Abstract
Purpose
Biological nitrogen fixation (BNF) plays an important role in nitrogen cycling by transferring atmospheric dinitrogen to the soil. BNF is performed by symbiotic and asymbiotic nitrogen-fixing microorganisms. However, the abundance, activity, and community structure of diazotrophs under different nitrogen fertilizer application rates and how root exudates influence diazotrophs remain unclear.
Materials and methods
15N-N2 and 13C-CO2 labeling, DNA-based stable isotope probing (SIP), and molecular biological techniques were used in this study. The abundance, activity, and structure of symbiotic and asymbiotic diazotrophs under different nitrogen fertilizer applications in paddy soil were investigated.
Results and discussion
We found that the nitrogen fixation capacity in milk vetch (Astragalus sinicus L.) and nifH gene abundance in the root nodules were significantly higher in the low-nitrogen treatment than in the control (zero) and high-nitrogen treatments. Nitrogen-fixing bacteria were abundant in the soils with a high biodiversity. Soil nifH gene sequences were dominated by α-, β-, and δ-proteobacteria, as well as by Cyanobacteria. The relative abundance of α-proteobacteria was lower, and the relative abundance of Cyanobacteria was higher under high nitrogen. Incubation of soil with 13CO2 and subsequent DNA-SIP analysis demonstrated that OTU65 from α-proteobacteria was relatively more abundant in heavy fractions of the 13C-labeled soils than that in the unlabeled soils, indicating that α-proteobacteria may prefer rhizodeposition carbon to other organic carbon. However, OTU24 and OTU73 from δ-proteobacteria had relatively high abundances in light fractions both in the 13C-labeled and unlabeled samples, indicating that δ-proteobacteria may prefer other soil organic carbon to rhizodeposition carbon.
Conclusions
The results suggested that soil N availability and rhizodeposition strongly modified the microbial communities of nitrogen-fixing bacteria. Moderate nitrogen application increased the symbiotic biological N fixing activity in the Astragalus sinicus L. rhizosphere. The BNF activity in the legume-rhizobia system is regulated by the exchange of organic C and N nutrient between the host plant and N-fixing bacteria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biological nitrogen fixation (BNF) is the enzymatic reduction of atmospheric dinitrogen to ammonium, which is an important part of the nitrogen cycle that contributes to the soil nitrogen pool. BNF-derived N plays an important role in substituting for chemical N fertilizer use in agricultural systems (Galloway et al. 2004). The amount of N fixed by BNF is estimated to be 2 × 1011 kg N year−1, which accounts for 75% of the N demand for plant growth in the world (FAO 1995).
Growing green manure legumes and recycling their biomass or harvesting residues and adding them to soil generally improve soil fertility, increase the yield of the subsequent rice crop, and reduce the requirement for chemical N fertilizer (Ladha and Reddy 2003; Choudhury and Kennedy 2004). The percentage of N derived from the air (% Ndfa) for green manure legumes is generally more than 80%, and the amount of N fixed by these legumes is as high as 450 kg N ha−1 (Ladha and Reddy 2003). Zhu et al. (2014) observed that approximately 82–88% of Astragalus sinicus L. N was released within the first 28 days after incorporation, and the released N could meet the N demand for rice plant growth. Many studies have shown that the amount of N provided by green manure alone is equivalent to 30–108% of that provided by inorganic fertilizer (Asagi and Ueno 2009). In addition to symbiotic nitrogen-fixing microorganisms, free-living soil diazotrophs can also contribute N nutrient, especially in paddy fields, which provide an optimal environment for biological nitrogen fixation (Roger and Ladha 1992).
The rate of biological nitrogen fixation is mainly dependent on soil nitrogen availability. Soil nitrogen deficiency can inhibit the synthesis of N-fixing enzymes, and high N fertilizer input decreases biological N fixation (Salvagiotti et al. 2008). Compared to no fertilizer, nodule number and soybean weight were higher when N was applied at low levels but lower in high N treatments (Wahab and AbdAlla 1996). Barbulova et al. (2007) observed inhibition of the symbiotic performance of rhizobia and nitrogen fixation under high N supply. Aeroponic culture experiments suggested that a high ammonium concentration (> 5 mM) inhibited nodulation, whereas a low concentration (0.4 mM) stimulated nodulation in an Acacia species (Weber et al. 2007). The abundance of nitrogen-fixing bacteria and N2-fixation activity is suppressed when nitrogen is abundant in the environment (Fuentes-Ramirez et al. 1999; Wang et al. 2018). Studies have shown that N fertilizer application significantly decreases the number of culturable diazotrophic bacteria in Indian and Brazilian sugarcanes (Muthukumarasamy et al. 1999; Junior et al. 2000).
Energy and substrate availability are also important factors for biological N fixation. Except for some asymbiotic nitrogen-fixing microorganisms that can derive their energy from photosynthesis and chemoautotrophy, all heterotrophic diazotrophs use organic material as an energy source (Halm et al. 2012). Previous studies have found that nitrogen-fixing efficiency is positively correlated to the available carbon content in the forest soil (Rosch and Bothe 2009). Plant straw application stimulates soil biological nitrogen fixation, and the application of organic material combined with inorganic fertilizer significantly improves soil N fixation efficiency compared to inorganic fertilizer alone (Liao et al. 2018).
Mutualistic cooperation between plants and microorganisms is ubiquitous, especially in symbiotic systems. Generally, leguminous plants provide C substrates to rhizobia and rhizobia supply some N nutrient to the plants. Mutual constraints can also occur in some symbiotic systems. Kiers et al. (2003) found that a decreased O2 supply from soybean is a possible mechanism to punish “cheating” rhizobia. In soil-plant systems, root exudates are regarded as the main driver of soil microbial communities. However, the effect of root exudates on symbiotic nitrogen fixation is still unclear. Recently, 13C-CO2 labeling combined with DNA stable isotope probing has been shown to be an effective technique to monitor C flux and plant-soil interactions.
Milk vetch (Astragalus sinicus L.) is the most common green manure for paddy soil and is grown prior to rice transplantation. In this study, 13C-CO2 and 15N-N2 labelings were conducted to study the response of symbiotic and asymbiotic nitrogen-fixing microorganisms to nitrogen fertilizer application. The main objectives were as follows: (1) determine whether nitrogen application affects biological N fixation and the diazotrophic bacterial community and (2) determine how rhizodeposition affects diazotrophic bacteria.
2 Materials and methods
2.1 Soil and plant material
Paddy soil was collected from the rice field in Taihu region (30°33′N, 116°20′E), Anhui Province, China, in August 2015. The cropping system was rice-green manure rotation. Twenty soil cores (10 cm diameter × 15 cm length) were taken from the plow layer. The soil samples were placed on ice and transported to the lab. Visible roots and stones in the soil were removed, and the soil was thoroughly mixed and sieved through a 2.0-mm mesh. The soil samples were separated into two portions. The first portion was air-dried for chemical analysis except that mineral-N was immediately analyzed. The second portion was stored at 4 °C for the pot experiment. The soil texture was sandy loam, soil pH was 6.2 (1:5 w/v, soil/water), and total C and total N contents were 10.9 g kg−1 and 0.81 g kg−1, respectively.
The cultivar of milk vetch (Astragalus sinicus L.) used in this study was Yijiangzi. The seeds were sterilized in 10% H2O2 for 10 min and then washed exhaustively with sterile water. A. sinicus seeds were set at 37 °C for pregermination in the dark.
2.2 13C-CO2 labeling experiment
Three treatments were conducted with three levels of nitrogen fertilizer: CK (without nitrogen), NL (nitrogen at 40 mg kg−1), and NH (nitrogen at 100 mg kg−1). Each pot (height 12 cm, diameter 6 cm) with 400 g soil (dry weight) was prepared to plant A. sinicus. All treatments were done in triplicate. The nitrogen fertilizer used in this experiment was urea. Monopotassium phosphate was used as P2O5 (120 mg kg−1 dry soil) and potassium as K2O (80 mg kg−1 dry soil). Similar gemmiparous seeds were chosen to plant in the pots, and 10 seeds were cultivated in each pot. A. sinicus was grown in a plant growth chamber, with an average light intensity of 295 UML, a 12.5-h photoperiod, a relative humidity of 60%, and a temperature of 24 °C in the day and 18 °C at night. The incubation conditions were kept consistent during the entire incubation period.
The 13C-CO2 continuous labeling experiment was carried out after 30 days of A. sinicus growth. The pots were incubated in two chambers with normal CO2 or 13C-CO2 (99.9 atom %). The total CO2 content was 350 mL m−3, the flow velocity of air without CO2 was 10 L min−1, and the flow velocity of CO2 was 3.8 mL min−1. Destructive sampling was conducted before labeling (30 days) and after 30-day labeling (60 days) from the 13C chamber and unlabeled chamber. The root samples were washed with deionized water, and the nodules were picked using tweezers and quickly frozen in liquid nitrogen and stored at − 80 °C. The shoots and roots were dried to measure the dry weight and nitrogen content. The soil used for DNA extraction was freeze-dried immediately after sampling, and soil samples for other chemical analyses were stored at 4 °C until analysis.
2.3 15N-N2 labeling experiment
A 15N-N2 labeling experiment was conducted after 53-day growth using the pulse labeling method, and the treatments were the same as described above in Section 2.2. The plants were grown in a sealed container (0.086 m3), and 10%-labeled 15N-N2 was injected to the container. Another set of plants were grown with ambient air as controls. The plants were labeled for 10 h each day. When 7-day labeling was complete, the nodules were freeze-dried, the abundance of 15N was measured, and the shoots and roots were dried to measure the 15N abundance. Soil with three nitrogen levels without plants was also labeled with 15N-N2 as a control, and the soil 15N abundance was measured after 7 days of labeling. 15N abundance was determined by a Delta V Advantage isotope ratio mass spectrometer (Thermo Finnigan, Germany).
2.4 Soil and nodule DNA extraction
Soil DNA was extracted using the FastDNA spin kit for soil (MP Biomedicals, USA) following the manufacturer’s instructions. Nodule DNA was extracted using CTAB according to the method of Kim et al. (1999). A NanoDrop spectrophotometer (ND-1000, Thermo Fisher Scientific, USA) was applied to assess DNA concentration and quality. All DNA samples were stored at − 80 °C for subsequent use.
2.5 Soil and nodule nifH gene abundance determination
The abundance of nifH was assessed in triplicate using a real-time quantitative PCR (qPCR) detection system (LightCycler 480, Roche, USA), with the primer sets PloF (5′-TGCGAYCCSAARGCBGACTC-3′) and PloR (5′-ATSGCCATCATYTCRCCGGA-3′) (Poly et al. 2001). Each reaction mixture contained 10 μL SYBR 2 Premix Ex Taq (Takara Shuzo, Shiga, Japan), 0.25 μM of each primer, and 2 μL of 10-fold diluted template DNA and made up to 20 μL with ddH2O. All standard curves were generated from 10-fold stepwise dilutions of plasmid DNA with the correct target genes. Negative controls were included, using water to replace the template DNA, in each plate. The quantitative amplification conditions were 95 °C for 4 min, 40 cycles of 95 °C for 45 s, 55 °C for 30 s, and 72 °C for 40 s. Quantitative PCR efficiencies between 90% and 110% were employed in this study.
2.6 DNA SIP fractionation
Both 13C-labeled and unlabeled soil DNA were used for gradient fractionation. The method was the same as described by Neufeld et al. (2007). Approximately 2-μg DNA was mixed with the CsCl solution to achieve the initial buoyant density gradient of 1.691 g mL−1 according to the refractive index (1.3999) measured by an AR200 hand-held refractometer (Reichert, Inc., Buffalo, NY). The speed of the ultracentrifugation was 45,000 rpm at 20 °C for 40 h (Beckman Coulter, German). LSP01-1A single-channel syringe pumps (Longer Precision Pump Co., Ltd., China) were used to fractionate the DNA samples; each sample was evenly separated to 16 layers. The collected DNA was purified and dissolved in 30 μL of sterile water. The copy number of the nifH gene in the fractionated DNA was measured by qPCR as described in Section 2.5.
2.7 Cloning and sequencing analyses
The whole soil sample DNA and the fractionated DNA (including 13C-labeled and unlabeled fractions) were amplified for cloning and sequencing. The obtained PCR products were gel-purified using the universal DNA purification kit (Tiangen Biotech, Beijing, China), and the purified products were ligated into pMD19-T vectors. For each of the three replicates, 80 positive clones were randomly selected and sequenced (240 clones for each treatment) (Shanghai Majorbio Bio-Pharm Technology Co., Shanghai, China). Chromas LITE software (version 2.01, Technelysium Pty Ltd., Australia) was used to check the sequence quality. All sequences were analyzed by the BLAST program in the NCBI GenBank database. Reference sequences from the GenBank database and the respective OTUs (98% similarity) sequences were selected to construct the phylogenetic tree (Long et al. 2018).
The gene sequences retrieved in this study were uploaded to the National Center for Biotechnology Information. The access numbers of the whole soil samples are KY011303-KY011835. The accession numbers of the unlabeled soils are KY046404-KY046914 and KY046916-KY047104, and the accession numbers of the 13C-labeled soils are KY121112-KY121811.
2.8 Statistical analysis
Quantitative PCR data were log-transformed before further analysis. To compare the abundances of nifH genes among all treatments, data were analyzed using ANOVA with SPSS 19.0 software (IBM, USA). The nucleic acid sequences were translated to protein sequences with MEGA software (version 6.0), and the operational taxonomic units (OTUs) of the protein sequences were classified with 95% similarity by DNAMAN (version 6.0). The representatives of each OTU and the reference sequences were aligned using the Clustal W program with MEGA software, and the phylogenetic tree was constructed using the neighbor-joining method. The confidence values of the tree nodes were estimated by a bootstrap analysis with 1000 replicates. Heat map plotting was performed in R 3.5.0 with the pheatmap package.
3 Results
3.1 Symbiotic nitrogen-fixing activity and microorganisms
Nitrogen fertilizer had a significant influence on plant biomass as well as the amount of total and labeled N. The shoot and root dry weights with the NH treatment were 2.18 and 0.86 g, respectively, which were much higher than those with the CK and NL treatments. The total N amounts in the shoots and roots with the NH treatment were 40.4% and 61.1% higher than CK, respectively, and 28.3% and 14.0% higher than the NL treatment, respectively (Table 1). However, the dry weight and total N of nodules with the NL treatment were the highest among the three treatments, and there was no significant difference between CK and NH treatments with respect to nodule dry weight and N content. The labeled N content of the shoots, roots, and nodules was significantly higher in the NL-treated plants, and there was no significant difference between the CK and NH treatments. Although the dry weight and total N content of nodules were much lower than the root, the labeled N content in these two parts was similar. The amount of labeled 15N in the soil was 0.14 ± 0.06, 0.11 ± 0.04, and 0.09 ± 0.04 mg in the CK, NL, and NH treatments, respectively, but there was no significant difference among the three treatments.
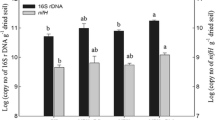
The abundance of the nifH gene in the nodule was 3.7 × 1010 copies g−1 (nodule dry weight) in plants under the CK treatment after 30 days of growth, and there was no significant difference between the two N fertilizer treatments. However, the nifH gene abundance in the NL treatment was 6.0 × 1010 copies g−1 dry nodule after 60 days of growth, which was significantly higher than that in the CK and NH treatments (Fig. 1).
A phylogenetic tree of the nifH gene of the nodule samples showed that the symbiotic nitrogen-fixing bacteria only included two gene types. More than 99% of the nifH genes belonged to Mesorhizobium, and less than 1% nifH genes belonged to Bradyrhizobium. All the Bradyrhizobium species were from the NH treatment.
3.2 Asymbiotic nitrogen-fixing activity and microorganisms
Compared to symbiotic nitrogen-fixing bacteria, the amount of nitrogen fixation by asymbiotic microorganisms was much lower. In the 15N-N2-labeled soil without plants, the labeled N content was only 0.05 ± 0.01 mg, which accounted for 5.1% of the labeled N fixed by symbiotic nitrogen-fixing organisms. The abundances of the nifH gene in these soils were 1.3 × 107 and 1.2 × 107 copies g−1 dry soil in the CK and NL treatments after 30 days of growth, whereas the nifH gene abundance in the NH treatment was much lower, at only 0.9 × 107 copies g−1 dry soil. The nifH gene abundance in soils significantly decreased to 6.9 to 8.8 × 106 copies g−1 dry soil after 60 days of growth among the three treatments (Fig. 2). These results suggested that the abundance of the nifH gene in the soil decreased with the application of N fertilizer.
Nitrogen-fixing bacteria were abundant in the soils with a high species biodiversity. In total, 107 OTUs were identified at 95% similarity using DNAMAN software. The nitrogen-fixing bacteria were mainly distributed among α-, β-, and δ-proteobacteria, as well as Cyanobacteria, which occupied more than 98% of the total sequences (Fig. 3). Compared to the CK treatment, N fertilizer application increased the relative abundance of δ-proteobacteria, which accounted for 25.0%, 27.2%, and 29.0% of CK, NL, and NH treatments, respectively. However, high-level N application significantly decreased the relative abundance of α-proteobacteria. The relative abundances of Cyanobacteria were 16.5%, 16.1%, and 21.3% in the CK, NL, and NH treatments, respectively, indicating that high nitrogen conditions favor Cyanobacteria growth. More specifically, the relative abundance of Burkholderiales was much higher with the CK treatment than that with the N application treatments, whereas the relative abundance of Desulfovibrionales was higher with N addition.
Both labeled and unlabeled soil DNA samples were distributed in 16 layers, and the buoyant density ranged from 1.578 to 1.745 g mL−1. A slight shift in the buoyant density was observed for the heavy layers, which indicated that 13C was incorporated into diazotrophic organism genomes. The abundances of the nifH gene in the 16 fractions were determined by qPCR, and the peak value was in layer 6 (Fig. 4). The nifH gene diversity was analyzed, comparing the lighter fractions (7–9 layers) and the heavy fractions (4–6 layers). All nifH sequences were translated to protein sequences and clustered at 95% similarity. As with the whole soil samples, α-, β-, δ-proteobacteria, and Cyanobacteria were the four most abundant phyla in both the light and heavy fractions. γ-proteobacteria, Actinobacteria, and Verrucomicrobia had low relative abundances, and each occupied less than 1.0% of the total sequences. The relative abundances of some genotypes/OTUs in the light and heavy fractions were shifted after 13C labeling. The heat map showed that OTU65, which belongs to α-proteobacteria, had a higher relative abundance in heavy fractions of the 13C-labeled soils than the unlabeled soils, which indicated that α-proteobacteria may prefer rhizodeposition carbon to other organic carbon. However, OTU24 and OTU73, which belong to the δ-proteobacteria, had relatively high abundances in the light fractions both in the 13C-labeled and unlabeled samples, which indicated that δ-proteobacteria may prefer other soil organic carbon to rhizodeposition carbon (Fig. 5).
Phylogenetic tree of soil nifH gene sequences retrieved from selected density fractions of the 13C-labeled and 12C-unlabeled treatments (protein sequences, 95% similarity). Their relative abundances are shown as a heat map distribution. The heat map colors represent the relative percentages of the OTU in different samples
4 Discussion
Nitrogen fertilizer application significantly altered symbiotic nitrogen fixation activity and N-fixing bacterial abundance in nodules. The 15N-N2 labeling experiment showed that the BNF activity in the moderate N application treatment was higher than that in the CK and the high N input treatment (NH). In the legume-rhizobia system, there is a symbiotic relationship of mutual cooperation and restraint between the host plant and N-fixing bacteria. The host legume can impose stimulation or sanction on rhizobia by increasing or decreasing the supply of a required resource for growth (Denison 2000; Kiers et al. 2003). Soil N content is one of the key constraints for this symbiotic system (Thrall et al. 2011). The moderate N application stimulated plant growth and increased the carbon and energy supply for nitrogen-fixing bacteria, which contributed to the BNF activity, because the N-fixing process is very energy-consuming (West et al. 2002; Kiers et al. 2003). When soil nitrogen availability is abundant, the host plant is more likely to use soil N directly rather than investing in symbiotic nitrogen fixation (Simms and Taylor 2002; Perez-Fernandez and Lamont 2016). Consequently, the BNF declines with increasing soil N availability, and high levels of N fertilizer can result in a decrease in nodulation rate and N-fixing efficiency (Caetanoanolles and Gresshoff 1991; Arias et al. 1999; Thomas et al. 2000). The symbiotic BNF activity in the legume-rhizobia system is regulated by the exchange of organic C and N nutrient between the host plant and N-fixing bacteria.
Like the BNF activity, the nifH gene abundance in nodules of the NL-treated plants was significantly higher than with the CK and NH treatments. However, no significant effect was found for the nifH gene types. The correlation between the N-fixing activities and nifH gene abundance suggests that the N-fixing efficiency of rhizobia is regulated by microbial abundance rather than microbial diversity. The increased substrate and energy supply from the legume to the rhizobia promoted the growth and reproduction of N-fixing bacteria. Not only microbial community abundance but also community diversity can be affected by substrate availability (Yao et al. 2011). The similar microbial genotype across the three treatments was consistent with the narrow host specificity of rhizobia (Yang et al. 2010). Some signaling molecules, including surface polysaccharides and secreted proteins, are regarded as the possible mechanisms for host specificity (Fauvart and Michiels 2008). In our study, the growth period of milk vetch was only 60 days, so this cannot be ruled out as a reason for the similar rhizobia genotypes.
The qPCR results suggested that nitrogen fertilization resulted in a decrease in soil nifH gene abundance, as previously reported for several agricultural soils (Coelho et al. 2009; Lindsay et al. 2010). Field experiments have also shown this suppressive effect of N fertilization and a significant negative relationship between inorganic N and nifH gene abundance (Lindsay et al. 2010; Silva et al. 2013). The nifH gene abundance in the N-related treatments was 3 to 4 times lower than that in the control treatment in long-term field experiments (Wang et al. 2017; Wang et al. 2018). Soil acidification and high inorganic N content were considered as two major impact factors (Feng et al. 2018). Asymbiotic N-fixing bacteria usually preferentially use soil available N instead of fixing it (Barron et al. 2009), due to the high energy demand of the latter process. However, some studies have reported the converse results of higher nifH gene abundance with N fertilization (Mergel et al. 2001; Liao et al. 2018). This may be due to the indirect effects of the N fertilizer on N-fixing bacteria because soil N availability can regulate the soil microbial community, plant growth, and root exudation patterns (Reis et al. 2000; Meng et al. 2012; Liao et al. 2018).
According to the soil nifH clone library, nifH genes are mainly distributed into α-, β-, and δ-proteobacteria, and Cyanobacteria. The results were consistent with previous studies (Ogilvie et al. 2008; Liao et al. 2018), which found a similar microbial distribution. Because different diazotrophic bacteria species have different sensitivities to N levels available in the soil (Harke et al. 2016), N fertilizer input altered the relative abundances of various diazotrophic bacteria. Wang et al. (2018) found that N input significantly increased the relative abundances of α-proteobacteria and Cyanobacteria, but decreased the relative abundance of δ-proteobacteria. The higher nifH gene abundance in the Cyanobacteria phylum in this study may suggest that photoautotrophic species require more nitrogen than other diazotrophic bacteria.
Desulfovibrio is an anaerobic sulfate-reducing bacterium. The high relative abundance of Desulfovibrionales indicated that the long-term flooding of the paddy field provides a suitable habitat for it. Soil N cycling is generally coupled with sulfur and iron cycling, and high N availability can increase the iron- or sulfur-related genes (Bao et al. 2014; Minamisawa et al. 2016). Here, we found that the relative abundance of Desulfovibrionales increased with N fertilizer input. This result was consistent with those of Sim et al. (2012), who reported that N limitation inhibited the growth of sulfate-reducing bacteria.
The genus Burkholderia represents a versatile group of organisms (Yabuuchi et al. 1992; Salles et al. 2006) and has been exploited for various purposes including N-fixation, plant growth promotion, and biological control (Coenye and Vandamme 2003). In this study, the relative abundance of Burkholderiales decreased with N fertilizer application based on the nifH gene analysis. The results suggest that the N-fixing function of Burkholderiales can be inhibited by high soil N (Kumar et al. 2018). The decrease in the relative abundance of Burkholderiales may be due to the direct inhibition by inorganic N of nitrogenase activity or through indirect effects on other microbial groups (Yoch and Whiting 1986).
The rhizosphere is the major soil ecological environment for plant-microorganism interactions (Meena et al. 2017; Li et al. 2018). Root exudates provide nutrients and energy sources to soil microorganisms (Bertin et al. 2003; Landi et al. 2006; Wang et al. 2016). The rhizospheric microbial communities are deeply affected by root exudates. On the other hand, rhizospheric microorganisms are the drivers of nutrient turnover and are beneficial for plant growth (Landi et al. 2006; Meena et al. 2017). Because root exudates are only a minor component of the total organic carbon sources in the soil (Nguyen 2003; Yao et al. 2012), understanding the effect of rhizodeposition on the rhizosphere microbial community should include consideration of other soil carbon pools (Dennis et al. 2010). 13C steady-state labeling is an efficient approach to understand the relative importance of rhizodeposition in determining microbial community diversity (Paterson et al. 2007; Li et al. 2016). Several studies (Wang et al. 2016; Liao et al. 2018) have confirmed that fungi and gram-negative bacteria are the main consumers of root exudates based on phospholipid fatty acid analysis. In this study, the soil microbial community was not only affected by N fertilizer but also root exudates. Continuous labeling results showed that α-proteobacteria were the main microbes incorporating native soil organic carbon. The findings suggested that asymbiotic nitrogen-fixing bacterial community diversity is strongly modified by rhizodeposition.
5 Conclusions
The results of this study demonstrated that low-nitrogen application increases the dry weight, nitrogen-fixation, and the abundance of the nifH gene in the root nodules of milk vetch (Astragalus sinicus L.). Additionally, the asymbiotic nitrogen-fixing bacterial community diversity is strongly influenced by root exudates and N fertilizer input. Further work is required to confirm the findings using field experiments and to understand the subtle changes in N-fixing bacterial species in the legume-rhizobia system.
References
Arias HOR, de la Vega L, Ruiz O, Wood K (1999) Differential nodulation response and biomass yield of Alexandria clover as affected by levels of inorganic nitrogen fertilizer. J Plant Nutr 22(8):1233–1239. https://doi.org/10.1080/01904169909365708
Asagi N, Ueno H (2009) Nitrogen dynamics in paddy soil applied with various 15N-labelled green manures. Plant Soil 322(1–2):251–262
Bao ZH, Okubo T, Kubota K, Kasahara Y, Tsurumaru H, Anda M, Ikeda S, Minamisawa K (2014) Metaproteomic identification of diazotrophic methanotrophs and their localization in root tissues of field-grown rice plants. Appl Environ Microbiol 80(16):5043–5052. https://doi.org/10.1128/AEM.00969-14
Barbulova A, Rogato A, D’Apuzzo E, Omrane S, Chiurazzi M (2007) Differential effects of combined N sources on early steps of the nod factor-dependent transduction pathway in Lotus japonicus. Mol Plant Microbe In 20(8):994–1003. https://doi.org/10.1094/MPMI-20-8-0994
Barron AR, Wurzburger N, Bellenger JP, Wright SJ, Kraepiel AML, Hedin LO (2009) Molybdenum limitation of asymbiotic nitrogen fixation in tropical forest soils. Nat Geosci 2(1):42–45. https://doi.org/10.1038/ngeo366
Bertin C, Yang XH, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256(1):67–83. https://doi.org/10.1023/A:1026290508166
Caetanoanolles G, Gresshoff PM (1991) Plant genetic-control of nodulation. Annu Rev Microbiol 45:345–382. https://doi.org/10.1146/annurev.mi.45.100191.002021
Choudhury ATMA, Kennedy IR (2004) Prospects and potentials for systems of biological nitrogen fixation in sustainable rice production. Biol Fertil Soils 39(4):219–227. https://doi.org/10.1007/s00374-003-0706-2
Coelho MRR, Marriel IE, Jenkins SN, Lanyon CV, Seldin L, O’Donnell AG (2009) Molecular detection and quantification of nifH gene sequences in the rhizosphere of sorghum (Sorghum bicolor) sown with two levels of nitrogen fertilizer. Appl Soil Ecol 42(1):48–53. https://doi.org/10.1016/j.apsoil.2009.01.010
Coenye T, Vandamme P (2003) Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ Microbiol 5(9):719–729. https://doi.org/10.1046/j.1462-2920.2003.00471.x
Denison RF (2000) Legume sanctions and the evolution of symbiotic cooperation by rhizobia. Am Nat 156(6):567–576. https://doi.org/10.1086/316994
Dennis PG, Miller AJ, Hirsch PR (2010) Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol Ecol 72(3):313–327. https://doi.org/10.1111/j.1574-6941.2010.00860.x
FAO (1995) Sustainable dryland cropping in relation to soil productivity––FAO soils bulletin, vol 72. Food and Agriculture Organization of the United Nations (FAO), Rome
Fauvart M, Michiels J (2008) Rhizobial secreted proteins as determinants of host specificity in the rhizobium-legume symbiosis. FEMS Microbiol Lett 285(1):1–9. https://doi.org/10.1111/j.1574-6968.2008.01254.x
Feng MM, Adams JM, Fan KK, Shi Y, Sun RB, Wang DZ, Guo XS, Chu HY (2018) Long-term fertilization influences community assembly processes of soil diazotrophs. Soil Biol Biochem 126:151–158. https://doi.org/10.1016/j.soilbio.2018.08.021
Fuentes-Ramirez LE, Caballero-Mellado J, Sepulveda J, Martinez-Romero E (1999) Colonization of sugarcane by Acetobacter diazotrophicus is inhibited by high N-fertilization. FEMS Microbiol Ecol 29(2):117–128. https://doi.org/10.1016/S0168-6496(98)00125-1
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vorosmarty CJ (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70(2):153–226. https://doi.org/10.1007/s10533-004-0370-0
Halm H, Lam P, Ferdelman TG, Lavik G, Dittmar T, LaRoche J, D’Hondt S, Kuypers MMM (2012) Heterotrophic organisms dominate nitrogen fixation in the South Pacific Gyre. ISME J 6(6):1238–1249
Harke MJ, Davis TW, Watson SB, Gobler CJ (2016) Nutrient-controlled niche differentiation of Western Lake Erie cyanobacterial populations revealed via metatranscriptomic surveys. Environ Sci Technol 50(2):604–615
Junior FBD, Reis VM, Urquiaga S, Dobereiner J (2000) Influence of nitrogen fertilisation on the population of diazotrophic bacteria Herbaspirillum spp. and Acetobacter diazotrophicus in sugar cane (Saccharum spp.). Plant Soil 219(1–2):153–159
Kiers ET, Rousseau RA, West SA, Denison RF (2003) Host sanctions and the legume-rhizobium mutualism. Nature 425(6953):78–81. https://doi.org/10.1038/nature01931
Kim HB, Lee SH, An CS (1999) Isolation and characterization of a cDNA clone encoding asparagine synthetase from root nodules of Elaeagnus umbellata. Plant Sci 149(2):85–94. https://doi.org/10.1016/S0168-9452(99)00124-7
Kumar U, Nayak AK, Shahid M, Gupta VVSR, Panneerselvam P, Mohanty S, Kaviraj M, Kumar A, Chatterjee D, Lal B, Gautam P, Tripathi R, Panda BB (2018) Continuous application of inorganic and organic fertilizers over 47 years in paddy soil alters the bacterial community structure and its influence on rice production. Agric Ecosyst Environ 262:65–75. https://doi.org/10.1016/j.agee.2018.04.016
Ladha JK, Reddy PM (2003) Nitrogen fixation in rice systems: state of knowledge and future prospects. Plant Soil 252(1):151–167. https://doi.org/10.1023/A:1024175307238
Landi L, Valori F, Ascher J, Renella G, Falchini L, Nannipieri P (2006) Root exudate effects on the bacterial communities, CO2 evolution, nitrogen transformations and ATP content of rhizosphere and bulk soils. Soil Biol Biochem 38(3):509–516. https://doi.org/10.1016/j.soilbio.2005.05.021
Li YY, Chapman SJ, Nicol GW, Yao HY (2018) Nitrification and nitrifiers in acidic soils. Soil Biol Biochem 116:290–301. https://doi.org/10.1016/j.soilbio.2017.10.023
Li YY, Wang J, Pan FX, Chapman SJ, Yao HY (2016) Soil nitrogen availability alters rhizodeposition carbon flux into the soil microbial community. J Soils Sediments 16(5):1472–1480. https://doi.org/10.1007/s11368-015-1337-6
Liao HK, Li YY, Yao HY (2018) Fertilization with inorganic and organic nutrients changes diazotroph community composition and N-fixation rates. J Soils Sediments 18(3):1076–1086. https://doi.org/10.1007/s11368-017-1836-8
Lindsay EA, Colloff MJ, Gibb NL, Wakelin SA (2010) The abundance of microbial functional genes in grassy woodlands is influenced more by soil nutrient enrichment than by recent weed invasion or livestock exclusion. Appl Environ Microbiol 76(16):5547–5555. https://doi.org/10.1128/AEM.03054-09
Long XE, Huang Y, Chi HF, Li YY, Ahmad N, Yao HY (2018) Nitrous oxide flux, ammonia oxidizer and denitrifier abundance and activity across three different landfill cover soils in Ningbo, China. J Clean Prod 170:288–297. https://doi.org/10.1016/j.jclepro.2017.09.173
Meena VS, Meena SK, Verma JP, Kumar A, Aeron A, Mishra PK, Bisht JK, Pattanayak A, Naveed M, Dotaniya ML (2017) Plant beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use efficiency: a review. Ecol Eng 107:8–32. https://doi.org/10.1016/j.ecoleng.2017.06.058
Meng XF, Wang L, Long XH, Liu ZP, Zhang ZH, Zed R (2012) Influence of nitrogen fertilization on diazotrophic communities in the rhizosphere of the Jerusalem artichoke (Helianthus tuberosus L.). Res Microbiol 163(5):349–356. https://doi.org/10.1016/j.resmic.2012.03.005
Mergel A, Kloos K, Bothe H (2001) Seasonal fluctuations in the population of denitrifying and N2-fixing bacteria in an acid soil of a Norway spruce forest. Plant Soil 230(1):145–160. https://doi.org/10.1023/A:1004826116981
Minamisawa K, Imaizumi-Anraku H, Bao ZH, Shinoda R, Okubo T, Ikeda S (2016) Are symbiotic methanotrophs key microbes for N acquisition in paddy rice root? Microbes Environ 31(1):4–10. https://doi.org/10.1264/jsme2.ME15180
Muthukumarasamy R, Revathi G, Lakshminarasimhan C (1999) Influence of N fertilisation on the isolation of Acetobacter diazotrophicus and Herbaspirillum spp. from Indian sugarcane varieties. Biol Fertil Soils 29(2):157–164. https://doi.org/10.1007/s003740050539
Neufeld JD, Vohra J, Dumont MG, Lueders T, Manefield M, Friedrich MW, Murrell JC (2007) DNA stable-isotope probing. Nat Protoc 2(4):860–866. https://doi.org/10.1038/nprot.2007.109
Nguyen C (2003) Rhizodeposition of organic C by plants: mechanisms and controls. Agronomie 23(5–6):375–396
Ogilvie LA, Hirsch PR, Johnston AWB (2008) Bacterial diversity of the Broadbalk ‘classical’ winter wheat experiment in relation to long-term fertilizer inputs. Microb Ecol 56(3):525–537. https://doi.org/10.1007/s00248-008-9372-0
Paterson E, Gebbing T, Abel C, Sim A, Telfer G (2007) Rhizodeposition shapes rhizosphere microbial community structure in organic soil. New Phytol 173(3):600–610. https://doi.org/10.1111/j.1469-8137.2006.01931.x
Perez-Fernandez MA, Lamont BB (2016) Competition and facilitation between Australian and Spanish legumes in seven Australian soils. Plant Spec Biol 31(4):256–271. https://doi.org/10.1111/1442-1984.12111
Poly F, Ranjard L, Nazaret S, Gourbiere F, Monrozier LJ (2001) Comparison of nifH gene pools in soils and soil microenvironments with contrasting properties. Appl Environ Microbiol 67:2255–2262. https://doi.org/10.1128/AEM.67.5.2255-2262.2001
Reis VM, Baldani JI, Baldani VLD, Dobereiner J (2000) Biological dinitrogen fixation in gramineae and palm trees. Crit Rev Plant Sci 19(3):227–247
Roger PA, Ladha JK (1992) Biological N2 fixation in wetland rice fields - estimation and contribution to nitrogen-balance. Plant Soil 141(1–2):41–55
Rosch C, Bothe H (2009) Diversity of total, nitrogen-fixing and denitrifying bacteria in an acid forest soil. Eur J Soil Sci 60(6):883–894. https://doi.org/10.1111/j.1365-2389.2009.01167.x
Salles JF, van Elsas JD, van Veen JA (2006) Effect of agricultural management regime on Burkholderia community structure in soil. Microb Ecol 52(2):267–279. https://doi.org/10.1007/s00248-006-9048-6
Salvagiotti F, Cassman KG, Specht JE, Walters DT, Weiss A, Dobermann A (2008) Nitrogen uptake, fixation and response to fertilizer N in soybeans: a review. Field Crop Res 108(1):1–13. https://doi.org/10.1016/j.fcr.2008.03.001
Silva MCPE, Schloter-Hai B, Schloter M, van Elsas JD, Salles JF (2013) Temporal dynamics of abundance and composition of nitrogen-fixing communities across agricultural soils. PLoS One 8(9):e74500. https://doi.org/10.1371/journal.pone.0073187
Sim MS, Ono S, Bosak T (2012) Effects of iron and nitrogen limitation on sulfur isotope fractionation during microbial sulfate reduction. Appl Environ Microbiol 78(23):8368–8376. https://doi.org/10.1128/AEM.01842-12
Simms EL, Taylor DL (2002) Partner choice in nitrogen-fixation mutualisms of legumes and rhizobia. Integr Comp Biol 42(2):369–380. https://doi.org/10.1093/icb/42.2.369
Thomas RB, Bashkin MA, Richter DD (2000) Nitrogen inhibition of nodulation and N2 fixation of a tropical N2-fixing tree (Gliricidia sepium) grown in elevated atmospheric CO2. New Phytol 145(2):233–243. https://doi.org/10.1046/j.1469-8137.2000.00577.x
Thrall PH, Laine AL, Broadhurst LM, Bagnall DJ, Brockwell J (2011) Symbiotic effectiveness of rhizobial mutualists varies in interactions with native Australian legume genera. PLoS One 6(8):e23545. https://doi.org/10.1371/journal.pone.0023545
Wahab AMA, AbdAlla MH (1996) Effect of different rates of N-fertilizers on nodulation, nodule activities and growth of two field grown cvs. of soybean. Fert Res 43(1–3):37–41
Wang C, Zheng MM, Song WF, Wen SL, Wang BR, Zhu CQ, Shen RF (2017) Impact of 25 years of inorganic fertilization on diazotrophic abundance and community structure in an acidic soil in southern China. Soil Biol Biochem 113:240–249. https://doi.org/10.1016/j.soilbio.2017.06.019
Wang J, Chapman SJ, Yao HY (2016) Incorporation of 13C-labelled rice rhizodeposition into soil microbial communities under different fertilizer applications. Appl Soil Ecol 101:11–19. https://doi.org/10.1016/j.apsoil.2016.01.010
Wang Q, Wang JL, Li YZ, Chen DW, Ao JH, Zhou WL, Shen AC, Li QW, Huang ZR, Jiang Y (2018) Influence of nitrogen and phosphorus additions on N2-fixation activity, abundance, and composition of diazotrophic communities in a Chinese fir plantation. Sci Total Environ 619-620:1530–1537
Weber J, Tham FY, Galiana A, Prin Y, Ducousso M, Lee SK (2007) Effects of nitrogen source on the growth and nodulation of Acacia mangium in aeroponic culture. J Trop For Sci 19(2):103–112
West SA, Kiers ET, Simms EL, Denison RF (2002) Sanctions and mutualism stability: why do rhizobia fix nitrogen? P Roy Soc B-Biol Sci 269(1492):685–694
Yabuuchi E, Kosako Y, Oyaizu H, Yano I, Hotta H, Hashimoto Y, Ezaki T, Arakawa M (1992) Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group-II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol Immunol 36(12):1251–1275. https://doi.org/10.1111/j.1348-0421.1992.tb02129.x
Yang SM, Tang F, Gao MQ, Krishnan HB, Zhu HY (2010) R gene-controlled host specificity in the legume-rhizobia symbiosis. Proc Nati Acad Sci USA 107(43):18735–18740
Yao H, Campbell CD, Qiao X (2011) Soil pH controls nitrification and carbon substrate utilization more than urea or charcoal in some highly acidic soils. Biol Fertil Soils 47(5):515–522. https://doi.org/10.1007/s00374-011-0554-4
Yao HY, Thornton B, Paterson E (2012) Incorporation of 13C-labelled rice rhizodeposition carbon into soil microbial communities under different water status. Soil Biol Biochem 53:72–77. https://doi.org/10.1016/j.soilbio.2012.05.006
Yoch DC, Whiting GJ (1986) Evidence for NH4 + switch-off regulation of nitrogenase activity by bacteria in salt-marsh sediments and roots of the grass Spartina alterniflora. Appl Environ Microbiol 51(1):143–149
Zhu B, Yi LX, Hu YG, Zeng ZH, Lin CW, Tang HM, Yang GL, Xiao XP (2014) Nitrogen release from incorporated 15N-labelled Chinese milk vetch (Astragalus sinicus L.) residue and its dynamics in a double rice cropping system. Plant Soil 374(1–2):331–344
Funding
This work was supported by the National Natural Science Foundation of China (41525002, 41877051, 41761134085), the National Key R & D Program of China (2017YFD0200102), and the Strategic Priority Research Program of Chinese Academy of Sciences (XDB15020301).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Yuan Ge
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Pan, F. & Yao, H. Response of symbiotic and asymbiotic nitrogen-fixing microorganisms to nitrogen fertilizer application. J Soils Sediments 19, 1948–1958 (2019). https://doi.org/10.1007/s11368-018-2192-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-018-2192-z