Leaves of Crataegus sanguinea Pall. afforded for the first time ergosterol 3-O-β-D-glucopyranoside, p-coumaric acid 4-O-β-D-glucopyranoside, trifolin, quercitrin, the new compound sanguineoside (5,7,3′,5′-tetrahydroxyflavanone 7-O-β-D-glucopyranoside), and compounds known from this species, e.g. oleanolic acid, caffeic acid, hyperoside, vitexin, and vitexin 2″-O-rhamnoside, which was the dominant flavonoid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Fruit and flowers of pharmacopoeial species of hawthorn (Crataegus L.) are widely used as cardiotonics and antiarrhythmics to treat cardiovascular conditions [1, 2]. C. sanguinea Pall. (Rosaceae) is a widely used pharmacopoeial species [1,2,3].
Leaves of C. sanguinea are known to contain flavonoids (hyperoside, vitexin, 2″-O-rhamnoside, apigenin, cosmosiin, luteolin, cynaroside, quercetin, rutin, fisetin, naringin, hesperidin, dihydroquercetin, catechin, and procyanidin); phenylpropanoids (caffeic acid, chlorogenic acid); saponins; vitamins; and many other secondary and primary metabolites [1,2,3]. Further studies of the chemical composition of C. sanguinea leaves as a promising domestic medicinal raw material are critical despite published data on their constituent composition. The goal of the present work was to study the constituent composition of C. sanguinea leaves.
Flavonoids 1–6, phenylpropanoids 7 and 8, saponin 9, and sterol 10 were isolated from hawthorn leaves during the studies.

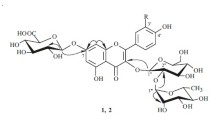
The PMR spectrum of 1 exhibited resonances for flavonoid aromatic protons at δ 6.15 (2H, d, J = 2.5 Hz, H-6,8), 6.88 (1H, s, H-4′), and 6.77 ppm (2H, s, H-2′,6′). The resonances for protons H-2ax (1H, dd, J = 4, 12 Hz), H-3ax (1H, dd, J = 12, 17 Hz), and H-3eq (1H, dd, J = 4, 17 Hz) at 5.43, 3.15, and 2.76 ppm, respectively, and UV spectral data characterized 1 as a flavanone [4]. Furthermore, the PMR spectrum of 1 had a 1H singlet at 12.07 ppm for the 5-OH group and a doublet for the glucose anomeric proton at 4.98 ppm with spin–spin coupling constant 7 Hz. The 2H doublet for H-6 and H-8 at 6.15 ppm (2H, d, J = 2.5 Hz) led to the conclusion that the 7-OH was glycosylated. Acid and enzymatic (β-glucosidase) hydrolysis of 1 formed glucose and the aglycon (5,7,3′,5′-tetrahydroxyflavanone), the structure of which was confirmed by mass-spectral data (M+ 288, 100%) and the 13C NMR spectrum.
Thus, 1 was called by us sanguineoside and had the structure 5,7,3′,5′-tetrahydroxyflavanone 7-O-β-D-glucopyranoside and was a new natural compound.
Flavonoids 2–6 were identified using UV, PMR, 13C NMR, and mass spectral data as vitexin (2), vitexin 2″-O-rhamnoside (3), trifolin (4), quercitrin (5), and hyperoside (6) [3, 5,6,7,8,9]. Flavonoids 4 and 5 were isolated from C. sanguinea leaves for the first time.
Phenylpropanoids 7 and 8 were identified as caffeic acid (7), which was reported earlier from C. sanguinea leaves [3], and p-coumaric acid 4-O-β-D-glucopyranoside, which was isolated earlier from Rhodiola rosea biomass [10].
Compound 9 was identified as oleanolic acid [11], which is known for Crataegus L. species [2, 3]. Compound 10 (ergosterol 3-O-β-D-glucopyranoside) was isolated for the first time from C. sanguinea leaves and other Crataegus species.
Experimental
PMR spectra were recorded on a Bruker AM 300 (300 MHz) instrument; 13C NMR spectra, on a Bruker DRX 500 (126.76 MHz) instrument. Mass spectra were taken on a Kratos MS-30 mass spectrometer. UV spectra were recorded using a Specord 40 spectrophotometer (Analytik Jena).
Extraction and Separation. Air-dried C. sanguinea leaves (100 g) that were collected in June 2016 in Samara Oblast were extracted with EtOH (70%) first with two extractions at room temperature for 24 h and then with heating on a boiling-water bath for 30 min. The combined aqueous EtOH extract was evaporated in vacuo to 50 mL, mixed with L 40/100 silica gel (30 g), and dried. The dried powder (dried extract + silica gel) was placed onto a layer of silica gel (8 cm diameter × 5 cm height) that was formed from a suspension in CHCl3. The chromatography column was eluted with CHCl3 and CHCl3–EtOH in various ratios (99:1, 98:2, 97:3, 95:5, 93:7, 90:10, 85:15, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70).
The separation was monitored using TLC on Sorbfil PTSKh-AF-A-UF plates and CHCl3–EtOH (9:1), CHCl3–EtOH–H2O (26:16:3), and n-BuOH–AcOH (glacial) –H2O (4:1:2). Fractions containing 1 as the dominant compound were combined. The precipitate that formed in them was separated and recrystallized from an H2O–Me2CO mixture to afford 1 in 0.6 mass% of air-dried raw material. Fractions containing 2 were combined. The precipitate that formed in them was separated and recrystallized from aqueous EtOH to afford 2 in 0.1 mass% of air-dried raw material. Fractions containing 3 were combined. The precipitate that formed in them was separated and recrystallized from aqueous EtOH to afford 3 in 0.05 mass% of air-dried raw material.
Fractions containing 4–6 were placed onto a Woelm polyamide column for further purification. Dried powder (extract + polyamide) was placed onto a chromatographic column (sorbent height 5.0 cm, diameter 4 cm) and eluted by H2O and aqueous EtOH (20, 40, 70, 96%) to produce 4 (40% EtOH) and 5 and 6 (70% EtOH), which were further purified by recrystallization from aqueous EtOH.
Fractions containing 7–10 were also purified over a column of silica gel with elution by CHCl3 and CHCl3–EtOH in various ratios (99:1, 98:2, 97:3, 95:5, 93:7, 90:10, 85:15). Compound 7 was finally purified by recrystallization from H2O; 8–10, from EtOH.
Sanguineoside (5,7,3′,5′-tetrahydroxyflavanone 7-O-β-D-glucopyranoside) (1). Light-yellow amorphous compound, C21H22O11, mass spectrum (70 eV, 200°C, m/z, %): aglycon M+ 288 (100%), 153 (67), 137 (8). UV spectrum (EtOH, λmax, nm): 290, 336 (sh); +A1C13 285, 304, 344, 394 (sh). 1H NMR spectrum (300 MHz, DMSO-d6, δ, ppm, J/Hz): 12.07 (1H, s, 5-OH), 9.17 (1H, s, 7-OH), 9.05 (2H, br.s, 3′, 5′-OH), 6.88 (1H, s, H-4′), 6,77 (2H, s, H-2′, 6′), 6.15 (2H, d, J = 2.5, H-6, 8), 5.43 (1H, dd, J = 4, 12, H-2ax), 3.15 (1Í, dd, J =12, 17, H-3ax), 2.76 (1H, dd, J = 4, 17, H-3eq), 4.98 (1H, d, J = 7, Glc H-1′′), 3.1–5.2 (6H, m, Glc). 13C NMR spectrum (126 MHz, DMSO-d6, δ, ppm): 197.17 (C-4), 78.71 (C-2), 165.30 (C-7), 145.81 (C-3′), 145.81 (C-5′), 118.07 (C-4′), 162.94 (C-5), 162.71 (C-9), 115.36, 94 (C-2′), 115.16 (C-6′), 129.22 (C-1′), 103.28 (C-10), 96.43 (C-8), 95.44 (C-6), 99.60 (Glc C-1′′), 78.71 (C-3′′), 77.09 (C-5′′), 73.03 (C-2′′), 70.06 (C-4′′), 60.78 (C-6′′), 42.19 (C-3).
Vitexin (5,7,4′-trihydroxyflavone 8-C-β-D-glucopyranoside) (2). Light-yellow crystalline powder, C21H20O10, mp 273–275°C (aqueous EtOH). Mass spectrum (ESI-MS, 180°C, m/z): M+ 433 (432 + H). 1H NMR spectrum (300 MHz, DMSO-d6, δ, ppm, J/Hz): 13.18 (1H, s, 5-OH), 10.37 (1H, br.s, 7-OH), 9.04 (1H, br.s, 4′-OH), 8.04 (2H, d, J = 9, H-2′, 6′), 6.89 (2H, d, J = 9, H-3′, 5′), 6.78 (1H, s, H-3), 6.27 (1H, s, H-6), 4.84 (1H, d, J = 9.9, Glc H-1′′), 3.1–4.6 (6H, m, Glc). 13C NMR spectrum (126 MHz, DMSO-d6, δ, ppm): 182.07 (C-4), 165.27 (C-2), 162.68 (C-7), 162.08 (C-4′), 161.11 (C-5), 160.36 (C-9), 128.94 (C-2′), 128.94 (C-6′), 121.59 (C-1′), 115.78 (C-3′), 115.32 (C-5′), 104.01 (C-10), 104.59 (C-8), 98.12 (C-6), 81.02 (C-3′′), 81.02 (C-5′′), 73.35 (Glc C-1′′), 70.82 (C-2′′), 70.52 (C-4′′), 61.27 (C-6′′).
Vitexin 2″-O-Rhamnoside (5,7,4′-trihydroxyflavone 2″-O-α-L-rhamnopyranosyl-8-C-β-D-glucopyranoside) (3). Light-yellow crystalline powder, C27H30O14, mp 213–215°C (aqueous Me2CO). Mass spectrum (ESI-MS, 180°C, m/z): 579 (M + H)+, 601 (M + Na)+, 617 (M + K)+. 1H NMR spectrum (300 MHz, DMSO-d6, δ, ppm, J/Hz): 13.21 (1H, s, 5-OH), 11.03 (1H, br.s, 7-OH), 10.37 (1H, br.s, 4′-OH), 8.21 (2H, d, J = 9, H-2′, 6′), 6.92 (2H, d, J = 9, H-3′, 5′), 6.83 (1H, s, H-3), 6.25 (1H, s, H-6), 5.33 (1H, br.s, Rha H-1′′′), 4.84 (1H, d, J = 9.9, Glc H-1′′), 3.2–5.25 (10H, m, Glc-H), 1.70 (3H, s, Rha CH3). 13C NMR spectrum (126 MHz, DMSO-d6, δ, ppm): 182.05 (C-4), C-2 (169.22), 164.05 (C-7), 162.08 (C-4′), 161.23 (C-5), 156.44 (C-9), 129.04 (C-2′), 129.04 (C-6′), 121.58 (C-1′), 116.21 (C-3′), 115.80 (C-5′), 104.01 (C-10), 103.98 (C-8), 97.81 (C-6), 102.4 5 (Rha C-1′′′), 97.81 (Glc C-2′′), 82.02 (C-5′′), 75.68 (C-3′′′), 75.68 (C-5′′), 72.52 (Glc C-1′′), 70.93 (C-2′′′), 70.93(C-4′′), 70.46 (C-5′′′), 60.99 (C-6′′), 20.44 (CH3).
Trifolin (3,5,7,4′-tetrahydroxyflavone 3-O-β-D-galactopyranoside) (4). Light-yellow crystalline compound, mp 240–242°C (aqueous EtOH), Mass spectrum (70 eV, 200°C, m/z, %): 286 (aglycon M+, 100 %), 153 (11), 152 (20), 121 (10). UV spectrum (EtOH, λmax, nm): 270, 338; + NaOAc 280, 371; + NaOAc + H3BO3 280, 371; +A1C13 И +A1C13 + HCl 275, 305, 396.
Quercitrin (3,5,7,3′,4′-pentahydroxyflavone 3-O-α-L-rhamnopyranoside) (5). Light-yellow crystalline compound, mp 224–226°C (aqueous EtOH), Mass spectrum (70 eV, 200°C, m/z, %): 302 (aglycon M+, 100%), 153 (12), 152 (43), 137 (8). 1H NMR spectrum (300 MHz, DMSO-d6, δ, ppm, J/Hz): 12.65 (1H, s, 5-OH), 9.82 (1H, s, 7-OH), 9.27 (1H, s, 4′-OH), 7.65 (1H, d, J = 2.5, H-2′), 7.61 (1H, dd, J = 2.5, 9, H-6′), 6.88 (1H, d, J = 9, H-5′), 6.82 (1H, d, J = 2.5, H-8), 6.42 (1H, d, J = 2.5, H-6), 5.58 (1H, d, J = 1.5, Rha H-1′′), 3.1–5.2 (4H, m, Rha-H), 1.25 (3H, d, J = 6, Rha CH3). 13C NMR spectrum (126 MHz, DMSO-d6, δ, ppm): 177.60 (C-4), 161.57 (C-7), 160.86 (C-5), 160.12 (C-9), 156.66 (C-2), 149.44 (C-4′), 148.65 (C-3′), 131.00 (C-3), 122.13 (C-6′), 121.71 (C-1′), 116.21 (C-2′), 115.23 (C-5′), 101.62 (C-10), 100.86 (Rha C-1′′), 98.41 (C-8), 94.30 (C-6), 116.21 (C-2′), 74.07 (C-3′′), 73.16 (C-5′′), 70.24 (C-4′′), 70.07 (C-2′′), 17.90 (C-6′′, CH3).
Hyperoside (3,5,7,3′,4′-pentahydroxyflavone 3-O-β-D-galactopyranoside) (6). Light-yellow amorphous compound, mp 230–232°C (aqueous Me2CO). Mass spectrum (70 eV, 200°C, m/z, %): 302 (aglycon M+, 100%), 153 (23), 137 (64). UV spectrum (EtOH, λmax, nm): 258, 266 sh, 363; + NaOAc 274, 381; + NaOAc + H3BO3 262, 378; +A1C13 275, 414; +A1C13 + HCl 271, 403.
Caffeic Acid (7). Light-yellow crystalline compound, C9H8O4, mp 200–202°C (H2O). Mass spectrum (70 eV, 200°C, m/z, %): 180 (M+, 100%).
p-Coumaric Acid 4-O-β-D-Glucopyranoside (8). White crystals, mp 206–208°C (EtOH). Mass spectrum (EI-MS, 180°C, m/z): aglycon M+ 164 (54%). UV spectrum (EtOH, λmax, nm): 285, 316 (sh). 1H NMR spectrum (300 MHz, DMSO-d6, δ, ppm, J/Hz): 7.68 (2H, d, J = 9, H-2, 6), 7.01 (2H, d, J = 9, H-3, 5), 6.85 (1H, d, J = 16, H-7), 5.83 (1H, d, J = 16, H-8), 4.88 (1H, d, J = 7, Glc H-1′), 3.1–4.6 (6H, m, Glc-H). 13C NMR spectrum (126 MHz, DMSO-d6, δ, ppm): 176.00 (C-9), 167.82 (C-4), 131.97 (C-2), 131.97 (C-6), 128.00 (C-3), 128.00 (C-5), 118.95 (C-7), 115.63 (C-8), 100.10 (Glc C-1′), 77.89 (C-3′), 77.15 (C-5′), 76.61 (C-2′), 72.53 (C-4′), 60.71 (C-6′).
Oleanolic Acid (9). White crystals (EtOH), C30H48O3, mp 303–305°C (EtOH). Mass spectrum (70 eV, 200°C, m/z, %): 456 (M+, 23), 341 (9), 248 (100), 219 (14), 208 (44), 203 (68), 135 (33), 121 (37), 107 (30), 81 (30), 55 (25).
Ergosterol 3-O-β-D-Glucopyranoside (10). White amorphous compound, UV spectrum (EtOH, λmax, nm): 271. Mass spectrum (70 eV, 200°C, m/z, %): 396 (M+, 18), 215 (5), 164 (23), 149 (19), 120 (31), 95 (24), 85 (17), 81 (33), 73 (58), 60 (100), 43 (84). 1H NMR spectrum (300 MHz, CDCl3, δ, ppm, J/Hz): 5.34 (2H, m, H-6, 7), 4.87 (2H, d, J = 7.7, H-22, 23), 4.22 (1H, d, J = 7, Glc H-1′), 3.65 (1H, m, H-3), 1.0–3.5 (26H, m, incl. Glc 6H), 0.65–0.95 (18H, m, CH3). 13C NMR spectrum (126 MHz, DMSO-d6, δ, ppm): 140.52 (C-5), 140.52 (C-8), 133.59 (C-22), 129.26 (C-23), 121.26 (C-6), 121.26 (C-7), 76.81 (C-3), 100.86 (Glc C-1′), 76.99 (C-3′), 76.84 (C-5′), 73.59 (C-2′), 70.19 (C-4′), 61.17 (C-6′), 56.24 (C-17), 54.50 (C-14), 49.67 (C-9), 45.21 (C-24), 41.92 (C-13), 40.51 (C-4), 40.51 (C-20), 38.38 (C-12), 36.28 (C-1), 35.53 (C-10), 33.42 (C-2), 31.49 (C-25), 28.78 (C-16), 22.68 (C-11), 20.66 (C-21), 19.77 (C-26), 19.16 (C-27), 19.00 (C-19), 18.68 (C-28), 11.50 (C-18).
References
Plant Resources of the USSR. Flowering Plants, Their Chemical Composition and Use. Families Hydrangeaceae–Haloragaceae [in Russian], Nauka, Leningrad, 1987, 326 pp.
A. L. Budantsev (chief ed.), Plant Resources of Russia: Wild Flowering Plants, Their Constituent Composition and Biological Activity, Vol. 2, Families Actinidiaceae, Malvaceae–Euphorbiaceae, Haloragaceae, Tovarishchestvo Nauchnykh Izdanii KMK, St. Petersburg, Moscow, 2009, 513 pp.
S. R. Khasanova, Author’s Abstract of a Doctoral Dissertation, Samara, 2016, 46 pp.
T. J. Mabry, K. R. Markham, and M. B. Thomas, The Systematic Identification of Flavonoids, Springer Verlag, Berlin–Heidelberg–New York, 1970, 354 pp.
M. G. Raghu and A. Pushpa, IOSR J. Pharm. Biol. Sci., 11 (6), 73 (2016).
J. Gupta and A. Gupta, Int. J. Chem. Stud., 4 (1), 14 (2016).
S. Thenmozhi and U. Subasini, Int. J. Res. Pharmacol. Pharmacother., 1, 84 (2016).
K. F. Taha and Z. T. Abd El Shakour, Int. J. Pharm. Sci. Res., 8 (3), 1081 (2017).
N. B. Begmatov, Kh. M. Bobakulov, X. Xin, and H. A. Aisa, Chem. Nat. Compd., 50, 1116 (2014).
V. A. Kurkin, G. G. Zapesochnaya, A. G. Dubichev, E. D. Vorontsov, I. V. Aleksandrova, and R. V. Panova, Chem. Nat. Compd., 27, 419 (1991).
H. C. Kwon, K. R. Lee, and O. P. Zee, Arch. Pharm. Res., 20 (2), 180 (1999).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2019, pp. 21–23.
Rights and permissions
About this article
Cite this article
Kurkin, V.A., Morozova, T.V., Pravdivtseva, O.E. et al. Constituents from Leaves of Crataegus sanguinea. Chem Nat Compd 55, 21–24 (2019). https://doi.org/10.1007/s10600-019-02606-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-019-02606-w