Abstract
Purpose
A number of studies have been conducted on the occurrence, transport, and fate of microplastics in soil environments. The complexity of matrices presents significant challenges in investigating microplastics in soil, highlighting the need for further research and development in this field. In this review, sampling and pretreatment methods available for detecting and further studying microplastics in soil environments are primarily focused with a minor discussion on their various sources and behavior. Finally, based on the current research findings, the directions of future research are proposed as well.
Methods
Based on a comprehensive search of the available database, we provide updated information on the sources and behavior of microplastics in the soil and the analytical techniques available for their study.
Results
Previous studies have predominantly focused on microplastic contamination and its levels in various environments. We propose that the focus of microplastic research needs to be redirected to allow a better understanding of the behavior and impact of soil microplastics. The novel approach involves modeling the behavior of microplastics in the soil and associated environmental impacts and risks and developing standardized testing methods. These tools will provide a comprehensive strategy for creating a healthy and safe environment.
Conclusions
As plastic production increases worldwide, the accumulation of microplastics in the soil also increases, with potentially adverse implications for food security, human health, and climate change. A comprehensive strategy for rational delineation of microplastic behavior in the soil, as presented here, is needed to counteract and control the environmental impact of microplastics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Plastics are highly versatile and functional materials. Global plastic production continually increases, with 356 million tons produced in 2018. Of these, only 29.1 million tons have been collected, with 32.5% recycled and 24.9% landfilled. The amount of plastic waste, that remains uncollected and continues to be used or improperly disposed of, exacerbates the environmental burden of plastics. Approximately 9 to 23 million tons of plastic waste enter rivers, lakes, and oceans each year, while 13 to 25 million tons of plastic waste are discharged into terrestrial environments (Lee and Cha 2022). By 2050, an estimated 260 million tons of plastic waste will be generated worldwide, with at least 45% expected to enter the environment without being recycled or incinerated (Geyer et al. 2017; IEA 2018).
Microplastics are high-molecular-weight solid particles (typically < 5 mm in size) that do not dissolve in water. There are two types of microplastics, primary and secondary. The former, such as microbeads used in cleansers and cosmetics, are intentionally produced, whereas the latter are generated through physical and chemical weathering (i.e., fragmentation and degradation) of larger plastics in the environment (Andrady 2011; Masura et al. 2015). The biodegradation rate of microplastics is overly low; they are widely distributed in our environment, including the soil.
Microplastics enter the soil and accumulate therein via various routes, e.g., resulting from agricultural activities, such as sludge and organic fertilizer use. They originate from different sources, such as tire dust, the atmosphere, and streams. Up to 90% of microplastics present in the sewage accumulate in the sludge, with microplastic sludge concentrations ranging from 1,500 to 56,400 particles/kg (Li et al. 2018; Mintenig et al. 2017). Further, organic fertilizers contain up to 895 microplastic particles/kg (Weithmann et al. 2018), suggesting that long-term use of sludge and organic fertilizers can lead to microplastic contamination of soils. A recent study revealed the presence of up to 15.2 polyethylene (PE) microplastic particles per liter of groundwater in karst regions, e.g., the Salem Plateau and Driftless Area (the United States) (Panno et al. 2019). As the soil is a repository of all substances in the Earth's environment, it likely contains substantial amounts of microplastics. These microplastics adversely impact the ecosystem via various pathways, e.g., ingestion of plastics by animals, biomagnification and bioaccumulation in the food chain, absorption and accumulation of endocrine-disrupting substances contained in plastic additives, and exposure to pollutants (Cole et al. 2011).
Several research studies have comprehensively reviewed the various sources and distribution, the migration, transformation, and ecological impacts of microplastics in soil (Sajjad et al. 2022; Yang et al. 2021; You et al. 2022; Zhao et al. 2022). However, the research progress of microplastics in the soil is restricted by inherent technological inconsistencies and difficulties in methods to identify and quantify their particles due to the complex matrices of soil. This review focuses sampling and pretreatment methods available for detecting and further studying microplastics in soil environments with a minor discussion on their various sources and behavior. We also discuss the implications of the current research findings and propose directions for future research for an improved understanding of the environmental impact of these pollutants.
2 Literature survey and discussion
2.1 Occurrence of microplastics and their behavior in the soil
2.1.1 Microplastic contamination in the soil
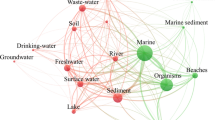
Microplastics are widely distributed in the environment, primarily attributed to the extensive production, consumption, and disposal of plastic products. They enter the soil due to various anthropogenic activities (Fig. 1). Corradini et al. (2019) broadly classified the sources of microplastics in the soil as industrial, agricultural, and others (Table 1). Industrial sources include tire dust, asphalt, various road and building paints, traffic safety facilities, artificial turf, and sports facility flooring (Dehghani et al. 2017; Dris et al. 2016; Dris et al. 2017; Henseler et al. 2019; Magnusson et al. 2016; Rezaei et al. 2019). In agriculture, the use of agricultural machinery, plastic mulch, polytunnels, agricultural waste, sewage sludge containing microplastics, organic fertilizers, controlled-release fertilizers, soil amendments, contaminated irrigation water, and flooding all contribute to large amounts of microplastics (Blasing and Amelung 2018; Carr et al. 2016; He et al. 2018; Rodríguez-Seijo et al. 2019; Weithmann et al. 2018). Microplastics are also present in a wide range of living environments, e.g., in clothing, furniture, or household items, and are released during waste incineration; they are also contributed by landfills and traffic (Dris et al. 2015, 2016; Liebezeit and Liebezeit 2015).
In soils, microplastics occur in various forms. High levels of PE, polypropylene (PP), polystyrene, and polyamide (PA) microplastics are detected in agricultural areas. In contrast, styrene-butadiene rubber is present in soils near roads and residential areas, primarily arising from the wear and tear of automobile tires (Choi et al. 2021). In industrial areas in Sydney (Australia), polyvinyl chloride (PVC) was detected in the soil (Fuller and Gautam 2016). Generally, the types of contaminating plastics vary with their sources, typically present on-site or located in adjacent areas.
Studies on soil microplastic distribution have been conducted in several countries (Table 2). Most such studies originate in China because of its large population, vast territory, and high plastic production. Another critical reason may be that plastic pollution control receives high attention and more studies are conducted. Further, most studies on soil microplastics have been conducted in agricultural areas, which are the main contaminated environments owing to the abundance of plastic sources, e.g., fertilizers and mulch, and their role as primary contributors to human and environmental microplastic exposure. In China, plastic concentrations of 40 ± 126 to 100 ± 141 particles/kg were detected in agricultural lands in the northern Loess Plateau (Han et al. 2019), and 10.3 ± 2.2 to 78.00 ± 12.91 particles/kg were detected in farming lands in Shanghai (Liu et al. 2018; Lv et al. 2019). The highest concentration of microplastics in agricultural soil was detected in the Wuhan region, with 4.3 × 104 to 6.2 × 105 particles/kg (Zhou et al. 2019). These studies indicate that the distribution of microplastics in agricultural soil varies regionally in China. The microplastic concentration in agricultural soils of Mittelfranken, Germany was reported to be 0.34 ± 0.36 particles/kg (Piehl et al. 2018). In South Korea's Yongin region, concentrations ranged from 81 to 18,870 particles/kg (Kim et al. 2021), and in the Yeoju region, it was 664 particles/kg (Choi et al. 2021). The variation in reported microplastic concentrations across regions and countries can be attributed to not only differences in the extent of soil microplastic contamination but also methods of sampling, pretreatment, and analysis.
Microplastics are present not only in agricultural lands but also in residential areas, roads, and forested areas. In forested regions in Wuhan, China, 9.6 × 104 to 6.9 × 105 microplastic particles/kg were detected (Zhou et al. 2019). In contrast, 184 ± 266 particles/kg of microplastics were detected in grasslands in the Santiago Province, Chile (Corradini et al. 2021). In the case of Yeoju (South Korea), 500 microplastic particles/kg were detected in residential areas and 1,108 particles/kg in road soils, indicating that human residential and living environments contain microplastics (Choi et al. 2021). Additionally, microplastics were detected in the soil of 29 floodplains in Switzerland (Scheurer and Bigalke 2018), and high concentrations of microplastics were detected in vacant lots in Wuhan, China (Zhou et al. 2019). Hence, microplastics are distributed widely in soils, such as soils of agricultural lands, residential areas, and roads, highly impacted by anthropogenic and industrial activities, and soils relatively less affected by these activities, such as those in forested areas.
2.1.2 Transport and fate of microplastics in the soil
The types of microplastic behavior in the subsurface soil environment can be divided into three categories, i.e., surface migration, infiltration in unsaturated zones, and transport in saturated media (Kim et al. 2019) (Table 3). Although microplastics are believed to be stored or show delayed mobility in soils and sediments, the possible mobility of extremely small (µm to nm) microplastic particles in the subsurface environment needs to be investigated in detail (Alimi et al. 2018).
The transport of microplastics accumulated in the soil via surface runoff into streams and groundwater during precipitation can be explained or predicted based on theories related to soil erosion and sediment transport (Nizzetto et al. 2016). Further, research on the transport of microplastics in unsaturated and saturated media, based on studies of colloid or nanoparticle behavior, has made considerable progress in recent decades (Alimi et al. 2018; Hüffer et al. 2017). The application of biosolids in agricultural fields is indeed a significant pathway for the transfer and accumulation of microplastics in soils. In Canada, the concentration of microplastics in biosolids used in agriculture was reported to range from 8.7 × 103 to 1.4 × 104 MP/kg and the annual loading of microplastics entering agricultural fields due to the application of biosolids was estimated to be 4.1 × 1011 to 1.3 × 1012 particles (Crossman et al. 2020). Furthermore, it has been observed that agricultural fields where biosolids are used more frequently and in larger quantities tend to have higher concentrations of microplastics. This can be explained by the fact that some of the microplastics present in biosolids were retained in the soil, leading to an accumulation of microplastics in those areas over time (Table 4).
Understanding the distribution of microplastics in the soil is crucial for assessing their potential impacts on soil health and the environment. Many studies have been conducted on the distribution of microplastics in the surface layer of the soil, with few investigations on their vertical or horizontal distribution. In one study, the authors focused on the distribution of plastics in three agricultural environments (agricultural land, orchard, and greenhouse) in the Loess Plateau (northern China) at depths of 0–10 cm (surface layer) and 10–30, suggested that the concentration of microplastics in the deeper layers was higher than in the surface layer (Han et al. 2019). However, in the orchard and greenhouse soils, the opposite tendency was observed as the concentrations of microplastics in the surface layer were 320 ± 329 and 100 ± 254 particles/kg, respectively, higher than those in the deeper layers, which indicates that the vertical distribution of microplastics varies with the cultivation method. Furthermore, agricultural activities, such as plowing, disturb both the topsoil and subsoil and organisms, such as earthworms and springtails, can transport microplastics (Kim and An 2019; Rillig et al. 2017). In contrast, no substantial differences were detected in the horizontal distribution of microplastics inside and outside polytunnel cultivation areas in Yongin, South Korea (Kim et al. 2021).
The fate of microplastics in soil environments and their interaction with organisms have also been investigated, with several possible scenarios identified (Ng et al. 2018). For instance, soil organisms and animals can ingest or propagate microplastics present in the soil, and plants can absorb nanoscale microplastics (Bandmann et al. 2012; Ng et al. 2018). Soil bacteria, earthworms, moles, and other underground organisms can break down microplastics into smaller particles, accelerating their transport within the soil (Rillig 2012). Soil disturbances, caused by plant roots, affect root movement and growth, and have a similar effect on the behavior of microplastics (Gabet et al. 2003). Further, the formation of large soil pores resulting from crop harvesting or plant root decomposition promotes the vertical movement of microplastics (Li et al. 2020).
Microplastics interact with metals, affecting their adsorption and distribution in the soil (Yu et al. 2021). Moreover, microplastics compete with soil organic matter for the adsorption of organic compounds and other substances (Ng et al. 2021). Interactions between microplastics and other substances in the soil can, in turn, adversely affect nitrogen and organic carbon cycling, nutrient delivery, and soil microbial activity (Dong et al. 2021a, b; Liu et al. 2018; Qi et al. 2020). According to recent studies, microplastics affect soil–plant systems and are ingested by various organisms at different trophic levels, ultimately accumulating in organisms along the food chain (Chai et al. 2020). For instance, as the concentration of microplastics in the soil increases, the ingestion of microplastics by exposed earthworms also increases (Guo et al. 2020). Pollutants, such as the adsorbed high-molecular-weight additives, accumulate in earthworms and can then be transferred to other organisms through the food chain (Li et al. 2020). Progressing through the food chain, microplastics from the soil could ultimately affect humans (De Falco et al. 2019). Microplastics can also disrupt soil nutrient cycling, impacting soil microbial activity, composition, and species diversity (Mbachu et al. 2021b), which could adversely impact plants and animals, also threatening food security. Notably, long-term disruption of soil microbial species diversity could adversely affect forest soil microbial communities and contribute to climate change (Ng et al. 2021).
2.2 Methods for soil microplastic analysis
2.2.1 Soil sample collection
Investigating environmental microplastics involves several stages, such as sampling, isolation, separation, identification, and quantification (Mai et al. 2018). However, no internationally recognized testing standards have yet been established for the investigation and analysis of microplastics in soil media, and the International Organization for Standardization (ISO) is working on standardizing test methods in this field (Jeong et al. 2018).
Although the soil sampling process is relatively simple, it can considerably impact the analysis results. This is because errors associated with soil sampling are generally larger than measurement errors in the analytical process. Therefore, soil sampling strategies must be carefully designed and consistent. For example, ISO 18400-104:2018 can be referenced to establish a soil sampling strategy (International Organization for Standardization 2018). Soil sampling for studying the distribution of soil microplastics requires a strategy for securing representative samples from the target site or area. Such a strategy must consider specific methods and criteria for selecting soil sampling points, sampling depth, and the number of samples collected at the target site or area. As the vertical or horizontal distribution of microplastics in the soil is not uniform, it is crucial to standardize sampling point selection with a consistent and comparable sampling depth to ensure the representativeness of the samples. The soil can be sampled using either grab sampling or composite sampling methods. Composite sampling is used for collecting representative samples at the target site. It involves selecting 2–6 sub-sites within the target site and combining the soil collected at each sub-site into a single sample. In South Korea, domestic standards for soil contamination testing provide specific guidelines for the soil sampling methodology for specific target areas (National Institute of Environmental Research 2017). For the agricultural land, 5–10 sub-sites are designated in a zigzag pattern within the target area, and the samples are collected at each sub-site and then combined. For factory areas, landfill sites, urban areas, and other areas, five sub-sites must be sampled, i.e., one in the center of the target area and one each 5–10 m from the center and in the four directions away from the center. The samples are then to be combined. When the distance between the points is insufficient because of the presence of facilities in the target area, appropriate changes are made to adjust this distance.
After collection, the samples are dried, sieved, divided into analytical samples, pretreated, and finally analyzed (Álvarez-Lopeztello et al. 2021; Amrutha and Warrier 2020; Harms et al. 2021). To ensure sample representativeness, sufficient sample mass should be obtained. Domestic standards for soil contamination testing recommend collecting approximately 0.5 kg of soil per sampling point. As the representative sample is a combination of 5–10 sub-site samples, the final sample mass is in the range of 2.5 to 5 kg (National Institute of Environmental Research 2017).
Soil samples are collected at various depths depending on the purpose of the investigation. The sampling depth would ideally be determined considering the vertical distribution of microplastics in the soil. Hitherto, few studies have been published on the vertical distribution and behavior of microplastics in the soil. Most studies focused on microplastics in the topsoil as the primary sources of soil microplastics are located above ground. Accordingly, a specific depth standard for the topsoil is critical, and the analytical outcomes may vary depending on the definition of the surface layer depth. For example, when microplastics are distributed mainly within a few centimeters from the soil surface, comparing microplastic concentrations at 5 and 30 cm sampling depths would reveal a lower concentration at the latter depth. Standardizing the topsoil depth is crucial for ensuring the consistency of the results and for comparing and evaluating the distribution of microplastics. In South Korea, according to the soil contamination testing standards, the topsoil is defined as the soil layer 0–15 cm from the surface. Hitherto, the sampling depth, in studies on microplastic occurrence in the soil, has ranged from 2 to 30 cm from the soil surface, with the most common sampling depth of up to 5 cm from the surface (Table 2). On agricultural land, the upper soil layer is disturbed by periodic plowings, such as paddy plowing and field plowing. During plowing, the soil is mixed to approximately 30 cm depth; hence, the soil sampled up to 30 cm from the surface is relatively homogeneous. Consequently, for ease of investigation, the depth of the topsoil layer in agricultural land, where periodic plowing is performed, could be set at up to 30 cm from the surface.
2.2.2 Organic matter decomposition
Soil samples often contain considerable amounts of organic matter, such as tree branches and plant roots. The organic matter interferes with the analytical separation of plastics from the soil. Specifically, it is difficult to spectrally distinguish microplastics from organic matter, and this reduces detection accuracy. In Raman and infrared spectroscopy, the organic matter can potentially distort the readings by visually interfering with the analysis (Blasing and Amelung 2018). Furthermore, it is difficult to optically distinguish microplastics from organic matter during analysis (Shaw and Day 1994). In addition, the densities of specific soil components (e.g., soil organic matter and organic fibers) and microplastics are similar, which can impact the accuracy of density separation (Zhang and Liu 2018). Hence, organic matter needs to be removed from soil samples before analysis.
The removal efficiency of organic matter by decomposing them can be improved by adjusting the reaction conditions, such as reagent concentration and reaction time and temperature. However, this is associated with the risk of microplastic degradation. Consequently, standardized pretreatment methods should be used to minimize plastic damage (Löder et al. 2017). Currently, no unified standard methods for organic matter decomposition are available, and there is a lack of systematic studies comparing the efficiency of various methods or providing established protocols and guidelines (Rocha-Santos and Duarte 2017). Below, we provide a brief critical overview of the currently available methods for organic matter decomposition.
Organic matter decomposition methods can be classified as acid-based, alkali-based, oxidizing agent-based, and enzymatic decomposition. Although various methods are available for decomposing organic matter, each has certain limitations, and more research is warranted to allow accurate separation of microplastics from soil samples. Table 5 summarizes the advantages and disadvantages of each method. Acid-based decomposition methods involve sulfuric acid, nitric acid, chlorous acid, and others (Munno et al. 2018; Scheurer and Bigalke 2018; Zou et al. 2019), with nitric acid being the most commonly used. Nitric acid-mediated decomposition of organic matter is robust, with a relatively short reaction time, from a few minutes to a few hours (Claessens et al. 2013; Scheurer and Bigalke 2018). However, acid treatment decomposes some plastics, such as acrylonitrile butadiene styrene, PA, and polyethylene terephthalate (PET) (Enders et al. 2017; Zou et al. 2019).
Alkali-based decomposition methods mostly involve sodium hydroxide and potassium hydroxide (Foekema et al. 2013). These methods effectively decompose animal tissues and soil humic acids (Dehaut et al. 2016; Prata et al. 2019). However, they do not decompose humins (Hurley and Nizzetto 2018). In contrast, they decompose plastics, such as polycarbonate (PC), PET, and cellulose acetate. Consequently, these methods are not recommended for research (Hamm et al. 2018; Karami et al. 2017).
Oxidizing agent-based methods are widely used as they do not alter microplastics (Han et al. 2020; Zhang et al. 2020). Hydrogen peroxide (H2O2) is the most commonly used oxidizer (He et al. 2018; Kumar et al. 2020). It effectively removes organic matter from various samples, such as soil (Wang et al. 2021), sludge (Li et al. 2018), water samples (Wang et al. 2017), and animal cells (Lv et al. 2019). However, the reaction time is long, over 24 h (Ding et al. 2021; Liu et al. 2018). Further, partial decomposition of certain plastics (PA, PS, PET, PVC, PC, polyurethane, PP, and low-density polyethylene) can occur during the reaction, making it unsuitable for microplastic analysis (Hurley and Nizzetto 2018; Nuelle et al. 2014).
Fenton oxidation [H2O2 + Fe(II)] reaction decomposes organic matter in the soil more effectively than H2O2 (Möller et al. 2022; Prata et al. 2019), with a relatively short reaction time (1 –2 h) (Hurley et al. 2018). However, the sample temperature rises as heat is released during the reaction, which could lead to the decomposition of microplastics in the sample. Accordingly, the reaction temperature needs to be maintained at 40 ℃ or less (Junhao et al. 2021). Further, the sample pH needs to be maintained at 3.0 or lower to suppress the generation of oxidized iron, which would fuel Fenton oxidation (Hurley et al. 2018).
Decomposition methods involving protease, pectinase, isozyme, cellulase, and other enzymes are popular (Cole et al. 2014) and have been used for organic matter decomposition in biological samples. According to recent studies, the organic matter reduction efficiency reaches approximately 90% when mixed enzymes are used (Löder et al. 2017; Mbachu et al. 2021b). However, the reaction times are long (at least 1 d) (Möller et al. 2022).
2.2.3 Density gradient separation
Density gradient separation is based on the density difference between microplastics present in a sample and other substances not removed by organic matter oxidation. High-density sand particles settle at the bottom during separation, and the supernatant containing low-density microplastics is preserved for analysis. The separation efficiency is determined by the density difference between the separation solution and plastic, such that the bigger the density difference, the better the separation. As shown in Table 6, for plastics, such as PVC and PET, where the density difference between the separation solution and plastic is low, the separation efficiency may vary (Junhao et al. 2021; Liu et al. 2019; Ruggero et al. 2020; Wang et al. 2018, 2020).
During density gradient separation, separation efficiency (recovery and reproducibility), cost, and hazards of the chemicals need to be considered (Yu et al. 2020). Till now, water, NaCl, ZnCl2, NaI, and ZnBr2 solutions have been mainly used in microplastic research. NaCl is widely used because of its low cost and non-toxicity (Corradini et al. 2019; Liu et al. 2018; Lv et al. 2019; Zhou et al. 2018). However, the recovery rate of small plastic particles (< 1 mm) may be as low as 40% because of the small density difference to general plastics (Li et al. 2018; Ruggero et al. 2020; Wang and Wang 2018). The density of Na2WO4, NaBr, 3Na2WO4·9WO3·H2O, and Li2WO4 solutions can reach 1.3–1.6 g/cm3 and these high-density reagents have high plastic separation efficiency; however, they are more expensive than other solvents, which increases the cost of testing (Eo et al. 2019; Liu et al. 2019). In contrast, CaCl2 solutions are relatively inexpensive and can be used at a density of 1.3 g/cm3 to efficiently separate microplastics. However, in some cases, CaCl2 interferes with Fourier-transform infrared spectroscopy (Stolte et al. 2015).
The most commonly used density gradient separation solutions for environmental sample pretreatment are 8 M ZnCl2 (Nuelle et al. 2014) and 10 M NaI (Imhof et al. 2012), with densities of 1.6 and 1.8 g/cm3 and pH of 8.8 and 2.4, respectively. The ZnCl2 solution with a density of 1.6 g/cm3 can be used for most microplastic separations (Junhao et al. 2021; Liu et al. 2019; Wang et al. 2020). At 1.8 g/cm3, the density of NaI solution is higher than that of other separating solutions, and it is often used for efficient microplastic separation (Junhao et al. 2021; Wang et al. 2020; Zhang and Liu 2018). However, both these solutions require caution during handling because of their toxicity.
3 Conclusions and perspectives
To date, research on microplastics in the soil environment has mainly focused on identifying their presence and levels. However, systematic studies are needed to understand the occurrence, distribution, and impact of microplastics specifically in the soil to allow their environmental management. It is necessary to understand the behavior and migration characteristics of microplastics in soil environments, but some critical gaps remain. Improved models for the prediction of microplastic behavior should be developed. Currently, colloid behavior models are used, but they do not reflect the characteristics of microplastics, which limits their ability to explain microplastic behavior in the environment. Accordingly, the characteristics of microplastics and environmental factors (e.g., exposure time) should be investigated, technologies to detect and quantify microplastics in soil environments should be developed, and predictive models should be iteratively verified and improved. Ultimately, such efforts would enable accurate assessment and prediction of microplastic behavior. In addition, studies on the impact and risk associated with the presence of microplastics in the soil environment are needed. Research regarding the risks of the exposure of humans as well as ecosystems to microplastics is currently at the stage of collection or confirmation of the evidence of toxicity. The specific toxicity mechanisms and impacts of microplastics on humans and ecosystems have not yet been elucidated, and research on the effects of microplastic contamination on ecosystems and human health is still in its infancy. Research on the risks of environmental exposure to microplastics needs to be conducted in stages, including the assessment of the effects of long-term exposure on the environment and humans in terms of toxicity.
The most urgent and essential issue for evaluating the status, exposure, and behavior of microplastics is the establishment of standardized testing and analysis techniques. Currently, no standardized testing and analysis protocols for microplastics in the soil are available. Similarly, the methodologies for sample collection, pretreatment, and instrument-based analyses need to be standardized to allow for the generation of consistent and reliable data. This would increase the reliability of the studies conducted on microplastic exposure and behavior, e.g., through comparative evaluations and verifications of research results, for a better understanding and management of microplastics.
In conclusion, as plastic production increases worldwide, the exposure to and accumulation of microplastics in the soil also increases, with potentially adverse implications for food security, human health, and climate change. A comprehensive strategy for rational delineation of microplastic behavior in the soil is needed, for example, as presented in this review, to counteract and control the environmental impact of microplastics.
Availability of data and material
All data generated or analyzed during this study are included in this published review article.
Code availability
Not applicable.
References
Alimi OS, Farner Budarz J, Hernandez LM, Tufenkji N (2018) Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environ Sci Technol 52:1704–1724. https://doi.org/10.1021/acs.est.7b05559
Álvarez-Lopeztello J, Robles C, del Castillo RF (2021) Microplastic pollution in neotropical rainforest, savanna, pine plantations, and pasture soils in lowland areas of Oaxaca, Mexico: Preliminary results. Ecol Indic 121:107084. https://doi.org/10.1016/j.ecolind.2020.107084
Amrutha K, Warrier AK (2020) The first report on the source-to-sink characterization of microplastic pollution from a riverine environment in tropical India. Sci Total Environ 739:140377. https://doi.org/10.1016/j.scitotenv.2020.140377
Andrady AL (2011) Microplastics in the marine environment. Mar Pollut Bull 62:1596–1605. https://doi.org/10.1016/j.marpolbul.2011.05.030
Bandmann V, Müller JD, Köhler T, Homann U (2012) Uptake of fluorescent nano beads into BY2-cells involves clathrin-dependent and clathrin-independent endocytosis. FEBS Lett 586:3626–3632. https://doi.org/10.1016/j.febslet.2012.08.008
Beriot N, Peek J, Zornoza R, Geissen V, Lwanga EH (2021) Low density-microplastics detected in sheep faeces and soil: a case study from the intensive vegetable farming in Southeast Spain. Sci Total Environ 755:142653
Blasing M, Amelung W (2018) Plastics in soil: Analytical methods and possible sources. Sci Total Environ 612:422–435. https://doi.org/10.1016/j.scitotenv.2017.08.086
Carr SA, Liu J, Tesoro AG (2016) Transport and fate of microplastic particles in wastewater treatment plants. Water Res 91:174–182. https://doi.org/10.1016/j.watres.2016.01.002
Chai B, Li X, Liu H, Lu G, Dang Z, Yin H (2020) Bacterial communities on soil microplastic at Guiyu, an E-Waste dismantling zone of China. Ecotoxicol Environ Saf 195:110521. https://doi.org/10.1016/j.ecoenv.2020.110521
Chia RW, Lee JY, Lee M, Lee S (2023) Comparison of microplastic characteristics in mulched and greenhouse soils of a major agriculture area. Korea J Polym Environ 31(5):2216–2229. https://doi.org/10.1007/s10924-022-02746-1
Choi YR, Kim Y-N, Yoon J-H, Dickinson N, Kim K-H (2021) Plastic contamination of forest, urban, and agricultural soils: a case study of Yeoju City in the Republic of Korea. J Soils Sediment 21:1962–1973. https://doi.org/10.1007/s11368-020-02759-0
Chouchene K, Nacci T, Modugno F, Castelvetro V, Ksibi M (2022) Soil contamination by microplastics in relation to local agricultural development as revealed by FTIR. ICP-MS and Pyrolysis-GC/MS Environ Poll 303:119016. https://doi.org/10.1016/j.envpol.2022.119016
Claessens M, Van Cauwenberghe L, Vandegehuchte MB, Janssen CR (2013) New techniques for the detection of microplastics in sediments and field collected organisms. Mar Pollut Bull 70:227–233. https://doi.org/10.1016/j.marpolbul.2013.03.009
Cole M, Lindeque P, Halsband C, Galloway TS (2011) Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull 62:2588–2597. https://doi.org/10.1016/j.marpolbul.2011.09.025
Cole M, Webb H, Lindeque PK, Fileman ES, Halsband C, Galloway TS (2014) Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci Rep 4:4528. https://doi.org/10.1038/srep04528
Corradini F, Casado F, Leiva V, Huerta-Lwanga E, Geissen V (2021) Microplastics occurrence and frequency in soils under different land uses on a regional scale. Sci Total Environ 752:141917. https://doi.org/10.1016/j.scitotenv.2020.141917
Corradini F, Meza P, Eguiluz R, Casado F, Huerta-Lwanga E, Geissen V (2019) Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci Total Environ 671:411–420. https://doi.org/10.1016/j.scitotenv.2019.03.368
Crossman J, Hurley RR, Futter M, Nizzetto L (2020) Transfer and transport of microplastics from biosolids to agricultural soils and the wider environment. Sci Total Environ 724:138334. https://doi.org/10.1016/j.scitotenv.2020.138334
De Falco F, Cocca M, Guarino V, Gentile G, Ambrogi V, Ambrosio L, Avella M (2019) Novel finishing treatments of polyamide fabrics by electrofluidodynamic process to reduce microplastic release during washings. Polym Degrad Stab 165:110–116. https://doi.org/10.1016/j.polymdegradstab.2019.05.001
Dehaut A, Cassone A-L, Frère L, Hermabessiere L, Himber C, Rinnert E, Rivière G, Lambert C, Soudant P, Huvet A, Duflos G, Paul-Pont I (2016) Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ Pollut 215:223–233. https://doi.org/10.1016/j.envpol.2016.05.018
Dehghani S, Moore F, Akhbarizadeh R (2017) Microplastic pollution in deposited urban dust, Tehran metropolis. Iran Environ Sci Pollut Res 24:20360–20371. https://doi.org/10.1007/s11356-017-9674-1
Ding L, Wang X, Ouyang Z, Chen Y, Wang X, Liu D, Liu S, Yang X, Jia H, Guo X (2021) The occurrence of microplastic in Mu Us Sand Land soils in northwest China: Different soil types, vegetation cover and restoration years. J Hazard Mater 403:123982. https://doi.org/10.1016/j.jhazmat.2020.123982
Ding L, Zhang S, Wang X, Yang X, Zhang C, Qi Y, Guo X (2020) The occurrence and distribution characteristics of microplastics in the agricultural soils of Shaanxi Province, in north-western China. Sci Total Environ 720:137525
Dong Y, Gao M, Qiu W, Song Z (2021a) Effect of microplastics and arsenic on nutrients and microorganisms in rice rhizosphere soil. Ecotoxicol Environ Saf 211:111899. https://doi.org/10.1016/j.ecoenv.2021.111899
Dong Y, Gao M, Qiu W, Song Z (2021b) Uptake of microplastics by carrots in presence of As (III): Combined toxic effects. J Hazard Mater 411:125055. https://doi.org/10.1016/j.jhazmat.2021.125055
Dris R, Gasperi J, Mirande C, Mandin C, Guerrouache M, Langlois V, Tassin B (2017) A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ Pollut 221:453–458. https://doi.org/10.1016/j.envpol.2016.12.013
Dris R, Gasperi J, Rocher V, Saad M, Renault N, Tassin B (2015) Microplastic contamination in an urban area: A case study in Greater Paris. Environ Chem 12:592–599. https://doi.org/10.1071/EN14167
Dris R, Gasperi J, Saad M, Mirande C, Tassin B (2016) Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar Pollut Bull 104:290–293. https://doi.org/10.1016/j.marpolbul.2016.01.006
Enders K, Lenz R, Beer S, Stedmon CA (2017) Extraction of microplastic from biota: recommended acidic digestion destroys common plastic polymers. ICES J Mar Sci 74:326–331. https://doi.org/10.1093/icesjms/fsw173
Eo S, Hong SH, Song YK, Han GM, Shim WJ (2019) Spatiotemporal distribution and annual load of microplastics in the Nakdong River, South Korea. Water Res 160:228–237. https://doi.org/10.1016/j.watres.2019.05.053
Feng S, Lu H, Tian P, Xue Y, Lu J, Tang M, Feng W (2020) Analysis of microplastics in a remote region of the Tibetan Plateau: implications for natural environmental response to human activities. Sci Total Environ 739:140087
Foekema EM, De Gruijter C, Mergia MT, van Franeker JA, Murk AJ, Koelmans AA (2013) Plastic in North Sea fish. Environ Sci Technol 47:8818–8824. https://doi.org/10.1021/es400931b
Fuller S, Gautam A (2016) A procedure for measuring microplastics using pressurized fluid extraction. Environ Sci Technol 50:5774–5780. https://doi.org/10.1021/acs.est.6b00816
Gabet EJ, Reichman O, Seabloom EW (2003) The effects of bioturbation on soil processes and sediment transport. Annu Rev Earth Planet Sci 31:249–273. https://doi.org/10.1146/annurev.earth.31.100901.141314
Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Sci Adv 3:1–5. https://science.org/doi/10.1126/sciadv.1700782
Guo J-J, Huang X-P, Xiang L, Wang Y-Z, Li Y-W, Li H, Cai Q-Y, Mo C-H, Wong M-H (2020) Source, migration and toxicology of microplastics in soil. Environ Int 137:105263. https://doi.org/10.1016/j.envint.2019.105263
Hamm T, Lorenz C, Piehl S (2018) Microplastics in aquatic systems–monitoring methods and biological consequences. YOUMARES 8–Oceans across boundaries: learning from each other. Proceedings of the 2017 conference for YOUng MARine RESearchers in Kiel, Germany 2018. Springer International Publishing, pp 179–195. https://doi.org/10.1007/978-3-319-93284-2_13
Han M, Niu X, Tang M, Zhang B-T, Wang G, Yue W, Kong X, Zhu J (2020) Distribution of microplastics in surface water of the lower Yellow River near estuary. Sci Total Environ 707:135601. https://doi.org/10.1016/j.scitotenv.2019.135601
Han X, Lu X, Vogt RD (2019) An optimized density-based approach for extracting microplastics from soil and sediment samples. Environ Pollut 254:113009. https://doi.org/10.1016/j.envpol.2019.113009
Harms IK, Diekötter T, Troegel S, Lenz M (2021) Amount, distribution and composition of large microplastics in typical agricultural soils in Northern Germany. Sci Total Environ 758:143615. https://doi.org/10.1016/j.scitotenv.2020.143615
He D, Luo Y, Lu S, Liu M, Song Y, Lei L (2018) Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. TrAC Trends Anal Chem 109:163–172. https://doi.org/10.1016/j.trac.2018.10.006
Henseler M, Brandes E, Kreins P (2019) Microplastics in agricultural soils: a new challenge not only for agro-environmental policy? 172nd EAAE Seminar, May 28–29, 2019, Brussels, Belgium. https://doi.org/10.22004/ag.econ.289746
Heuchan SM, Fan B, Kowalski JJ, Gillies ER, Henry HA (2019) Development of fertilizer coatings from polyglyoxylate–polyester blends responsive to root-driven pH change. J Agric Food Chem 67(46):12720–12729
Hüffer T, Praetorius A, Wagner S, von der Kammer F, Hofmann T (2017) Microplastic exposure assessment in aquatic environments: Learning from similarities and differences to engineered nanoparticles. Environ Sci Technol 51:2499–2507. https://doi.org/10.1021/acs.est.6b04054
Hurley RR, Lusher AL, Olsen M, Nizzetto L (2018) Validation of a method for extracting microplastics from complex, organic-rich, environmental matrices. Environ Sci Technol 52:7409–7417. https://doi.org/10.1021/acs.est.8b01517
Hurley RR, Nizzetto L (2018) Fate and occurrence of micro(nano)plastics in soils: Knowledge gaps and possible risks. Curr Opin Environ Sci Health 1:6–11. https://doi.org/10.1016/j.coesh.2017.10.006
IEA (2018) The future of petrochemicals: Towards more sustainable plastics and fertilisers. Inter Energy Agency (IEA), Paris. https://doi.org/10.1787/9789264307414-en
Imhof HK, Schmid J, Niessner R, Ivleva NP, Laforsch C (2012) A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol Oceanogr: Methods 10:524–537. https://doi.org/10.4319/lom.2012.10.524
International Organization for Standardization (2018) Standard ISO 18400–104:2018 - Soil Quality — Sampling. https://www.iso.org/standard/65223.html. Accessed Oct 2018
Jabeen K, Su L, Li J, Yang D, Tong C, Mu J, Shi H (2017) Microplastics and mesoplastics in fish from coastal and fresh waters of China. Environ Pollut 221:141–149
Jeong DH, Ju B, Lee W, Chung H, Park J, Kim C (2018) A mini-review on discharge characteristics and management of microplastics in sewage treatment plants. J Korean Soc Water Wastewater 32:337–348. https://doi.org/10.11001/jksww.2018.32.4.337
Junhao C, Xining Z, Xiaodong G, Li Z, Qi H, Siddique KH (2021) Extraction and identification methods of microplastics and nanoplastics in agricultural soil: a review. J Environ Manag 294:112997. https://doi.org/10.1016/j.jenvman.2021.112997
Karami A, Golieskardi A, Choo CK, Romano N, Ho YB, Salamatinia B (2017) A high-performance protocol for extraction of microplastics in fish. Sci Total Environ 578:485–494. https://doi.org/10.1016/j.scitotenv.2016.10.213
Kim S-K, Kim J-S, Lee H, Lee H-J (2021) Abundance and characteristics of microplastics in soils with different agricultural practices: Importance of sources with internal origin and environmental fate. J Hazard Mater 403:123997. https://doi.org/10.1016/j.jhazmat.2020.123997
Kim SW, An Y-J (2019) Soil microplastics inhibit the movement of springtail species. Environ Int 126:699–706. https://doi.org/10.1016/j.envint.2019.02.067
Kim Y, Han WS, Yoon H (2019) Mobility of microplastics in subsurface environments: Current knowledge and perspectives. J Soil Groundw Environ 24:1–12. https://doi.org/10.7857/JSGE.2019.24.3.001
Kumar M, Xiong X, He M, Tsang DC, Gupta J, Khan E, Harrad S, Hou D, Ok YS, Bolan NS (2020) Microplastics as pollutants in agricultural soils. Environ Pollut 265:114980. https://doi.org/10.1016/j.envpol.2020.114980
Lee JY, Cha JH (2022) Current status of researches on microplastics in groundwater and perspectives. J Geol Soc Korea 58:233–241. https://doi.org/10.14770/jgsk.2022.58.2.233
Li J, Liu H, Paul Chen J (2018) Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res 137:362–374. https://doi.org/10.1016/j.watres.2017.12.056
Li J, Song Y, Cai Y (2020) Focus topics on microplastics in soil: Analytical methods, occurrence, transport, and ecological risks. Environ Pollut 257:113570. https://doi.org/10.1016/j.envpol.2019.113570
Liebezeit G, Liebezeit E (2015) Origin of synthetic particles in honeys. Pol J Food Nutr Sci 65:143–147. https://doi.org/10.1515/pjfns-2015-0025
Liu F, Olesen KB, Borregaard AR, Vollertsen J (2019) Microplastics in urban and highway stormwater retention ponds. Sci Total Environ 671:992–1000. https://doi.org/10.1016/j.scitotenv.2019.03.416
Liu M, Lu S, Song Y, Lei L, Hu J, Lv W, Zhou W, Cao C, Shi H, Yang X (2018) Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ Pollut 242:855–862. https://doi.org/10.1016/j.envpol.2018.07.051
Löder MG, Imhof HK, Ladehoff M, Löschel LA, Lorenz C, Mintenig S, Piehl S, Primpke S, Schrank I, Laforsch C, Gerdts G (2017) Enzymatic purification of microplastics in environmental samples. Environ Sci Technol 51:14283–14292. https://doi.org/10.1021/acs.est.7b03055
Lu S, Zhu K, Song W, Song G, Chen D, Hayat T, Alharbi NS, Chen C, Sun Y (2018) Impact of water chemistry on surface charge and aggregation of polystyrene microspheres suspensions. Sci Total Environ 630:951–959. https://doi.org/10.1016/j.scitotenv.2018.02.296
Lusher A, Buenaventura NT, Eidsvoll D, Thrane JE, Økelsrud A, Jartun M (2018) Freshwater microplastics in Norway: a first look at sediment, biota and historical plankton samples from Lake Mjøsa and Lake Femunden. NIVA-rapport
Lv W, Zhou W, Lu S, Huang W, Yuan Q, Tian M, Lv W, He D (2019) Microplastic pollution in rice-fish co-culture system: A report of three farmland stations in Shanghai, China. Sci Total Environ 652:1209–1218. https://doi.org/10.1016/j.scitotenv.2018.10.321
Magnusson K, Eliaeson K, Fråne A, Haikonen K, Olshammar M, Stadmark J, Hultén J (2016) Swedish sources and pathways for microplastics to the marine environment. http://urn.kb.se/resolve?urn=urn:nbn:se:ivl:diva-362
Mai L, Bao L-J, Shi L, Wong CS, Zeng EY (2018) A review of methods for measuring microplastics in aquatic environments. Environ Sci Pollut Res 25:11319–11332. https://doi.org/10.1007/s11356-018-1692-0
Masura J, Baker J, Foster G, Arthur C (2015) Laboratory methods for the analysis of microplastics in the marine environment: recommendations for quantifying synthetic particles in waters and sediments. NOAA Marine Debris Division, Silver Spring, MD, p 31. https://doi.org/10.25607/OBP-604 (NOAA Technical Memorandum NOS-OR&R-48)
Mbachu O, Jenkins G, Kaparaju P, Pratt C (2021a) The rise of artificial soil carbon inputs: Reviewing microplastic pollution effects in the soil environment. Sci Total Environ 780:146569. https://doi.org/10.1016/j.scitotenv.2021.146569
Mbachu O, Jenkins G, Pratt C, Kaparaju P (2021b) Enzymatic purification of microplastics in soil. MethodsX 8:101254. https://doi.org/10.1016/j.mex.2021.101254
Mintenig SM, Int-Veen I, Löder MGJ, Primpke S, Gerdts G (2017) Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res 108:365–372. https://doi.org/10.1016/j.watres.2016.11.015
Möller JN, Heisel I, Satzger A, Vizsolyi EC, Oster SDJ, Agarwal S, Laforsch C, Löder MGJ (2022) Tackling the challenge of extracting microplastics from soils: A protocol to purify soil samples for spectroscopic analysis. Environ Toxicol Chem 41:844–857. https://doi.org/10.1002/etc.5024
Munno K, Helm PA, Jackson DA, Rochman C, Sims A (2018) Impacts of temperature and selected chemical digestion methods on microplastic particles. Environ Toxicol Chem 37:91–98. https://doi.org/10.1002/etc.3935
National Institute of Environmental Research (2017) Soil contamination process test standards. NIER, Incheon
Ng E-L, Huerta Lwanga E, Eldridge SM, Johnston P, Hu H-W, Geissen V, Chen D (2018) An overview of microplastic and nanoplastic pollution in agroecosystems. Sci Total Environ 627:1377–1388. https://doi.org/10.1016/j.scitotenv.2018.01.341
Ng EL, Lin SY, Dungan AM, Colwell JM, Ede S, Huerta Lwanga E, Meng K, Geissen V, Blackall LL, Chen D (2021) Microplastic pollution alters forest soil microbiome. J Hazard Mater 409:124606. https://doi.org/10.1016/j.jhazmat.2020.124606
Nizzetto L, Bussi G, Futter MN, Butterfield D, Whitehead PG (2016) A theoretical assessment of microplastic transport in river catchments and their retention by soils and river sediments. Environ Sci Process Impacts 18:1050–1059. https://doi.org/10.1039/c6em00206d
Nizzetto L, Norling M et al (2022) Catchment-scale mechanistic predictions of microplastic transport and distribution across land and water. Research Square
Nuelle M-T, Dekiff JH, Remy D, Fries E (2014) A new analytical approach for monitoring microplastics in marine sediments. Environ Pollut 184:161–169. https://doi.org/10.1016/j.envpol.2013.07.027
Orona-Návar C, García-Morales R, Loge FJ, Mahlknecht J, Aguilar-Hernández I, Ornelas-Soto N (2022) Microplastics in Latin America and the Caribbean: a review on current status and perspectives. J Environ Manag 309:114698
Panno SV, Kelly WR, Scott J, Zheng W, McNeish RE, Holm N, Hoellein TJ, Baranski EL (2019) Microplastic contamination in karst groundwater systems. Groundwater 57:189–196. https://doi.org/10.1111/gwat.12862
Piehl S, Leibner A, Löder MGJ, Dris R, Bogner C, Laforsch C (2018) Identification and quantification of macro- and microplastics on an agricultural farmland. Sci Rep 8:17950. https://doi.org/10.1038/s41598-018-36172-y
Prata JC (2018) Airborne microplastics: consequences to human health? Environ Pollut 234:115–126
Prata JC, da Costa JP, Girão AV, Lopes I, Duarte AC, Rocha-Santos T (2019) Identifying a quick and efficient method of removing organic matter without damaging microplastic samples. Sci Total Environ 686:131–139. https://doi.org/10.1016/j.scitotenv.2019.05.456
Qi R, Jones DL, Li Z, Liu Q, Yan C (2020) Behavior of microplastics and plastic film residues in the soil environment: A critical review. Sci Total Environ 703:134722. https://doi.org/10.1016/j.scitotenv.2019.134722
Rezaei M, Riksen MJPM, Sirjani E, Sameni A, Geissen V (2019) Wind erosion as a driver for transport of light density microplastics. Sci Total Environ 669:273–281. https://doi.org/10.1016/j.scitotenv.2019.02.382
Rillig MC (2012) Microplastic in terrestrial ecosystems and the soil? Environ Sci Technol 46:6453–6454. https://doi.org/10.1021/es302011r
Rillig MC, Ziersch L, Hempel S (2017) Microplastic transport in soils by earthworms. Sci Rep 7:1362. https://doi.org/10.1038/s41598-017-01594-7
Rocha-Santos TA, Duarte AC (2017) Characterization and analysis of microplastics. Elsevier, Amsterdam (ISBN: 9780444638984)
Rodríguez-Seijo A, Santos B, Ferreira da Silva E, Cachada A, Pereira R (2019) Low-density polyethylene microplastics as a source and carriers of agrochemicals to soil and earthworms. Environ Chem 16:8–17. https://doi.org/10.1071/EN18162
Ruggero F, Gori R, Lubello C (2020) Methodologies for microplastics recovery and identification in heterogeneous solid matrices: A review. J Polym Environ 28:739–748. https://doi.org/10.1007/s10924-019-01644-3
Sajjad M, Huang Q, Khan S, Khan MA, Liu Y, Wang J, Lian F, Wang Q, Guo G (2022) Microplastics in the soil environment: A critical review. Environ Technol Innov 27:102408. https://doi.org/10.1016/j.eti.2022.102408
Scheurer M, Bigalke M (2018) Microplastics in Swiss floodplain soils. Environ Sci Technol 52:3591–3598. https://doi.org/10.1021/acs.est.7b06003
Shaw DG, Day RH (1994) Colour-and form-dependent loss of plastic micro-debris from the North Pacific Ocean. Mar Pollut Bull 28:39–43. https://doi.org/10.1016/0025-326X(94)90184-8
Sommer F, Dietze V, Baum A, Sauer J, Gilge S, Maschowski C, Gieré R (2018) Tire abrasion as a major source of microplastics in the environment. Aerosol Air Qual Res 18:2014–2028
Steinmetz Z, Wollmann C, Schaefer M, Buchmann C, David J, Tröger J, Schaumann GE (2016) Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci Total Environ 550:690–705
Stolte A, Forster S, Gerdts G, Schubert H (2015) Microplastic concentrations in beach sediments along the German Baltic coast. Mar Pollut Bull 99:216–229. https://doi.org/10.1016/j.marpolbul.2015.07.022
Tagg AS, Harrison JP, Ju-Nam Y, Sapp M, Bradley EL, Sinclair CJ, Ojeda JJ (2017) Fenton’s reagent for the rapid and efficient isolation of microplastics from wastewater. Chem Commun 53(2):372–375
Unice KM, Weeber MP, Abramson M et al (2019) Characterizing export of land-based microplastics to the estuary-Part I: application of integrated geospatial microplastic transport models to assess tire and road wear particles in the Seine watershed. Sci Total Environ 646:1639–1649
Wang F, Wong CS, Chen D, Lu X, Wang F, Zeng EY (2018) Interaction of toxic chemicals with microplastics: A critical review. Water Res 139:208–219. https://doi.org/10.1016/j.watres.2018.04.003
Wang F, Wu H, Wu W, Wang L, Liu J, An L, Xu Q (2021) Microplastic characteristics in organisms of different trophic levels from Liaohe Estuary. China Sci Total Environ 789:148027. https://doi.org/10.1016/j.scitotenv.2021.148027
Wang J, Peng J, Tan Z, Gao Y, Zhan Z, Chen Q, Cai L (2017) Microplastics in the surface sediments from the Beijiang River littoral zone: Composition, abundance, surface textures and interaction with heavy metals. Chemosphere 171:248–258. https://doi.org/10.1016/j.chemosphere.2016.12.074
Wang W, Ge J, Yu X, Li H (2020) Environmental fate and impacts of microplastics in soil ecosystems: Progress and perspective. Sci Total Environ 708:134841. https://doi.org/10.1016/j.scitotenv.2019.134841
Wang W, Wang J (2018) Investigation of microplastics in aquatic environments: An overview of the methods used, from field sampling to laboratory analysis. TrAC Trends in Anal Chem 108:195–202. https://doi.org/10.1016/j.trac.2018.08.026
Weithmann N, Möller JN, Löder MGJ, Piehl S, Laforsch C, Freitag R (2018) Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci Adv 4:eaap8060. https://doi.org/10.1126/sciadv.aap8060
Yang L, Kang S, Wang Z, Luo X, Guo J, Gao T, Zhang Y (2022) Microplastic characteristic in the soil across the Tibetan Plateau. Sci Total Environ 828:154518. https://doi.org/10.1016/j.scitotenv.2022.154518
Yang L, Zhang Y, Kang S, Wang Z, Wu C (2021) Microplastics in soil: A review on methods, occurrence, sources, and potential risk. Sci Total Environ 780:146546. https://doi.org/10.1016/j.scitotenv.2021.146546
You X, Wang S, Li G, Du L, Dong X (2022) Microplastics in the soil: A review of distribution, anthropogenic impact, and interaction with soil microorganisms based on meta-analysis. Sci Total Environ 832:154975. https://doi.org/10.1016/j.scitotenv.2022.154975
Yu H, Zhang Z, Zhang Y, Fan P, Xi B, Tan W (2021) Metal type and aggregate microenvironment govern the response sequence of speciation transformation of different heavy metals to microplastics in soil. Sci Total Environ 752:141956. https://doi.org/10.1016/j.scitotenv.2020.141956
Yu H-W, Kim YS, Lee S, Yoo J, Choi J (2020) A review on analytical methods and occurrences for microplastics in freshwater. J Environ Anal Health Toxicol 23:180–193. https://doi.org/10.36278/jeaht.23.4.180
Zhang GS, Liu YF (2018) The distribution of microplastics in soil aggregate fractions in southwestern China. Sci Total Environ 642:12–20. https://doi.org/10.1016/j.scitotenv.2018.06.004
Zhang S, Liu X, Hao X, Wang J, Zhang Y (2020) Distribution of low-density microplastics in the mollisol farmlands of northeast China. Sci Total Environ 708:135091. https://doi.org/10.1016/j.scitotenv.2019.135091
Zhao S, Zhang Z, Chen L, Cui Q, Cui Y, Song D, Fang L (2022) Review on migration, transformation and ecological impacts of microplastics in soil. App Soil Ecol 176:104486. https://doi.org/10.1016/j.apsoil.2022.104486
Zhou Q, Zhang H, Fu C, Zhou Y, Dai Z, Li Y, Tu C, Luo Y (2018) The distribution and morphology of microplastics in coastal soils adjacent to the Bohai Sea and the Yellow Sea. Geoderma 322:201–208. https://doi.org/10.1016/j.geoderma.2018.02.015
Zhou Y, Liu X, Wang J (2019) Characterization of microplastics and the association of heavy metals with microplastics in suburban soil of central China. Sci Total Envirn 694:133798. https://doi.org/10.1016/j.scitotenv.2019.133798
Zhou B, Wang J, Zhang H, Shi H, Fei Y, Huang S, Barceló D (2020) Microplastics in agricultural soils on the coastal plain of Hangzhou Bay, east China: multiple sources other than plastic mulching film. J Hazard Mater 388:121814
Zou Y-D, Xu Q-Q, Zhang G, Li F-Y, Li F-M (2019) Influence of six digestion methods on the determination of polystyrene microplastics in organisms using the fluorescence intensity. Huan Jing Ke Xue 40:496–503. https://doi.org/10.13227/j.hjkx.201804072
Acknowledgements
This work was supported by grants funded by the Ministry of Environment (MOE) of the Republic of Korea (RE202101055) and the National Institute of Environment Research (NIER), the Ministry of Environment (MOE) of the Republic of Korea (NIER-SP2022-063).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. Material preparation, data collection, and analysis were performed by SJY, BTL, SOK, and SHP. The first draft of the manuscript was written by SJY and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible editor: Dong-Mei Zhou
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Sj., Lee, BT., Kim, SO. et al. Review of microplastics in soils: state-of-the-art occurrence, transport, and investigation methods. J Soils Sediments 24, 779–792 (2024). https://doi.org/10.1007/s11368-023-03689-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-023-03689-3