuximab, and (−) epigallocatechin-3-gallate plus 5-fluorouracil erlotinib/gefitinib (Table 1). Furthermore, natural products can reduce the side effects of standard anticancer therapy on normal organs as shown by the combination of curcumin and gefitinib, which led to reduced gastrointestinal side effects compared to gefitinib alone in xenograft tumor-bearing mice (Table 1).
Molecular docking results for the compounds showing the strongest interaction with HER1/EGFR tyrosine kinase domain (PDB ID = 3W2O) and HER2 tyrosine kinase domain (PDB ID = 3PP0). Residues labeled bold at the tables were stated in the literature to reside at the site where the known EGFR inhibitors bind
Inhibitors of HER2/HER3 signaling
Although the inhibition of other EGFR family members was much less investigated, several studies provided results for the inhibition of HER2 and HER3 and their related downstream signaling routes. As can be seen in Table 2, most evidence has been gathered for (−) epigallocatechin-3-gallate, one of the active ingredients of green tea (Camelia sinensis), the flavonoid genistein from soy (Glycine max), and curcumin from Curcuma longa.
Few publications investigated other compounds such as houttuyninum, 11,11′-dideoxy-verticcillin, ZH-4B, resveratrol, pterostilbene, dihydrocalcones, quercetin, and apigenin. The mechanisms of actions how these phytochemicals affect HER2 and HER3 are comparable with those observed for EGFR. They include inhibition of HER2/HER3 phosphorylation and expression as well as inhibition of downstream signal transducers, e.g., ERK1/2, AKT, STAT3, p38MAPK, cRAF, Elk-1, and PI3K (Table 2). Further evaluation of the compounds mentioned in Table 2 in terms of HER2 inhibition was performed with in silico molecular docking analyses on human HER2 tyrosine kinase domain. Molecular docking analyses in silico on human HER2 tyrosine kinase domain revealed curcumin to interact with comparable binding energies as the known inhibitor, lapatinib with similar docking poses (Fig. 3). The results for the in silico molecular docking analyses on EGFR and HER2 tyrosine kinase domains are represented in Table 3 and Table 4, respectively. The inhibition of HER2/HER3 and related signal transduction pathways led to growth inhibition and induction of apoptosis as well as to the inhibition of human tumor xenograft growth in vivo. Comparable to EGFR inhibition, HER2 and HER3 inhibition by curcumin also caused synergistic growth inhibition with 5-fluorouracil/oxaliplatin (Table 2).
Conclusions and perspectives
The identification of tumor target molecules with prognostic relevance for patients opened avenues for the development of more specific treatment options. Important examples in current cancer biology and pharmacology are epidermal growth factor receptors and specific small molecules inhibiting their signaling in tumors. Nevertheless, resistance can also occur towards targeted therapies and novel drugs attacking these receptors are needed. Natural products have been identified as possible novel drug candidates specifically inhibiting EGFR in tumor cells. An important perspective for EGFR/HER2/HER3 inhibiting natural products is their use for personalized treatment options. The individual testing of the mutational status would allow selecting the right EGFR/HER2/HER3 inhibitor for the right patient. In this respect, natural products may represent valuable tools for the development of personalized therapy in the years to come.
The reliable prediction of resistance development is still a major unresolved issue. Deep sequencing and next-generation sequencing have great potentials in monitoring the development of drug resistance in individual tumors and thus offer a new dimension in personalized medicine. In addition to monitoring clinical course of tumor diseases upon drug treatment, whole genome sequencing techniques may be useful to measure the modulation of drug resistance by natural compounds. In addition to studies with large numbers of patient samples taken before and after treatment, longitudinal studies monitoring the same patients at the beginning of and during therapy may provide better insight into the individual mechanisms of resistance development in each individual tumor. This kind of research opens avenues for the prediction of individual response of a tumor patient to therapy. It would be of great value for patients to know whether or not a tumor will respond to the proposed therapy [31]. If a tumor is resistant, therapy will only cause toxic effects in normal tissues without effect on the tumor. Then, another more effective regimen could be applied or natural products alleviating the adverse side effects of chemotherapy could be applied.
Abbreviations
- ART:
-
Artesunate
- CET:
-
Cephalotaxine
- EGFR:
-
Epidermal growth factor receptor
- GBM:
-
Glioblastoma multiforme
- HER:
-
Human epidermal growth factor receptor
- HHT:
-
Homoharringtonine
- NSCLC:
-
Non-small cell lung carcinoma
- SCLC:
-
Small cell lung carcinoma
References
Scagliotti GV, Selvaggi G, Novello S, Hirsch FR (2004) The biology of epidermal growth factor receptor in lung cancer. Clin Cancer Res 10:4227s–4232s
Riese DJ 2nd, van Raaij TM, Plowman GD, Andrews GC, Stern DF (1995) The cellular response to neuregulins is governed by complex interactions of the erbB receptor family. Mol Cell Biol 15:5770–5776
Beerli RR, Hynes NE (1996) Epidermal growth factor-related peptides activate distinct subsets of ErbB receptors and differ in their biological activities. J Biol Chem 271:6071–6076
Olayioye MA, Neve RM, Lane HA, Hynes NE (2000) The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 19:3159–3167
Earp HS, Dawson TL, Li X, Yu H (1995) Heterodimerization and functional interaction between EGF receptor family members: a new signaling paradigm with implications for breast cancer research. Breast Cancer Res Treat 35:115–132
Oliveira S, van Bergen en Henegouwen PM, Storm G, Schiffelers RM (2006) Molecular biology of epidermal growth factor receptor inhibition for cancer therapy. Expert Opin Biol Ther 6:605–617
Astsaturov I, Cohen RB, Harari P (2006) Targeting epidermal growth factor receptor signaling in the treatment of head and neck cancer. Expert Rev Anticancer Ther 6:1179–1193
Perea S, Hidalgo M (2004) Predictors of sensitivity and resistance to epidermal growth factor receptor inhibitors. Clin Lung Cancer 6(Suppl 1):S30–S34
Efferth T, Volm M (1993) Reversal of doxorubicin-resistance in sarcoma 180 tumor cells by inhibition of different resistance mechanisms. Cancer Lett 70:197–202
Efferth T, Volm M (1992) Immunocytochemical detection of oncoproteins in animal and human tumor lines with acquired or inherent multidrug resistance. Cancer Detect Prev 16:237–243
Volm M, Koomagi R, Efferth T (2004) Prediction of drug sensitivity and resistance of cancer by protein expression profiling. Cancer Genomics Proteomics 1:157–166
el-Deiry WS (1997) Role of oncogenes in resistance and killing by cancer therapeutic agents. Curr Opin Oncol 9:79–87
Volm M, Efferth T, Mattern J (1992) Oncoprotein (c-myc, c-erbB1, c-erbB2, c-fos) and suppressor gene product (p53) expression in squamous cell carcinomas of the lung. Clinical and biological correlations. Anticancer Res 12:11–20
Efferth T, Volm M (2004) Protein expression profiles indicative for drug resistance of kidney carcinoma. Cancer Genomics Proteomics 1:17–22
Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK et al (2001) Malignant glioma: genetics and biology of a grave matter. Genes Dev 15:1311–1333
Nagane M, Huang HJ, Cavenee WK (1997) Advances in the molecular genetics of gliomas. Curr Opin Oncol 9:215–222
Nagane M, Narita Y, Mishima K, Levitzki A, Burgess AW, Cavenee WK et al (2001) Human glioblastoma xenografts overexpressing a tumor-specific mutant epidermal growth factor receptor sensitized to cisplatin by the AG1478 tyrosine kinase inhibitor. J Neurosurg 95:472–479
Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ (1998) Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci U S A 95:5724–5729
Huang PH, Mukasa A, Bonavia R, Flynn RA, Brewer ZE, Cavenee WK et al (2007) Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc Natl Acad Sci U S A 104:12867–12872
Han J (1988) Traditional Chinese medicine and the search for new antineoplastic drugs. J Ethnopharmacol 24:1–17
Efferth T, Davey M, Olbrich A, Rucker G, Gebhart E, Davey R (2002) Activity of drugs from traditional Chinese medicine toward sensitive and MDR1- or MRP1-overexpressing multidrug-resistant human CCRF-CEM leukemia cells. Blood Cells Mol Dis 28:160–168
Efferth T, Sauerbrey A, Halatsch ME, Ross DD, Gebhart E (2003) Molecular modes of action of cephalotaxine and homoharringtonine from the coniferous tree Cephalotaxus hainanensis in human tumor cell lines. Naunyn Schmiedebergs Arch Pharmacol 367:56–67
Klayman DL (1985) Qinghaosu (artemisinin): an antimalarial drug from China. Sci 228:1049–1055
Efferth T (2007) Willmar Schwabe Award 2006: antiplasmodial and antitumor activity of artemisinin—from bench to bedside. Planta Med 73:299–309
Efferth T, Giaisi M, Merling A, Krammer PH, Li-Weber M (2007) Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells. PLoS One 2:e693
Efferth T, Li PC, Konkimalla VS, Kaina B (2007) From traditional Chinese medicine to rational cancer therapy. Trends Mol Med 13:353–361
Mitsudomi T, Yatabe Y (2010) Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J 277:301–308
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225–249
Volm M, Koomagi R, Mattern J, Efferth T (2002) Protein expression profiles indicative for drug resistance of non-small cell lung cancer. Br J Cancer 87:251–257
Efferth T (2001) The human ATP-binding cassette transporter genes: from the bench to the bedside. Curr Mol Med 1:45–65
Efferth T, Volm M (2005) Pharmacogenetics for individualized cancer chemotherapy. Pharmacol Ther 107:155–176
Volm M, Koomagi R, Mattern J, Efferth T (2002) Expression profile of genes in non-small cell lung carcinomas from long-term surviving patients. Clin Cancer Res 8:1843–1848
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S et al (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353:123–132
Volm M, Kastel M, Mattern J, Efferth T (1993) Expression of resistance factors (P-glycoprotein, glutathione S-transferase-pi, and topoisomerase II) and their interrelationship to proto-oncogene products in renal cell carcinomas. Cancer 71:3981–3987
Earp HS 3rd, Calvo BF, Sartor CI (2003) The EGF receptor family—multiple roles in proliferation, differentiation, and neoplasia with an emphasis on HER4. Trans Am Clin Climatol Assoc 114:315–333, discussion 33–4
Menard S, Tagliabue E, Campiglio M, Pupa SM (2000) Role of HER2 gene overexpression in breast carcinoma. J Cell Physiol 182:150–162
Figueroa-Magalhaes MC, Jelovac D, Connolly RM, Wolff AC (2014) Treatment of HER2-positive breast cancer. Breast 23:128–136
Kurebayashi J (2001) Biological and clinical significance of HER2 overexpression in breast cancer. Breast Cancer 8:45–51
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Sci 235:177–182
Paik S, Hazan R, Fisher ER, Sass RE, Fisher B, Redmond C et al (1990) Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol 8:103–112
Koutras AK, Fountzilas G, Kalogeras KT, Starakis I, Iconomou G, Kalofonos HP (2010) The upgraded role of HER3 and HER4 receptors in breast cancer. Crit Rev Oncol Hematol 74:73–78
Srinivasan R, Gillett CE, Barnes DM, Gullick WJ (2000) Nuclear expression of the c-erbB-4/HER-4 growth factor receptor in invasive breast cancers. Cancer Res 60:1483–1487
Suo Z, Risberg B, Kalsson MG, Willman K, Tierens A, Skovlund E et al (2002) EGFR family expression in breast carcinomas. c-erbB-2 and c-erbB-4 receptors have different effects on survival. J Pathol 196:17–25
Mendelsohn J (1997) Epidermal growth factor receptor inhibition by a monoclonal antibody as anticancer therapy. Clin Cancer Res 3:2703–2707
Roskoski R Jr (2014) ErbB/HER protein-tyrosine kinases: structures and small molecule inhibitors. Pharmacol Res 87C:42–59
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477
Efferth T, Koch E (2011) Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr Drug Targets 12:122–132
Wu J, Zuo F, Du J, Wong PF, Qin H, Xu J (2013) Icariside II induces apoptosis via inhibition of the EGFR pathways in A431 human epidermoid carcinoma cells. Mol Med Rep 8:597–602
Du H, Xu B, Wu C, Li M, Ran F, Cai S et al (2012) Effects of CS-1 on A431 cell proliferation, cell cycle, and epidermal growth factor receptor signal transduction. Acta Biochim Biophys Sin (Shanghai) 44:136–146
Kim S, Han J, Kim JS, Kim JH, Choe JH, Yang JH et al (2011) Silibinin suppresses EGFR ligand-induced CD44 expression through inhibition of EGFR activity in breast cancer cells. Anticancer Res 31:3767–3773
Leeman-Neill RJ, Cai Q, Joyce SC, Thomas SM, Bhola NE, Neill DB et al (2010) Honokiol inhibits epidermal growth factor receptor signaling and enhances the antitumor effects of epidermal growth factor receptor inhibitors. Clin Cancer Res 16:2571–2579
Zhang YX, Chen Y, Guo XN, Zhang XW, Zhao WM, Zhong L et al (2005) 11,11′-Dideoxy-verticillin: a natural compound possessing growth factor receptor tyrosine kinase-inhibitory effect with anti-tumor activity. Anticancer Drugs 16:515–524
Zhang Y, Zheng L, Zhang J, Dai B, Wang N, Chen Y et al (2011) Antitumor activity of taspine by modulating the EGFR signaling pathway of Erk1/2 and Akt in vitro and in vivo. Planta Med 77:1774–1781
Sun M, Ren J, Du H, Zhang Y, Zhang J, Wang S et al (2010) A combined A431 cell membrane chromatography and online high performance liquid chromatography/mass spectrometry method for screening compounds from total alkaloid of Radix caulophylli acting on the human EGFR. J Chromatogr B Analyt Technol Biomed Life Sci 878:2712–2718
Wang S, Sun M, Zhang Y, Du H, He L (2010) A new A431/cell membrane chromatography and online high performance liquid chromatography/mass spectrometry method for screening epidermal growth factor receptor antagonists from Radix sophorae flavescentis. J Chromatogr A 1217:5246–5252
Peterson G, Barnes S (1993) Genistein and biochanin A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation. Prostate 22:335–345
Peterson G, Barnes S (1996) Genistein inhibits both estrogen and growth factor-stimulated proliferation of human breast cancer cells. Cell Growth Differ 7:1345–1351
Yang EB, Wang DF, Mack P, Cheng LY (1996) Genistein, a tyrosine kinase inhibitor, reduces EGF-induced EGF receptor internalization and degradation in human hepatoma HepG2 cells. Biochem Biophys Res Commun 224:309–317
Theodorescu D, Laderoute KR, Calaoagan JM, Guilding KM (1998) Inhibition of human bladder cancer cell motility by genistein is dependent on epidermal growth factor receptor but not p21ras gene expression. Int J Cancer 78:775–782
Shao ZM, Wu J, Shen ZZ, Barsky SH (1998) Genistein inhibits both constitutive and EGF-stimulated invasion in ER-negative human breast carcinoma cell lines. Anticancer Res 18:1435–1439
Salvatori L, Caporuscio F, Coroniti G, Starace G, Frati L, Russo MA et al (2009) Down-regulation of epidermal growth factor receptor induced by estrogens and phytoestrogens promotes the differentiation of U2OS human osteosarcoma cells. J Cell Physiol 220:35–44
Gadgeel SM, Ali S, Philip PA, Wozniak A, Sarkar FH (2009) Genistein enhances the effect of epidermal growth factor receptor tyrosine kinase inhibitors and inhibits nuclear factor kappa B in nonsmall cell lung cancer cell lines. Cancer 115:2165–2176
El-Rayes BF, Ali S, Ali IF, Philip PA, Abbruzzese J, Sarkar FH (2006) Potentiation of the effect of erlotinib by genistein in pancreatic cancer: the role of Akt and nuclear factor-kappaB. Cancer Res 66:10553–10559
Shushan A, Ben-Bassat H, Mishani E, Laufer N, Klein BY, Rojansky N (2007) Inhibition of leiomyoma cell proliferation in vitro by genistein and the protein tyrosine kinase inhibitor TKS050. Fertil Steril 87:127–135
Nakamura H, Wang Y, Kurita T, Adomat H, Cunha GR (2011) Genistein increases epidermal growth factor receptor signaling and promotes tumor progression in advanced human prostate cancer. PLoS One 6:e20034
Oh HY, Leem J, Yoon SJ, Yoon S, Hong SJ (2010) Lipid raft cholesterol and genistein inhibit the cell viability of prostate cancer cells via the partial contribution of EGFR-Akt/p70S6k pathway and down-regulation of androgen receptor. Biochem Biophys Res Commun 393:319–324
Park SJ, Kim MJ, Kim YK, Kim SM, Park JY, Myoung H (2010) Combined cetuximab and genistein treatment shows additive anti-cancer effect on oral squamous cell carcinoma. Cancer Lett 292:54–63
Anastasius N, Boston S, Lacey M, Storing N, Whitehead SA (2009) Evidence that low-dose, long-term genistein treatment inhibits oestradiol-stimulated growth in MCF-7 cells by down-regulation of the PI3-kinase/Akt signalling pathway. J Steroid Biochem Mol Biol 116:50–55
Bhatia N, Agarwal R (2001) Detrimental effect of cancer preventive phytochemicals silymarin, genistein and epigallocatechin 3-gallate on epigenetic events in human prostate carcinoma DU145 cells. Prostate 46:98–107
Li D, Wu LJ, Tashiro S, Onodera S, Ikejima T (2007) Oridonin-induced A431 cell apoptosis partially through blockage of the Ras/Raf/ERK signal pathway. J Pharmacol Sci 103:56–66
Liang YC, Lin-shiau SY, Chen CF, Lin JK (1997) Suppression of extracellular signals and cell proliferation through EGF receptor binding by (−)-epigallocatechin gallate in human A431 epidermoid carcinoma cells. J Cell Biochem 67:55–65
Masuda M, Suzui M, Weinstein IB (2001) Effects of epigallocatechin-3-gallate on growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin Cancer Res 7:4220–4229
Sah JF, Balasubramanian S, Eckert RL, Rorke EA (2004) Epigallocatechin-3-gallate inhibits epidermal growth factor receptor signaling pathway. Evidence for direct inhibition of ERK1/2 and AKT kinases. J Biol Chem 279:12755–12762
Zhang X, Zhang H, Tighiouart M, Lee JE, Shin HJ, Khuri FR et al (2008) Synergistic inhibition of head and neck tumor growth by green tea (−)-epigallocatechin-3-gallate and EGFR tyrosine kinase inhibitor. Int J Cancer 123:1005–1014
Adachi S, Nagao T, To S, Joe AK, Shimizu M, Matsushima-Nishiwaki R et al (2008) (−)-Epigallocatechin gallate causes internalization of the epidermal growth factor receptor in human colon cancer cells. Carcinog 29:1986–1993
Adachi S, Shimizu M, Shirakami Y, Yamauchi J, Natsume H, Matsushima-Nishiwaki R et al (2009) (−)-Epigallocatechin gallate downregulates EGF receptor via phosphorylation at Ser1046/1047 by p38 MAPK in colon cancer cells. Carcinog 30:1544–1552
Milligan SA, Burke P, Coleman DT, Bigelow RL, Steffan JJ, Carroll JL et al (2009) The green tea polyphenol EGCG potentiates the antiproliferative activity of c-Met and epidermal growth factor receptor inhibitors in non-small cell lung cancer cells. Clin Cancer Res 15:4885–4894
Lim YC, Cha YY (2011) Epigallocatechin-3-gallate induces growth inhibition and apoptosis of human anaplastic thyroid carcinoma cells through suppression of EGFR/ERK pathway and cyclin B1/CDK1 complex. J Surg Oncol 104:776–780
Chang CM, Chang PY, Tu MG, Lu CC, Kuo SC, Amagaya S et al (2012) Epigallocatechin gallate sensitizes CAL-27 human oral squamous cell carcinoma cells to the anti-metastatic effects of gefitinib (Iressa) via synergistic suppression of epidermal growth factor receptor and matrix metalloproteinase-2. Oncol Rep 28:1799–1807
Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB (2005) (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res 11:2735–2746
Hou Z, Sang S, You H, Lee MJ, Hong J, Chin KV et al (2005) Mechanism of action of (−)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res 65:8049–8056
Pal HC, Sharma S, Strickland LR, Agarwal J, Athar M, Elmets CA et al (2013) Delphinidin reduces cell proliferation and induces apoptosis of non-small-cell lung cancer cells by targeting EGFR/VEGFR2 signaling pathways. PLoS One 8:e77270
Fridrich D, Teller N, Esselen M, Pahlke G, Marko D (2008) Comparison of delphinidin, quercetin and (−)-epigallocatechin-3-gallate as inhibitors of the EGFR and the ErbB2 receptor phosphorylation. Mol Nutr Food Res 52:815–822
Singh F, Gao D, Lebwohl MG, Wei H (2003) Shikonin modulates cell proliferation by inhibiting epidermal growth factor receptor signaling in human epidermoid carcinoma cells. Cancer Lett 200:115–121
Hashimoto S, Xu Y, Masuda Y, Aiuchi T, Nakajo S, Uehara Y et al (2002) Beta-hydroxyisovalerylshikonin is a novel and potent inhibitor of protein tyrosine kinases. Jpn J Cancer Res 93:944–951
Soung YH, Chung J (2011) Curcumin inhibition of the functional interaction between integrin alpha6beta4 and the epidermal growth factor receptor. Mol Cancer Ther 10:883–891
Lee JY, Lee YM, Chang GC, Yu SL, Hsieh WY, Chen JJ et al (2011) Curcumin induces EGFR degradation in lung adenocarcinoma and modulates p38 activation in intestine: the versatile adjuvant for gefitinib therapy. PLoS One 6:e23756
Wang S, Yu S, Shi W, Ge L, Yu X, Fan J et al (2011) Curcumin inhibits the migration and invasion of mouse hepatoma Hca-F cells through down-regulating caveolin-1 expression and epidermal growth factor receptor signaling. IUBMB Life 63:775–782
Jiang AP, Zhou DH, Meng XL, Zhang AP, Zhang C, Li XT et al (2014) Down-regulation of epidermal growth factor receptor by curcumin-induced UBE1L in human bronchial epithelial cells. J Nutr Biochem 25:241–249
Li S, Liu Z, Zhu F, Fan X, Wu X, Zhao H et al (2014) Curcumin lowers erlotinib resistance in non-small cell lung carcinoma cells with mutated EGF receptor. Oncol Res 21:137–144
Chadalapaka G, Jutooru I, Burghardt R, Safe S (2010) Drugs that target specificity proteins downregulate epidermal growth factor receptor in bladder cancer cells. Mol Cancer Res 8:739–750
Hung CM, Su YH, Lin HY, Lin JN, Liu LC, Ho CT et al (2012) Demethoxycurcumin modulates prostate cancer cell proliferation via AMPK-induced down-regulation of HSP70 and EGFR. J Agric Food Chem 60:8427–8434
Qiu P, Xu L, Gao L, Zhang M, Wang S, Tong S et al (2013) Exploring pyrimidine-substituted curcumin analogues: design, synthesis and effects on EGFR signaling. Bioorg Med Chem 21:5012–5020
Castillo-Pichardo L, Dharmawardhane SF (2012) Grape polyphenols inhibit Akt/mammalian target of rapamycin signaling and potentiate the effects of gefitinib in breast cancer. Nutr Cancer 64:1058–1069
Lee MF, Pan MH, Chiou YS, Cheng AC, Huang H (2011) Resveratrol modulates MED28 (Magicin/EG-1) expression and inhibits epidermal growth factor (EGF)-induced migration in MDA-MB-231 human breast cancer cells. J Agric Food Chem 59:11853–11861
Wang Y, Romigh T, He X, Orloff MS, Silverman RH, Heston WD et al (2010) Resveratrol regulates the PTEN/AKT pathway through androgen receptor-dependent and -independent mechanisms in prostate cancer cell lines. Hum Mol Genet 19:4319–4329
Stewart JR, O’Brian CA (2004) Resveratrol antagonizes EGFR-dependent Erk1/2 activation in human androgen-independent prostate cancer cells with associated isozyme-selective PKC alpha inhibition. Invest New Drugs 22:107–117
Qiu L, Wang Q, Di W, Jiang Q, Schefeller E, Derby S et al (2005) Transient activation of EGFR/AKT cell survival pathway and expression of survivin contribute to reduced sensitivity of human melanoma cells to betulinic acid. Int J Oncol 27:823–830
Teller N, Roth M, Esselen M, Fridrich D, Boettler U, Blust V et al (2013) Apple procyanidins affect several members of the ErbB receptor tyrosine kinase family in vitro. Food Funct 4:689–697
Huang CY, Chan CY, Chou IT, Lien CH, Hung HC, Lee MF (2013) Quercetin induces growth arrest through activation of FOXO1 transcription factor in EGFR-overexpressing oral cancer cells. J Nutr Biochem 24:1596–1603
Senthilkumar K, Arunkumar R, Elumalai P, Sharmila G, Gunadharini DN, Banudevi S et al (2011) Quercetin inhibits invasion, migration and signalling molecules involved in cell survival and proliferation of prostate cancer cell line (PC-3). Cell Biochem Funct 29:87–95
Jung JH, Lee JO, Kim JH, Lee SK, You GY, Park SH et al (2010) Quercetin suppresses HeLa cell viability via AMPK-induced HSP70 and EGFR down-regulation. J Cell Physiol 223:408–414
Raspaglio G, Ferrandina G, Ferlini C, Scambia G, Ranelletti FO (2003) Epidermal growth factor-responsive laryngeal squamous cancer cell line Hep2 is more sensitive than unresponsive CO-K3 one to quercetin and tamoxifen apoptotic effects. Oncol Res 14:83–91
Lee LT, Huang YT, Hwang JJ, Lee AY, Ke FC, Huang CJ et al (2004) Transinactivation of the epidermal growth factor receptor tyrosine kinase and focal adhesion kinase phosphorylation by dietary flavonoids: effect on invasive potential of human carcinoma cells. Biochem Pharmacol 67:2103–2114
Lee LT, Huang YT, Hwang JJ, Lee PP, Ke FC, Nair MP et al (2002) Blockade of the epidermal growth factor receptor tyrosine kinase activity by quercetin and luteolin leads to growth inhibition and apoptosis of pancreatic tumor cells. Anticancer Res 22:1615–1627
Huang YT, Hwang JJ, Lee PP, Ke FC, Huang JH, Huang CJ et al (1999) Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br J Pharmacol 128:999–1010
Richter M, Ebermann R, Marian B (1999) Quercetin-induced apoptosis in colorectal tumor cells: possible role of EGF receptor signaling. Nutr Cancer 34:88–99
Mehta R, Katta H, Alimirah F, Patel R, Murillo G, Peng X et al (2013) Deguelin action involves c-Met and EGFR signaling pathways in triple negative breast cancer cells. PLoS One 8:e65113
Kern M, Tjaden Z, Ngiewih Y, Puppel N, Will F, Dietrich H et al (2005) Inhibitors of the epidermal growth factor receptor in apple juice extract. Mol Nutr Food Res 49:317–328
Prasad R, Katiyar SK (2012) Bioactive phytochemical proanthocyanidins inhibit growth of head and neck squamous cell carcinoma cells by targeting multiple signaling molecules. PLoS One 7:e46404
Lee EJ, Oh SY, Sung MK (2012) Luteolin exerts anti-tumor activity through the suppression of epidermal growth factor receptor-mediated pathway in MDA-MB-231 ER-negative breast cancer cells. Food Chem Toxicol 50:4136–4143
Lee DE, Lee KW, Song NR, Seo SK, Heo YS, Kang NJ et al (2010) 7,3′,4′-Trihydroxyisoflavone inhibits epidermal growth factor-induced proliferation and transformation of JB6 P+ mouse epidermal cells by suppressing cyclin-dependent kinases and phosphatidylinositol 3-kinase. J Biol Chem 285:21458–21466
Ma H, Yao Q, Zhang AM, Lin S, Wang XX, Wu L et al (2011) The effects of artesunate on the expression of EGFR and ABCG2 in A549 human lung cancer cells and a xenograft model. Mol 16:10556–10569
Chun J, Kim YS (2013) Platycodin D inhibits migration, invasion, and growth of MDA-MB-231 human breast cancer cells via suppression of EGFR-mediated Akt and MAPK pathways. Chem Biol Interact 205:212–221
Wang L, Cao H, Lu N, Liu L, Wang B, Hu T et al (2013) Berberine inhibits proliferation and down-regulates epidermal growth factor receptor through activation of Cbl in colon tumor cells. PLoS One 8:e56666
Thoennissen NH, O’Kelly J, Lu D, Iwanski GB, La DT, Abbassi S et al (2010) Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and -negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene 29:285–296
Zhou NN, Tang J, Chen WD, Feng GK, Xie BF, Liu ZC et al (2012) Houttuyninum, an active constituent of Chinese herbal medicine, inhibits phosphorylation of HER2/neu receptor tyrosine kinase and the tumor growth of HER2/neu-overexpressing cancer cells. Life Sci 90:770–775
Guo XN, Zhong L, Zhang XH, Zhao WM, Zhang XW, Lin LP et al (2004) Evaluation of active recombinant catalytic domain of human ErbB-2 tyrosine kinase, and suppression of activity by a naturally derived inhibitor, ZH-4B. Biochim Biophys Acta 1673:186–193
Sakla MS, Shenouda NS, Ansell PJ, Macdonald RS, Lubahn DB (2007) Genistein affects HER2 protein concentration, activation, and promoter regulation in BT-474 human breast cancer cells. Endocr 32:69–78
Li Y, Mi C, Wu YZ, Yang SF, Yang ZQ (2004) [The effects of genistein on epidermal growth factor receptor mediated signal transduction pathway in human ovarian carcinoma cells lines SKOV3 and its xenograft in nude mice]. Zhonghua Bing Li Xue Za Zhi 33:546–549
Seo HS, Choi HS, Choi YK, Um JY, Choi I, Shin YC et al (2011) Phytoestrogens induce apoptosis via extrinsic pathway, inhibiting nuclear factor-kappaB signaling in HER2-overexpressing breast cancer cells. Anticancer Res 31:3301–3313
Pan MH, Lin CC, Lin JK, Chen WJ (2007) Tea polyphenol (−)-epigallocatechin 3-gallate suppresses heregulin-beta1-induced fatty acid synthase expression in human breast cancer cells by inhibiting phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase cascade signaling. J Agric Food Chem 55:5030–5037
Kushima Y, Iida K, Nagaoka Y, Kawaratani Y, Shirahama T, Sakaguchi M et al (2009) Inhibitory effect of (−)-epigallocatechin and (−)-epigallocatechin gallate against heregulin beta1-induced migration/invasion of the MCF-7 breast carcinoma cell line. Biol Pharm Bull 32:899–904
Pianetti S, Guo S, Kavanagh KT, Sonenshein GE (2002) Green tea polyphenol epigallocatechin-3 gallate inhibits Her-2/neu signaling, proliferation, and transformed phenotype of breast cancer cells. Cancer Res 62:652–655
Masuda M, Suzui M, Lim JT, Weinstein IB (2003) Epigallocatechin-3-gallate inhibits activation of HER-2/neu and downstream signaling pathways in human head and neck and breast carcinoma cells. Clin Cancer Res 9:3486–3491
Shimizu M, Deguchi A, Joe AK, McKoy JF, Moriwaki H, Weinstein IB (2005) EGCG inhibits activation of HER3 and expression of cyclooxygenase-2 in human colon cancer cells. J Exp Ther Oncol 5:69–78
Patel BB, Sengupta R, Qazi S, Vachhani H, Yu Y, Rishi AK et al (2008) Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer 122:267–273
Patel BB, Gupta D, Elliott AA, Sengupta V, Yu Y, Majumdar AP (2010) Curcumin targets FOLFOX-surviving colon cancer cells via inhibition of EGFRs and IGF-1R. Anticancer Res 30:319–325
Sun SH, Huang HC, Huang C, Lin JK (2012) Cycle arrest and apoptosis in MDA-MB-231/Her2 cells induced by curcumin. Eur J Pharmacol 690:22–30
Catania A, Barrajon-Catalan E, Nicolosi S, Cicirata F, Micol V (2013) Immunoliposome encapsulation increases cytotoxic activity and selectivity of curcumin and resveratrol against HER2 overexpressing human breast cancer cells. Breast Cancer Res Treat 141:55–65
Meiyanto E, Putri DD, Susidarti RA, Murwanti R, Sardjiman, Fitriasari A et al (2014) Curcumin and its analogues (PGV-0 and PGV-1) enhance sensitivity of resistant MCF-7 cells to doxorubicin through inhibition of HER2 and NF-kB activation. Asian Pac J Cancer Prev 15:179–184
Pan MH, Lin YT, Lin CL, Wei CS, Ho CT, Chen WJ (2011) Suppression of Heregulin-beta1/HER2-modulated invasive and aggressive phenotype of breast carcinoma by pterostilbene via inhibition of matrix metalloproteinase-9, p38 kinase cascade and Akt activation. Evid Based Complement Alternat Med 2011:562187
Huynh H, Nguyen TT, Chan E, Tran E (2003) Inhibition of ErbB-2 and ErbB-3 expression by quercetin prevents transforming growth factor alpha (TGF-alpha)- and epidermal growth factor (EGF)-induced human PC-3 prostate cancer cell proliferation. Int J Oncol 23:821–829
Way TD, Kao MC, Lin JK (2004) Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem 279:4479–4489
Conflict of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kadioglu, O., Cao, J., Saeed, M.E.M. et al. Targeting epidermal growth factor receptors and downstream signaling pathways in cancer by phytochemicals. Targ Oncol 10, 337–353 (2015). https://doi.org/10.1007/s11523-014-0339-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-014-0339-4