Abstract
Ceramicines are a series of limonoids that were isolated from the bark of Malaysian Chisocheton ceramicus (Meliaceae) and were known to show various biological activity. Four new limonoids, ceramicines Q–T (1–4) were isolated from the barks of C. ceramicus, and their structures were determined on the basis of the 1D and 2D NMR analyses in combination with calculated 13C chemical shift data. Ceramicines Q–T (1–4) were established to be new limonoids with a cyclopentanone[α]phenanthren ring system with a β-furyl ring at C-17, and without a tetrahydrofuran ring like ceramicine B, which is characteristic of known ceramicines. Ceramicine R (2) exhibited potent antimalarial activity against Plasmodium falciparum 3D7 strain with IC50 value of 2.8 µM.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The plants belonging to Meliaceae have been reported to produce limonoids [1]. In our search for new bioactive compounds from medicinal plants, we have reported the isolation of new limonoids from plants of this genus [2,3,4,5,6,7,8,9,10,11,12,13,14,15], and alkaloids [16,17,18,19,20,21,22,23] and coumarins [24] showing antimalarial activity or inhibiting acetylcholinesterase. Ceramicine B, in particular, has been reported to show a strong lipid droplets accumulation (LDA) inhibitory activity on mouse pre-adipocyte cell line (MC3T3-G2/PA6) [6,7,8] and also anti-melanin deposition activity [9]. With the purpose of discovering limonoids with biological activity, we further investigated the constituents of C. ceramicus which led to the isolation of four new limonoids, ceramicines Q–T (1–4), and ceramicine R (2) showed potent antimalarial activity (Fig. 1). Their structures were determined on the basis of NMR and MS spectroscopic analyses in combination with NMR chemical shifts calculations.
Results and discussions
Compounds 1–4 were obtained as optically active white amorphous solids. The 1H and 13C NMR data (Tables 1 and 2) suggested the identity of 1–4 as ceramicine derivatives.
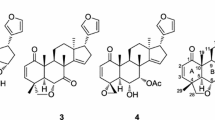
Ceramicine Q (1) was obtained as an optically active, \([{\upalpha }]_{{\text{D}}}^{27}\) + 119 (c 1.0, CHCl3), white amorphous solid and was revealed to have the molecular formula C25H30O5, by HRESITOFMS [m/z 433.1991 (M + Na)+, ∆ − 1.4 mmu]. IR absorptions implied the presence of α,β-unsaturated ketone (1683 cm−1) and hydroxy (3461 cm−1) groups. 1H and 13C NMR data (Table 1) revealed 25 carbon resonances due to one carbonyl, two sp2 quaternary carbons, four sp3 quaternary carbons, six sp2 methines, five sp3 methines, four sp3 methylenes, and three methyls. Among them, four sp3 carbons (δC60.4, 60.4, 66.7, and 74.3) and two sp2 methines (δC 139.7 and 142.5) were ascribed to those bearing an oxygen atom.
Analyses of the HSQC and 1H-1H COSY spectra (Fig. 2) revealed the presence of four partial structures; a (C-2 and C-3), b (C-5 ~ C-7), c (C-9, C-11, and C-12), d (C-15 ~ C-17), and e (C-22 ~ C-23). HMBC correlations of H3-18 to C-12, C-13, C-14 and C-17 suggested the connectivity of c, d, and C-14 through C-13. HMBC correlations of H-17 to C-21, H-16 to C-20, and H-23 to C-20 and C-21 suggested the presence of β-furyl at C-17, and the correlation of H2-16 to C-13 and C-14 completed the structure of ring D. The presence of ring C was deduced from the HMBC cross-peaks of H3-30 to C-7, C-8, C-9 and C-14, and the connectivity of b, C-1, C-5, and C-19 through C-10 was suggested by the HMBC correlations of H3-19 to C-1, C-5, C-9 and C-10. HMBC correlations of H-2 to C-10 and C-4, and H-3 to C-1 and C-5 suggested the presence of ring A. Finally, HMBC correlations of H-3 to C-29 (δC 60.4) suggested the presence of 1-oxaspiro[2.5]oct-4-ene of 1 as shown in Fig. 2.
The relative configuration of 1 was assigned by analyses of the 1H-1H coupling constant data and the NOESY correlations (Fig. 2). First, H-6, H-17, CH3-19, and CH3-30 were assigned to be β-axially oriented from the NOESY correlations of H-6/H3-19 and H3-30/H-17 and H3-19, while H-5, H-9 and CH3-18 were deduced to possess α-orientation from the NOESY correlations of H-9/H-5 and H3-18. Both H-6 and H-7 should possess β-orientation because of the multiplicity pattern of H-6 (dd, 10.8, 2.4) and H-7 (d, 2.4). The stereochemistry of epoxy ring was elucidated to be as shown in Fig. 2 by the presence of NOESY correlations of H-29a/H-3 and H3-19, and H-29b/H3-19.
Ceramicine R (2), \([\upalpha ]_{{\text{D}}}^{28}\) 126 (c 0.5, CHCl3), was revealed to have the molecular formula C25H30O5 by HRESITOFMS. Its NMR data are highly similar to 1. However, the signals for oxymethylene of H2-29 in 1 are not observed in 2, and a methyl signal (δH 1.86) and two sp3 quaternary carbons bearing an oxygen atom (δC 55.6 and 68.0) are observed instead. Analysis of the 2D NMR data including HMBC and NOESY (Fig. 3) correlations supported the structure of 2 to be 4,5-epoxy derivative as shown in Fig. 1. Specifically, the HMBC correlations of H3-29 to C-3, C-4 (δC 55.6) and C-5 (δC 68.0), and H3-19 to C-5 supported its functionality in the structure of 2.
NOESY correlations of H-6/H3-19 and H3-30, H-7/H3-30 and H-15, and H-9/H3-18 supported the stereochemistry of 2. Configurations of H-6 and H-7 taking β-orientation like as in 1 also supported by proton 3J coupling constant (J = 2.7 Hz).
The remaining problem regarding stereochemistry is that of epoxy in positions 4 and 5. Only the presence of two NOESY correlations of H-3/H3-29 and H-6/H3-19 is difficult to determine unequivocally. Therefore, we compared the predicted NMR chemical shifts of the two possible diastereomers, 2a (4,5-α-epoxy) and 2b (4,5-β-epoxy) as shown below (Fig. 4), calculated for the ωB97X-V/6-311+G* optimized conformations using ωB97X-V/6-311+G*(2df,2p).
Conformational searches and chemical shift calculations for compounds 2a and 2b were performed with the Spartan’20 software [25]. Stable conformers up to 40 kJ/mol for 2a and 2b were initially searched using the Merck molecular force field (MMFF) method. Stable conformers suggested were optimized using Hartree–Fock (HF)/3-21G (40 kJ/mol) and ωB97X-D/6-31G* (15 kJ/mol). Final energy optimization for the Boltzmann distribution was conducted at the ωB97X-V/6-311+G*(2df,2p) level of theory. NMR properties were calculated at the ωB97X-V/6-311+G*(2df,2p) level of theory and scaled. The obtained chemical shifts were corrected using the Boltzmann distribution to give calculated 13C chemical shifts.
The 13C chemical shift differences of predicted and experimental data are shown as graph in Fig. 5. Two atoms of C-6 and C-9 in 2b shows a deviation of greater than 5 ppm, but the average deviation is less than 2 ppm in 2a. Overall, the 13C chemical shifts predicted for structure 2a (Fig. 4) correlate very well with those observed for ceramicine R (2).
Thus, we propose that the correct structure of ceramicine R (2) is the structure 2a. The prediction of 13C chemical shifts by DFT calculation is a very powerful tool for screening proposed structures and should be used more widely.
Ceramicine S (3), \([\upalpha ]_{{\text{D}}}^{27}\) + 34 (c 1.0, CHCl3), was revealed to have the molecular formula C28H36O6 by HRESITOFMS. Its 1H and 13C NMR data are highly similar to ceramicine R (2) (Table 2). However, the NMR data of 3 suggested that the oxymethylene moiety [δH 4.03 (d, 10.8), 4.59 (d, 10.8); δC 72.0] with acetyl group [δH 2.01 (3H, s); δC 21.0 and 171.3] was observed instead of the epoxide function in 2. IR absorptions implied the presence of α,β-unsaturated ketone (1684 cm−1), hydroxy (3502 cm−1), and acetyl (1733 cm−1) groups. The HMBC correlations of H2-28 and H3-2’ to C-1’ (δC 171.3) supported its functionality in the structure of 3. Finally, the NOESY correlations confirmed the position of the oxymethylene moiety with the acetyl group at C-28 and the α orientation of the hydroxy at C-6 and C-7 (See Supplementary Information).
By HRESITOFMS, ceramicine T (4), \([\upalpha ]_{{\text{D}}}^{28}\) + 67 (c 0.5, CHCl3), was revealed to have the molecular formula C29H36O7. Their NMR data are also highly similar to 3, differing only on the signals assigned to the presence of one acetyl and one methoxy carbonyl groups in 4 and the disappearance of oxy-methylene protons of 3. IR absorptions implied the presence of α,β-unsaturated ketone (1683 cm−1), hydroxy (3534 cm−1), and acetyl and/or methoxycarbonyl (1732 cm−1) groups. Analysis of the NMR data revealed the presence of a cyclopentanone[α]phenanthren ring system with a β-furyl ring at C-17.
The planar structure of 4 was deduced from the 1H-1H COSY correlations and the especially HMBC correlations of H3-29 and H3-3’ to C-28 and H3-30 to C-7. The relative configuration of 4 was then deduced from the NOESY correlations of H-6/H3-19, H3-29 and H3-30, H-9/H-5 and H3-18, H-7/H3-30, and H-12a/H3-30 and H-17 (See Supplementary Information). Thus, the structure of 4 was proposed to be as shown in Fig. 1.
Considering that 1–4 were isolated from the same extract as ceramicine B [3], their absolute configurations were assumed to be similar to ceramicine B based on the biogenetic relationships. Ceramicines Q and R might be generated from a cyclopentanone[α]phenanthren ring system followed by oxidative decarboxylation at C-28.
Antimalarial activity
Ceramicines Q–T (1–4) were tested for antimalarial activity against Plasmodium falciparum 3D7 strain. The assay showed that 2 had potent in vitro antimalarial activity [the half-maximal (50%) inhibitory concentration (IC50) = 2.8 µM, whereas 1, 3, and 4 did not (> 5.0 µM).].
Previously, we also reported some limonoids, ceramicines A–D with a cyclopentanone[α]phenanthren ring system with a tetrahydrofuran ring and a β-furyl ring from the barks of C. ceramicus, exhibited antimalarial activity against P. falciparum 3D7 in vitro [2, 3].
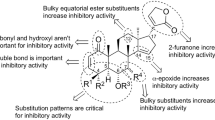
The activity might be depending on their unique cyclopentanone[α]phenanthren ring system and also be influenced by the presence of a tetrahydrofuran ring and its substituent patterns around rings A and B.
Experimental section
General experimental procedures
Optical rotations were measured on a JASCO DIP-1000 polarimeter. UV spectra were recorded on a Shimadzu UVmini-1240 spectrophotometer and IR spectra on a JASCO FT/IR-4100 spectrophotometer. High-resolution ESI MS were obtained on a JMS-T100LP (JEOL). 1H and 2D NMR spectra were measured on a 400 MHz or 600 MHz spectrometer at 300 K, while 13C NMR spectra were on a 100 MHz or 150 MHz spectrometer. The residual solvent peaks were used as internal standards (δH 7.26 and δC 77.0 for CDCl3, δH 3.31 and δC 49.0 for CD3OD).
Material
The barks of C. ceramicus were collected in Terengganu, Malaysia in July 2013. The botanical identification was made by Prof. A. Hamid A. Hadi, University of Malaya. Voucher specimens (No. HOSHI13CCB) are deposited in the department of Pharmacognosy Hoshi University.
Extraction and isolation
The barks of C. ceramicus (8 kg) were extracted with MeOH to obtain 1.43 kg of extract. The MeOH extract was successively partitioned with n-hexane, EtOAc, n-BuOH and water. The n-hexane-soluble materials were separated further by silica gel column chromatography (n-hexane/EtOAc 1:0→1:1, CHCl3/MeOH 1:0→0:1) to obtain 10 fractions (A–J). Fraction I was separated further with a ODS silica gel column (MeOH/H2O 7:3→1:0, acetone) to obtain 6 fractions (I-1–I-6). Fraction I-3 was also separated by HPLC (Shiseido ODS MGII 30 × 250 mm, 85% aqueous MeOH at 8.0 mL/min, UV detection at 210 nm) into 7 fractions (I-3-a–I-3-g). Fraction I-3-c, I-3-d, and I-3-f are ceramicine F, G and B, respectively. Separation of fraction I-3-a by HPLC (Nacalai tesque Cholester 10 × 250 mm, 65% aqueous MeCN at 2.0 mL/min, UV detection at 210 nm) yielded ceramicine Q (1) (6.0 mg, 0.000075%, tR 18.6 min) and ceramicine R (2) (0.8 mg, 0.00001%, tR 21.0 min).
Separation of fraction I-3-e by HPLC (Nacalai tesque Cholester 10 × 250 mm, 60% aqueous MeCN at 2.0 mL/min, UV detection at 210 nm) yielded ceramicine S (3) (10.6 mg, 0.00013%, tR 44.0 min) and ceramicine T (4) (1.0 mg, 0.000012%, tR 50.0 min).
Ceramicine Q (1): white amorphous solid; \([\upalpha ]_{{\text{D}}}^{27}\) + 119 (c 1.0, CHCl3); UV (MeOH) λmax (ε) 204 (10,000) nm; CD (MeOH) λmax (Δε) 337 (− 1.59) and 216 (10.3) nm; IR (Zn-Se) νmax 3461 and 1683 cm–1; 1H and 13C NMR data (Table 1); ESIMS m/z 433 (M + Na)+; HRESIMS m/z 433.1977 (M + Na)+ [calcd for C25H30O5Na (M + Na)+: 433.1991].
Ceramicine R (2): white amorphous solid; \([\upalpha ]_{{\text{D}}}^{28}\) + 126 (c 0.5, CHCl3); UV (MeOH) λmax (ε) 204 (10,700) nm; CD (MeOH) λmax (Δε) 336 (− 2.36), 250 (2.18) and 230 (1.52) nm; IR (Zn-Se) νmax 3450 and 1697 cm–1; 1H and 13C NMR data (Table 1); ESIMS m/z 433 (M + Na)+; HRESIMS m/z 433.1982 (M + Na)+ [calcd for C25H30O5Na (M + Na)+: 433.1991].
Ceramicine S (3): white amorphous solid; \([\upalpha ]_{{\text{D}}}^{27}\) + 34 (c 1.0, CHCl3); UV (MeOH) λmax (ε) 204 (11,900) nm; CD (MeOH) λmax (Δε) 333 (− 2.96), 249 (Δ 0.36), 230 (Δ − 0.35) and 212 (Δ 3.79) nm; IR (Zn-Se) νmax 3502, 1733 and 1684 cm–1; 1H and 13C NMR data (Table 1); ESIMS m/z 491 (M + Na)+; HRESIMS m/z 491.2417 (M + Na)+ [calcd for C28H36O6Na (M + Na)+: 491.2410].
Ceramicine T (4): white amorphous solid; \([\upalpha ]_{{\text{D}}}^{28}\) + 67 (c 0.5, CHCl3); UV (MeOH) λmax (ε) 204 (12,000) nm; CD (MeOH) λmax (Δε) 336 (− 2.21), 234 (− 12.6), 206 (17.0) nm; IR (Zn-Se) νmax 3534, 1732 and 1683 cm–1; 1H and 13C NMR data (Table 2); ESIMS m/z 519 (M + Na)+; HRESIMS m/z 519.2361 (M + Na)+ [calcd for C29H36O7Na (M + Na)+: 519.2359].
13C chemical shift calculations
Conformational searches and chemical shift calculations for compounds 2a and 2b were performed with the Spartan’20 software [25]. Stable conformers up to 40 kJ/mol for 2a and 2b were initially searched using the Merck molecular force field (MMFF) method. Stable conformers suggested were optimized using Hartree–Fock (HF)/3-21G (40 kJ/mol) and ωB97X-D/6-31G* (15 kJ/mol). Final energy optimization for the Boltzmann distribution was conducted at the ωB97X-V/6-311+G*(2df,2p) level of theory. NMR properties were calculated at the ωB97X-V/6-311+G*(2df,2p) level of theory and scaled. The obtained chemical shifts were corrected using the Boltzmann distribution to give calculated 13C chemical shifts.
Parasite strain culture
P. falciparum laboratory strain 3D7 was obtained from Prof. Masatsugu Kimura (Osaka City University, Osaka, Japan). For the assessment of antimalarial activity of the compounds in vitro, the parasites were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 0.5 g/L l-glutamine, 5.96 g/L HEPES, 2 g/L sodium bicarbonate (NaHCO3), 50 mg/L hypoxanthine, 10 mg/L gentamicin, 10% heat-inactivated human serum, and red blood cells (RBCs) at a 3% hematocrit in an atmosphere of 5% CO2, 5% O2, and 90% N2 at 37 °C as previously described [26]. Ring-form parasites were collected using the sorbitol synchronization technique [27]. Briefly, the cultured parasites were collected by centrifugation at 840 g for 5 min at room temperature, suspended in a fivefold volume of 5% D-sorbitol (Nacalai Tesque, Kyoto, Japan) for 10 min at room temperature, and then they were washed twice with RPMI 1640 medium to remove the D-sorbitol. The utilization of blood samples of healthy Japanese volunteers for the parasite culture was approved by the institutional review committee of the Research Institute for Microbial Diseases (RIMD), Osaka University (approval number: 22-3).
Antimalarial activity
Ring-form-synchronized parasites were cultured with compounds 1–4 at sequentially decreasing concentrations (50, 15, 5, 1.5, 0.5, and 0.15 µM) for 48 h for the flow cytometric analysis using an automated hematology analyzer, XN-30. The XN-30 analyzer was equipped with a prototype algorithm for cultured falciparum parasites [prototype; software version: 01-03, (build 16)] and used specific reagents (CELLPACK DCL, SULFOLYSER, Lysercell M, and Fluorocell M) (Sysmex, Kobe, Japan) [28, 29]. Approximately 100 µL of the culture suspension diluted with 100 µL phosphate-buffered saline was added to a BD Microtainer MAP Microtube for Automated Process K2 EDTA 1.0 mg tube (Becton Dickinson and Co., Franklin Lakes, NJ, USA) and loaded onto the XN-30 analyzer with an auto-sampler as described in the instrument manual (Sysmex). The parasitemia (MI-RBC%) was automatically reported [25]. Then 0.5% DMSO alone or containing 5 µM artemisinin was used as the negative and positive controls, respectively. The growth inhibition (GI) rate was calculated from the MI-RBC% according to the following equation:
The IC50 was calculated from GI (%) using GraphPad Prism version 5.0 (GraphPad Prism Software, San Diego, CA, USA) [30].
References
Zhang Y, Xu H (2017) Recent progress in the chemistry and biology of limonoids. RSC Adv 7:35191–35220
Mohamad K, Hirasawa Y, Lim CS, Awang K, Hadi AHA, Takeya K, Morita H (2008) Ceramicine A and walsogyne A, novel limonoids from two species of Meliaceae. Tetrahedron Lett 49:4276–4278
Mohamad K, Hirasawa Y, Litaudon M, Awang K, Hadi AHA, Takeya K, Ekasari W, Widyawaruyanti A, Zaini NC, Morita H (2009) Ceramicine B-D, a new antiplasmodial limonoids from Chisocheton ceramicus. Bioorg Med Chem 17:727–730
Wong CP, Shimada M, Nagakura Y, Nugroho AE, Hirasawa Y, Kaneda T, Awang K, Hadi AHA, Mohamad K, Shiro M, Morita H (2011) Ceramicine E-I, new limonoids from Chisocheton ceramicus. Chem Pharm Bull 59:407–411
Wong C, Shimada M, Nugroho AE, Hirasawa Y, Kaneda T, Hadi AHA, Osamu S, Morita H (2012) Ceramicines J-L, new limonoids from Chisocheton ceramicus. J Nat Med 66:566–570
Nugroho AE, Hashimoto A, Wong C-P, Yokoe H, Tsubuki M, Kaneda T, Hadi AHA, Morita H (2018) Ceramicines M-P from Chisocheton ceramicus: isolation and structure–activity relationship study. J Nat Med 72:64–72
Wong CP, Deguchi J, Nugroho AE, Kaneda T, Hadi AHA, Morita H (2013) Ceramicines from Chisocheton ceramicus as lipid-droplets accumulation inhibitors. Bioorg Med Chem Lett 23:1786–1788
Wong CP, Kaneda T, Hadi AHA, Morita H (2014) Ceramicine B, a limonoid with anti-lipid droplets accumulation activity from Chisocheton ceramicus. J Nat Med 68:22–30
Iijima C, Wong CP, Nugroho AE, Sotozono Y, Someya S, Hirasawa Y, Kaneda T, Hadi AHA, Morita H (2016) Anti-melanin deposition activity of ceramicines from Chisocheton ceramicus. J Nat Med 70:702–707
Nugroho AE, Okuda M, Yamamoto Y, Hirasawa Y, Wong C-P, Kaneda T, Shirota O, Hadi AHA, Morita H (2013) Walsogynes B – G, limonoids from Walsura chrysogyne. Tetrahedron 69:4139–4145
Nugroho AE, Nakajima S, Wong C-P, Hirasawa Y, Kaneda T, Shirota O, Tougan T, Horii T, Hadi AHA, Morita H (2022) Walsogynes H – O from Walsura chrysogyne. J Nat Med 76:94–101
Prema WCP, Awouafack MD, Nugroho AE, Win YY, Win NN, Ngwe H, Morita H, Morita H (2019) Two new quassinoids and other constituents from the Picrasma javanica wood and their biological activities. J Nat Med 73:589–596
Prema WCP, Kodama T, Nugroho AE, El-Desoky AH, Awouafack MD, Win YY, Ngwe H, Abe I, Morita H, Morita H (2020) Three new quassinoids isolated from the wood of Picrasma javanica and their anti-Vpr activities. J Nat Med 74:571–578
Nugroho AE, Hirasawa Y, Kaneda T, Shirota O, Matsuno M, Mizukami H, Morita H (2021) Triterpenoids from Walsura trichostemon. J Nat Med 75:415–422
Nugroho AE, Tange M, Kusakabe Y, Hirasawa Y, Kaneda T, Shirota O, Matsuno M, Mizukami H, Tougan T, Horii T, Morita H (2022) Triterpenoids from Walsura trichostemon. J Nat Med 76:756–764
Nugroho AE, Zhang W, Hirasawa Y, Tang Y, Wong CP, Kaneda T, Hadi AHA, Morita H (2018) Bisleuconothines B-D, modified eburnane-aspidosperma bisindole alkaloids from Leuconotis griffithii. J Nat Prod 81:2600–2604
Nugroho AE, Ono Y, Jin E, Hirasawa Y, Kaneda T, Rahman A, Kusumawati I, Tougan T, Horii T, Zaini NC, Morita H (2021) Bisindole alkaloids from Voacanga grandifolia leaves. J Nat Med 75:408–414
Tang Y, Nugroho AE, Hirasawa Y, Tougan T, Horii T, Hamid A, Hadi A, Morita H (2019) Leucophyllinines A and B, bisindole alkaloids from Leuconotis eugeniifolia. J Nat Med 73:533–540
Amelia P, Nugroho AE, Hirasawa Y, Kaneda T, Tougan T, Horii T, Morita H (2019) Indole alkaloids from Tabernaemontana macrocarpa Jack. J Nat Med 73:820–825
Amelia P, Nugroho AE, Hirasawa Y, Kaneda T, Tougan T, Horii T, Morita H (2021) Two new bisindole alkaloids from Tabernaemontana macrocarpa Jack. J Nat Med 75:633–642
Hirasawa Y, Yasuda R, Minami W, Hirata M, Nugroho AE, Tougan T, Uchiyama N, Hakamatsuka T, Horii T, Morita H (2021) Divaricamine A, a new anti-malarial trimeric monoterpenoid indole alkaloid from Tabernaemontana divaricata. Tetrahedron Lett 83:153423
Hirasawa Y, Agawa-Kakimoto M, Zaima K, Uchiyama N, Goda Y, Morita H (2021) Complanadine F, a novel dimeric alkaloid from Lycopodium complanatum. J Nat Med 75:403–407
Hirasawa Y, Tanaka T, Hirasawa S, Wong CP, Uchiyama N, Kaneda T, Goda Y, Morita H (2022) Cliniatines A-C, new Amaryllidaceae alkaloids from Clivia miniata, inhibiting Acetylcholinesterase. J Nat Med 76:171–177
Ogasawara A, Noguchi R, Shigi T, Nugroho AE, Hirasawa Y, Kaneda T, Tougan T, Horii T, Hamid A, Hadi A, Morita H (2022) Caloforines A-G, coumarines from the bark of Calophyllum scriblitifolium. J Nat Med 76:645–653
Spartan’20 (2020) WaveFunction, Inc., Irvine, CA
Trager W, Jensen JB (1976) Human malaria parasites in continuous culture. Science 193:673–675. https://doi.org/10.1126/science.781840
Lambros C, Vanderberg JP (1979) Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 65:418–420
Tougan T, Suzuki Y, Itagaki S, Izuka M, Toya Y, Uchihashi K, Horii T (2018) An automated haematology analyzer XN-30 distinguishes developmental stages of falciparum malaria parasite cultured in vitro. Malar J 17:59. https://doi.org/10.1186/s12936-018-2208-6
Toya Y, Tougan T, Horii T, Uchihashi K (2021) Lysercell M enhances the detection of stage-specific Plasmodium-infected red blood cells in the automated hematology analyzer XN-31 prototype. Parasitol Int 80:102206. https://doi.org/10.1016/j.parint.2020.102206
Tougan T, Toya Y, Uchihashi K, Horii T (2019) Application of the automated haematology analyzer XN-30 for discovery and development of anti-malarial drugs. Malar J 18:8. https://doi.org/10.1186/s12936-019-2642-0
Acknowledgements
We thank Prof. Masatsugu Kimura (Osaka City University, Osaka, Japan) for the kind gift of the 3D7 strain, Mr. Yuji Toya and Dr. Kinya Uchihashi (Sysmex) for the setting of the XN-30 analyzer and Ms. Toshie Ishisaka and Ms. Sawako Itagaki for their technical assistance. This work was partly supported by JSPS KAKENHI (JP 19K07152 and JP 22K06671 to MH) and (JP 16K08309 to AEN), Japan.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nugroho, A.E., Wong, C.P., Hirasawa, Y. et al. Antimalarial ceramicines Q-T from Chisocheton ceramicus. J Nat Med 77, 596–603 (2023). https://doi.org/10.1007/s11418-023-01706-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-023-01706-w