Abstract
Understanding the mechanism and key controlling factors of nitrification in highly acidic soils is important from both ecological and environmental perspectives. Many acid soils are also characterized by vegetation that produces polyphenolic and terpene compounds that inhibit microbial activity. We investigated the potentially ameliorative effects of lime, charcoal, and urea additions on soil nitrification and carbon substrate utilization (using the MicroResp method). Four soils were studied from widely different environments but with similar pH and inputs of phytochemicals to determine the relative effects of these potentially controlling factors. The addition of charcoal had no significant effect on net nitrification, but charcoal significantly increased soil basal respiration and altered C substrate utilization in the two Scottish soils. Urea greatly increased nitrification in both the Chinese soils, but there was no effect of urea on nitrification in the two Scottish soils. Lime application increased nitrification in all the soils except for the Chinese mixed forest soil. Multivariate analysis of the C source utilization data revealed that lime altered C substrate utilization more than urea or charcoal in these highly acidic soils. Our results suggest that acid-tolerant nitrifiers do exist in these soils and have potential for high activity, and pH (lime addition) and N-substrate (urea) most often increased nitrification. However, no single factor controlled nitrification in every soil, suggesting an interaction between abiotic and nitrifier community composition as a result of land use and soil type interactions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrification is a crucial N cycling process in the soil ecosystem and has great agricultural and environmental importance as it controls the release of nitrate for plant uptake and/or leaching to water courses. Nitrification appears to be absent in some highly acidic soils (pH < 4.5) where nitrate concentrations are low and accumulation of nitrate does not occur unless the pH is first raised (De Boer and Kowalchuk 2001). However, high concentrations of nitrate and high nitrification rates have been found in a wide range of acid soils including tea orchards, forests, and grasslands (Pennington and Ellis 1993; Pansombat et al. 1997; Xue et al. 2006).
The nitrification rate is driven by the abundance and activity of nitrifier populations (Stark and Firestone 1996), which can be affected by various environmental factors such as substrate concentration (Staley et al. 1990), pH (De Boer et al. 1996), and allelopathic inhibition (White 1994). Natural or low input managed soil ecosystems are often considered to be substrate-limited and respond rapidly to the addition of nitrogen fertilizer (DeLuca et al. 2006), and indeed several studies have shown that fertilized soils commonly display higher nitrifying activity due to the stimulation of nitrification by N fertilizers (Aarnio and Martikainen 1996; Mendum et al. 1999; Xue et al. 2008). Soil pH is clearly an important factor related to nitrification activity (De Boer et al. 1996; Baggs et al. 2010), and lime application can increase nitrification activity in some acidic soils (Arora et al. 1986). However, many acid soils are also associated with vegetation that produces compounds such as phenolic acids and terpenes that might inhibit microbial activity. Phytochemical-induced inhibition of nitrification has also been proposed in ecosystems such as forests where trees or shrubs produce C substrates that may be toxic to nitrifiers and/or reduce the available N-substrate when carbon-rich terpenes are mineralized by the microbial community and N is immobilized (White 1994). As charcoal or activated carbon has the capacity to deactivate phytotoxic compounds, via adsorption, charcoal additions may stimulate net nitrification, and this has been shown in some forest soils (Berglund et al. 2004; Deluca et al. 2002). However, it is still unclear which, if any, factor is the key controlling factor and what is the relative importance of these factors for nitrification in such acidic soils.
Consequently, we selected four contrasting highly acidic soils with similar pH (in the range 3.5–4.4) which also had vegetation characterized by potentially allelopathic compounds such as phenolics and/or terpenes, but were from different environments (China and Scotland). The soils had different land uses and organic matter and nitrate content, and so we sought to further explore the relative importance of the effects of lime, charcoal, and urea application on soil nitrification and whether they were overriding effects on soils from such very different environments. The soils were also selected to cover a wide range of nitrification rates. Nitrification was measured, and since it is associated with mineralization of soil organic matter and microbial carbon source utilization (Wheatley et al. 1991), the effects of pH (lime), charcoal, and urea application on substrate utilization patterns (including phytochemicals implicated in allelopathy) were determined using the MicroResp system (Campbell et al. 2003; Chapman et al. 2007). The MicroResp system using a 96-well format made it feasible to test several soils for several selected factors simultaneously and was also suitable for testing volatile C compounds (Chapman et al. 2007; Kaufmann et al. 2006). The objectives of this study were to determine the relative effects of these factors for nitrification in these acidic soils and to understand the mechanisms that the amendments might have on the soil microbial functional diversity and nitrifying activity. We hypothesized that in soils with inhibitory or allelopathic compounds, pH and substrate concentration would still be the first and second most important factors and that charcoal addition would also stimulate nitrification and C source utilization.
Materials and methods
Soils
Soil samples for the study were collected from three sites. The first study site was located in West Lake district of Hangzhou, Zhejiang Province in Southeast China. The soils were Ultisols with kaolinite, chlorite, Fe, and Al oxides as the dominant clay minerals. The soils were developed on quaternary red earth. Composite samples of a 90-year-old tea orchard and neighboring forest were collected. The tea orchard received two or three applications of urea-N per year, averaging ∼900 kg N ha−1 year−1. The forest was a deciduous–conifer mixed forest with no management or fertilizer input. The main tree species include Lithocarpus (Lithocarpus glaber), Cunninghamina (Cunninghamina lanceolata), and Chinese red pine (Pinus massoniana Lamb). Soils were also collected from two sites located in Scotland and consisted of the O horizon from a peaty podzol (Podzols) under native Scots pine woodland (Pinus sylvestris L.) at Ballochbuie forest and mineral, sandy loam soil (Alluvial soils) from under Rhododendron (Rhododendron simsil Planch) shrubs at the Macaulay Land Use Research Institute. All the soils were taken from the surface layer (0–10 cm) using augers to get composite samples from several locations and pooled into a single representative sample. Field moist soils were sieved <2 mm and visible pieces of plant material and soil animals were removed before use. The vegetation, location, mean annual temperature, and some physicochemical properties of the soils are shown in Table 1.
Soil chemical analysis
Soil pH was measured by a combination glass electrode (soil/water, 1:2.5). Total N was determined by Kjeldahl digestion (Keeney and Nelson 1982) and quantified using a continuous flow analyzer (Skalar, the Netherlands), and the total organic C was determined by dichromate oxidation (Nelson and Sommers 1982). Inorganic N (NH +4 –N and NO −3 –N) was extracted with 2 M KCl and KCl-extracted N was determined colorimetrically in a SPECTRAmax 190-microplate spectrophotometer (Molecular Device Corporation, California, USA; Shand et al. 2008).
Net N mineralization and nitrification
Net soil N mineralization and nitrification were determined with a 31-day incubation study. The soils were amended with one of the following treatments: (1) 0 or 200 mg kg−1 urea-N; (2) 0 or lime as appropriate to raise the soil pH to a target pH of 6.3–6.8 corresponding to 6, 10, 20, and 50 g CaCO3 kg−1 dry soil, respectively, for the forest, tea orchard, Rhododendron, and pine soils; and (3) 0 or 2,000 kg ha−1 charcoal (Alder wood lump, pH 8.9, Sigma Aldrich, UK). The charcoal was ground to pass a 100-μm sieve before use. The combination of treatments resulted in a 2 × 2 × 2 factorial experiment of six replicates for each soil. About 350 mg soil (100 mg soil for the Scottish pine) was placed in 96-deep well microplates and the microplates covered by parafilm. The plates were incubated under conditions of high humidity, and regular weighing showed no change in weight and no water was added. All the soil samples were adjusted to the moisture content of 50% water holding capacity and incubated at room temperature (20 ± 1°C). Soil pH and inorganic N were measured after 4, 10, 17, 23, and 31 days by sampling additional replicate plates for each harvest. Nitrogen mineralization was calculated by the difference in soil inorganic N at the end and beginning of the incubation.
Soil substrate utilization pattern
The MicroResp system (Campbell et al. 2003) was used to measure substrate-induced respiration and basal respiration of a variety of C sources including potentially inhibitory phytochemicals (phenolic acids and monoterpenes). Briefly, the system involves the use of a carbon dioxide detection microplate attached to a 1.2-ml deep well plate which contains the soil and a selection of C sources. The two plates are connected with a rubber gasket and a seal formed when clamped together. The soils were pre-incubated at room temperature (about 25°C) for 2 weeks in a high-humidity atmosphere and then placed in the deep well plate with a MicroResp filling device. All measurements were done in triplicate. Two soils were tested per plate, and treatments and their six replicates were randomized across the plates. Basal respiration was measured in wells with water only added (25 μl). Substrate-induced respiration was measured by adding one of 15 different C sources, which were applied in 25 μl aliquots to achieve a final concentration of 30 mg g−1 soil water except for alpha pinene and alpha 3-carene, which were applied in 1 μl solution with the addition of a further 24 μl water. The other 13 C sources used were protocatechuic acid, caffeic acid, glucosamine, trehalose, arabinose, d-glucose, d-galactose, l-alanine, oxalic acid, l-malic acid, arginine, l-cysteine HCl, and citric acid (all Sigma Aldrich).
Statistics
Means and least significant differences at the 5% level were calculated by a three-way (lime × urea × charcoal) ANOVA using SPSS version 10.0 for Windows (SPSS Inc., Chicago, USA). The MicroResp C source profiles were analyzed by canonical variate analysis after first reducing the dimensionality by principal component analysis using Genstat 5.3 (NAG Ltd., Oxford, UK).
Results
The control soils with no amendments did not change in pH over time (Table 2). Target pH values where lime was added were achieved and maintained until at least day 4 in all treatments. Although the amount of lime added was sufficient to reach the target pH (6.3–6.8) in tea bush soils, pH in these soils significantly declined by day 31 to 5.7–6.0 (Table 2), but this was still significantly greater than the starting pH. Urea increased pH in all soils at day 4, but later decreased the pH after 31 days to less than the control soil. Amendment of soils with charcoal only also slightly increased soil pH (Table 2).
The only addition of ground charcoal to any soil had no significant effect on net nitrification (Table 3). However, there was a significant synergistic increase in nitrification with charcoal, urea, and lime in the Chinese tea orchard soil compared to lime and urea together (Table 3). Urea without lime added greatly increased nitrification in both the Chinese soils, but there was no effect of urea addition on nitrification in the Scottish pine or rhododendron soils. The rhododendron soil with the application of both urea and lime showed a significantly higher nitrification rate compared with that of the addition of lime alone (Table 3). The addition of lime significantly increased nitrification in all the soils except for the forest soil. Interestingly, lime application greatly decreased average net nitrification in the forest soil, even with the application of both lime and urea treatment which had a very high NH +4 –N concentration (>230 mg kg−1). In the last week of incubation, nitrification in the forest soil with lime treatment was similar to the control, but still much lower than urea application alone.
Almost all the urea applied to the pine, rhododendron, and tea orchard soils was transformed into NH +4 –N within the first 4 days. But urea applied to the forest soil was not entirely transformed into NH +4 –N within the first 4 days since soil NH +4 –N concentration still increased quickly after a 4-day incubation and lime application continued to increase urea hydrolysis in this soil (data not shown). Based on a 31-day laboratory incubation, the addition of charcoal to soil had no significant effect on inorganic N content, but the addition of lime resulted in a slight yet significant increase in soil inorganic N and net mineralization for all soils except for the pine soil (Table 4).
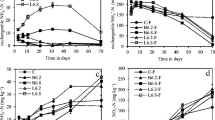
The average basal respiration (water only added) measured using MicroResp ranged from 0.17 to 3.94 μg CO2–C g−1 h−1, with the large differences between the different soils consistent with their organic matter content. Amendment of the pine soil with charcoal significantly increased soil basal respiration (Fig. 1). Lime application significantly increased basal respiration in the two Chinese soils, but decreased it in the two Scottish soils. Urea without lime added significantly decreased basal respiration in the mixed forest and pine soils, but with no significant effect in the other soils.
Substrate-induced respiration (SIR) rates for all C sources were above the basal respiration, with the exception of arginine for the forest soil in which lime had been added. In fact, lime application significantly decreased arginine SIR in all soils except the tea orchard soil. The highest SIR respiration was found with malic acid, and the SIR was four to eight times higher than basal respiration. Urea without lime added significantly decreased alanine SIR in all the tested soils (Fig. 1). Amendment of the two Scottish soils with charcoal alone significantly increased the SIR for alpha pinene, which is one of the main phytochemical compounds in pine soil (Bremner and McCarty 1988).
Due to the huge difference in SIR between different soils, the canonical analysis of the MicroResp profiles was carried out separately using each soil with different treatments (Fig. 2). The results showed that lime application altered substrate utilization pattern more than urea or charcoal in these highly acidic soils where all the lime treatments were distinct from the no lime treatments on canonical variate 1. Charcoal showed the smallest effect on substrate utilization pattern, and nearly all the charcoal treatments and controls were clustered together. There was some separation of the control and urea treatment in the pine, rhododendron, and tea orchard soils, but there was no significant separation between the forest control and the urea treatment.
Ordination plot of the first two canonical axes produced by canonical analysis of principal coordinates of MicroResp C source utilization profiles. Control (empty square); Charcoal (empty diamond); Urea (empty circle); Urea + Charcoal (empty triangle); Lime (filled square); Lime + Charcoal (filled diamond); Lime + Urea (filled circle); Lime + Urea + Charcoal (filled triangle). Each value is the mean ± SE of three replicates
Discussion
Charcoal adsorption of organic compounds and the aggregation of nitrifiers around charcoal particles are possible mechanisms by which charcoal influences soil net nitrification (Berglund et al. 2004). Some studies have found that charcoal can promote soil nitrification in the presence of a N substrate (Berglund et al. 2004; Deluca et al. 2002, 2006). However, a small synergy with charcoal to nitrification was found in only the Chinese tea orchard soil in our study. The addition of ground charcoal had no significant effect on net nitrification in any of the four acidic soils, even if the soils with the application of both lime and urea had very high NH +4 –N concentrations. A possible reason for these differences between our and other studies may be due to the different land uses investigated. MacKenzie and DeLuca (2006) also found that the effect of charcoal was dependent on plant type, and their studies revealed that the addition of charcoal and glycine leads to a significant increase in net nitrification in shrub (Arctostaphylos uva-ursi) litter microcosms, but not sedge (Carex geyeri) litter microcosms. They also found that charcoal had no effect on nitrification in some soils that had naturally high rates of nitrifier activity. Since plant species have a large effect on microbial community structure, the quantity, species, and activity of soil nitrifiers are likely to be different in different land uses. Our results suggested that the quantity and activity in some acidic soil systems may not change after charcoal application. Of course, charcoal type and the particle size (active surface area) may be other factors which affect the adsorption of organic compounds and soil nitrification. The charcoal in our study was added at a concentration of 2000 kg ha−1, which was chosen because some studies showed that activated carbon at the rate of 1,000–10,000 kg ha−1 or 1% (w/w) field-collected charcoal can increase soil net nitrification (Berglund et al. 2004; Deluca et al. 2002, 2006). It was also ground to <0.1 mm and was therefore similar to other studies (MacKenzie and DeLuca 2006). In our study, charcoal was not specifically activated and was added in a lower amount (2,000 kg/ha), so this cannot be ruled out as a reason for the difference between other studies using higher amounts.
Although charcoal had no significant effect on nitrification in this study at the concentrations used, it did affect soil microbial activity at this concentration. Amendment of the pine soil with charcoal significantly increased soil basal respiration, and alpha-pinene SIR also increased in the two Scottish soils with charcoal treatment. It is probable that charcoal is reducing the inhibitory effects of allelopathic compounds in these soils on the microbial populations responsible for their degradation, but not for the nitrifier populations. This is interesting because terpenes have been hypothesized to be inhibitory to nitrifiers (White 1994), but although charcoal stimulated respiration in these soils, nitrification was not increased, suggesting that such inhibitory compounds are not as important as pH or ammonium concentration. Multivariate analysis of our MicroResp data also confirmed that charcoal can significantly alter substrate utilization patterns in the pine, rhododendron, and tea orchard soils, although the effect is relatively small compared to lime and urea application.
Some studies showed a strong correlation between the NH +4 –N concentration and NO −3 –N conversion, and nitrification is thought to be substrate-limited (Currie 1999; De Boer and Kowalchuk 2001), but some other studies showed that the addition of substrate alone to soils had absolutely no effect on nitrification (Berglund et al. 2004; DeLuca et al. 2006). In this study, we found that urea application greatly increased nitrification in the two Chinese soils, but had no effect in the Scottish pine and rhododendron soils. The two Chinese acidic soils had relatively high nitrification; in contrast, the two Scottish acidic soils had very low nitrification, although almost all the applied urea was transformed by urea hydrolysis. This suggested that N substrate can promote nitrification only in the soils which already have activated nitrifiers to some extent. This was further emphasized by the observation that the rhododendron soil with the application of both urea and lime showed a significantly higher nitrification compared to that with the addition of lime alone.
Soil pH is an important factor controlling nitrification activity (De Boer et al. 1996; Sauvé et al. 1999; SteMarie and Pare 1999). Many studies showed that liming and other pH-raising treatments can promote nitrification and induce nitrate accumulation in some acidic soils (De Boer and Kowalchuk 2001). The lime application significantly increased soil net nitrification in all the acidic soils except for the forest soil. The result confirmed the general concept that the nitrification activity is higher in neutral or slightly alkaline conditions. However, lime application greatly decreased net nitrification in the forest soil. The result is very surprising, and it may be due to the lag effect of lime in raising the pH. By examining the soil net nitrification separately each week, we saw that the nitrifying activity did show some increase at the end of the incubation, but it was still much lower than that in the treatment of urea alone. The results may suggest that nitrification in the forest soil is mainly driven by some acid-tolerant nitrifiers which have higher activity in the low pH condition than in the high pH condition. Measurements of net nitrification rates in soils have usually been performed by extended sample incubation (2–8 weeks), either in the field or in the lab (Ross et al. 2006). Our incubation experiment was 31 days, and this is a limitation to test the long-term effect of lime application in the forest soil. However, urea treatments without lime showed high nitrification rates in the tea and forest soils, which suggests that acid-tolerant nitrifiers do exist in highly acidic soils and have high activity.
The ability to utilize a range of C sources is fundamental to all the ecological functions and may reflect the activity of organic matter mineralization and microbial functional diversity. Arginine mineralization appears to be a fast and rapid method for estimating soil microbial biomass, and arginine is an amino acid often found in soil. Arginine mineralization has been proven to be a useful discriminator of treatment differences in previous studies (Campbell et al. 2008). In our study, the arginine SIR was low or even lower than basal respiration in the forest soil, and lime application significantly decreased arginine SIR in all soils except for the tea orchard. The result suggested that neutral condition can decrease the activity of arginine utilization. Campbell et al. (2008) also found very low arginine SIR in some frequently burned forest soils which were close or equal to basal respiration. They tested for ammonia production and ruled this out and suggested that the effect was due to either small but significant changes in soil pH and/or the effects of residual charcoal arising from burning. Our results suggest that pH exerts a large control on arginine utilization. Since the adsorption of amino acids by soil is sensitive to soil pH, their availability as substrates to microbes is dependent on soil pH change, and differences observed in sole C source tests might reflect differences in availability rather than differences in the total amounts (Yao et al. 2000).
All three amendments—lime, urea, and charcoal—can alter the substrate utilization pattern. It is not surprising that lime had the biggest effect on microbial functional diversity since a key factor in determining soil microbial community structure is soil pH. Many studies have observed a significant change in the microbial community diversity as it responds to pH change (Pennanen et al. 1998; Bååth and Anderson 2003; Kennedy et al. 2005; Rousk et al. 2010). Consequently, the change in microbial community structure after lime application can affect soil organic matter mineralization and increase the activity of nitrifiers, except perhaps some acid-tolerant nitrifiers in these highly acidic soils. Charcoal was shown to have no major effect on nitrification in this study, suggesting in acid soils with potentially inhibitory compounds such as phenolics and terpenes that these are not major controls on nitrification.
In conclusion, soil pH had the greater effect on nitrification and substrate utilization more than urea or charcoal in the highly acidic soils, but acid-tolerant nitrifers do exist in these soils and have high activity. Nitrification was not controlled by any single factor in these soils, and the low pH and potentially high allelopathic compounds did not account for all differences between the soils from different environments. There was an interaction between abiotic and nitrifier community composition as a result of land use and soil type interactions. Further studies should pay attention to the changes in abundance and diversity of nitrifier population in these soils.
References
Aarnio T, Martikainen PJ (1996) Mineralization of carbon and nitrogen, and nitrification in Scots pine forest soil treated with fast- and slow-release nitrogen fertilizers. Biol Fertil Soils 22:214–220
Arora Y, Mulongoy K, Juo ASR (1986) Nitrification and mineralization potentials in a limed ultisol in the humid tropics. Plant Soil 92:153–157
Bååth E, Anderson TH (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol Biochem 35:955–963
Baggs EM, Smales CL, Bateman EJ (2010) Changing pH shifts the microbial source as well as the magnitude of N2O emission from soil. Biol Fertil Soils 46:793–806
Berglund L, DeLuca TH, Zackrisson O (2004) Activated carbon amendments to soil alters nitrification rates in Scots pine forests. Soil Biol Biochem 36:2067–2073
Bremner JM, McCarty GW (1988) Effects of terpenoids on nitrification in soil. Soil Sci Soc Am J 52:1630–1633
Campbell CD, Chapman SJ, Cameron CM, Davidson MS, Potts JM (2003) A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl Environ Microbiol 69:3593–3599
Campbell CD, Cameron CM, Bastias BA, Chen C, Cairney JWG (2008) Long term repeated burning in a wet sclerophyll forest reduces fungal and bacterial biomass and responses to carbon substrates. Soil Biol Biochem 40:2246–2252
Chapman SJ, Campbell CD, Artz RRE (2007) Assessing CLPPs using MicroResp (TM)—a comparison with Biolog and multi-SIR. J Soils Sediments 7:406–410
Currie WS (1999) The responsive C and N biogeochemistry of the temperate forest floor. Trends Ecol Evol 14:316–320
De Boer W, Kowalchuk GA (2001) Nitrification in acid soils: micro-organisms and mechanisms. Soil Biol Biochem 33:853–866
De Boer W, Klein Gunnewiek PJA, Parkinson D (1996) Variability of N mineralization and nitrification in a simple, simulated microbial forest soil community. Soil Biol Biochem 28:203–211
DeLuca TH, Nilsson MC, Zackrisson O (2002) Nitrogen mineralization and phenol accumulation along a fire chronosequence in northern Sweden. Oecologia 133:206–214
DeLuca TH, MacKenzie MD, Gundale MJ, Holben WE (2006) Wildfire-produced charcoal directly influences nitrogen cycling in ponderosa pine forests. Soil Sci Soc Am J 70:448–453
Kaufmann K, Chapman SJ, Campbell CD, Harms H, Hohener P (2006) Miniaturized test system for soil respiration induced by volatile pollutants. Environ Pollut 140:269–278
Keeney DR, Nelson DW (1982) Nitrogen-inorganic forms. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. American Society of Agronomy, Madison, pp 643–698
Kennedy N, Connolly J, Clipson N (2005) Impact of lime, nitrogen and plant species on fungal community structure in grassland microcosms. Environ Microbiol 7:780–788
MacKenzie MD, DeLuca TH (2006) Charcoal and shrubs modify soil processes in ponderosa pine forests of western Montana. Plant Soil 287:257–266
Mendum TA, Sockett RE, Hirsch PR (1999) Use of molecular and isotopic techniques to monitor the response of autotrophic ammonia-oxidizing populations of the β subdivision of the class Proteobacteria in arable soils to nitrogen fertilizer. Appl Environ Microbiol 66:4155–4162
Nelson DW, Sommers LE (1982) Total carbon, organic carbon and organic matter. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. American Society of Agronomy, Madison, pp 539–580
Pansombat K, Kanazawa S, Horiguchi T (1997) Microbial ecology in tea soils. 1. Soil properties and microbial populations. Soil Sci Plant Nutr 43:317–327
Pennanen T, Fritze H, Vanhala P, Kiikkilä O, Neuvonen S, Bååth E (1998) Structure of a microbial community in soil after prolonged addition of low levels of simulated acid rain. Appl Environ Microbiol 64:2173–2180
Pennington PI, Ellis RC (1993) Autotrophic and heterotrophic nitrification in acidic forest and native grassland soils. Soil Biol Biochem 25:1399–1408
Ross DS, Fredriksen G, Jamison AE, Wemple BC, Bailey SW, Shanley JB, Lawrence GB (2006) One-day rate measurements for estimating net nitrification potential in humid forest soils. For Ecol Manag 230:91–95
Rousk J, Brookes PC, Bååth E (2010) The microbial PLFA composition as affected by pH in an arable soil. Soil Biol Biochem 42:516–520
Sauvé S, Dumestre A, McBride M, Gillett JW, Berthelin J, Hendershot W (1999) Nitrification potential in field-collected soils contaminated with Pb or Cu. Appl Soil Ecol 12:29–39
Shand CA, Williams BL, Coutts G (2008) Determination of N-species in soil extracts using microplate techniques. Talanta 74:648–654
Staley TE, Caskey WH, Boyer DG (1990) Soil denitrification and nitrification potentials during the growing-season relative to tillage. Soil Sci Soc Am J 54:1602–1608
Stark JM, Firestone MK (1996) Kinetic characteristics of ammonium-oxidizer communities in a California oak woodland-annual grassland. Soil Biol Biochem 28:1307–1317
SteMarie C, Pare D (1999) Soil, pH and N availability effects on net nitrification in the forest floors of a range of boreal forest stands. Soil Biol Biochem 31:1579–1589
Wheatley RE, Griffiths BS, Ritz K (1991) Variations in the rates of nitrification and denitrification during the growth of potatoes (Solanum tuberosum L.) in soil with different carbon inputs and the effect of these inputs on soil nitrogen and plant yield. Biol Fertil Soils 11:57–162
White CS (1994) Monoterpenes: their effects on ecosystem nutrient cycling. J Chem Ecol 20:1381–1406
Xue D, Yao HY, Ge DY, Huang CY (2006) Microbial biomass, N mineralization and nitrification, enzyme activities, and microbial community diversity in tea orchard soils. Plant Soil 288:319–331
Xue D, Yao HY, Ge DY, Huang CY (2008) Soil microbial community structure in diverse land use systems: a comparative study using BIOLOG, DGGE, and PLFA analysis. Pedosphere 18:653–663
Yao H, He Z, Wilson MJw, Campbell CD (2000) Microbial community structure in a sequence of soil with increasing fertility and changing land use. Microb Ecol 40:223–237
Acknowledgments
This work was financially supported by the National Basic Research Program of China (973 Program, no. 2007CB109305), the Royal Society of Edinburgh, and the National Science Foundation of China (nos. 30871600, 30971859, 31071869). Duncan White, Clare Cameron, Charlie Shand, and Renate Wendler are gratefully acknowledged for technical assistance. We also thank Stephen Chapman for helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yao, H., Campbell, C.D. & Qiao, X. Soil pH controls nitrification and carbon substrate utilization more than urea or charcoal in some highly acidic soils. Biol Fertil Soils 47, 515–522 (2011). https://doi.org/10.1007/s00374-011-0554-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-011-0554-4