Abstract
From the fruits of Phaleria macrocarpa, icariside C3 (1), phalerin (2), and mangiferin (3) were isolated and their structures were identified on the basis of spectroscopic data. Icariside C3 (1) showed a slow vasorelaxant activity against noradrenaline-induced contraction of isolated rat aorta. The structure of phalerin (2) was revised as 2,4′,6-trihydroxy-4-methoxybenzophenone-2-O-β-d-glucoside.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phaleria macrocarpa (Thymelaeaceae), known as Mahkota Dewa, has been used traditionally for treatment of cancers in Indonesia [1]. So far, 3,4,5-trihydroxy-4′-methoxybenzophenone-3-O-β-d-glucoside, named as phalerin, has been isolated from the leaves of P. macrocarpa, which showed cytotoxicity against myeloma cell line (NS-1) [2].
On the other hand, phenylpropanoids in many plants exhibit various bioactivities such as anti-bacterial, cytotoxicity, anti-oxidant, enzyme inhibition, and immuno-modulator properties. We reported that some phenylpropanoids, alkaloids, and peptides in plants showed the vasodilator activity on rat aorta [3, 4]. On continuing the search for chemical constituents with vasorelaxant activity in medicinal plants, we examined isolation of chemical constituents with vasorelaxant activity from the fruits of Phaleria macrocarpa. The extract prepared from P. macrocarpa showed a moderate vasorelaxant activity. The present paper deals with the isolation and identification of chemical constituents from the fruits of P. macrocarpa.
The dried fruits of P. macrocarpa (400 g) were extracted with CHCl3 and then MeOH to yield CHCl3 (15.4 g) and MeOH (96 g) extracts, respectively. The CHCl3 extract (8 g), which showed a moderate vasorelaxant activity against isolated rat aortic strips, was subjected to a silica gel column (CHCl3/MeOH, 1:0 → 0:1) and then purified by HPLC (MeOH/H2O) to give compounds 1 (10.3 mg) and 2 (1.6 mg).
Compound 1 showed the pseudomolecular ion peak at m/z 441 (M+Na)+ in ESIMS. Compared the NMR data of 1 with those of a known sesquiterpene glycoside, icariside C3, isolated from Epimedium grandiflorum var. thunbergianum [5], the similar chemical shift pattern was observed. Detailed analysis of HSQC and HMBC correlations, compound 1 was identified with icariside C3 [5] (Fig. 1).
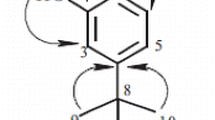
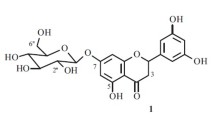
Compound 2 showed the pseudomolecular ion peak at m/z 423 (M+H)+ in ESIMS, and the molecular formula, C20H22O10, was established by HRESIMS [m/z 423.1290 (M+H)+ Δ −0.1 mDa]. 1H and 13C NMR data suggested the presence of one carbonyl, six sp2 quaternary carbons, six sp2 methines, one sp3 methyl, one sp3 methylene, and five sp3 methines. Among them, signals due to six oxygen-bearing carbons at δc 62.6, 71.3, 74.8, 77.8, 78.4, and 102.7, which were assignable to glucose carbons appeared. Detailed analysis of 2D NMR correlations, 2 was identified with 2,4′,6-trihydroxy-4-methoxybenzophenone-2-O-β-d-glucoside, which has been isolated from Gnidia involucrate belonging to Thymelaeaceae [6]. So far, phalerin was isolated as a benzophenone glycoside from the leaves of Phaleria macrocarpa [2]. Compared the NMR data of 2 with those of phalerin, the same chemical shifts were observed and therefore 2 and phalerin were the same compounds, although the proposed two structures were not identical as shown in Fig. 2. The difference between 2 and phalerin was the substituent pattern of the aromatic rings of the benzophenone. HMBC correlations as shown in Fig. 3 and NOESY correlations between the methoxy methyl and two aromatic protons of H-3 and H-5 clearly indicated the structure of 2 and phalerin to be 2,4′,6-trihydroxy-4-methoxybenzophenone-2-O-β-d-glucoside.
The MeOH extract was subjected to a Diaion HP-20 column (H2O/MeOH, 1:0 → 0:1) and then purified by HPLC (MeOH/H2O) to give a C-glucosylxantone (3) together with a benzophenone glycoside (2). Compound 3 was identified with mangiferin by comparison of spectral data with those described in the literature [7].
The CHCl3 extract from P. macrocarpa showed a moderate vasorelaxant activity. When noradrenaline (NA) 3×10−7 M was applied to thoracic aortic rings with endothelium after achieving a maximal response, we added 1–3 at 10−4 M [8]. Icariside C3 showed a slow vasorelaxant effect, whereas 2 and 3 were found to have no vasorelaxant effect.
Calcium ion can contract aortic rings concentration dependently in Ca2+-free Krebs–Henseleit solution (KHS) after depolarization with isotonic high K+ (60 mM) by the influx via voltage-dependent Ca2+ channels (VDCs); this contraction was not inhibited by icariside C3.
Before NA 3×10−7 M was applied to thoracic aortic rings, the aortic rings were exposed to icariside C3 (1:10−4 M) for one hour. The NA-induced contractions were divided into two phases. The first contraction is called the phasic phase, characterized by an initial rapid and transient contraction, and the second is the tonic phase, exhibiting a long-lasting contraction. Compound 1 did not inhibit the phasic contractions, while it clearly inhibited the tonic contractions, indicating 1 did not affect receptor of NA (NAα1) (Figs. 4, 5).
On the other hand, concentration-response relationships for relaxant responses of isoproterenol on the aortic rings precontracted with 3×10−7 M NA was shown in Fig. 6. As icariside C3 (1) enhanced vasorelaxant responses of isoproterenol, 1 inhibited NA-induced vasocontractions, which might be in part attributable to the involvement of an increase of the second messenger such as cAMP and cGMP, inhibition of phosphodiesterase, and activation of adenylate cyclase.
Experimental section
General experimental procedures
Optical rotations were measured on a JASCO DIP-4 polarimeter. UV spectra were recorded on a Shimadzu UV1600PC spectrophotometer and IR spectra on a Perkin–Elmer 1710 spectrophotometer. 1H and 2D NMR spectra were recorded on a 600 MHz spectrometer at 300 K, while 13C NMR spectra were measured on a 150-MHz spectrometer. Chemical shifts were reported using residual CD3OD (δH 3.31 and δC 49.0) as internal standard. Standard pulse sequences were employed for the 2D NMR experiments. Mass spectra were obtained using a Micromass LCT spectrometer.
Plant material
The mature fruits of Phaleria macrocarpa (Thymelaeaceae) were collected at Pasuruan (ca. 300 m a.s.l), East Java, Indonesia in 2005. The botanical identification was made by Mr. Abdul Rahman, MSi, Department of Natural Products, Faculty of Pharmacy, Airlangga University, Indonesia. Voucher specimens have been deposited at the Department of Natural Products, Faculty of Pharmacy, Airlangga University.
Extraction and isolation
The sun-dried fruits of Phaleria macrocarpa (400 g) was crushed and macerated with CHCl3 (24 h) at room temperature (800 ml×6), then the residue was macerated (24 h) with MeOH (800 ml×6). The extracts were then evaporated in rotary evaporator (40°C) yield CHCl3 extract (15.4 g) and MeOH extract (96 g). The CHCl3 extract was chromatographed on silica gel using CHCl3/MeOH (5:2) and then the fourth fraction was purified by HPLC (60% MeOH/H2O) to give icariside C3 (1, 0.005%) [5]. The first fraction was purified by HPLC (35% MeOH/H2O) to give 2,4′,6-trihydroxy-4-methoxybenzophenone-2-O-β-d-glucoside (2, 0.0008%). The MeOH extract was chromatgraphed on Diaion HP-20 using MeOH/H2O and then the fraction eluted from 60% MeOH was purified by HPLC (35% MeOH/H2O) to give 2,4′,6-trihydroxy-4-methoxybenzophenone-2-O-β-d-glucoside (2, 0.007%) and mangiferin (3, 0.008%) [7].
2,4′,6-Trihydroxy-4-methoxybenzophenone-2-O-β-d-glucoside (2)
Colorless solid; [α]20 D +4° (c 0.9, MeOH); UV (MeOH) λmax 205 nm (ε 3,400), 225 nm (ε 1,300), and 290 nm (ε 1,200); IR (KBr) νmax 3,380, 2,940, 2,880, 1,620, 1,510, 1,420, 1,390, 1,320, 1,280, 1,200, 1,160, 1,080, and 920 cm−1; 1H NMR (CD3OD); δ3.25 (1H, dd, J =9.2, 9.2 Hz, H-4″), 3.38 (2H, m, H-3″ and H-5″), 3.64 (1H, dd, J =6.0, 11.9 Hz, H-6″), 3.80 (3H, s, OMe), 3.85 (2H, dd, J=2.3, 11.9 Hz, H-2″ and H-6″), 4.86 (1H, d, J =7.8 Hz, H-1″), 6.16 (1H, d, J =2.0 Hz, H-5), 6.38 (1H, d, J = 2.0 Hz, H-3), 6.68 (2H, d, J =8.7 Hz, H-3′ and H-5′), and 7.65 (2H, d, J =8.7 Hz, H-2′ and H-6′). 13C NMR (CD3OD); δ55.9 (OMe), 62.6 (C-6″), 71.3 (C-4″), 74.8 (C-2″), 77.8 (C-3″), 78.4 (C-5″), 94.8 (C-5), 97.0 (C-3), 102.7 (C-1″), 112.4 (C-1), 117.5 (C-3′ and C-5′), 129.3 (C-1′), 134.0 (C-2′ and C-6′), 158.3 (C-6), 158.9 (C-2), 163.9 (C-4), 169.0 (C-4′), and 196.7 (C=O); ESIMS m/z 423 (M+H)+; HRESIMS m/z 423.1290 (M+H; calcd for C20H23O10, 423.1291).
Vasodilator assay
A male Wistar rat weighting 340 g was killed by bleeding from carotid arteries under an anesthetization [8]. A section of the thoracic aorta between the aortic arch and the diaphragm was removed and placed in oxygenated, modified Krebs-Henseleit solution (KHS: 118.0 mM NaCl, 4.7 mM KCl, 25.0 mM NaHCO3, 1.8 mM CaCl2, 1.2 mM NaH2PO4, 1.2 mM MgSO4, and 11.0 mM glucose). The aorta was cleaned of loosely adhering fat and connective tissue and cut into ring preparations 3 mm in length. The tissue was placed in a well-oxygenated (95% O2, 5% CO2) bath of 10 ml KHS solution at 37°C with one end connected to a tissue holder and the other to a force-displacement transducer (Nihon Kohden, TB-611T). The tissue was equilibrated for 60 min under a resting tension of 1.0 g. During this time, the KHS in the tissue bath was replaced every 20 min.
After equilibration, each aortic ring was contracted by treatment with 3×10−7 M NA. The presence of functional endothelial cells was confirmed by demonstrating relaxation to 10−5 M acetylcholine (Ach), and aortic ring in which 80% relaxation occurred, were regard as tissues with endothelium. When the NA-induced contraction reached plateau, each sample was added.
These animal experimental studies were conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals, Hoshi University, and under the supervision of the Committee on Animal Research of Hoshi University, which is accredited by the Ministry of Education, Science, Sports Culture, and Technology of Japan.
References
Harmanto N (1998) Makutodewa Obat Pusaka Para Dewa. Agro Media Pustaka, Jakarta, pp 7–13
Hartati WMS, Mubarika S, Gandjar IG, Hamann MT, Rao KV, Wahyuono S (2005) Phalerin, a new benzophenoic glucoside isolated from the methanolic extract of Mahkota Dewa (Phaleria macrocarpa) leaves. Majalah Farmasi Indones 16:51–57
Morita H, Eda M, Iizuka T, Hirasawa Y, Sekiguchi M, Yun YS, Itokawa H, Takeya K (2006) Structure of a new cyclic nonapeptide, segetalin F and vasorelaxant activity of segetalins from Vaccaria segetalis. Bioorg Med Chem Lett 17:4458–4461
Morita H, Iizuka T, Choo CY, Chan KL, Takeya K, Kobayashi J (2006) Vasorelaxant activity of cyclic peptide, cyclosquamosin B, from Annona squamosa. Bioorg Med Chem Lett 16:4609–4611
Miyase T, Ueno A, Takizawa N, Kobayashi H, Karasawa H (1987) Studies on the glycosides of Epimedium grandiflorum Morr. var. thunbergianum (Miq.) Nakai. I. Chem Pharm Bull 35:1109–1117
Ferrari J, Terreaux C, Sahpaz SD, Msonthi J, Wolfender JL, Hostettmann K (2000) Benzophenone glycosides from Gnidia involucrata. Phytochemistry 54:883–889
Fujita M, Inoue T (1982) Studies on the constituents of Iris flo-. rentina L. II. C-glucosides of xanthones and flavones from the leaves. Chem Pharm Bull 30:2342–2348
Nagai M, Noguchi M, Iizuka T, Otani K, Kamata K (1996) Vasodilator effects of des(α-carboxy-3,4-dihydroxyphenethyl)lithospermic acid (8-epiblechnic acid), a derivative of lithospermic acids in salviae miltiorrhizae radix. Biol Pharm Bull 19:228–233
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and a grant by the Open Research Center Project. We gratefully acknowledge the financial support provided by Assessment Service Unit, Faculty of Pharmacy, Airlangga University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oshimi, S., Zaima, K., Matsuno, Y. et al. Studies on the constituents from the fruits of Phaleria macrocarpa . J Nat Med 62, 207–210 (2008). https://doi.org/10.1007/s11418-007-0209-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-007-0209-9