Abstract
Natural compounds have been studied as a source of countless bioactive compounds with diverse activities. Among them, many dietary phytochemicals have been thoroughly studied for their cytotoxic or apoptotic effects in several cellular models in order to explain their anticancer capacity. Curcumin and resveratrol are two natural compounds with a large body of evidence showing their cytotoxic activity against a wide variety of cancer cells; however, their poor absorption, bioavailability, and low selectivity have limited their clinical use. With the aim of improving bioavailability and selectivity, the antiproliferative effects of free-, liposomed-, and immunoliposomed-curcumin and/or resveratrol formulations have been compared in two human breast cancer cell lines with different HER2 expression levels. The results demonstrate that when HER2-targeted immunoliposomes are coupled to trastuzumab there is a dramatic increase in the antiproliferative effects of curcumin and resveratrol in HER2 positive human breast cancer cells in comparison to regular liposomed or free forms, indicating an increase of its therapeutic effect. The enhancement of the cytotoxic effects was also correlated to the uptake of curcumin at intracellular level, as shown by using ImageStream technique. The striking efficacy of the immunoliposomed formulation containing both resveratrol and curcumin suggests a multitargeted mechanism of action that deserves further study. These findings show the potential of HER2-targeted nanovesicles to develop new drug delivery systems for cancer therapy based on these compounds and justify further preclinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human epidermal growth factor receptor-2 (HER2) is a 185-kDa transmembrane receptor tyrosine kinase that belongs to the epidermal growth factor receptor (EGFR) family [1, 2]. HER2 is normally expressed at low levels in some adult cell types [3], but it is overexpressed in ~25–30 % of breast and ovarian cancers; where up to two million receptor molecules can be expressed at the breast tumor cell surface [4]. HER2-overexpression also frequently occurs in a number of other carcinomas [5] and it is pathologically associated with large tumor size, lack of estrogen and progesterone-receptor expression, the presence of nodal metastasis and also clinically associated with aggressive disease, increased risk of relapse, and poor long-term survival [6, 7].
The high incidence of HER2 and its relevance in some forms of breast cancer make it an attractive target for the development of therapeutic agents. Trastuzumab (Herceptin®, Genentech, Inc., San Francisco, CA), the first HER2-targeted agent approved for clinical use in breast cancer patients, is a humanized monoclonal antibody (mAb) that binds the extracellular domain of the HER2 receptor [8]. Trastuzumab can induce antitumor responses as a single agent [9], but it presents more efficacy when combined with chemotherapy [10].
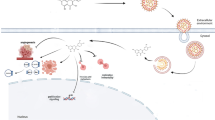
Curcumin and resveratrol are natural bioactive compounds with multitargeting properties that have demonstrated clinical benefits in cancer treatments [11, 12]. Although these compounds have shown significant efficacy in cell culture studies, their introduction into the clinical setting is hindered largely by their poor absorption and rapid metabolism, resulting in poor bioavailability upon oral administration [13, 14]. Efficacy of these compounds is also limited when they are used intravenously, intraperitoneally, or through the oral mucosa due to metabolism. One of the strategies utilized to overcome these limitations is the use drug delivery systems, such as liposomes, to deliver compounds into the systemic circulation and increase the compounds stability [15, 16]. Alternatively, immunoliposomes may be an improved strategy bearing targeted action.
Liposomes entrap drugs, thereby prolonging circulation time and also changing the drugs’ distribution in vivo. Moreover, pegylated liposomes modified with polyethylene glycol (PEG) have decreased uptake by the macrophages of the reticulo-endothelial system (RES), and hence stay in circulation for a relatively long period of time [17, 18]. The main disadvantage of liposomes is their lack of selectivity because they do not specifically interact with tumor cells. Consequently, a selective form of drug delivery to target cells can be achieved by using immunoliposomes, i.e., a liposome in which a monoclonal or polyclonal antibody is attached to the liposomal surface that recognizes and binds to a specific antigen on the surface of the targeted tumor cells [19, 20]. Immunoliposomes have the potential to transfer large quantities of drug molecules directly to tumor cells, thereby reducing their potential systemic side effects. In addition, drugs delivered via immunoliposomes have shown antitumor activities similar to, or greater, than those of the drug alone [21].
Active targeting of drugs’ carriers is expected to improve their safety and clinical parameters. It has been shown that the use of anti-HER2 immunoliposomes increased the cytotoxicity drug effects with a severe reduction in cell viability in a panel of human breast cancer cell lines. These results directly correlated to the cells’ level of HER2 expression [22]. In addition, this strategy has been successfully tested with alternative overexpressed target surface molecules [23].
In the present study, compounds that have shown promising in vitro anticancer properties, such as curcumin, resveratrol, or a combination of both, have been used as anticancer agents loaded into immunoliposomes. The efficacy and selectivity of this system against two cell lines with different HER2 expression levels have been compared to the use of regular liposomes and the compounds in their free form. The correlation of the uptake of curcumin and its cytotoxic activity on the cancer cell models was also studied.
Materials and methods
Materials
Egg-yolk phosphatidylcholine (EYPC) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-PEG-2000 (DSPE-PEG) were obtained from Lipoid GmbH (Ludwigshafen, Germany). Cholesterol (Chol) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide (polyethylene glycol) 2000] (D2000 M) were purchased from Avanti Polar Lipids (Alabaster, AL, USA). The antibody Herceptin™ (Trastuzumab) was purchased from Roche. Curcumin extract (95 % curcuminoids, HPLC) was kindly provided by Monteloeder, SL (Elche, Alicante, Spain), and resveratrol (98 % HPLC) was obtained from Sigma-Aldrich Corporation (St. Louis, MO, USA). All other chemicals and solvents were of analytical grade, purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA) and used without further purification.
Preparation of liposomes
Two types of lipid compositions were used in the present study, one containing only EYPC for the preparation of regular liposomes and one used for the immunoliposomes preparation, containing adequate amounts of each lipid combined in a molar ratio of 74.5:20:5:0.5 (EYPC:Chol:DSPE-PEG:D2000 M), which has been optimized in previous studies [22].
For all preparations, a lipid film was obtained by removing the organic solvent by evaporation under a stream of nitrogen (N2) and then further vacuum-dried for 3–4 h to remove any residual organic solvent. The film was hydrated with histidine buffer (THIS) (1.2 g/L histidine–HCl, 0.78 g/L histidine, pH 7.4). The resultant liposomal suspension was formed by vigorous vortexing at 37 °C and a homogeneous milky suspension of MLVs was obtained. The liposomal suspension was filter-extruded through a 100-nm polycarbonate membrane, Track-Etch Nuclepore membrane (Whatman, UK), to obtain large unilamellar vesicles (LUVs). Size reduction was performed by 15 extrusion cycles performed by hand with a syringe extruder LiposofastTM (Avestin Inc., Canada) [24] or by LIPEXTM Extruder (Northern Lipids Inc) [24]. The resulting products were stored at 4 °C until use.
Preparation of immunoliposomes
Lipids were combined and LUVs were prepared as mentioned above. Then, the antibody trastuzumab was derivatized as previously described [22]. Briefly, the antibody was thiolated by reacting with 2-iminothiolane (Traut’s reagent) for 2 h, and then incubated with the unilamellar vesicles in an Argon inert atmosphere for 12 h at room temperature with gentle agitation. During the reaction, the thiol group of the derivatized antibody reacted with the maleimide group of the D2000 M lipid present in the liposome, yielding the immunoliposome with the antibody covalently attached (the technique is thoroughly reviewed in [25]). After derivatization, the unbound antibody was separated by size exclusion chromatography using Sephadex® G-25.
Incorporation of compounds into liposomes or immunoliposomes
After several trials, optimum synthesis process was achieved by antibody conjugation followed by the incorporation of the compounds. We observed that loading of the compounds prior to antibody conjugation decreased both conjugation efficacy and incorporation of the drugs. Natural compounds (curcumin and/or resveratrol) in 5 % DMSO were added and incubated at 37 °C for 1 h with gentle agitation to obtain the final liposome or immunoliposome suspension. Then, the non-encapsulated compounds were eliminated using an Amicon ultra centrifugal tube (Millipore, Europe). Liposomed preparations contained a molar percentage of total lipid ranging from 83 to 86 % and HPLC determined percentages of drugs were 14.1 % for curcumin, 17.2 % for resveratrol, and 3.5 and 12.7 % for curcumin and resveratrol, respectively, for the combined system. In the immunoliposomed system, molar percentage of total lipid including cholesterol ranged from 77 to 94 %, and determined percentages of drugs were 6.5 % for curcumin, 5.8 % for resveratrol, and 5.7 and 1.7 % for curcumin and resveratrol, respectively, for the combined system. In case of the coadministration of the compounds, the final molar ratio of curcumin:resveratrol was different than 1:1 in the different formulations due to the different solubility and lipid affinity properties of the compounds. The final obtained ratios measured by HPLC–DAD were 1:1.6 in the free system, 1:3.6 for liposomed system and 1:3.1 for immunoliposomed system (values obtained from three independent preparations).
Liposome size determination
The Zetasizer Nano ZS (Malvern Instruments Ltd, UK) was used to determine the mean vesicle size, size distribution, and polydispersity of the liposomes with the following conditions: 80 s sampling time, 1.33 refractive index, 173° scattering angle, and a temperature of 37 °C. The measurements were performed using LUVs suspensions of either liposomes, liposomes containing the compounds or immunoliposomes. Final results derived from three independent series of measurements made by 12 replicates. Only the results that showed a polydispersity index (PDI) <0.2 were considered as valid. The typical Nano software DTS version 5.00 was utilized.
HPLC analysis
The amounts of curcumin and resveratrol encapsulated into liposomes were analyzed and quantified, according to previously reported methods [26, 27], using an Agilent LC 1100 series (Agilent Technologies, Inc., Palo Alto, CA, USA) controlled by the Chemstation software and equipped with a pump, autosampler, column oven, and fluorescence (FL) and UV–diode array detector. For curcuminoids, an isocratic method was used: the mobile phase consisted of methanol, isopropyl alcohol, water, and acetic acid in the proportions 20:27:48:5 v/v. The flow rate was 0.5 ml/min, and the samples were run for 25 min for peak identification. The isocratic elution was monitored with fluorescence detector at a wavelength of excitation of 420 nm and emission of 540 nm. For trans-resveratrol, the analysis was performed using a linear gradient between 10 % methanol in water (solvent A) and 90 % methanol in water (solvent B). The flow rate was 0.6 ml/min with a linear gradient from 0 to 90 % B in 25 min. Diode-array detection was set at 304 nm. The software ChemStation for LC 3D (Agilent Technologies Life Sciences and Chemical Analysis, Waldbronn, Germany) was used for quantization purposes. Quantitative evaluation of the compounds was performed by means of a six-point regression curve (R 2 > 0.996) in a concentration range between 0.02 and 0.8 mM, using external standards of purity ≥90 % (Sigma-Aldrich Co.) at known concentrations. Representative chromatograms for curcumin or resveratrol detection and quantitation are included in Supplementary information.
Cell cultures
JIMT1 cell line, a high-grade invasive ductal carcinoma positive to HER-2 receptor, but resistant to the conventional therapy with trastuzumab [28, 29], was kindly provided by Institut Català d’Oncologia (Girona, Spain). The MCF7 breast cancer cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA). Both cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with Glutamax (GIBCO), supplemented with 50 units/ml penicillin, 50 μg/ml streptomycin (GIBCO), and 10 % of heat-inactivated fetal bovine serum (FBS) (GIBCO). The cells were subcultured every 2–3 days, maintained in a monolayer in T25 or T75 flasks (Sarstedt, Spain), and incubated at 37 °C in an atmosphere of 95 % air and 5 % CO2.
Cytotoxicity assay
Cytotoxicity of curcumin and/or resveratrol as free drugs, liposomed, and immunoliposomed preparations against JIMT1 and MCF7 cells was studied. Cell suspensions were seeded into 96-well microplates at a density of 1.5–2.0 × 104 cells/well; the cells were then incubated for 24 h, until approximately 50 % confluence and treated with the different formulations. After 72 h of additional incubation at 37 °C, 5 % CO2, the medium was aspirated, and the cytotoxicity of the various preparations was evaluated by the addition of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide (MTT) solution (total vol. was 100 μl/well). After 3 h of incubation at 37 °C, 5 % CO2, cell survival was estimated using an absorbance spectrophotometer microplate reader (SPECTROstar Omega, BMG LABTECH) measuring absorbance at 570 nm with background correction at 620 nm. The absorbance was proportional to the number of living cells in each well. The cytotoxic IC50 values (inhibitory concentration 50 %) for the drugs were determined from log concentration–effect curves in GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA), using nonlinear regression analysis. The data presented are the mean of three data sets in which each condition was assayed in eight replicates. The values of standard deviation (±SD) are presented as error bars.
Western blot analysis of HER2 expression
The cells were washed twice with PBS, scraped from the plate and centrifuged at 1,500 rpm for 5 min. The cellular pellets were lysed in lysis buffer containing 50 mM Tris pH 7.4, 1 % Igepal CA-630, 150 mM NaCl, 5 mM EDTA, and 10 mg/ml protease inhibitor cocktail (Sigma-Aldrich, Europe). The cells were kept on ice for 20 min and, after a freezing/thawing cycle, they were centrifuged at 12,000 rpm for 5 min. The cellular pellets were discarded, and the protein content of the cell extracts (supernatant) was measured by Bradford assay (Bio-Rad, Richmond, CA, USA). Then, 50 μg of protein from each lysate was used for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), transferred to nitrocellulose membranes, incubated with primary monoclonal antibodies against HER2 (sc-284, Santa Cruz Biotechnology, INC) and incubated with horseradish peroxidase-linked secondary antibodies (Sigma-Aldrich, Europe). Proteins were detected by the enhanced chemiluminescence (ECL) method (Amersham International, Buckinghamshire, UK). Densitometric analyses were performed using the SigmaGel gel analysis software (Jandel Scientific, Chicago, IL, USA).
Flow cytometry and ImageStream analysis
MCF7 and JIMT1 cells were trypsinized and washed repeatedly with PBS-EDTA 0.5 % to obtain single cell suspensions. Then, 1 × 106 cells of each cell line were fixed in 3.5 % Formalin (Sigma-Aldrich Co., St. Louis, MO) during 5 min at 4 °C. After three cycles of washing with PBS-EDTA 0.5 %, the cells were blocked for 30 min in PBS+BSA 2 % at RT. Once the blocking solution was removed, the cells were incubated overnight at 4 °C with gentle agitation with the anti-human HER2/neu antibody (sc-284, Santa Cruz Biotechnology, INC) at a concentration of 0.5 μg/105 cells. The cells were washed three times with PBS before proceeding with the secondary antibody staining using anti-rabbit FITC-conjugated IgG (F-2765, Molecular Probes) at a dilution of 1:25 for 30 min at RT. As a control, the secondary antibody alone was used after blocking to show nonspecific binding of the IgG to cells. After washing and recollecting the cells in PBS, the FITC-stained cells were quantitated by flow cytometry in an Epics XL instrument (Beckman Coulter Co., Miami, Florida) by analyzing the intensity of the green fluorescence associated to cells.
To assess the cell binding/interaction and selectivity of the immunoliposomes to the cells bearing different HER2 expression levels, flow cytometry (FACS) analysis was performed. For immunoliposomes, MCF7 and JIMT1 cells were plated into a T25 flask (1 × 106 cells/ml); and 24 h later, they were incubated with free curcumin, liposomed curcumin and curcumin encapsulated into immunoliposomes labeled with rhodamine-PE. After 2–3 h of incubation, the medium was discarded, and the cells were washed three times with cold PBS. The cells were detached following 5 min of incubation with a trypsin solution (0.25 %) at 37 °C, centrifuged at 1,000 rpm and resuspended in PBS.
The cellular uptake/attachment efficiency was determined by the ImageStream multispectral imaging flow cytometer (Amnis Corporation, Seattle, WA) using 488-nm laser excitation. Classifiers were set when needed, such as to eliminate the collection of debris (based on the low area in the bright-field imagery), to eliminate clusters of cells (based on the high area in bright-field imagery), and also camera saturating events (based in the presence of peak intensities >1022). For all samples, imagery excluded by classifiers was observed prior to data collection to ensure that events of interest were not lost. Typical files contained imagery for 5,000–9,000 cells, with each cell imaged with side-scatter, bright-field, and a channel of fluorescence (curcumin stain). Images of cells collected on the ImageStream were analyzed using ImageStream Data Exploration and Analysis Software (IDEAS). The quantitative measurement of liposome cell binding was calculated using features available in IDEAS. The analysis of the fluorescence intensity of curcumin incorporated to cells was conducted in focus-single cells. This classification was performed based on their small bright-field area, high bright-field aspect ratio, and high nuclear contrast (as measured by the gradient max feature).
Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA). The results are presented as the mean ± standard deviation. The statistical significance of each experiment was determined using Student’s t test. Differences between two or more groups were compared using non-parametric tests and were considered statistically significant when p < 0.05.
Results
The aim of this study was to test whether the cytotoxicity of known chemopreventive compounds, such as curcumin and resveratrol, was enhanced by vehiculization into liposomes and to determine if selectivity was achieved by liposome immunolabeling in HER2 overexpressing breast cancer cells. For that purpose, curcumin, resveratrol, and a mixture of both components were tested in their free from, incorporated into liposomes or incorporated into immunoliposomes against JIMT1 and MCF7 cell lines. These cell lines were chosen as cellular models for two main reasons: for their particular clinical relevance and for their different level of HER2-expression. Many breast cancers depend on estrogenic hormones for their development and growth, i.e., the MCF7 cell line shows a positive estrogen receptor expression and supposes a model for these clinical cases. On the other hand, the JIMT1 cell line, lacking expression of both estrogen and progesterone receptors, represents clinical hormone-independent model. JIMT1 shows gene amplification and overexpression of HER2 [29], but at the same time, it is resistant to the conventional therapy used for HER2-overexpressing breast cancers based on trastuzumab and other HER2 inhibiting drugs [28, 29]. In contrast, the MCF7 cell line presents a very low HER2 expression level.
Expression of HER2 receptor on breast cancer cell lines
The first step was to study the differential HER2 expression in the two human breast cancer cells: JIMT1 and MCF7. Although both cell lines are from tumorigenic origin, they differ in their hormone dependence, growth conditions, appearance, and HER2 expression. Therefore, HER2 expression levels were checked first using western blotting. Protein extracts were obtained from cell lysates and processed as described in the methods. Figure 1a shows the blot results of the quantification of the relative expression levels; the HER2 expression is approximately 20 times higher in JIMT1 than in MCF7 (p < 0.05). Furthermore, to demonstrate that the HER2 receptor detected by western assays was present on cell surface, FACS was used. JIMT1 and MCF7 cells were stained with an anti-HER2/neu antibody that binds HER2 as described in the methods. The results for HER2 surface detection by flow cytometry are shown in Fig. 1b, and confirm that the higher HER2 expression observed in JIMT1 cells (gray histogram) is accompanied with the higher membrane localization of the receptor when compared with MCF7 (black histogram). Control cells stained only with the secondary antibody showed a similar pattern for both cell lines, as previously reported [22]. This substantial difference in the expression of the receptor in the two cell types was used to test an immune-directed delivery system based on using immunoliposomes to deliver curcumin and/or resveratrol in these cancer cells.
a Whole protein lysates from JIMT1 and MCF7 breast cancer cells were prepared and analyzed by Western blot and densitometric analysis to quantify total HER2 expression. Asterisk represents significant difference compared to MCF7 cells (*p < 0.05). β-Actin was used as loading control. b Flow cytometry analysis on the same cell lines stained with an anti-HER2/neu antibody, JIMT1 (gray histogram) and MCF7 (black histogram)
Design and characterization of the liposomes and immunoliposomes formulations
Preparations of liposomes and immunoliposomes were obtained following the protocols described in the methods and characterized in size using a dynamic light scattering technique. All immunoliposome preparations were characterized in size, and also in lipid, antibody, and compounds content. Size distribution of the immunoliposomes yielded an average size of ~100 nm on the day of the preparation with a polydispersity index of <0.2 (Supplementary information, Fig. 1). The amount of antibody present in the immunoliposomes and the antibody/liposome ratio were determined as previously reported [22], showing an average of 31.5 ± 3.2 ng Ab/nmol lipid (~0.216 nmol Ab/μmol lipid). The stability of the liposomes and immunoliposomes was also tested by determining size, lipid integrity, and total antibody amount coupled to the liposomes (data not shown for briefness). No significant changes were observed after 2 weeks of storage at 4 °C compared to the day of the preparation. The amount of the compounds encapsulated or bound to the liposomes or immunoliposomes were determined in every assay by liquid chromatography analysis (Supplementary information, Fig. 2). Control experiments showing the absence of effect of treatments with trastuzumab or immunoliposomes lacking drugs on MCF7 and JIMT1 cell lines were obviated, since this has been already proven [22].
Cytotoxic effects of free-, liposomed-, and immunoliposomed-formulations containing anticancer compounds
Curcumin formulations
The different formulations were prepared as described in the methods, and the cells were incubated for 72 h. Then, cytotoxicity was measured, and IC50 values were obtained for all the treatments (Table 1).
Free curcumin showed a similar cytotoxicity value against both cell lines, as observed from their calculated IC50 values, independently of the HER2 expression level. The encapsulation of curcumin into liposomes improved the IC50 value by almost twice for both the MCF7 and JIMT1 cell lines, once again, independently of HER2 expression. However, the antibody attachment to liposomes led to different results, depending on HER2 expression. First, cells with higher HER2 expression had a more dramatic IC50 reduction. The IC50 value for JIMT1 dropped 75 % of its value when liposomes and immunoliposomes carrying curcumin were compared (p < 0.001), while a 30 % decrease was observed for MCF7 cells (p < 0.01), see Table 1 for detailed values. These numeric results can be easily confirmed when survival plots for the different treatments are compared (Fig. 2a). The encapsulation of curcumin into liposomes induced a left-shift of the curves in both cell lines (pink trace compared with orange trace), but the use of immunoliposomes (red trace) carrying HER2 antibody induced an additional left-shift compared to liposomes only in the case of JIMT1 cells, indicating a higher efficacy and, therefore, bigger selectivity of the immunoliposomes in correlation to the HER2 expression of the cells.
Resveratrol formulations
Free resveratrol behaved differently compared to free curcumin. Its cytotoxic activity was a little stronger in MCF7 cells compared to JIMT1 cells. This was most likely due to some mechanistic differences between these compounds, as resveratrol is implicated in cell estrogenic response [30], which is more relevant in MCF7 cells (estrogen-dependent) than in JIMT1 cells (estrogen-independent). Similar to the results observed with curcumin, resveratrol encapsulation into liposomes reduced the IC50 values for both cell lines (see Table 1; Fig. 2b), i.e., decreased to 31 % in JIMT1 cells and to 42 % in MCF7 cells, compared to free compounds. In contrast, different results were obtained between the two cell types when resveratrol was immunoliposomed. No significant differences were observed between liposomed and immunoliposomed resveratrol in MCF7 cells (p > 0.01). In contrast, a significant decrease (35 %) was observed between liposomes and immunoliposomes when compared in JIMT1 cells (p < 0.01) (Table 1; Fig. 2b). Therefore, the encapsulation of resveratrol into immunoliposomes also increased its efficacy and selectivity against HER2 overexpressing cells, similar to curcumin, but this effect was not observed in the MCF7 low HER2 expression cells.
Combined curcumin/resveratrol formulations
Combinations containing both curcumin and resveratrol were intended to bear a 1:1 molar ratio of the two compounds. Nevertheless, due to the different lipid affinity of the compounds, after the incubation of the compounds with the phospholipid vesicles, the final obtained ratios measured by HPLC–DAD were 1:1.6 for the free system, 1:3.6 for the liposomed system and 1:3.1 for the immunoliposomed system; these findings denote the higher binding capacity of resveratrol for the phospholipid vesicles.
When combined in their free form, curcumin and resveratrol showed a similar effect to those previously observed for individual compounds, i.e., a higher cytotoxic effect on MCF7 cells (Table 1; Fig. 2c), suggesting the higher contribution of resveratrol in this effect. Liposome encapsulation of the two compounds slightly changed the cytotoxicity in JIMT1 cells, but it decreased the IC50 value to almost 50 % of its original value for MCF7 cells. When both compounds were immunoliposomed, the IC50 value decreased ~14 times compared to the liposomed system in JIMT1 cells (p < 0.01), indicating a drastic enhancement of their efficacy in this system. In contrast, no significant change (p > 0.05) (Table 1) was observed in the cytotoxicity of both compounds when MCF7 HER2-negative cells were treated with liposomes or immunoliposomes.
Internalization and uptake studies of curcumin on breast cancer cell lines
Cytotoxicity results indicated that the system using immunoliposomes resulted in a significant lowering of IC50 values, indicating a higher toxicity in cell populations overexpressing the target HER2. To further examine the selectivity of the immunoliposomes, a comparison of the in vitro cell binding and internalization analyses of the formulations were performed on the HER2-positive cells, JIMT1, and on the HER2-negative control cell line, MCF7. Curcumin was preferred over resveratrol due to its strong auto-fluorescence that allows its internalization to be easily measured by the ImageStream multispectral imaging flow cytometry (MIFC) technique. To evaluate the level of internalization of curcumin, cultured cells were incubated with the three different (delivery) formulations of curcumin. The drug’s accumulation in the different cell lines was measured using a novel image-based flow cytometric approach (MIFC), which has greater specificity and sensitivity than standard flow cytometry methods. We previously reported that immunoliposomes bearing trastuzumab exhibited a much higher level of binding in HER2 overexpressing cell lines than the liposome formulations lacking the antibody [22].
The cellular uptake of curcumin was measured through the determination of curcumin fluorescence intensity associated to cells after the cells were incubated for 2 h with either free curcumin, liposomed curcumin, or curcumin incorporated into immunoliposomes (Table 2; Fig. 3). First, MCF7 cells showed an enhanced capacity to accumulate curcumin compared to JIMT1 cells (Table 2; Fig. 3). Further uptake analysis revealed that liposomed curcumin showed a higher accumulation of curcumin fluorescence in both cell lines when compared to free curcumin. Moreover, no significant change of the curcumin fluorescence uptake was observed in MCF7 cells treated with immunoliposomed curcumin compared to those treated with liposomed curcumin (Table 2; Fig. 3a). In contrast, when curcumin was loaded into HER2-targeted immunoliposomes, a significant increase of the percentage of gated cells (curcumin stained) was observed only in the HER2 overexpressing JIMT1 breast cancer cells (Table 2; Fig. 3b).
Representative images of a MCF7 and b JIMT1 cells treated with either free curcumin, liposomed curcumin or curcumin incorporated into anti-HER2-immunoliposomes. Images were selected randomly from files of 5,000–9,000 images. Channels represent images of darkfield (DF), bright-field (BF) and curcumin (Curc) fluorescence
Discussion
In this work, the potential anticancer capacity of two natural compounds, curcumin, and resveratrol, against two different human breast cancer cell lines has been studied using different drug delivery systems: free compounds, compounds loaded into liposomes or compounds loaded into immunoliposomes bearing Herceptin, an anti-HER2 antibody.
Liposomes and other specialized nanostructures, such as immunoliposomes, have been successfully used in previous studies [22, 23, 31–33] to improve the bioavailability, efficacy, and/or selectivity of potential anticarcinogenic compounds. When compared with free compounds, non-targeted liposomes offer the advantage of a long plasma half-life, and the ability to release the encapsulated drug over an extended period of time [17, 18]. However, they only passively target diseased tissues, mainly due to differences in their vascular architecture and permeability. Non-targeted liposomes preferentially localize in the tumor stroma and in tissue macrophages, but rarely reach the tumor cells [34, 35]. Moreover, efficacy of non-targeted liposomes is also compromised because drug uptake by tumor cells mainly occurs following the release of the encapsulated drug from the liposomes into the tumor interstitial space, from where drug uptake into non-target cells may also occur, as well as drug redistribution out of the tumor. In contrast, targeted immunoliposomes are predominantly found internalized in tumor cells within a broad area of distribution in the tumor tissue [35]. Tumor-targeted drug delivery, mediated by the binding of the liposomes to a tumor-associated antigen with a high rate of endocytosis, such as HER2, represents a rational approach, not only to allow more of the encapsulated drug into target cells, but also to increase the specificity of drug uptake. In the present study, we selected HER2 as the target of choice for human breast cancer cells to build immunoliposomes containing anticancer compounds for several reasons: it is overexpressed in 20–30 % of breast and ovarian cancers [4] and is pathologically associated with aggressive disease, increased risk of relapse and poor long-term survival [6, 7]. Immunoliposomes have successfully been used in preclinical studies to deliver anticancer agents to different tumor types by binding to various antigens on the cell surface [34, 36–38]. Additionally, the successful large-scale production of HER2-specific immunoliposomes has finally paved the way for the clinical use of tumor-targeted nanovesicles [39].
First, in our cellular system, free curcumin was more effective at inhibiting cell proliferation than resveratrol despite its poor solubility. Whereas curcumin had an almost identical effect in both cell lines, resveratrol was more effective against the hormone-dependent MCF7 cell line. This is most likely because the mechanism of action of resveratrol is related to the modulation of ER-dependent cellular growth. However, resveratrol has shown the capability to decrease proliferation in both ER-positive and ER-negative breast cancer cells, which suggests the participation of additional effects, such as activation of sirtuin-1 (SIRT1). SIRT1 functions as a novel upstream regulator for AMPK signaling (AMP activated protein kinase) that suppresses mTOR (mammalian target of rapamycin) oncogenic transformation, as has been recently reported [40, 41]. The combinations of the two compounds in their free form did not improve the cytotoxic effect. On the contrary, the incorporation of curcumin or resveratrol into liposomed formulations significantly increased the cytotoxic effect in both cell lines, indicating an enhanced intracellular release of the compounds most likely through membrane fusion events.
The incorporation of the compounds into liposomes decreased the IC50 values ~1/2–1/3 of the original value obtained with the compounds in their free form. The use of immunoliposomes dropped these values even more when compared to those obtained with the liposomed forms, more so for curcumin than for resveratrol, and especially for the HER2 positive JIMT1 cells. The immunoliposomed combination of curcumin and resveratrol was extremely effective in the HER2-positive JIMT1 cell line compared to liposomed or free forms (IC50 value decreased to 7 % when comparing liposomed and immunoliposomed forms), revealing that this system allows both compounds to reach their intracellular antiproliferative targets. Therefore, our results also confirm that the anticancer drugs, when loaded onto HER2-immunoliposomes, are selectively targeted to HER2-positive JIMT1 cells in vitro, resulting in a significantly greater inhibition of cell proliferation than that obtained for non-targeted liposomes or free drugs. In contrast, no significant differences on the inhibition of cellular proliferation were observed when liposomal formulations and immunoliposomes were compared for the HER2-negative cell line MCF7.
The specificity of immunoliposomes to HER2-overexpressing cells was also correlated using the novel ImageStream technique, a multispectral imaging cytometer which allows the user to obtain numeric results of curcumin accumulation in the different cell lines (Table 2) as well as darkfield, bright-field, and fluorescent images of each analyzed cell (Fig. 3). The percentage of curcumin-stained cells after the treatment with liposomed curcumin was between 2.6 and 4.4-fold higher, in MCF7 and JIMT1 cells, respectively, than those for free curcumin treatments; these findings suggest that the incorporation of curcumin into the two breast cancer cell models was more effective when this compound was administered in liposomed form, in correlation to its higher cytotoxicity (Fig. 2; Table 1). When immunoliposomed curcumin was used, curcumin accumulation into MCF7 cells did not increase when compared to its liposomed form. In contrast, accumulated curcumin increased 1.7 times when liposomes and immunoliposomes were compared on the HER2-positive JIMT1 cell line. This was monitored by the intracellular curcumin location in the fluorescence images of isolated cells (Fig. 3). These results are also in agreement with the cytotoxicity results of the different formulations using curcumin (Fig. 2; Table 1).
It has been reported that curcumin exerts is antiproliferative and/or apoptotic anticancer effects through the modulation of the ERK or NF-κB-mediated pathways as well as the PI3 K/Akt signaling pathway [14, 42], which are closely related to tumor formation and progression. However, resveratrol seems to be related to the activation of anti-aging/cellular stress-like genes, such as sirtuin-1 and NRF2 signaling, which leads to activation of AMPK, a suppressor of mTOR [40, 41]. Most diseases, and especially cancer, are a result of the disregulation of many different gene products [43]; hence, the modulation of multiple signaling pathways is the most suitable strategy for treatment. Considering the abovementioned complementary actions of these phytochemicals, their combination into nanoformulations may be a promising cancer therapy alternative [31, 33].
In conclusion, the present study demonstrates that HER2-targeted immunoliposomes coupled to trastuzumab dramatically increase the antiproliferative effects of curcumin and resveratrol in HER2 positive human breast cancer cells compared to regular liposomed or free forms, indicating an increase of the therapeutic effect of this special delivery nano-formulation. The enhancement of the cytotoxic effects also correlated to the uptake of curcumin at an intracellular level. The efficacy and selectivity of the combination of both compounds in the same immunoliposomes formulated against HER2 positive breast cancer cells revealed an intriguing mechanism of action that deserves further attention. This study clearly shows the potential of HER2-targeted nanovesicles to enhance the antitumor effect of drugs at low, well-tolerated dose levels and suggests further in vivo investigations using xenograft mice to assess the clinical potential of this nanovesicular drug delivery system for cancer therapy.
Abbreviations
- Chol:
-
Cholesterol
- Curc:
-
Curcumin
- D200M:
-
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide (PEG) 2000]
- DLS:
-
Dynamic light scattering
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DMSO:
-
Dimethylsulfoxide
- DSPE-PEG:
-
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-PEG-2000
- ER:
-
Estrogen receptor
- EYPC:
-
Egg yolk phosphatidylcholine
- FACS:
-
Fluorescent activated cell sorting
- FBS:
-
Fetal bovine serum
- FL:
-
Fluorescence
- HER2:
-
Human epidermal growth factor receptor 2
- HPLC:
-
High performance liquid chromatography
- IC50 :
-
Inhibitory concentration 50 %
- MIFC:
-
Multispectral imaging flow cytometry
- MLVs:
-
Multilamellar vesicles
- LUVs:
-
Unilamellar vesicles
- mAb:
-
Monoclonal antibody
- MTT:
-
(3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide)
- N2 :
-
Nitrogen
- PDI:
-
Polydispersity index
- PEG:
-
Polyethylene glycol
- RES:
-
Reticuloendothelial system
- Resv:
-
Resveratrol
- SD:
-
Standard deviation
- THIS:
-
Histidine buffer
References
Yamamoto T et al (1986) Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature 319(6050):230–234
Bargmann CI, Hung MC, Weinberg RA (1986) The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature 319(6050):226–230
Press MF, Cordon-Cardo C, Slamon DJ (1990) Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene 5(7):953–962
Venter DJ, Fau-Tuzi NL et al (1987) Overexpression of the c-erbB-2 oncoprotein in human breast carcinomas: immunohistological assessment correlates with gene amplification. Lancet 2:69–72
Mitchell MS, Press MF (1999) The role of immunohistochemistry and fluorescence in situ hybridization for HER-2/neu in assessing the prognosis of breast cancer. Semin Oncol 26(4 Suppl. 12):108–116
Slamon DJ, Clark GM, Wong SG (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182
Fau PM et al (2000) The molecular and cellular biology of HER2/neu gene amplification/overexpression and the clinical development of herceptin (trastuzumab) therapy for breast cancer. Cancer Treat Res 13:57–75
Carter P et al (1992) Humanization of an anti-p185(HER2) antibody for human cancer therapy. Proc Natl Acad Sci USA 89(10):4285–4289
Cobleigh MA et al (1999) Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17(9):2639–2648
Slamon DJ et al (2001) Use of chemotherapy plus a monoclonal antibody against her2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792
Filomeni G et al (2007) Trans-resveratrol induces apoptosis in human breast cancer cells MCF-7 by the activation of MAP kinases pathways. Genes Nutr 2(3):295–305
Karunagaran D, Rashmi R, Kumar TRS (2005) Induction of apoptosis by curcumin and its implications for cancer therapy. Curr Cancer Drug Targets 5(2):117–129
Amri A et al (2012) Administration of resveratrol: what formulation solutions to bioavailability limitations? J Control Release 158(2):182–193
Kunnumakkara AB, Anand P, Aggarwal BB (2008) Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett 269(2):199–225
Coimbra M et al (2011) Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int J Pharm 416(2):433–442
Coimbra M et al (2012) Critical factors in the development of tumor-targeted anti-inflammatory nanomedicines. J Control Release 160(2):232–238
Klibanov AL et al (1990) Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett 268(1):235–237
Papahadjopoulos D et al (1991) Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci USA 88(24):11460–11464
Ahmad I et al (1993) Antibody-targeted delivery of doxorubicin entrapped in sterically stabilized liposomes can eradicate lung cancer in mice. Cancer Res 53(7):1484–1488
Huwyler J, Yang J, Pardridge WM (1997) Receptor mediated delivery of daunomycin using immunoliposomes: pharmacokinetics and tissue distribution in the rat. J Pharmacol Exp Ther 282(3):1541–1546
Reddy KR (2000) Controlled-release, pegylation, liposomal formulations: new mechanisms in the delivery of injectable drugs. Ann Pharmacother 34(7–8):915–923
Barrajon-Catalan E et al (2010) Selective death of human breast cancer cells by lytic immunoliposomes: correlation with their HER2 expression level. Cancer Lett 290(2):192–203
Barrajón-Catalán E et al (2011) Immunoliposomes: A Multipurpose Strategy in Breast Cancer Targeted Therapy, in Breast Cancer—Current and Alternative Therapeutic Modalities, Intech, Editor, p 435–452
Vemuri S, Rhodes CT (1995) Preparation and characterization of liposomes as therapeutic delivery systems: a review. Pharm Acta Helv 70(2):95–111
Hansen CB et al (1995) Attachment of antibodies to sterically stabilized liposomes: evaluation, comparison and optimization of coupling procedures. Biochim Biophys Acta 1239(2):133–144
Green CE et al (2008) Extraction, processing, and storage effects on curcuminoids and oleoresin yields from Curcuma longa L. grown in Jamaica. J Agric Food Chem 56(10):3664–3670
Chen L et al (2001) High-speed counter-current chromatography separation and purification of resveratrol and piceid from Polygonum cuspidatum. J Chromatogr A 907(1–2):343–346
Rennstam K et al (2007) Cytogenetic characterization and gene expression profiling of the trastuzumab-resistant breast cancer cell line JIMT-1. Cancer Genet Cytogenet 172(2):95–106
Tanner M et al (2004) Characterization of a novel cell line established from a patient with Herceptin-resistant breast cancer. Mol Cancer Ther 3(12):1585–1592
Bhat KPL et al (2001) Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res 61(20):7456–7463
Falco A et al (2013) Melittin-loaded immunoliposomes against viral surface proteins, a new approach to antiviral therapy. Antiviral Res 97(2):218–221
Munin A, Edwards-Lévy F (2011) Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 3(4):793–829
Narayanan NK et al (2009) Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer 125(1):1–8
Huang SK et al (1992) Microscopic localization of sterically stabilized liposomes in colon carcinoma-bearing mice. Cancer Res 52(19):5135–5143
Park JW et al (2002) Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res 8(4):1172–1181
Kirpotin D et al (1997) Sterically stabilized anti-HER2 immunoliposomes: design and targeting to human breast cancer cells in vitro. Biochemistry 36(1):66–75
Mamot C et al (2005) Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res 65(24):11631–11638
Sapra P et al (2004) Improved therapeutic responses in a xenograft model of human B lymphoma (Namalwa) for liposomal vincristine versus liposomal doxorubicin targeted via Anti-CD19 IgG2a or Fab fragments. Clin Cancer Res 10(3):1100–1111
Nellis DF et al (2005) Preclinical manufacture of an anti-HER2 scFv-PEG-DSPE, liposome-inserting conjugate. 1. Gram-scale production and purification. Biotechnol Prog 21(1):205–220
Lin JN et al (2010) Resveratrol modulates tumor cell proliferation and protein translation via SIRT1 -dependent AMPK activation. J Agric Food Chem 58(3):1584–1592
Menendez JA, Fau-Joven J et al (2013) Xenohormetic and anti-aging activity of secoiridoid polyphenols present in extra virgin olive oil: A new family of gerosuppressant agents. Cell Cycle 12(4):555–578
Reuter S et al (2008) Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochem Pharmacol 76(11):1340–1351
Vogelstein B, Kinzler KW (2004) Cancer genes and the pathways they control. Nat Med 10(8):789–799
Acknowledgments
This investigation has been supported by Grants AGL2011-29857-C03-03 from MICINN, PROMETEO/2012/007 and ACOMP/2013/093 from GV, and CIBER (CB12/03/30038, Fisiopatología de la Obesidad y la Nutrición, CIBERobn, Instituto de Salud Carlos III), Spain. A. Catania was supported by Ministero della Pubblica Istruzione and sponsored under the Joint Doctorate Program in Biotechnology between Catania University and Institute of Molecular and Cell Biology. We thank the personnel of Elche University Hospital for their advice and help in the ImageStream analysis and for providing us with the commercial antibody. We also thank Dra. Inmaculada Jiménez from Elche University Hospital and Dr. Jose Solla from Alicante University for their invaluable help.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All the experiments comply with the current laws of the country in which they were performed (Spain).
Author information
Authors and Affiliations
Corresponding author
Additional information
Angela Catania and Enrique Barrajón-Catalán have contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Catania, A., Barrajón-Catalán, E., Nicolosi, S. et al. Immunoliposome encapsulation increases cytotoxic activity and selectivity of curcumin and resveratrol against HER2 overexpressing human breast cancer cells. Breast Cancer Res Treat 141, 55–65 (2013). https://doi.org/10.1007/s10549-013-2667-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2667-y