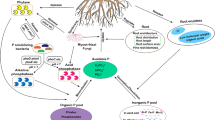
Abstract
Purpose
Soil available phosphate (AP) is largely dependent on phoD-harboring bacteria, which can release alkaline phosphatase (ALP) to transform insoluble P for plant absorption. However, the way of phoD-harboring bacterial communities responding to restoration measures in alpine ecosystems, which are among the least studied and most vulnerable ecosystems, remains largely unknown. This knowledge is fundamental for formulating effective ecosystem management and soil conservation policies.
Materials and methods
By combining quantitative PCR and amplicon sequencing, we examined the alterations in phoD-harboring bacterial communities across four distinct meadow types, and explored the potential environmental drivers of alpine soil P availability.
Results and discussion
The results indicated that the fenced and fenced + reseeded meadows exhibited higher ALP activity and soil AP content compared to the grazed meadow, but lower than that of the undegraded meadow. The fenced meadow had the highest phoD-harboring bacterial community diversity. Rare genera such as Rhizobium, Breoghania, and Actinomadura, played a critical role in regulating ALP activity. A structural equation model demonstrated that soil pH, nutrient supply (e.g., soil organic carbon, NO3−-N), and vegetation together drove the improvement of soil P availability by enhancing ALP activity, which was closely related to phoD-harboring bacterial communities. These findings suggest that fencing could promote alpine soil P availability by adjusting phoD-harboring bacteria and ALP activity.
Conclusions
Our results demonstrate the beneficial impacts of grazing-to-fencing conversion on meadows in alpine ecosystems and can contribute to the development of sustainable management strategies for degraded alpine ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Phosphorus (P) plays a vital role as a crucial nutrient for growth in living organisms, serving as both a structural and functional element. It is among the most essential nutrients required for supporting various biological processes and promoting overall organismal development (Cordell and White 2015). Although P is present in plants at only 0.2% of their dry weight, it remains the most important limiting element for plant productivity (Holtan et al. 1988), with the same level of significance as carbon (C) and nitrogen (N). However, P does not exist as an element in the soil but is dissolved in the soil solution as orthophosphates (H2PO4−, HPO42−, and PO43−) or is bound to soil particles and organic matter (Liu et al. 2020a), making it difficult for direct use by plants. Therefore, researchers are increasingly focusing on P mineralization and enhancing the efficiency of P utilization.
Alkaline phosphatase (ALP), a phosphomonoesterase which can convert organic P into available P (AP), has been widely used to evaluate organic P mineralization within agroecosystems (Nannipieri et al. 2011). For example, the study conducted by Luo et al. (2017) reported a higher ALP activity in soils that were amended with organic matter compared to soils that received chemical-only fertilization. In addition, compared to orthophosphate fertilizer applied in conventional agriculture, animal manure used in organic farming may enhance ALP activity and overall microbial community activity through the inclusion of humic-enzyme complexes with manure (Watts et al. 2010). Some soil factors, such as pH and organic matter content, have been reported to regulate the activity of ALP (Dai et al. 2020; Luo et al. 2019). Nevertheless, the regulatory mechanisms underlying ALP activity in grassland ecosystems remain unclear (Mencel et al. 2022). Some studies, such as Chmolowska et al. (2017) and Futa et al. (2017), have demonstrated that grazing can increase ALP activity. On the other hand, Gilmullina et al. (2020) found a decrease in ALP activity associated with grazing. Additionally, N addition has been reported to significantly suppress ALP activity in semi-arid grassland soils (Tian et al. 2016). Further research is necessary to assess the impacts of ecological restoration measures on ALP activity in grassland ecosystems.
Bacteria, especially Actinobacteria and Proteobacteria, are known to have a predominant role in ALP secretion (Chen et al. 2017). In the presence of P deficiency, they promote AP release and recovery by increasing ALP secretion and activity (Chen et al. 2021). Bacteria carrying genes encoding ALP can be categorized into different families, namely phoA, phoD, and phoX (Gou et al. 2020). Among these families, phoD has been found in various soil types and is particularly abundant compared to phoX and phoA (Tan et al. 2013). As a result, understanding the changes or fluctuations in community of bacteria harboring phoD gene during ecosystem restoration and their association with P cycling is integral to formulating effective ecosystem management and conservation policies. Several studies have provided insights into the impacts of phosphate fertilization on phoD gene abundance and community diversity of bacteria harboring phoD gene in different soil types. For instance, Ikoyi et al. (2018) found that short-term phosphate fertilization in grassland soil could decrease phoD gene abundance. On the other hand, Tan et al. (2013) observed that phoD gene abundance was increased in pasture soil due to long-term phosphate fertilizer addition. In addition to phoD-harboring bacteria that can promote ALP to mineralize P from organic compounds, it has been reported that other microorganisms are capable of solubilizing inorganic P through soil acidification (Miller et al. 2010; Rice et al. 2012). Meanwhile, the diversity of rare phoD taxa has been reported to be more strongly related to ALP activity than the diversity of abundant taxa in Chinese steppe and agricultural ecosystems (Wei et al. 2019). Therefore, it is important to study the rare bacterial communities in phoD to enhance the effectiveness of soil P.
Alpine ecosystems cover approximately 20% of the Earth’s terrestrial surface area and play vital roles in climate stabilization, food provision, and biodiversity conservation. Owing to the extreme environmental stresses in alpine ecosystems (e.g., low temperatures, freezing cycles, high winds, strong UV irradiance, and low oxygen), they are one of the most understudied terrestrial ecosystems and are one of the most vulnerable to climate change and human disturbance. Alpine ecosystems are threatened by overgrazing, which can affect the alpine ecosystem structure and function by altering plant and soil properties. Fencing and replanting grass seeds have been extensively applied to restore grazing–induced alpine meadows, and have been reported to be beneficial to primary productivity, soil aggregate status, organic C, and available N levels. Nevertheless, there is still limited knowledge regarding how grazing-to-fencing conversion influences the availability of soil P. Given the P limitation in alpine meadows, it is crucial to have comprehensive knowledge about the availability of P in alpine for the formulation of effective alpine ecosystem management and soil conservation policies. The main focus of this study was to investigate the influence of grazing-to-fencing conversion on soil AP content, ALP activity, and phoD-harboring bacterial communities (pHBC) in the alpine meadows. The study utilized quantitative PCR, high-throughput sequencing, and multiple statistical analyses to investigate these effects, based on a 5-year field experiment on the Tibetan Plateau. Our hypothesis was that grazing-to-fencing conversion could improve AP, possibly by increasing ALP activity as well as changing pHBC, which are strongly interconnected with plant biomass, soil nutrients, and pH. Our results enhance the comprehension of how soil P availability in alpine ecosystems can be enhanced by promoting the activity of phoD-harboring microbes. This knowledge could provide valuable insights for sustainably managing degraded alpine ecosystems with guiding efforts toward their restoration and conservation.
2 Materials and methods
2.1 Field experiment
The field experiment was located at the Bangjietang Alpine Meadow Ecosystem Research Station’s experimental site of Tibetan Plateau (32°21′N, 91°40′E, 4672 m ASL) (Fig. 1). The temperature is −3.1 °C, and the precipitation is 400 mm on average (Yang et al. 2019). The soil in this area is categorized as a Mattic-Cryic Cambisol according to the FAO/UNESCO taxonomy. The main plant communities include Kobresia pygmaea, Kobresia robusta, Stipa purpurea, Poa cymophila, Kengyilia thoroldiana, Elymus nutans, and Roegneria thoroldiana (Wang et al. 2020b).
2.2 Sample collection
The field experiment comprised four restoration treatments, covering an approximate area of 200 hectares (Wang et al. 2021c): (1) Undegraded meadow (UM): without human disturbance or livestock trampling and nibbling, and primitive vegetation was rich in variety; (2) Grazed meadow (GM): 4 yaks grazing per hectare since 1988; (3) Fenced meadow (FM): fenced with barbed wire in May 2009 to prevent livestock from entering (before fencing, the meadow was managed in the same manner as GM); (4) Fenced + reseeded meadow (FRM): The FRM was reseeded based on the GM; the seeds of the local dominant plants, Elymus nutans Griseb., Kengyilia thoroldiana (Oliver) J. L and Poacymophila Keng. were sown in May 2009. The sowing depth was 3 cm, with a spacing of 20 cm, and sowing densities of 3 g·m−2, 5 g·m−2, and 8 g·m−2, respectively.
In October 2018, five replicated plots covering 1 hm2 were randomly established in each meadow, and 20 plots were totally set up (Berman et al. 2018; Guo et al. 2017). For soil sampling as well as vegetation surveys, nine 10 m × 10 m blocks were established along the diagonal line with a separation distance of 10–20 m between each plot. Within each plot, three 1 m × 1 m quadrats were randomly set up, and an S-shaped pattern was followed within each quadrat for soil sampling. Five soil cores were collected at depths ranging from 0 to 15 cm, and were then mixed together to form a composite soil sample. Stones, plant litter, root residues, and other debris were removed from the soil samples. Finally, soil samples collected from the nine 10 m × 10 m blocks were combined to create a composite sample as a repetition for each treatment (Chen et al. 2021; Tang et al. 2019). Soil samples were sieved using a 2.0 mm mesh and were subsequently divided into three parts. The first part was placed at 4 °C for dissolved nutrients, microbial biomass P (MBP), and ALP activity measurement. The second was immediately placed in − 80 °C for microbial composition analysis, and the third was air-dried for soil physicochemical determination. Aboveground biomass (AGB) and belowground biomass (BGB) were obtained by collecting all plant tissues in each quadrat and oven-dried at 60 °C for 48 h (Wang et al. 2021c). The species richness of vegetation was estimated by recording the number of species (Zhang et al. 2016); The Shannon diversity of vegetation community was calculated using the following equation:
where pi represents the relative abundance of each species, and n represents the number of species.
2.3 Soil physicochemical analysis
Soil properties, including pH, moisture (SM), organic carbon (SOC), total nitrogen (TN), Dissolved organic C (DOC), dissolved organic N (DON), total phosphorus (TP), AP, nitrate nitrogen (NO3−-N) and ammonium nitrogen (NH4+-N), ALP activity, MBP, exchangeable calcium (Ca-ex) were measured by standard methods, and were shown in Supplementary Material.
2.4 phoD gene abundance quantification
Total DNA was extracted from 0.5 g soil using the E.Z.N.A. Soil DNA Kit (Omega, CT, USA). The quality and concentration of the DNA were assessed using a NanoDrop2000 UV spectrometer (Thermo Fisher Scientific, USA). The phoD gene abundance was determined by quantitative real-time PCR (qPCR) using an ABI 7300 Real-Time PCR System. Primers phoD-F730 (TGGGAYGATCAYGARGT) and phoD-R1083 (CTGSGCSAKSACRTTCCA) were used to amplify the phoD gene (Ragot et al. 2015). The qPCR reaction was conducted in triplicate using a 2X Taq Plus Master Mix (Thermo Fisher Scientific Inc., MA, USA) consisting of 10 μL, along with 0.8 μL of each primer, 1 μL of DNA solution, and 7.4 μL of double distilled H2O. The qPCR reaction was conducted under the following temperature and time conditions: Initially, a pre-denaturation cycle was performed for 5 minutes at 95 °C. A denaturation cycle is then carried out at the same temperature for 30 seconds, and 35 annealing cycles were subsequently performed. Finally, an extension step was completed for 1 minute at 72 °C. For the construction of the plasmid standard containing the phoD genes, we utilized the pMD18-T plasmid (TaKaRa, Japan). The concentration of the constructed plasmids was measured using a NanoDrop2000 UV spectrophotometer (Thermo Fisher Scientific, USA) and then converted to the copy number of DNA molecules (copies/µl). The standard curves were generated by performing 10-fold serial dilutions of the plasmids. The concentration of the plasmid and the number of base pairs were measured to calculate the copy number (Hu et al. 2018). The amplification efficiency was assessed to be 91.06%, with an R2 value of 0.9988.
2.5 phoD gene amplicons sequencing and data processing
Primers of phoD-F733 and phoD-R1083 were used to amplify the phoD gene. The PCR reactions were carried out in triplicate, starting with an initial denaturation step at 95 °C for 3 minutes, followed by denaturation at 95 °C for 5 seconds, annealing at 58 °C for 30 seconds, and extension at 72 °C for 1 minute. After the three reactions were combined, the amplicons were purified using the AxyPrepDNA Gel Extraction Kit (Axygen, USA) and were paired-end sequenced using the Illumina MiSeq (Illumina Inc., San Diego, CA). The raw sequencing data have been deposited in the Genome Sequence Archive at the BIG Data Center (accession number CRA010112).
The QIIME-1.9.1 pipeline was used to analyze the raw sequences, following the methodology described by Hu et al. (2018). The low-quality and chimeric sequences were excluded prior to analysis. The remaining high-quality sequences were clustered into operational taxonomic units (OTUs). For each OTU, a representative sequence was selected using UCLUST 1.2.22q, as outlined by Chen et al. (2021). OTUs with a singleton sequence were excluded. Taxonomic assignment was determined using the RDP classifer 2.2 against the FunGene database. The α-diversity, Shannon and Chao1 richness indexes of pHBC were calculated using the Mothur 1.30.2 software (Chen et al. 2017).
2.6 Data treatment
A one-way analysis of variance (ANOVA) was utilized to analyze variations in vegetation characteristics, soil properties, ALP activity, phoD gene abundance, the pHBC diversity, and taxa abundance among the four different meadow types. Nonmetric multidimensional scaling (NMDS) and similarities analysis (Anosim) were employed to investigate the variations in the pHBC structure across the different meadows. Random forest modeling (RFM) was used for assessing the critical genera affecting ALP activity. Spearman’s coefficient was used to evaluate the correlations among vegetation characteristics, soil properties, the pHBC structure, and phoD gene abundance. Redundancy analysis (RDA) was utilized to analyze associations among soil properties, vegetation characteristics, as well as the pHBC structure. The structural equation modeling (SEM) was utilized for establishing relationships among environmental factors, ALP activity, the pHBC structure, as well as phoD gene abundance, with respect to soil AP. ANOVA was conducted using SPSS 26.0 (IBM Corp., Somers, USA). SEM was conducted using the AMOS 26. NMDS, Anosim, Spearman’s correlations, and RDA were performed using the ‘ggplot2’ (Wickham 2016), ‘vegan’ (Dixon 2003), ‘rfPermute’ (Archer 2022), and ‘plspm’ (Tenenhaus et al. 2005) packages in R 4.2.1 (https://www.r-project.org/). Abundant OTUs were identified based on their relative abundances exceeding 1% of the total sequences, while rare OTUs were characterized by abundances lower than 1%.
3 Results
3.1 TP, AP, C/P, N/P, MBP, and ALP activity
Grazing-to-fencing conversion significantly affected the soil P-related properties (Fig. 2). Among the four meadow types, the UM site had the highest TP content, and the FM site showed a significantly higher AP content than that of the GM site, but lower than that of the UM and FRM sites (P < 0.05). Besides, the FM site exhibited higher soil C/P and N/P ratios compared to FRM and GM sites, although still lower than the UM site, significantly (P < 0.05). The UM site exhibited the highest levels of MBP content and ALP activity, whereas the GM sites had the lowest levels. Furthermore, the FM sites showed significantly higher MBP content and ALP activity compared to the FRM sites (P < 0.05).
a TP, soil total phosphorus, b AP, soil available phosphorus, c C/P, the ratio of SOC and TP, d N/P, the ratio of TN and TP, e MBP, microbial biomass phosphorus, f ALP: alkaline phosphatase activity, g phoD gene abundance (copy number g−1 soil), h Shannon index of the phoD-harboring bacteria community, i Chao1 index of the phoD-harboring bacteria community. Different letters indicate significant differences between different treatments (Duncan’s test, P < 0.05), UM: undegraded meadow; GM: grazed meadow; FM: fenced meadow; FRM: fenced + reseeded meadow
3.2 phoD gene abundance and pHBC
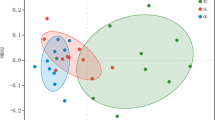
The response of phoD gene abundance and pHBC varied among the four restoration measures (Fig. 2g). Among the meadow types, the UM site displayed the greatest abundance of phoD gene, which showed 2.87–4.14 times greater than that observed in the other three meadow types, and ranked in the following order: FM, FRM, and GM sites. Remarkably, fencing led to an increase in the diversity of pHBC. The FM site exhibited higher Shannon and Chao 1 indices compared to the UM and GM sites. (Fig. 2h, i). The ordination of NMDS showed that the different meadow types had distinct pHBC compositions, manifested by the separation of cluster from each other in each treatment (Fig. 3), indicating that restoration measures remarkably influenced the β-diversity of pHBC. This finding was supported by Anosim analyses (R2 = 0.71, P < 0.001) (Fig. 3). The phoD gene sequences belonged to 19 bacterial phyla and 255 genera. The dominant phyla were Actinobacteria (32.57%), Proteobacteria (12.25%), Plantomycetes (2.04%), and Cyanobacteria (0.20%), which accounted for 47.06% of all sequences (Fig. 4a). When comparing the FM and GM sites, the relative abundance of Proteobacteria was significantly higher in the FM site (P < 0.05). The relative abundances of Actinobacteria, Plantomycetes and Cyanobacteria showed no significant differences among all sites (P > 0.05). At the genus level, Rubrobacter (6.77%), Streptomyces (6.31%), Frankia (5.30%), Pseudomonas (3.31%), and Micromonospora (3.28%) were the dominant genera (Fig. 4b). Compared to fencing and fencing + reseeding, grazing resulted in a significant increase in the relative abundance of Micromonospora, while causing significant decreases in the relative abundances of Rubrobacter and Frankia (P < 0.05). Moreover, the UM site exhibited a higher relative abundance of Streptomyces than the GM site.
Community compositions at the phylum (a) and genus (b) level in four meadows. Only the relative abundance of the phylum and genus in one sample > 1% was shown. Different letters indicate significant differences between different treatments (Duncan’s test, P < 0.05). UM: undegraded meadow; GM: grazed meadow; FM: fenced meadow; FRM: fenced + reseeded meadow
Results from RFM indicated that 42.9% of the variance in ALP activity could be explained by 255 bacterial genera, with only 18 genera as significant predictors (Fig. 5; P < 0.05). Most of these predictors were rare genera (relative abundance < 1%; Fig. S1), with Rhizobium, Breoghania and Actinomadura being the most significant. These rare genera responded differently to the restoration measures, with a higher relative abundance of Rhizobium at the FRM sites, and the highest abundance of Breoghania and Actinomadura in the UM, followed by FM, FRM, and GM sites (Fig. S1).
Random forest modeling analysis identified the main taxa predicting the changes in ALP activity (showing only the significant results). Rare genus, the relative abundance of which were less than 1%; abundant genus, the relative abundance of which were more than 1%. Significance levels of each predictor are as follows: *P < 0.05 and **P < 0.01
3.3 Potential environmental drivers of soil P availability
For uncovering the underlying mechanism behind soil P availability, we conducted an investigation on the modifications in vegetation characteristics and soil properties (Tables S1 and S2) among the four meadow types and evaluated their associations with ALP activity and pHBC. In general, the implementation of fencing and fencing + seeding led to noticeable improvements in vegetation cover, diversity, and biomass. This was evident through higher coverage, increased plant species richness, and enhanced AGB and BGB observed in the FRM and FM sites compared to the GM sites. Regarding soil nutrient variables, there were significant differences observed among the meadows. The FM site had higher levels of SOC, TN, and NO3−-N contents than the FRM and GM sites (P < 0.01). However, it should be noted that these nutrient levels in the FM site were still lower than those observed in the UM site. Soil pH values were highest in FRM site, ranging from 8.45 to 8.82. Among the four meadows, the highest NH4+-N content and lowest C/N concentration were found in the FM site. The results from the RDA indicated that the pHBC variation was accounted for by the first two axes, explaining a total of 46.87% of the variation (Fig. 6). Soil nutrient contents, especially SOC, TP, and NO3−-N, contributed more to the changes in pHBC (Table S3). Additionally, the SEM model provided additional insights into the influences of soil and vegetation characteristics on P availability (Figs. 7 and S2). Soil AP content was directly affected by ALP activity, which was regulated by the β-diversity of pHBC and phoD gene abundance. Soil pH directly and negatively impacted ALP activity as well as soil SOC, TP, and NO3−-N content, and BGB could be the predominant driver of phoD gene abundance. Additionally, soil TP content significantly affected the variation in the β-diversity of pHBC. Together, these findings indicate that grazing-to-fencing conversion directly affects soil P availability by altering ALP activity, which is regulated by the pHBC diversity and phoD gene abundance.
Redundancy analysis (RDA) for the phoD-harboring bacteria community structure and environmental factors. SOC, soil organic carbon; TP, total phosphorus; NO3−-N, nitrate nitrogen; BGB, belowground biomass; P-S, Shannon diversity index of plants; Ca-ex, exchangeable Ca; AP, soil available phosphorus; MBP, microbial biomass phosphorus. The green arrows indicate vegetation properties and brown arrows soil properties
Structural equation model (SEM) of vegetation character (P-S and BGB), soil properties (pH, SOC, NO3−-N, TP, and MBP), microbial attributes (phoD gene abundance and phoD-harboring bacteria community diversity), and ALP activity as predictors of AP a Larger path coefficients are reflected in the width of the arrow; red indicates a positive effect and blue a negative effect. Path coefficients are calculated after 1000 bootstraps. Coefficients of inner model differing significantly from 0 are indicated by *P < 0.05, **P < 0.01, and ***P < 0.001. The model is assessed using the goodness of fit statistic. Path coefficients that are not significantly different from 0 are shown as dashed lines. χ2 = 1.035, df = 2, P = 0.596, CFI = 1.000, RMSEA = 0.000. b Standardized total effects (direct plus indirect effects) of these factors derived from the SEM on AP are shown. Std. coeff.: standard coefficient; AP, available P; P-S, Shannon diversity index of plants; BGB, below-ground biomass; SOC, soil organic carbon; Ca-ex, exchangeable Ca; TP, total phosphorus; NO3−-N, nitrate nitrogen; β, the β-diversity of the phoD-harboring bacteria community; Chao1, the Chao1 index of phoD-harboring bacteria community; Gene abundance, phoD gene abundance; ALP, alkaline phosphatase activity
Because of the important roles of ALP activity, the pHBC diversity, and phoD gene abundance in regulating soil AP content, Spearman’s coefficient was further utilized to test their associations with the detected environmental variables (Figs. 8 and S3, Table S4). Remarkably, ALP activity exhibited a positive association with phoD gene abundance. Additionally, both ALP activity and phoD gene abundance were positively associated with plant coverage, BGB, and soil nutrients (e.g., SOC, TN, and DOC) but negatively associated with pH and plant diversity, significantly (P < 0.05). Nevertheless, we found no significant association between α-diversity (e.g., Shannon diversity and Chao1 estimator) with plant and soil factors (P > 0.05), while β-diversity was positively associated with pH and plant species diversity, and negatively correlated with most soil nutrient properties, such as SOC, NO3−-N, and TP (P < 0.05). Taxonomically, the relative abundances of Breoghania, Actinomadura and Streptomyces exhibited positive associations with SOC, NO3−-N, and TP (P < 0.05).
Correlation heat maps between the genus of phoD-harboring bacteria and environmental factors. Only the relative abundance of the genus in one sample > 1% was shown. SM, soil moisture; SOC, soil organic carbon; TN, soil total nitrogen; DOC, dissolved organic carbon; DON, dissolved organic nitrogen; NO3−-N, nitrate nitrogen; NH4+-N, ammonia nitrogen; TP, total phosphorus; AP, soil available phosphorus; ALP activity, alkaline phosphatase activity; MBP, microbial biomass P; C/N, the ratio of SOC and TN; C/P, the ratio of SOC and TP; N/P, the ratio of TN and TP; P-S, Shannon diversity index of plants; P-R, the richness of plants; AGB, aboveground biomass; BGB, belowground biomass. *P < 0.05; **P < 0.01; ***P < 0.001
4 Discussion
4.1 Grazing-to-fencing conversion increases the soil AP contents and ALP activity
Grazing prohibited by fencing or a combination of fencing and seeding have been widely applied to improve grassland soil P fertility (Han et al. 2020). Consistent with previous studies on long-term grazing effects, the soil AP content exhibited a significant increase in both fencing and fencing + reseeding treatments compared to the grazing treatment. This finding demonstrated the positive impact of fencing on soil P availability. The increased soil AP content is due to the mineralization of P-containing organic matter. In general, there are two forms in which plant P released into the soil (Liu et al. 2020b). Water-soluble P, as the first form, is easily released into the soil following the decomposition of plant residues and the secretion of roots. The second form involves organic substances generated through microbial metabolism. The improvement of soil AP content after feeding and reseeding is likely due to the greater organic substances presence resulting from plant biomass decomposition, which facilitates the release of inorganic P, such as FePO4 and AlPO4. The availability of P from native sources has been suggested to be increased by organic amendments (Cheng et al. 2016; Li et al. 2019). This phenomenon can be explained by the capacity of organic substances to enhance the concentration of exchangeable Ca, thereby facilitating the formation of available Ca-P. Exchangeable Ca levels have indeed been shown to play a pivotal role in regulating the phases of Ca-P in alkaline soils. Supporting this view, exchangeable Ca was highly and significantly associated with AP content in this study (r = 0.843, P < 0.05). Furthermore, the presence of organic matter has a dual impact on P availability in alkaline soils. It not only inhibits the deposition of hydroxyapatite but also promotes the generation of dicalcium phosphate dihydrate (Luo et al. 2019). This suggests that organic matter potentially enhance P availability by influencing the mineral phases in the soil.
Phosphatase activity has the potential to influence the P status and availability in soil, which is achieved through the catalysis of ester phosphate bond hydrolysis and subsequent release of phosphate anions from phosphate esters (Chen et al. 2017). It has been estimated that phosphatase have the capability to convert 20% to 80% of organic P into AP, which can be absorbed and utilized by microorganisms (Wang et al. 2021d). As a result, P-soluble bacteria obtain the necessary P for their growth by secreting phosphatase enzymes. This study proposed that the implementation of fencing and fencing + reseeding resulted in increased ALP activity, thereby enhancing P availability through the mineralization of organic P, as evidenced by the higher ALP activity in the FM treatment in comparison to that of the GM treatment. This could be attributed to the fact that increased organic matter from aboveground and belowground biomass provides C resources for phoD bacteria and increases their abundance and diversity, thereby facilitating ALP secretion. This inference is supported by the higher phoD gene abundance and the pHBC diversity in the FM treatment. The relationship between soil ALP and AP has been controversial; for example, Fraser et al. (2015) identified a negative correlation between ALP activity and Olsen-P concentration in agricultural ecosystems, while Hu et al. (2018) found a significantly positive relationship in long-term fertilized cropland, which was in agreement with our study. This discrepancy could be due to the dependency of the P availability–ALP relationship on soil P levels. In P-limited soil, phosphatase would be stimulated to express and meet the microbial needs, whereas in P-abundant soil, the increase in microbial population led to higher phosphatase secretion. This enhanced phosphatase secretion promoted the mineralization of organic P, resulting in a high retention of Olsen-P (Hu et al. 2018). Higher levels of soil phosphatase activity also indicate a strong capacity of soil microbes to mineralize complex P compounds.
4.2 Grazing-to-fencing conversion increases the phoD genes abundance and pHBC diversity
Previous studies have reported a shift in soil pHBC during grazing prohibition (Dong et al. 2020). However, understanding about the pHBC structure in alpine ecosystems is still limited. In this study, a higher pHBC α-diversity (e.g., Shannon and Chao1 estimator) was observed in fenced and fenced + reseeded meadows compared to grazed meadows. This finding aligns with previous studies that a positive impact of fencing on microbial species richness (Fan et al. 2020). The higher plant diversity observed in the fenced site is believed to contribute to this effect (Eldridge and Delgado-Baquerizo 2018). It has been documented that a more diverse plant community leads to higher microbial diversity due to the presence of a wider range of organic substrates (Wang et al. 2020a). It is important to note that reseeding plant species has been found to increase soil bacterial diversity, as mentioned in the study by Hu et al. (2018). However, no significant difference in the pHBC diversity was observed between the fencing-only treatments and the fencing combined with reseeding treatments. Additionally, our findings indicated that reseeding on the fenced site could not enhance the richness of pHBC. This suggested that the introduction of new plant species has a negligible impact on the diversity of pHBC. One possible explanation for this is that the newly introduced species have poor adaptability to the soil environment, making them less competitive than local species. Besides, we observed that fencing improved the phoD gene abundance compared to grazing. Furthermore, we found significantly positive associations among BGB, SOC, and phoD gene abundance. This could be attributed to the increased availability of organic C resulting from root decomposition and exudate release in the two fencing treatments, which fosters a higher quantity and growth of microbes harboring the phoD gene (Zhang et al. 2018).
Our investigation, consistent with previous studies (Lagos et al. 2016; Randall et al. 2019), identified Actinobacteria, Proteobacteria, and Cyanobacteria as the predominant bacteria. These consistent findings suggest that these microbial groups, which are essential for the decomposition of organic P, may dominate various soil types and environmental conditions. However, the implementation of restoration measures resulted in a significant alteration of the pHBC composition. (Dong et al. 2020; Wang et al. 2021b). In a recent research by Wang et al. (2021b), it was found that afforestation increased the diversity of pHBC more than natural restoration on the Loess Plateau. In our own observations, we noted that both fencing and fencing + reseeding stimulated the growth of Rubrobacter, Frankia and Streptomyces. These particular bacteria, Rubrobacter and Streptomyces, are known to thrive in C-rich environments due to their ability to secrete extracellular enzyme complexes (Wang et al. 2021a). Frankia have also been reported to exhibit rapid growth by utilizing hemicellulose and lignin as C sources (Lemanowicz et al. 2016). It is worth noting that these three genera, Rubrobacter, Frankia, and Streptomyces, encompass numerous bacterial species that are capable of solubilizing and mineralizing P (Hamdali et al. 2008). Therefore, it would be valuable for future studies to investigate the interplay between C and P cycling in different soil conditions, such as those characterized by P limitation, C limitation, or both. In addition, the results from RFM indicated that 18 taxa were identified as contributors to ALP activity, and most of them were rare genera (relative abundance < 1%). This finding suggests a critical role of the rare bacterial groups, rather than common taxa, in contributing to ALP release. Notably, Rhizobium, Breoghania, and Actinomadura were among the significant contributors, exhibiting a higher relative abundance in the FM and FRM sites compared to the GM site, indicating that fencing and fencing + reseeding effectively promote the restoration of soil P-solubilizing bacterial communities. Moreover, this observation aligns with the positive relationship observed between these bacteria abundance and soil AP content. These findings suggest that the management practices of fencing and reseeding can enhance the presence and activity of P-solubilizing bacteria, leading to improving soil P availability. It is worth mentioning that Actinomadura has been shown to serve as a significant role in solubilization of P, making it more available for plants to uptake (Nimnoi et al. 2014), while Breoghania is not well-documented, which also has potential contribution to soil P removal (Gallego et al. 2010). Comparing with the grazing treatment, the fencing treatment increased the abundance of Rhizobium, a rhizobacterium known for its ability to solubilize soil P (Cheng et al. 2019; Yousafi et al. 2019). Additionally, the practice of fencing, along with fencing + reseeding, was shown to specifically enhance the population of certain P-solubilizing bacteria, thereby promoting the P availability.
4.3 Soil nutrient and pH are responsible for the increased soil P availability by regulating pHBC and ALP activity
The soil P availability is regulated by various biochemical processes, and the link between ALP activity and P availability could be confusing. According to the RDA and SEM models, ALP activity directly regulated soil P availability, which was influenced by phoD gene abundance and the pHBC structure. Besides, our observations indicated that phoD gene abundance played a more significant role in regulating soil ALP activity compared to the pHBC structure, indicating that the increased abundance of phoD gene resulting from fencing made a greater influence on P availability when comparing with the changes in the pHBC structure. Previous research has demonstrated the importance of SOC as a primary driver of phosphorolysis in soil. Increased SOC levels, as promoted by fencing, enhance the accumulation of exchangeable Ca, which further influencing the interaction between exchangeable Ca and P, leading to forming more available Ca-P (Hu et al. 2018). The SEM model showed that the pHBC structure was mainly affected by C and N, rather than P. This finding aligns with the research conducted by Ragot et al. (2016), who investigated strong correlations among dissolved organic C, pH, and the pHBC structure in grassland ecosystems. Similarly, Hu et al. (2018) identified soil organic C as an influential factor of the pHBC structure. These studies confirm the crucial function of C and N in regulating P availability, although P is also essential for microorganisms. Abundant C and N resources also provide substrates for microbiota and increase their diversity, growth, and metabolic activities. Consequently, in our study, we observed direct effects of SOC and NO3−-N on phoD gene abundance and the pHBC diversity. The NO3−-N can might affect soil P through N-rich phosphatases secreted by bacteria and roots. In addition, nitrate accumulation can cause soil acidification, which might aggravate the transformation and migration of soil P. Although soil P availability has been found to be enhanced by soil acidification (Luo et al. 2017), our study did not find a direct effect of pH on soil P availability. Instead, soil pH can indirectly impact AP content by exerting influence on both ALP activity and the pHBC structure, which aligns with previous research indicating that soil pH is a key factor driving microbial community activity and structure (Dai et al. 2020).
Notably, BGB also has a significant impact on soil AP. Root exudates and organic acids contribute to the mineralization of soil organic P. Additionally, the presence of root biomass promotes the pHBC growth and reduces rhizosphere pH in alpine meadows. This is supported by the direct effect of BGB on phoD gene abundance.
5 Conclusion
Our research findings demonstrated that grazing-to-fencing conversion could improve alpine soil P availability by regulating pHBC, especially through increasing the rare genera abundance such as Rhizobium, Breoghania, and Actinomadura, and enhancing ALP activity. Among the various factors examined, SOC and NO3−-N were observed to regulate pHBC more strongly than other factors. Soil pH, nutrient supply (e.g., SOC, NO3−-N), and changes of plant community together drive the enhancement of soil P availability by enhancing ALP activity, which is closely associated with phoD gene abundance as well as the pHBC structure. Our study confirmed the positive effect of fencing or fencing + reseeding on soil P availability and revealed the underlying mechanisms, which could be recommended for the restoration of grazing-induced degraded alpine meadows.
References
Archer E (2022) rfPermute: Estimate permutation p-values for random forest importance metrics. https://CRAN.R-project.org/package=rfPermute
Berman TS, Laviad-Shitrit S, Lalzar M, Halpern M, Inbar M (2018) Cascading effects on bacterial communities: cattle grazing causes a shift in the microbiome of a herbivorous caterpillar. ISME J 12:1952–1963. https://doi.org/10.1038/s41396-018-0102-4
Chen X, Condron LM, Dunfield KE, Wakelin SA, Chen L (2021) Impact of grassland afforestation with contrasting tree species on soil phosphorus fractions and alkaline phosphatase gene communities. Soil Biol Biochem 159:108274. https://doi.org/10.1016/j.soilbio.2021.108274
Chen XD, Jiang N, Chen ZH, Tian JH, Sun N, Xu MG, Chen LJ (2017) Response of soil phoD phosphatase gene to long-term combined applications of chemical fertilizers and organic materials. Appl Soil Ecol 119:197–204. https://doi.org/10.1016/j.apsoil.2017.06.019
Cheng JM, Jing GH, Wei L, Jing ZB (2016) Long-term grazing exclusion effects on vegetation characteristics, soil properties and bacterial communities in the semi-arid grasslands of China. Ecol Eng 97:170–178. https://doi.org/10.1016/j.ecoleng.2016.09.003
Cheng X, Ji X, Ge Y, Li J, Qi W, Qiao K (2019) Characterization of antagonistic bacillus methylotrophicus isolated from rhizosphere and its biocontrol effects on maize stalk rot. Phytopathology 109:571–581. https://doi.org/10.1094/PHYTO-07-18-0220-R
Chmolowska D, Elhottová D, Krištůfek V, Kozak M, Kapustka F, Zubek S (2017) Functioning grouped soil microbial communities according to ecosystem type, based on comparison of fallows and meadows in the same region. Sci Total Environ 599–600:981–991. https://doi.org/10.1016/j.scitotenv.2017.04.220
Cordell D, White S (2015) Tracking phosphorus security: indicators of phosphorus vulnerability in the global food system. Food Secur 7:337–350. https://doi.org/10.1007/s12571-015-0442-0
Dai Z, Liu G, Chen H, Chen C, Wang J, Ai S, Wei D, Li D, Ma B, Tang C, Brookes PC, Xu J (2020) Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J 14:757–770. https://doi.org/10.1038/s41396-019-0567-9
Dixon P (2003) VEGAN, a package of R functions for community ecology. J Veg Sci 14:927–930. https://doi.org/10.1111/j.1654-1103.2003.tb02228.x
Dong SK, Li Y, Ganjurjav H, Gao QZ, Gao XX, Zhang J, Yan YL, Zhang Y, Liu SL, Hu GZ, Wang XX, Wu HB, Li S (2020) Grazing promoted soil microbial functional genes for regulating C and N cycling in alpine meadow of the Qinghai-Tibetan Plateau. Agr Ecosyst Environ 303. https://doi.org/10.1016/j.agee.2020.107111
Eldridge DJ, Delgado-Baquerizo M (2018) Grazing reduces the capacity of landscape function analysis to predict regional-scale nutrient availability or decomposition, but not total nutrient pools. Ecol Ind 90:494–501. https://doi.org/10.1016/j.ecolind.2018.03.034
Fan DD, Kong WD, Wang F, Yue LY, Li XZ (2020) Fencing decreases microbial diversity but increases abundance in grassland soils on the Tibetan Plateau. Land Degrad Dev 31:2577–2590. https://doi.org/10.1002/ldr.3626
Fraser TD, Lynch DH, Bent E, Entz MH, Dunfield KE (2015) Soil bacterial phoD gene abundance and expression in response to applied phosphorus and long-term management. Soil Biol Biochem 88:137–147. https://doi.org/10.1016/j.soilbio.2015.04.014
Futa B, Patkowski K, Bielińska EJ, Gruszecki TM, Pluta MP, Kulik M, Chmielewski SJPJOSS (2017) Sheep and horse grazing in a large-scale protection area and its positive impact on chemical and biological soil properties. Pol J Soil Sci 49:111. https://doi.org/10.17951/PJSS.2016.49.2.111
Gallego S, Vila J, Nieto JM, Urdiain M, Rossello-Mora R, Grifolla M (2010) Breoghania corrubedonensis gen. nov sp nov., a novel alphaproteobacterium isolated from a Galician beach (NW Spain) after the Prestige fuel oil spill, and emended description of the family Cohaesibacteraceae and the species Cohaesibacter gelatinilyticus. Syst Appl Microbiol 33:316–321. https://doi.org/10.1016/j.syapm.2010.06.005
Gilmullina A, Rumpel C, Blagodatskaya E, Chabbi A (2020) Management of grasslands by mowing versus grazing – impacts on soil organic matter quality and microbial functioning. Appl Soil Ecol 156:103701. https://doi.org/10.1016/j.apsoil.2020.103701
Gou X, Cai Y, Wang C, Li B, Zhang Y, Tang X, Shen J, Cai Z (2020) Effects of different long-term cropping systems on phosphorus adsorption and desorption characteristics in red soils. J Soils Sediments 20:1371–1382. https://doi.org/10.1007/s11368-019-02493-2
Guo H, Ye C, Zhang H, Pan S, Ji Y, Li Z, Liu M, Zhou X, Du G, Hu F, Hu S (2017) Long-term nitrogen & phosphorus additions reduce soil microbial respiration but increase its temperature sensitivity in a Tibetan alpine meadow. Soil Biol Biochem 113:26–34. https://doi.org/10.1016/j.soilbio.2017.05.024
Hamdali H, Hafidi M, Virolle MJ, Ouhdouch Y (2008) Rock phosphate-solubilizing Actinomycetes: screening for plant growth-promoting activities. World J Microb Biot 24:2565–2575. https://doi.org/10.1016/B978-0-444-63987-5.00002-5
Han X, Li YH, Du XF, Li YB, Wang ZW, Jiang SW, Li Q (2020) Effect of grassland degradation on soil quality and soil biotic community in a semi-arid temperate steppe. Ecol Process 9. https://doi.org/10.1186/s13717-020-00256-3
Holtan H, Kamp-Nielsen L, Stuanes AO (1988) Phosphorus in soil, water and sediment: an overview. Hydrobiologia 170:19–34. https://doi.org/10.1007/BF00024896
Hu YJ, Xia YH, Sun Q, Liu KP, Chen XB, Ge TD, Zhu BL, Zhu ZK, Zhang ZH, Su YR (2018) Effects of long-term fertilization on phoD-harboring bacterial community in Karst soils. Sci Total Environ 628–629:53–63. https://doi.org/10.1016/j.scitotenv.2018.01.314
Ikoyi I, Fowler A, Schmalenberger A (2018) One-time phosphate fertilizer application to grassland columns modifies the soil microbiota and limits its role in ecosystem services. Sci Total Environ 630:849–858. https://doi.org/10.1016/j.scitotenv.2018.02.263
Lagos LM, Acuna JJ, Maruyama F, Ogram A, Mora MD, Jorquera MA (2016) Effect of phosphorus addition on total and alkaline phosphomonoesterase-harboring bacterial populations in ryegrass rhizosphere microsites. Biol Fert Soils 52:1007–1019. https://doi.org/10.1007/s00374-016-1137-1
Lemanowicz J, Bartkowiak A, Breza-Boruta B (2016) Changes in phosphorus content, phosphatase activity and some physicochemical and microbiological parameters of soil within the range of impact of illegal dumping sites in Bydgoszcz (Poland). Environ Earth Sci 75. https://doi.org/10.1007/s12665-015-5162-4
Li JW, Liu YL, Hai XY, Shangguan ZP, Deng L (2019) Dynamics of soil microbial C:N:P stoichiometry and its driving mechanisms following natural vegetation restoration after farmland abandonment. Sci Total Environ 693. https://doi.org/10.1016/j.scitotenv.2019.133613
Liu J, Li CY, Xing YW, Wang Y, Xue YL, Wang CR, Dang TH (2020a) Effects of long-term fertilization on soil organic phosphorus fractions and wheat yield in farmland of Loess Plateau. J Appl Ecol 31:157–164. https://doi.org/10.13287/j.1001-9332.202001.028
Liu J, Ma Q, Hui X, Ran J, Ma Q, Wang X, Wang Z (2020b) Long-term high-P fertilizer input decreased the total bacterial diversity but not phoD-harboring bacteria in wheat rhizosphere soil with available-P deficiency. Soil Biol Biochem 149. https://doi.org/10.1016/j.soilbio.2020.107918
Luo G, Sun B, Li L, Li M, Liu M, Zhu Y, Guo S, Ling N, Shen Q (2019) Understanding how long-term organic amendments increase soil phosphatase activities: Insight into phoD- and phoC-harboring functional microbial populations. Soil Biol Biochem 139. https://doi.org/10.1016/j.soilbio.2019.107632
Luo GW, Ling N, Nannipieri P, Chen H, Raza W, Wang M, Guo SW, Shen QR (2017) Long-term fertilisation regimes affect the composition of the alkaline phosphomonoesterase encoding microbial community of a vertisol and its derivative soil fractions. Biol Fert Soils 53:375–388. https://doi.org/10.1007/s00374-017-1183-3
Mencel J, Mocek-Plociniak A, Kryszak A (2022) Soil microbial community and enzymatic activity of grasslands under different use practices: a review. Agronomy 12. https://doi.org/10.3390/agronomy12051136
Miller SH, Browne P, Prigent-Combaret C, Combes-Meynet E, Morrissey JP, O’Gara F (2010) Biochemical and genomic comparison of inorganic phosphate solubilization in Pseudomonas species. Env Microbiol Rep 2:403–411. https://doi.org/10.1111/j.1758-2229.2009.00105.x
Nannipieri P, Giagnoni L, Landi L, Renella G (2011) Role of phosphatase enzymes in soil. In: Bünemann E, Oberson A, Frossard E (eds) Phosphorus in action: Biological processes in soil phosphorus cycling. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 215–243. https://doi.org/10.1007/978-3-642-15271-9_9
Nimnoi P, Pongsilp N, Lumyong SJJOPN (2014) Co-inoculation of soybean (glycine max) with actinomycetes and bradyrhizobium japonicum enhances plant growth, nitrogenase activity and plant nutrition. J Plant Nutr 37:432–446. https://doi.org/10.1080/01904167.2013.864308
Ragot SA, Huguenin-Elie O, Kertesz MA, Frossard E, Bunemann EK (2016) Total and active microbial communities and phoD as affected by phosphate depletion and pH in soil. Plant Soil 408:15–30. https://doi.org/10.1007/s11104-016-2902-5
Ragot SA, Kertesz MA, Bunemann EK (2015) phoD alkaline phosphatase gene diversity in soil. Appl Environ Microb 81:7281–7289. https://doi.org/10.1128/AEM.01823-15
Randall K, Brennan F, Clipson N, Creamer R, Griffiths B, Storey S, Doyle E (2019) Soil bacterial community structure and functional responses across a long-term mineral phosphorus (Pi) fertilisation gradient differ in grazed and cut grasslands. Appl Soil Ecol 138:134–143. https://doi.org/10.1016/j.apsoil.2019.02.002
Rice O, Miller SH, Morrissey JP, O’Gara F (2012) Exploitation of glucose catabolic gene fusions to investigate in situ expression during Pseudomonas–plant interactions. Biol Fert Soils 48:235–238. https://doi.org/10.1007/s00374-011-0586-9
Tan H, Barret M, Mooij MJ, Rice O, Morrissey JP, Dobson A, Griffiths B, O’Gara F (2013) Long-term phosphorus fertilisation increased the diversity of the total bacterial community and the phoD phosphorus mineraliser group in pasture soils. Biol Fert Soils 49:661–672. https://doi.org/10.1007/s00374-012-0755-5
Tang L, Zhong L, Xue K, Wang S, Xu Z, Lin Q, Luo C, Rui Y, Li X, Li M, Liu W-T, Yang Y, Zhou J, Wang Y (2019) Warming counteracts grazing effects on the functional structure of the soil microbial community in a Tibetan grassland. Soil Biol Biochem 134:113–121. https://doi.org/10.1016/j.soilbio.2019.02.018
Tenenhaus M, Vinzi VE, Chatelin Y-M, Lauro C (2005) PLS path modeling. Comput Stat Data an 48:159–205. https://doi.org/10.1016/j.csda.2004.03.005
Tian J, Wei K, Condron LM, Chen Z, Xu Z, Chen L (2016) Impact of land use and nutrient addition on phosphatase activities and their relationships with organic phosphorus turnover in semi-arid grassland soils. Biol Fert Soils 52:675–683. https://doi.org/10.1007/s00374-016-1110-z
Wang BR, An SS, Liang C, Liu Y, Kuzyakov Y (2021a) Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol Biochem 162. https://doi.org/10.1016/j.soilbio.2021.108422
Wang H, Bu L, Song F, Tian J, Wei G (2021b) Soil available nitrogen and phosphorus affected by functional bacterial community composition and diversity as ecological restoration progressed. Land Degrad Dev 32:183–198. https://doi.org/10.1002/ldr.3707
Wang J, Liu G, Zhang C, Wang G (2020a) Effect of long-term destocking on soil fungal functional groups and interactions with plants. Plant Soil 448:495–508. https://doi.org/10.1007/s11104-020-04452-0
Wang J, Wang X, Liu G, Wang G, Wu Y, Zhang C (2020b) Fencing as an effective approach for restoration of alpine meadows: Evidence from nutrient limitation of soil microbes. Geoderma 363. https://doi.org/10.1016/j.geoderma.2019.114148
Wang J, Wang X, Liu G, Wang G, Zhang C (2021c) Grazing-to-fencing conversion affects soil microbial composition, functional profiles by altering plant functional groups in a Tibetan alpine meadow. Appl Soil Ecol 166. https://doi.org/10.1016/j.apsoil.2021.104008
Wang Y, Huang Q, Gao H, Zhang R, Yang L, Guo Y, Li H, Awasthi MK, Li G (2021d) Long-term cover crops improved soil phosphorus availability in a rain-fed apple orchard. Chemosphere 275:130093. https://doi.org/10.1016/j.chemosphere.2021.130093
Watts DB, Torbert HA, Feng YC, Prior SA (2010) Soil microbial community dynamics as influenced by composted dairy manure, soil properties, and landscape position. Soil Sci 175:474–486. https://doi.org/10.1097/SS.0b013e3181f7964f
Wei XM, Hu YJ, Razavi BS, Zhou J, Shen JL, Nannipieri P, Wu JS, Ge TD (2019) Rare taxa of alkaline phosphomonoesterase-harboring microorganisms mediate soil phosphorus mineralization. Soil Biol Biochem 131:62–70. https://doi.org/10.1016/j.soilbio.2018.12.025
Wickham H (2016) ggplot2: Elegant graphics for data analysis. https://ggplot2.tidyverse.org
Yang F, Niu K, Collins CG, Yan X, Ji Y, Ling N, Zhou X, Du G, Guo H, Hu S (2019) Grazing practices affect the soil microbial community composition in a Tibetan alpine meadow. Land Degrad Dev 30:49–59. https://doi.org/10.1002/ldr.3189
Yousafi Q, Kanwal S, Rashid H, Khan MS, Saleem S, Aslam M (2019) In silico structural and functional characterization and phylogenetic study of alkaline phosphatase in bacterium, Rhizobium leguminosarum (Frank 1879). Comput Biol Chem 83:107142. https://doi.org/10.1016/j.compbiolchem.2019.107142
Zhang C, Liu G, Xue S, Wang G (2016) Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol Biochem 97:40–49. https://doi.org/10.1016/j.soilbio.2016.02.013
Zhang C, Liu GB, Song ZL, Wang J, Guo L (2018) Interactions of soil bacteria and fungi with plants during long-term grazing exclusion in semiarid grasslands. Soil Biol Biochem 124:47–58. https://doi.org/10.1016/j.soilbio.2018.05.026
Funding
This work was financially supported by the National Natural Sciences Foundation of China (No. 42130717, No. 42177449), National Key Research and Development Program of China (2022YFF1300802, 2023YFF1315103) and the West Light Foundation of Chinese Academy of Science (XAB2020YN05); and the Interdisciplinary Research Center for Soil Microbial Ecology and Land Sustainable Productivity in Dry Areas at Northwest A&F University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no competing interests.
Additional information
Responsible editor: Qiaoyun Huang
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bie, Y., Wang, J., Wang, X. et al. Grazing-to-fencing increases alpine soil phosphorus availability by promoting phosphatase activity and regulating the phoD-harboring bacterial communities. J Soils Sediments 24, 1260–1273 (2024). https://doi.org/10.1007/s11368-023-03709-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-023-03709-2