Abstract
Amentoflavone is a bioflavonoid found in a variety of traditional Chinese medicines such as Gingko and Selaginella tamariscina. It has been reported that amentoflavone has anti-inflammatory, antioxidant, antiviral and anticancer effects. However, the effect of amentoflavone on osteogenic differentiation of human mesenchymal stem cells (hMSCs) has not been studied. In this study, we aim to explore the effect of amentoflavone on the proliferation and osteogenic differentiation of hMSCs. The results showed that amentoflavone significantly enhanced the proliferation, alkaline phosphatase (ALP) activity and mineralization in hMSCs. Western blot analysis revealed that the expression of runt-related transcription factor 2 and osterix proteins was upregulated in amentoflavone-treated hMSCs. Furthermore, we investigated the possible signaling pathways responsible for osteogenic differentiation of hMSCs by amentoflavone. We found that amentoflavone significantly increased the levels of phosphorylated JNK and p-p38. The amentoflavone-induced increases of ALP and mineralization were significantly diminished when the JNK and p38 MAPK pathways were blocked by selected inhibitors (SP600125, SB203580) in hMSCs. Furthermore, in vivo evidence indicated that amentoflavone protected against the dexamethasone-induced inhibition of osteoblast differentiation in tg(sp7:egfp) zebrafish larvae. Thus, we showed for the first time that amentoflavone improves the osteogenesis of hMSCs through the JNK and p38 MAPK pathway. Amentoflavone may be beneficial in treating bone-related disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis, characterized by a high risk of fragility fractures and skeletal pain, is one of the most common bone disorders and results in low bone mineral density and the deterioration of bone microarchitecture [1]. What causes osteoporosis is the imbalance of function between osteoblasts and osteoclasts [2]. Drugs, such as bisphosphonates, calcitonin and selective estrogen receptor modulators, have been used in osteoporosis treatments to inhibit bone resorption. They are effective in stabilizing bone architecture, but they cannot increase bone formation and restore the bone structure and have obvious side effects [3–5]. Thus, it is very important to develop anabolic agents to treat osteoporosis.
Mesenchymal stem cells (MSCs) are multipotent cells that can differentiate into osteoblasts, adipocytes, chondrocytes, myoblasts, neurons, muscle cells and endothelial cells in response to different stimuli [6, 7]. The multipotent property of MSCs makes it possible for them to be used in cell therapy for musculoskeletal applications including bone tissue engineering and osteoporosis [8]. In recent years, MSCs showed promising results for healing bone-related diseases such as bone fracture and segmental bone defects [9–11]. In addition, the osteogenic differentiation potential of MSCs was weak in osteoporotic patients [12]. Therefore, it could be a potential therapy to enhance osteogenesis of MSCs for bone tissue engineering and osteoporotic patients [13].
In recent years, the zebrafish has become an ideal model for bone research. The cortical bone transgenic zebrafish line, tg(sp7:egfp), which labels osteoblast cells with green fluorescent protein, makes the skeleton structure visible during the development of the zebrafish embryo [14]. Fleming et al. successfully used glucocorticoid to establish an osteoporosis-like phenotype in zebrafish. This model has become popular in high-throughput screening for bone anabolic compounds and is used to study bone-related diseases [15–17].
Amentoflavone is a bioflavonoid found in a variety of traditional Chinese medicines such as Gingko and Selaginella tamariscina. It has been reported to have anti-inflammatory, antioxidant, antiviral and anticancer effects [18–21]. In addition, studies have reported that flavonoids have an anabolic effect on bone metabolism [22]. However, the effect of amentoflavone on the osteogenic differentiation of human mesenchymal stem cells (hMSCs) has not been reported.
In the present study, we investigated the effect of amentoflavone on the osteogenic differentiation of hMSCs. The results showed that amentoflavone could promote the osteogenic differentiation of hMSCs by activating the JNK and p38 MAPK pathways. In vivo results also proved that amentoflavone protected against the dexamethasone (Dex)-induced inhibition of osteoblast differentiation in tg(sp7:egfp) zebrafish larvae.
Materials and methods
Materials
Amentoflavone (>99 % purity) was purchased from Chengdu Best Reagent Co., Ltd. (Chengdu, China). Human fetal bone marrow-derived MSCs were offered from the Stem Cell Bank in the Prince of Wales Hospital (Hong Kong, China). Human ethics approval was obtained from the Joint CUHK-NTEC Clinical Research Ethics Committee of the Chinese University of Hong Kong (reference no. CRE-2011.383). Tg(sp7:egfp) zebrafish line was kindly provided by Dr. Chung-Der Hsiao’s group from Chung Yuan Christian University, Taiwan. Minimum essential medium α (α-MEM), MSC qualified fetal bovine serum (FBS) and 0.25 % trypsin-EDTA were purchased from Invitrogen (Carlsbad, CA, USA). Paraformaldehyde was purchased from ChengDu Kelong Chemical Reagent Co. (Chengdu, China). Agarose was purchased from Biowest (Spain). Methyl thiazolyl tetrazolium (MTT), SP600125 and SB203580, NBT-BCIP solution, l-ascorbic acid 2-phosphate, β-glycerophosphate, Dex and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Alkaline Phosphatase Assay Kits were purchased from the Beyotime Institute of Biotechnology. Anti-GAPDH, anti-runt-related transcription factor 2 (Runx2) and anti-osterix (Osx) antibodies were purchased from Abcam. Anti-p38, anti-phosphorylated-p38 (p-p38), anti-JNK and anti-phosphorylated-JNK (p-JNK) antibodies were purchased from Cell Signaling Technology.
Cell proliferation assay
The hMSCs were cultured in 96-well flat-bottomed plates with 5 × 103 cells per well. After 24 h, amentoflavone was added to the culture medium at different concentrations (0.1–10 µM). Then, the cells were incubated for 1, 2, 3, 7 and 14 days. The effect of amentoflavone on hMSC cell proliferation was determined by MTT assay. After incubation, the hMSCs were treated with 10 µl MTT solution of 0.5 mg/ml at 37 °C for 4 h. Then, the medium was abandoned, and the dark blue formazan crystals were subsequently dissolved in 200 µl DMSO at 37 °C for shaking 10 min. The absorbance at 490 nm was measured with a microplate reader.
Cell culture and osteogenic differentiation of hMSCs
The hMSCs were kept in α-MEM supplemented with 10 % FBS and 1 % penicillin-streptomycin (Gibco) and cultured at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air. After reaching 80 % confluence, the hMSCs were rinsed twice with phosphate-buffered saline (PBS), trypsinized with 0.25 % trypsin-EDTA for 2 min and then using serum-containing medium to end the trypsinization immediately. The mixed fluid was collected and centrifuged with 900g for 3 min. Next, leaving the supernatant gently, the precipitate was resuspended in 10 % serum-containing medium. Finally, the cells were subcultured 1:3. After reaching 80 % confluence, the cells were stimulated by osteogenic induction medium (OIM) consisting of 10 % serum-containing medium with 100 nM Dex, 10 mM β-glycerophosphate and 0.05 mM l-ascorbic acid 2-phosphate. The medium was replaced every 3 days.
ALP activity assay
Alkaline phosphatase (ALP) activity was measured by ALP Assay Kits (Beyotime, China). After 7 days of osteogenic induction, the cells in 24-well flat-bottomed plates were washed lightly with PBS and lysed in 0.1 % (v/v) Triton X-100 in PBS for 30 min. The activity was then assessed under the protocol of ALP Assay Kits. The concentration of cell lysate protein was determined by using bicinchoninic acid (BCA) protein assay reagent (Beyotime, China). The absorbance was measured at 562 nm with a microplate reader. ALP activity was normalized to the total protein concentration.
ALP staining
After 7 days of osteogenic induction, the cells were rinsed twice with PBS and fixed with 500 µl 70 % ethanol at room temperature for 10 min. After aspirating the ethanol, the cells were equilibrated by 500 µl ALP buffer (0.15 M NaCl, 0.15 M Tris-HCl, 1 mM MgCl2, pH9.0) twice for 10 min and stained with NBT/BCIP solution at 37 °C for about 20 min in dark. Upon the appearance of purple-blue precipitates, the reaction was stopped by distilled water. Photographs were taken of all samples.
Alizarin Red S staining and quantification
After 14 days of osteogenic induction, the cells in 24-well flat-bottomed plates were washed twice with PBS, fixed with 500 µl 70 % ethanol for 15 min and then incubated with 0.5 % Alizarin Red S (pH 4.1) for 5 min at 37 °C. The samples were then washed three times with deionized water to stop the reaction. After taking photos, the samples were extracted with 10 % cetylpyridinium chloride (CPC, Sigma) for 10 min, and the absorbance at 562 nm was measured with a microplate reader.
Western blot analysis
Western blot analysis was performed as previously described [23]. Briefly, equal proteins were fractionated by 10 % SDS-PAGE, and proteins were transferred to a polyvinylidene difluoride (PVDF) membrane. Then, the membrane was blocked with 5 % skim milk for 1 h at room temperature. Specific antibodies against Runx2, Osx, p38, p-p38, JNK, p-JNK and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used. The band intensity was quantified by Quantity One software.
Zebrafish skeleton development assay
Zebrafish skeleton development assay was performed as previous described [17]. In brief, tg(sp7:egfp) embryos were raised in embryo medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4) at 28.5 °C for 2 days. The embryos were then randomly divided into five groups: vehicle control (0.1 % DMSO), 10 µM Dex, Dex + 0.1 µM amentoflavone, Dex + 1 µM amentoflavone and Dex + 5 µM amentoflavone. All embryos were kept in 24-well flat-bottomed plates with ten fish per well, and the medium was half changed every day. After 6 days, the fish were fixed in 4 % paraformaldehyde in PBS for 2 h and fixed with 1 % low melting agarose (LMA, Invitrogen, USA). Images were scanned using a confocal laser scanning microscope. The fluorescent area and integrated optical density (IOD) were calculated by Image Pro Plus 6.0.
Statistical analysis
All statistical data were presented as the mean ± SD. Statistical significance was assessed by one-way analysis of variance (ANOVA) with a Student-Newman-Keuls post hoc test for multiple-group comparison. Probability values of P < 0.05 were considered to represent statistical significance.
Results
The effect of amentoflavone on hMSC proliferation
In order to test the effect of amentoflavone on hMSC proliferation, the MTT assay was performed. The result showed that amentoflavone enhanced hMSC proliferation in a dose-dependent manner, and the most effective dose was 5 μM (Fig. 1).
Amentoflavone increased the ALP activity of hMSCs
As ALP is a sign of osteogenic differentiation of hMSCs, we evaluated whether amentoflavone would increase the ALP activity. The hMSCs were treated with different concentrations of amentoflavone (0.1, 1, 5 μM) in OIM for 7 days. The ALP staining and quantitative data showed amentoflavone increased ALP activity in a dose-dependent manner, and the concentration of 5 μM was the peak (Fig. 2).
Amentoflavone increased ALP activity of hMSCs. a The ALP activity was tested after hMSCs had been treated with different concentrations of amentoflavone in OIM for 7 days (n = 4). b ALP of hMSCs was stained with BCIP/NBT kit after culturing in the same conditions for 7 days. Scale bars represent 200 µm. **P < 0.01 compared with Con; ## P < 0.01 compared with OIM
Amentoflavone increased the mineralization of hMSCs
Alizarin Red staining (ARS) was carried out to estimate whether hMSCs incubated with amentoflavone resulted in an increase of extracellular calcium deposition. The result showed that samples had no calcium deposition formation in the absence of OIM at 14 days, while in the presence of OIM and amentoflavone, they had remarkable calcium nodule formation (Fig. 3). Furthermore, quantification of calcium deposition showed that amentoflavone significantly promoted calcium deposition in a dose-dependent manner, and 5 μM was the best (Fig. 3).
Amentoflavone promoted the mineralization of hMSCs. a The hMSCs were treated with amentoflavone in OIM for 14 days, and the mineralized nodules of hMSCs were stained by Alizarin Red S. b The mineralization, for three independent experiments, was quantified by extraction of Alizarin Red S dye with 10 % CPC. Scale bars represent 200 µm. **P < 0.01 compared with Con; ## P < 0.01 compared with OIM
Amentoflavone increased the phosphorylation of JNK and p38 in hMSCs
It is well known that the JNK and p38 MAPK pathways play an important role in regulating the osteogenesis of MSCs [24, 25]. In order to check whether amentoflavone stimulated the osteogenesis of hMSCs by activating the JNK and p38 MAPK pathways, we detected the levels of p-JNK and p-p38 in hMSCs through Western blot. The results showed that amentoflavone significantly increased the levels of p-p38 and p-JNK, while SB203580 and SP600125 eliminated this effect of amentoflavone (Fig. 4).
Amentoflavone induced activation of the JNK and p38 MAPK signaling pathways. The hMSCs were pretreated with SP600125 or SB203580 for 1 h and incubated with amentoflavone (5 μM) in OIM for 3 days. The proteins were then separated by 10 % SDS-PAGE and analyzed by Western blot with specific antibodies. a The level of p-JNK was detected by Western blot. b Quantification of three independent Western blot experiments was shown with the mean level of p-JNK normalized to total JNK. *P < 0.05; # P < 0.05. c The level of p-p38 was detected by Western blot. d Quantification of three independent Western blot experiments was shown with the mean level of p-p38 normalized to total p38. *P < 0.05; # P < 0.05
SB203580 and SP600125 eliminated the increasing of ALP activity induced by amentoflavone
SB203580 and SP600125 are selective inhibitors of the p38 and JNK pathway, respectively [25]. ALP staining showed SB203580 and SP600125 strongly inhibited ALP activity increased by amentoflavone (Fig. 5). This was demonstrated by the ALP activity assay (Fig. 5).
SB203580 and SP600125 inhibited the increasing of ALP activity induced by amentoflavone. a, b The hMSCs were pretreated with SP600125 or SB203580 for 1 h and incubated with amentoflavone (5 μM) in OIM for 7 days. Then, ALP was stained with BCIP/NBT. c, d The ALP activity was measured by ALP Assay Kits. The experiments were repeated three times. Scale bars represent 200 µm. **P < 0.01; # P < 0.05; ## P < 0.01
SB203580 and SP600125 reduced the extracellular calcium deposition induced by amentoflavone
As expected, SB203580 and SP600125 also significantly reduced the calcium deposition stimulated by amentoflavone (Fig. 6). Quantification of calcium nodules also proved this (Fig. 6).
SB203580 and SP600125 inhibited the effect of amentoflavone on mineralized nodules. a, b The hMSCs were pretreated with SP600125 or SB203580 for 1 h and incubated with amentoflavone (5 μM) in OIM for 14 days. Then, Alizarin Red S stained the mineralized nodules. c, d The mineralization was quantified by extraction of Alizarin Red S dye with 10 % CPC. Scale bars represent 200 µm. The experiments were repeated three times. **P < 0.01; ## P < 0.01
SB203580 and SP600125 abolished Runx2 and Osx protein expression induced by amentoflavone
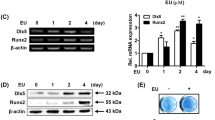
We performed Western blot analysis to test Runx2 and Osx protein expression in cells treated with SB203580 and SP600125. The results showed amentoflavone increased the expression of Runx2 and Osx proteins. At the same time, SP600125 and SB203580 blocked the effect of amentoflavone on the expression of Runx2 and Osx proteins (Fig. 7).
SP600125 and SB203580 inhibited the effect of amentoflavone on the expression of Runx2 and Osx protein in hMSCs. The hMSCs were pretreated with SB203580 (a–c) or SP600125 (d–f) for 1 h and then incubated in OIM with amentoflavone (5 μM) for 7 days. The total proteins were extracted for Western blot to analyze the expression level of Runx2 and Osx. GAPDH was used as loading control. The experiments were repeated three times. **P < 0.01
Amentoflavone protected against the Dex-induced inhibition of osteoblast differentiation in tg(sp7:egfp) zebrafish larvae
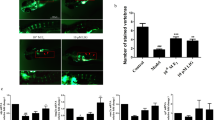
We further examined the effect of amentoflavone on bone formation in tg(sp7:egfp) zebrafish larvae. The results showed that Dex exhibited a reduction in the fluorescence area and IOD. Amentoflavone alleviated the inhibition of Dex in the fluorescence area and IOD in a dose-dependent manner in tg(sp7:egfp) zebrafish larvae (Fig. 8).
The protection of amentoflavone against dexamethasone (Dex)-induced inhibition of osteoblast differentiation in tg(sp7:egfp) zebrafish larvae. a Fluorescence imaging of zebrafish larvae fixed in turn with 4 % paraformaldehyde and 1 % low-melting-point agarose. a Control; b Dex; c Dex + 0.1 μM amentoflavone; d Dex + 1 μM amentoflavone; e Dex + 5 μM amentoflavone. b, c The fluorescent area and integrated optical density (IOD) calculations of mean pixel number (n = 10). Scale bars represent 200 µm. *P < 0.05; **P < 0.01
Discussion
MSCs are multipotent cells with the capacity to differentiate into osteoblasts, adipocytes and chondrocytes [6, 7]. MSC differentiation into osteoblasts is an important part of the bone growth and remodeling process [9, 11]. Recently, hMSCs have be used extensively as a source of osteogenic cells for bone tissue engineering [10, 11]. Thus, it is important to promote osteogenic differentiation of hMSCs.
The osteogenic differentiation of MSCs is characterized by cell proliferation, differentiation and mineralization. First, we studied the effect of amentoflavone on the proliferation of hMSCs. Our results showed that amentoflavone promoted the proliferation of hMSCs in a dose-dependent manner, suggesting that amentoflavone is advantageous to osteogenic differentiation of hMSCs in a wide range of concentrations.
As ALP activity is an early osteogenic marker and plays an important role in bone formation [26], we investigated the role of amentoflavone on ALP activity to evaluate the effect of amentoflavone on osteogenic differentiation of hMSCs. Our results indicated that amentoflavone significantly increased the activity of ALP. As expected, amentoflavone also strengthened mineralized nodule formation, a marker for a later stage of osteogenic differentiation [27]. These results revealed that amentoflavone promoted osteogenic differentiation of hMSCs.
Runx2 plays a pivotal role in the regulation of osteogenic differentiation. Osx, a zinc finger-containing transcription factor required for osteoblastic differentiation, acts as a downstream factor of Runx2 [28–30]. In this research, we found that amentoflavone significantly increased the expression levels of Runx2 and Osx. The results suggested that amentoflavone might enhance the osteogenic differentiation of hMSCs via mediating the transcription factors Runx2 and Osx.
JNK and p38 MAPK signaling pathways play a vital role in the regulation of osteogenesis [24, 25]. The activation of JNK and p38 MAPK signaling pathways has been shown to be involved in both genistein and myocilin-promoted osteogenic differentiation [25, 31]. To explore whether JNK and p38 MAPK signaling pathways were also involved in the amentoflavone-mediated osteogenic differentiation in hMSC, we incubated cells with amentoflavone and OIM. We found amentoflavone significantly increased the levels of p-JNK and p-p38 in a dose-dependent manner. To further confirm our findings, we used inhibitors of p38 MAPK (SB203580) or JNK (SP600125) to check whether the stimulation of amentoflavone on osteogenic differentiation in hMSCs was abolished. The results showed that SB203580 or SP600125 significantly reduced the ALP activity and mineralized nodule formation induced by amentoflavone in the process of osteogenic differentiation. At the same time, SB203580 or SP600125 also efficiently decreased the expression of Runx2 and Osx protein. These data clearly demonstrated that the JNK and p38 MAPK signaling pathways played a crucial role during amentoflavone-induced osteogenesis in hMSCs (Fig. 9).
The zebrafish is an ideal model for bone research because of the rapid generation time, prolific fecundity, external development, optically transparent embryos, and genomic conservation between zebrafish and humans [32–35]. In particular, zebrafish has great similarity to humans in terms of bone development [36–38]. Osx (sp7) is a transcription factor expressed in osteoblasts and a marker for studying osteoblasts. In transgenic zebrafish tg(sp7:egfp), the sp7 gene is expressed accompanying GFP expression during osteoblast differentiation, and GFP-positive osteoblasts are visible in zebrafish. Therefore, tg(sp7:egfp) provides an excellent tool for investigating bone formation directly [15–17]. We found that Dex inhibited the bone formation, while amentoflavone alleviated the inhibition of Dex on osteoblast differentiation in tg(sp7:egfp) zebrafish larvae, suggesting amentoflavone protected against the Dex-induced inhibition of osteoblast differentiation.
In conclusion, this study demonstrated that amentoflavone stimulated the osteogenesis of hMSCs at least partially by activating the JNK and p38 MAPK signaling pathways. More importantly, amentoflavone protected against the Dex-induced inhibition of osteoblast differentiation in larval zebrafish. These results suggest that amentoflavone may be useful for treating bone-related disorders.
References
Raisz LG (2005) Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Investig 115:3318–3325
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377:1276–1287
Marie PJ, Kassem M (2011) Osteoblasts in osteoporosis: past, emerging, and future anabolic targets. Eur J Endocrinol 165:1–10
Sandhu SK, Hampson G (2011) The pathogenesis, diagnosis, investigation and management of osteoporosis. J Clin Pathol 64:1042–1050
Suthon S, Jaroenporn S, Charoenphandhu N, Suntornsaratoon P, Malaivijitnond S (2016) Anti-osteoporotic effects of Pueraria candollei var. mirifica on bone mineral density and histomorphometry in estrogen-deficient rats. J Nat Med 70:225–233
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Jiang YH, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41–49
Deans RJ, Moseley AB (2000) Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol 28:875–884
Wang X, Wang Y, Gou W, Lu Q, Peng J, Lu S (2013) Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int Orthop 37:2491–2498
Cao L, Liu G, Gan Y, Fan Q, Yang F, Zhang X, Tang T, Dai K (2012) The use of autologous enriched bone marrow MSCs to enhance osteoporotic bone defect repair in long-term estrogen deficient goats. Biomaterials 33:5076–5084
Shekkeris AS, Jaiswal PK, Khan WS (2012) Clinical applications of mesenchymal stem cells in the treatment of fracture non-union and bone defects. Curr Stem Cell Res Ther 7:127–133
Park WW, Suh KT, Kim JI, Kim SJ, Lee JS (2009) Decreased osteogenic differentiation of mesenchymal stem cells and reduced bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J 18:1920–1926
Pino AM, Rosen CJ, Rodriguez JP (2012) In osteoporosis, differentiation of mesenchymal stem cells (MSCs) improves bone marrow adipogenesis. Biol Res 45:279–287
DeLaurier A, Eames BF, Blanco-Sanchez B, Peng G, He X, Swartz ME, Ullmann B, Westerfield M, Kimmel CB (2010) Zebrafish sp7:EGFP: a transgenic for studying otic vesicle formation, skeletogenesis, and bone regeneration. Genesis 48:505–511
Fleming A, Sato M, Goldsmith P (2005) High-throughput in vivo screening for bone anabolic compounds with zebrafish. J Biomol Screen 10:823–831
De Vrieze E, Van Kessel MA, Peters HM, Spanings FA, Flik G, Metz JR (2014) Prednisolone induces osteoporosis-like phenotype in regenerating zebrafish scales. Osteoporos Int 25:567–578
Luo S, Yang Y, Chen J, Zhong Z, Huang H, Zhang J, Cui L (2016) Tanshinol stimulates bone formation and attenuates dexamethasone-induced inhibition of osteogenesis in larval zebrafish. J Orthop Transl 4:35–45
Lin YM, Anderson H, Flavin MT, Pai YH, Mata-Greenwood E, Pengsuparp T, Pezzuto JM, Schinazi RF, Hughes SH, Chen FC (1997) In vitro anti-HIV activity of biflavonoids isolated from Rhus succedanea and Garcinia multiflora. J Nat Prod 60:884–888
Zhang Z, Sun T, Niu JG, He ZQ, Liu Y, Wang F (2015) Amentoflavone protects hippocampal neurons: anti-inflammatory, antioxidative, and antiapoptotic effects. Neural Regen Res 10:1125–1133
Park NH, Lee CW, Bae JH, Na YJ (2011) Protective effects of amentoflavone on Lamin A-dependent UVB-induced nuclear aberration in normal human fibroblasts. Bioorg Med Chem Lett 21:6482–6484
Tarallo V, Lepore L, Marcellini M, Dal Piaz F, Tudisco L, Ponticelli S, Lund FW, Roepstorff P, Orlandi A, Pisano C, De Tommasi N, De Falco S (2011) The biflavonoid amentoflavone inhibits neovascularization preventing the activity of proangiogenic vascular endothelial growth factors. J Biol Chem 286:19641–19651
Weaver CM, Alekel DL, Ward WE, Ronis MJ (2012) Flavonoid intake and bone health. J Nutr Gerontol Geriatr 31:239–253
Xu D, Xu L, Zhou C, Lee WY, Wu T, Cui L, Li G (2014) Salvianolic acid B promotes osteogenesis of human mesenchymal stem cells through activating ERK signaling pathway. Int J Biochem Cell Biol 51:1–9
Greenblatt MB, Shim JH, Zou W, Sitara D, Schweitzer M, Hu D, Lotinun S, Sano Y, Baron R, Park JM, Arthur S, Xie M, Schneider MD, Zhai B, Gygi S, Davis R, Glimcher LH (2010) The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J Clin Investig 120:2457–2473
Kwon HS, Johnson TV, Tomarev SI (2013) Myocilin stimulates osteogenic differentiation of mesenchymal stem cells through mitogen-activated protein kinase signaling. J Biol Chem 288:16882–16894
Harrison G, Shapiro IM, Golub EE (1995) The phosphatidylinositol-glycolipid anchor on alkaline phosphatase facilitates mineralization initiation in vitro. J Bone Miner Res 10:568–573
Orimo H, Shimada T (2008) The role of tissue-nonspecific alkaline phosphatase in the phosphate-induced activation of alkaline phosphatase and mineralization in SaOS-2 human osteoblast-like cells. Mol Cell Biochem 315:51–60
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755–764
Fakhry M, Hamade E, Badran B, Buchet R, Magne D (2013) Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J Stem Cells 5:136–148
Nishio Y, Dong Y, Paris M, O’Keefe RJ, Schwarz EM, Drissi H (2006) Runx2-mediated regulation of the zinc finger Osterix/Sp7 gene. Gene 372:62–67
Liao QC, Xiao ZS, Qin YF, Zhou HH (2007) Genistein stimulates osteoblastic differentiation via p38 MAPK–Cbfa1 pathway in bone marrow culture. Acta Pharmacol Sin 28:1597–1602
Metscher BD, Ahlberg PE (1999) Zebrafish in context: uses of a laboratory model in comparative studies. Dev Biol 210:1–14
Trede NS, Langenau DM, Traver D, Look AT, Zon LI (2004) The use of zebrafish to understand immunity. Immunity 20:367–379
Amatruda JF, Shepard JL, Stern HM, Zon LI (2002) Zebrafish as a cancer model system. Cancer Cell 1:229–231
Dooley K, Zon LI (2000) Zebrafish: a model system for the study of human disease. Curr Opin Genet Dev 10:252–256
Du SJ, Frenkel V, Kindschi G, Zohar Y (2001) Visualizing normal and defective bone development in zebrafish embryos using the fluorescent chromophore calcein. Dev Biol 238:239–246
Kim SN, Bae SJ, Kwak HB, Min YK, Jung SH, Kim CH, Kim SH (2012) In vitro and in vivo osteogenic activity of licochalcone A. Amino Acids 42:1455–1465
Pasqualetti S, Banfi G, Mariotti M (2012) The zebrafish scale as model to study the bone mineralization process. J Mol Histol 43:589–595
Acknowledgments
This project was supported by the National Natural Science Foundation of China (30772768, 81102450), Science and Technology Planning Project of Guangdong Province (2013B031800013), Natural Science Foundation of Guangdong Province (2014A030313534), Administration of Traditional Chinese Medicine of Guangdong Province (20151263), Social Science and Technology Development Project of Dongguan (2014108101052), Science and Technology Planning Project for Medical Treatment and Public Health of Dongguan (2014105101294) and Initiating Fund of Scientific Research for Doctors of Guangdong Medical University (B2013016).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
X. Zha and Z. Xu contributed equally.
Rights and permissions
About this article
Cite this article
Zha, X., Xu, Z., Liu, Y. et al. Amentoflavone enhances osteogenesis of human mesenchymal stem cells through JNK and p38 MAPK pathways. J Nat Med 70, 634–644 (2016). https://doi.org/10.1007/s11418-016-0993-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-016-0993-1