Abstract
Purpose
Contaminated sediments are an important exposure pathway for the aquatic fauna in the Rhine River. We applied bioassays with the aim to characterize the ecotoxicological hazard potential of sediments of an oxbow lake of the Rhine River, especially to fish. Potential effects on fish and water flea were evaluated indirectly by applying in vitro and in vivo bioassays in the laboratory. Results were compared with those of the official German risk assessment of dredged sediments.
Materials and methods
Sediments taken from 13 sites along a 600-m transect line were tested for acute toxicity to water flea (Daphnia magna immobilization test), teratogenicity, and embryotoxicity (sediment contact test with Danio rerio), as well as for cytotoxicity (neutral red retention assay with RTL-W1 cells) and estrogenic effects (lyticase-assisted yeast estrogen screen (L-YES) assay). The tests were conducted using pore water, organic extracts, or native sediments. Spatial patterns of the measured effects were also assessed.
Results and discussion
Virtually all samples induced estrogenic, teratogenic, embryotoxic, and cytotoxic effects, but no acute toxicity on D. magna was observed. Cytotoxicity was in accordance with previous studies on the Rhine, Neckar, and Danube Rivers. Estrogenic effects were in the range of estradiol equivalent (EEQ) values detected in UK estuaries. Although sediment contact tests with D. rerio embryos showed virtually no mortality, sublethal effects were common. Some of the effects increased with increasing distance to the main channel.
Conclusions
The test with D. magna is, along with bacteria and algae toxicity assays, an important part of the German standard risk assessment for sediments. However, it failed to identify the ecological hazard of our sediment samples to fish. Our results indicate that adverse effects on fish are possible and suggest the need for revising risk assessment procedures in order to address the risk for this important organism group in aquatic ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Contaminated sediments are known to be a major source of heavy metals and persistent organic pollutants to aquatic ecosystems (IKSR 2003). Contaminants are bioavailable to fishes through surface contact, ingestion, and biomagnification (Leppänen 1995; Legler et al. 2002) and can severely affect their populations (Keiter et al. 2006; Braunbeck et al. 2009). Polluted sediments can be remobilized during flood events and dredging activities (Wölz et al. 2009). Under a climate change scenario, more frequent flooding events may intensify these processes in the upcoming decades (Kay et al. 2006; Solomon et al. 2007).
The Rhine River is one of the largest and most anthropogenically influenced rivers in Europe (IKSR 2002, 2003; LUA LR-P 2006). Radical, large-scale constructions including the disconnection of many river branches from the main channel, which started at the beginning of the nineteenth century, have forced it into an artificial, rectified bed. The Rhine River used to be highly polluted with nutrients, pesticides, and wastewaters. During the 1960s–1980s, poor water quality led to massive fish mortality (Hünemörder 2004). Since then, water quality has improved and the biodiversity of the aquatic biota has increased (IKSR 2009b). Today, some of the cutoff meanders form oxbow lakes with characteristics of both flowing and standing water bodies. They form a very special, structurally rich ecosystem and are ideal feeding and spawning habitats for many fish species (Admiraal et al. 1993; Van den Brink et al. 1994). While some species use the backwaters temporarily (Molls 1999), other species spend most of their lives in these oxbow lakes (Brühne and Scharbert 2003). Presently, the populations of most fish species that existed in the Rhine River prior to rectification have recovered, but the structure of the fish community has undergone modifications (IKSR 2007). In contrast to the pre-rectification time, Rhine River oxbow lakes are now dominated by euryoecious species, which tolerate various habitats, while stagnophilic species in particular, preferring standing waters, are remarkably rare (Tittizer and Krebs 1996; UBA 2007).
One of these oxbow lakes is the Karlskopf situated at Rhine kilometer 375.4. The Karlskopf is an artificial quarry pond of 28 ha resulting from continuous dredging activities until 1985. It is characterized by a steep shoreline and a lack of shallow water zones (LUWG et al. 1999). Today, the Karlskopf and the surrounding area are declared a natural protection area and human activities are prohibited. Though protected, information on the toxicity of its sediments and their potential impacts on its ichthyofauna is still fragmented (MUFV R 2006). One reason for this may be that the quality assessment of sediment samples is challenging.
Chemical analysis of priority pollutants, as it is done in the Rhine River monitoring programs (IKSR 2003, 2009a), does not provide sufficient information on sediment contamination and potential toxicity because each sample may contain a complex mixture of compounds whose effects might be additive, synergistic, antagonistic, or chronic (Charles et al. 2002; Brian et al. 2005; Hayes et al. 2006). Additionally, not every compound detected is necessarily bioavailable (Ahlf 1995; Ahlf et al. 2002). Also, metabolites can be more toxic than the initial compound (Huberman et al. 1976; Lund et al. 1988). Therefore, it is logistically impossible to control such a great variety of substances and little is known about their toxic effects. In this regard, ecotoxicological bioassays can provide insight into the potential effects of contaminated sediment and can be used to identify the areas of highest concern, although they will fail to identify the responsible substances and their potential sources (Ahlf et al. 2002).
Contamination of sediments is correlated to the particle size, where the surface area and thus number of binding sites for pollutants increase with decreasing particle size (Kukkonen and Landrum 1996; Duong et al. 2009). Small particles settle slowly and are therefore able to reach parts of the water body that are relatively far away from the main river channel (Asselman and Middelkoop 1995). Previous studies at the quarry pond Karlskopf did not find a spatial gradient of dioxin-like effects from sediments, most likely because sediments are distributed homogeneously during flood events (Heimann et al. 2011). However, different types of pollutants behave differently and vary in their effects. Some of these effects may remain undetected by ecotoxicological bioassays aimed at assessing dioxin-like compounds.
Since official risk assessment protocols typically consist of only one or a few biotests (BfG 2000), it is clear that potentially harmful effects may remain unaccounted for in environmental risk management. In our study, we applied two in vivo and two in vitro bioassays with sediment extracts, pore water, and native sediment with the aim to examine the ecotoxicological hazard potential of the Karlskopf sediments, especially to fish. Potential effects on fish and water flea were evaluated indirectly with laboratory bioassays. Specifically, we assessed Karlskopf sediments for acute toxicity to water flea (Daphnia magna immobilization test, OECD 2004), teratogenicity, embryotoxicity (sediment contact test with Danio rerio, Hollert et al. 2003), cytotoxicity (neutral red retention assay with RTL-W1 cells, Babich and Borenfreund 1992), and estrogenic effects (lyticase-assisted yeast estrogen screen (L-YES) assay, Routledge and Sumpter 1996). Ethanolic and acetonic extracts were used to represent a worst case scenario, while the actual bioavailable fraction of the contaminants was examined in tests using pore water and native sediments. The results were compared with those of the official German risk assessment of dredged sediments in the same area. Furthermore, spatial patters of estrogenic, cytotoxic, embryotoxic, and teratotoxic effects were assessed.
2 Materials and methods
2.1 Sampling
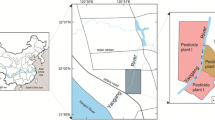
Between April and May 2009, sediment samples were taken from the bottom of the Karlskopf quarry pond (Fig. 1). A total of 10 samples were taken every 60 m along a transect and used to conduct the L-YES, the sediment contact assay with D. rerio embryos, and the neutral red retention assay. Three extra samples from the entrance, middle, and end section of the quarry pond (A, B, and C) were used in the acute pore water toxicity test with D. magna according to the official German risk assessment of dredged sediments. Water depth was kept constant (4.8–6.8 m) across sampling sites (Fig. 1). Each sample was obtained by collecting 3.5 kg from the upper 10 cm of the sediment using a sediment corer. All samples were stored in dark and cool (3–8 °C) conditions and processed within 24 h after sampling.
2.2 Sample processing and bioassays
2.2.1 Sample processing and extraction
For all samples (except A, B, and C), a part of the fresh sample was stored in the dark at 3–8 °C and used in the sediment contact test with D. rerio within 5 days. The remaining sample was freeze-dried (Alpha 1-2 LDplus, Christ, Osterode, Germany) and stored until extraction (in the dark, −24 °C). Pore water from samples A, B, and C was immediately obtained and used for tests with D. magna.
Lyticase-assisted yeast estrogen screen
We extracted freeze-dried sediments according to a modification of Lopez de Alda and Barceló (2001). Briefly, 10 g of freeze-dried sediment was extracted with 200 mL ethanol by 20-min sonication (Ultrasonic bath, unheated 230 V, 50 Hz, 1.75 L, Fisher Scientific, Loughborough, UK) and 1-h horizontal shaking with 150 rpm (2345Q Orbital Shaker, Thermo Fisher Scientific, Waltham, USA), followed by 30-min centrifugation at 3000 rpm (Heraeus Multifuge 4KR, DJB Labcare, Buckinghamshire, UK). The clear supernatant was decanted, rotary evaporated (300 mbar, 35 °C) (Laborota 4011 digital rotary evaporator, Heidolph, Kehlheim, Germany), and concentrated close to dryness with a gentle stream of nitrogen. The extract was then redissolved in 1 mL ethanol to a resulting concentration of 10 g dry weight (dw) sediment equivalents (SEQ)/mL ethanol and stored at −24 °C in the dark. As a process control, 200 mL of ethanol was extracted simultaneously.
Neutral red retention assay (NR)
A total of 40 g of freeze-dried sediment was extracted using acetone (Acetone, p.a.; Sigma-Aldrich) at 8–10 cycles/h for 14 h in a Soxhlet extractor (Schott, Mainz, Germany) according to the procedure given by Rocha et al. (2009). An empty extraction thimble was treated similarly as a process control. The extracts were rotary evaporated and concentrated close to dryness under a gentle stream of nitrogen. The samples were re-dissolved in 2 mL dimethyl sulfoxide (DMSO) to a resulting concentration of 20 g dw SEQ/mL DMSO. Elemental sulfur was removed using activated copper powder following the US EPA guideline 3660B (USEPA 1996). The extracts were stored in the dark at −24 °C until use in the assays.
Immobilization test with D. magna
Pore water from samples A, B, and C was obtained following a standard operation procedure of the German Federal Institute of Hydrology (BfG 2007), with slight modifications. Briefly, 650 g of sediment were centrifuged for 20 min at 8 °C and 4400 rpm (Heraeus Multifuge 4KR, DJB Labcare, Buckinghamshire, UK). Due to unexpectedly high turbidity of the supernatant, decanted pore water was filtered through a 20-μm cellulose filter (Whatman filter paper Grade 41, Sigma-Aldrich, Deisenhofen, Germany) to reduce turbidity and used immediately for the test.
2.2.2 Lyticase-assisted yeast estrogen screen
The estrogenic potency of the sediment samples was assessed using the L-YES, a lyticase-assisted modification of the YES assay established by Routledge and Sumpter (1996). The YES utilizes yeast cells (Saccharomyces cerevisiae) stably transfected with the gene coding for the human estrogen receptor α and incorporating a β-galactosidase reporter system (lac-z). The test was conducted according to Schultis and Metzger (2004) with modifications following Schmitt et al. (2008) and Wagner and Oehlmann (2009). Test concentrations were determined using preliminary range finding tests, which suggested initial test concentrations of 0.05 mg dw SEQ/mL test medium. The final test concentrations were 6.4, 3.2, 1.6, 0.8, 0.4, 0.2, 0.1, and 0.05 mg dw SEQ/mL test medium. The extracted samples and process control were serially diluted with test medium in 96-well microtiter plates. Blank wells (containing only test medium and ethanol) and negative controls (containing medium, ethanol, and yeast cells) were run on every plate. On a separate plate, a 17β-estradiol standard (E2; p.a., Sigma-Aldrich, Deisenhofen, Germany) serving as a positive control was tested in concentrations of 1 nM, 300 pM, 100 pM, 30 pM, 10 pM, 3 pM and 1 pM. The extracts and the E2 standard were tested in eight technical replicates on a 96-well microtiter plate. The test was repeated four times (n = 4 biological replicates). Plates were incubated at 30 °C for 24 h, after which cell numbers were determined photometrically by measuring the absorbance at 595 nm. To each well, 100 μL of lacZ solution containing lacZ buffer, chlorophenol red-β-d-galactopyranoside (CPRG, Roche Diagnostics, Mannheim, Germany), lyticase, and β-mercaptoethanol (Sigma-Aldrich, Deisenhofen, Germany) was added and plates were incubated for 30 min (Titramax 1000 + Incubator 1000, Heidolph, Schwabach, Germany). Then, absorbance was measured photometrically at 540 nm in intervals of 30 min (Infinite M200, Tecan, Crailsheim, Germany). Subsequently, the measurement at which the concentration–response relationship of E2 showed a 50 % effect concentration (EC50) close to 1 × 10−10 M, a bottom value lower than 1 absorbance units (a.u.) and a top value greater than 3 a.u. were used for evaluation (Wagner and Oehlmann 2009). Absorbance was corrected for blank values and cell number.
2.2.3 Sediment contact assay with D. rerio embryos
The test was conducted according to Hollert et al. (2003) and the German Institute for Standardization (Deutsche Institut für Normung, DIN) guideline DIN 38415-6 (DIN 2001). We opted to use native sediments in the test, since freeze-drying sediments can increase their toxicity (Seiler et al. 2008) and acetonic extracts can overestimate toxicity by exposing embryos to substances that are not bioavailable (Burton 1991; Ahlf 1995). The sediment contact assay (SCA) with native sediments is a very realistic scenario, and thus, the results have high ecological relevance (Hallare et al. 2011). Maintenance of zebrafish and egg production was carried out according to Braunbeck et al. (2005). Briefly, zebrafish were kept in 30-L aquaria in groups of 12 males and 8 females. Photoperiod was adjusted to 10 h of darkness and 14 h of light. Water temperature was maintained at 26 ± 1 °C. Fish were fed daily with dry flakes (TetraMin™, Tetra GmbH, Melle, Germany) and Artemia sp. nauplii (Great Salt Lake Artemia Cysts, Sanders, Ogden, USA). For spawning, glass dishes were transferred into the tanks to collect the eggs. Spawning occurred within 0.5–1 h after the onset of illumination.
Teratogenicity and embryo toxicity were evaluated by exposing five D. rerio eggs at the eight-cell stage to 3 g of native sediments covered with artificial water in polystyrene six-well plates (TPP, Zurich, Switzerland). Each sample was tested using five eggs per well and a total of 20 eggs per sample (n = 4 technical replicates). 3,4-Dichloroaniline (DCA, Sigma-Aldrich, Deisenhofen, Germany) was tested as an aqueous positive control in a concentration of 3.7 mg/L, which is equal to 70 % effect concentration (EC70) of DCA (DIN 2001). In total, 20 and 40 eggs were used for the positive and negative controls, respectively (n = 4 or 8 technical replicates). The test plates were sealed with clear polyester adhesive sealing tape (Nunc, Roskilde, Denmark) and incubated for 48 h at 26 °C in the dark. As suggested by Strecker et al. (2011), the samples were agitated on a horizontal shaker to avoid development retardation due to hypoxia. After incubation, embryos were collected from the sediments, rinsed in artificial water, and evaluated for lethal and sublethal effects using an inverse microscope (Eclipse TS100, Nikon, Düsseldorf, Germany). Mortality criteria were (a) coagulation, (b) lack of heartbeat, (c) missing somite development, and (d) failure of tail detachment from the yolk sack. According to the test guideline, the test was considered valid if the negative and positive controls showed an effect <10 % and ≥10 %, respectively. Mortalities >10 % were considered indicative of an effect. Sublethal effects, such as lack of blood circulation and lack of pigmentation, were also recorded (Nagel 2002). Criteria are listed in Table 1. The test was repeated independently four times (n = 4 biological replicates).
2.2.4 Neutral red retention assay
The neutral red retention assay was conducted according to Babich and Borenfreund (1992) with modifications described by Klee et al. (2004) and Heger et al. (2012). Briefly, RTL-W1 cells were cultured in 75 cm2 plastic culture flasks (Cellstar, Greiner Bio-one, Frickenhausen, Germany) in Leibowitz’s L15 medium supplemented with 9 % fetal bovine serum (Biochrom, Berlin, Germany) and 1 % penicillin/streptomycin solution (10,000 U/mL in 0.9 % NaCl, Sigma-Aldrich, Deisenhofen, Germany) at 20 °C. In a 96-well microtiter plate (TPP, Zurich, Switzerland), RTL-W1 cells were exposed to the extracts in seven serial dilutions (1:2) ranging from 200 to 3.13 mg dw SEQ/mL medium; 3,5-dichlorophenol at a concentration of 40 mg/L served as a positive control. Cells were exposed for 48 h at 20 °C and subsequently incubated with neutral red (2-methyl-3-amino-7-dimethylamino-phenazine, concentration 0.005 %) for 3 h. The retention of neutral red by viable cells was determined photometrically by measuring the absorption at 540 nm corrected for the absorption at 690 nm reference wavelength (Infinite M200, Tecan, Crailsheim, Germany). Negative and positive controls (n = 12 and n = 6 technical replicates, respectively) were run on each test plate. Four independent assay repetitions were conducted for each sample and three for the process control (n = 4 and n = 3 biological replicates, respectively).
2.2.5 Immobilization test with D. magna
A 48-h toxicity assay was conducted following OECD guideline 202 (OECD 2004). Daphnid neonates aged less than 24 h were obtained from a laboratory culture of D. magna of the UBA V strain at the University of Landau, Germany. Neonates were exposed to pure pore water and serial dilutions (1:2, 1:4, 1:8, and 1:16) in M4-Elendt medium. Exposures were completed in a static non-renewal manner without aeration and food provision during the exposure period. Four replicates of each of the test concentrations and a negative control of pure M4-Elendt medium were tested in 50-mL beakers containing five individuals each. Since pore water needs to be used freshly, this series of experiments was performed on the same day. Test beakers were kept at 20 °C on a cycle of 16 h light/8 h dark. The ecotoxicological endpoint was immobility after 24 and 48 h. Following completion of the 48-h acute toxicity exposure recommended in the guideline, experimental exposures were extended to 96 h with the number of immobile individuals being recorded every 24 h to assess potential chronic toxicity.
2.3 Data analysis
2.3.1 Lyticase-assisted yeast estrogen screen
We calculated estradiol equivalent (EEQ) to compare the sediments’ estrogenic effects among sites and with other European rivers. The EEQ quantifies the amount of estrogenic compounds, expressed as estradiol equivalents per g sample sediment. The EEQs were calculated using:
where E2-EC20 is the concentration of E2 reaching 20 % of its maximum induction and Extract-EC20 is the concentration of sediment extract that causes an induction equivalent to 20 % of the maximum E2 induction. The EEQs were calculated by inserting the corrected optical densities in a second-order polynomial function fitted with the curve parameters from the E2 standard. Excel 2003 (Microsoft, Redmond, USA) and GraphPad Prism software (GraphPad Prism 5 Software Inc., La Jolla, CA, USA) were used for the calculations. The limit of detection (LOD) and the limit of quantification (LOQ) were calculated as the reporter gene activity caused by the solvent control plus three (10) times the standard deviation, respectively.
2.3.2 Sediment contact test with D. rerio embryos
Our dataset did not meet the statistical assumptions for parametric tests. Therefore, a Kruskal–Wallis test (α = 0.05) followed by a Dunn’s test (P < 0.0001) was used to detect significant differences between observed lethal and sublethal effects of each sample and the negative and positive controls across all sampling sites.
2.3.3 Neutral red retention assay and immobilization test with D. magna
The EC50 and lowest observed effect concentration (LOEC) values were interpolated using the four-parameter logistic hill equation fitted to the concentration–response curves using the least squares method where possible (GraphPad Prism). The LOECs were determined using an ANOVA (α = 0.001) followed by a Dunnett’s test. In the neutral red assay, the EC50 of sediment toxicity is expressed as the concentration of 50 % neutral red retention by viable cells (NR50).
To provide a toxicity value that was independent from the methodology employed, we calculated toxic potency (pT) according to de Zwart and Slooff (1993) with modifications by Krebs (1999). The pT value indicates the number of times a sample must be diluted at a ratio of 1:2 with a standardized medium before adverse effects on the test organisms can no longer be measured. The first nontoxic dilution stage was derived by choosing the dilution stage below the LOEC value (i.e., NOEC). When no effect was observed at any of the concentrations assayed, the highest concentration was used as the first nontoxic dilution stage. The pT values were calculated for both the neutral red retention and D. magna immobilization assays and were compared with the official German risk assessment method for dredged sediments (BfG 2007).
Unless specified differently, a significance level of α = 0.05 was used for all statistical analyses. The L-YES, SCA, and neutral red retention assay were examined for correlation between the test results using Spearman’s correlation analysis (GraphPad Prism). We also performed correlation analysis to examine the relationship between the effect size of each sample and the distance to the main river.
3 Results
3.1 Lyticase-assisted yeast estrogen screen
All samples with the exception of sample 8 (480 m from the main river channel) showed a significantly higher estrogenic activity than the negative controls. The EEQs of the samples ranged from 1.03 to 5.14 ng E2 equivalents/g dw SEQ (samples 4 and 6, respectively; Table 2). Overall, no significant geographical trend in the estrogenic activity was detected with an increase in distance to the main river channel (r (Spearman) = 0.29, P > 0.05). The detection limit was 0.001 nM E2, and the limit of quantification was 0.002 nM E2.
3.2 Sediment contact test with D. rerio embryos
The controls met the validity criteria as established in the guideline. Only samples from sites 6, 9, and 10 exhibited a mean mortality value above the DIN guideline effect level of 10 % (Fig. 2). On the contrary, sublethal effects, such as the absence of blood circuit and pigmentation, occurred more commonly in the samples. The lack of blood circulation was especially pronounced in the samples 6–10 and could also be observed in the positive controls. To evaluate the effect of missing pigmentation, we only considered completely unpigmented (neither eyes, nor body) embryos (Fig. 3). None of the controls showed an incomplete pigmentation above the 10 % threshold. In contrast, virtually all samples caused this effect, again especially common in samples 6–10. A positive correlation was found between the strength of the effects and the distance from the main river channel (Fig. 2) (r (Spearman) = 0.67 and 0.77 for lack of blood circuit and lack of pigmentation, respectively, P < 0.05).
Results from the sediment contact test with Danio rerio embryos (n = 4). Values expressed as mean ± SEM, regression line with 95 % CI. a No pigmentation, (r (Spearman) = 0.77, P < 0.05), b no blood circuit (r (Spearman) = 0.67, P < 0.05), and c mortality. Mortality was mainly below the 10 % threshold established in the guideline. Sublethal effects increase with distance to the main river channel
3.3 Neutral red retention cytotoxicity assay
Concentration-dependent cytotoxic effects (membrane damage measured as decreased neutral red retention) on RTL-W1 cells were detected for all extracts. The NR50 values ranged from 33.3 to 53.5 mg dw SEQ/mL medium (sample 10 and sample 1, respectively). The LOECs ranged from 12.5 to 50 mg dw SEQ/mL medium in samples 10, 8, and 6, respectively. A median NR50 value of 45.28 mg dw SEQ/mL medium was calculated from all samples (Fig. 4). We found a significant increase in toxicity with increasing distance from the main river (Fig. 5; r (Spearman) = −0.64, P < 0.05). The pT values calculated from the cytotoxicity assay were as high as 3 and 4 (moderately and distinctively toxic), except for sample 2, which was less toxic (Table 3). There was no correlation between the effect values from the L-YES, SCA, and neutral red retention cytotoxicity test (r (Spearman) = 0.40; P > 0.05).
Comparison of the NR50 values from the neutral red retention assays with RTL-W1 cells. Data for sediment extracts from Karlskopf (n = 10) in comparison to sediment extracts from the Danube (n = 4, Keiter et al. 2006) and Rhine River (surface and core, n = 9, Kosmehl et al. 2007), infrequently (IS, n = 7) and frequently (FS, n = 7) inundated floodplains, and suspended particulate matter (SPM, n = 6) (Schulze et al. 2015). Data are represented as box–whisker plots including the 25 and 75 % percentiles (box) as well as the minimum and maximum values (whiskers). The central solid line represents the median
Cytotoxicity as measured in the neutral red retention assay (n = 4): values are expressed as mean 50 % neutral red retention ± SEM, regression line with 95 % confidence intervals. Correlation analysis showed an increasing toxicity with increasing distance to the main river (r (Spearman) = −0.64, P < 0.05)
3.4 Immobilization test with D. magna
No D. magna neonate toxicity was observed for any of the Karlskopf pore water exposures after 48 h. Due to the absence of any significant toxicity, a concentration–response curve could not be generated and the EC50 could not be calculated. Moreover, after the extended exposure time of 96 h, daphnids of all test groups remained mobile (Fig. 6). With the results from the acute 48-h test, the Karlskopf sediment material can be assigned a pT value of 0 (no toxicity observed) (Table 3).
4 Discussion
While sediments can act as a source of contamination (Heise and Förstner 2006) and may also affect fish in the Rhine River, the official German risk assessment of sediments (BfG 2000), based on toxicity tests conducted on D. magna, as well as bacteria and algae ecotoxicity assays, might be underestimating this risk. The D. magna test resulted in no risk (pT = 0), but three alternative biotests showed a hazardous potential of the sediment samples to fish. A battery of bioassays evaluating various contaminants and effects, as performed in the present study, was able to detect ecotoxicological effectiveness of a wider variety of contaminants for a potentially wider range of organisms, especially fish.
The Federal Institute for Hydrology (BfG) performs three biotests with pore water and eluates in their official, standard assessment of dredged material in German Federal Waterways (HABA-WSV, BfG 2000): (1) algae growth test with Desmodesmus subspicatus (DIN 38412 part 33); (2) immobilization test with D. magna (DIN 38412 part 30); and (3) bacteria test with Vibrio fischeri (DIN EN ISO 11348-3). Based on these tests, the BfG assigned surface sediments from lakes, harbors, and hydrological barrages on the Upper Rhine River pT values from 0 to 2. These values allow unrestrained relocation of sediments during dredging activities (BfG 2004, 2005). So far, sediments with pT values greater than 2 were only found in deeper (i.e., older) sediment layers of the Upper Rhine (BfG 2004). These pT values are considered critical and imply restrictions for the relocation of dredged sediments (BfG 2000). In accordance with these results, the present study did not find any significant toxicity of the Karlskopf sediment pore waters to D. magna neonates. Neither in the 48-h acute toxicity test nor in the prolonged test could we observe immobilization of daphnids in the treatment groups. However, the mandatory test gives only information on daphnids. The three other biotests in the present study indicated effects on ichthyofauna (SCA), cell viability (NR), and the endocrine system (L-YES). The lack of correlation between the effects of these tests suggests a diversity of effects and contaminants which may not be properly assessed by any single test.
4.1 Different tests may estimate the bioavailability of pollutants differently
The pT values calculated from the neutral red retention assay (performed with sediment extracts) were higher than the ones from the test with D. magna (performed with pore water). There are several possible reasons for this finding. Many pollutants are lipophilic and hence are not found in the water phase but bound to particles (Ahlf et al. 2002). Karlskopf contaminants may be sorbed to the sediment rather than dissolved in the pore water. This suggests that hydrophobic compounds, rather than hydrophilic contaminants, play an important role in sediment toxicity. On the other hand, acetonic and ethanolic extracts as used in the cytotoxicity assay and the L-YES are more exhaustive for the specific organic substances to be assessed with either test system, but may overestimate the hazardous potential of the organic pollutants since not all of them might be bioavailable (Burton 1991; Ahlf 1995). Neither pore water nor aqueous eluates allow the ingestion of particles by the test organism. Therefore, tests with aqueous eluates and pore water often underestimate the toxic potential of the sediments (Harkey et al. 1994). It has been shown by König (2002) and Ulrich (2002) that aqueous eluates of suspended particles from the Upper Rhine gave hardly any cytotoxicity, but, on the contrary, cytotoxic effects were detected in acetonic extracts from the same samples.
4.2 Effects on invertebrates vs. fish
Contaminants can have a different impact on different taxonomic groups depending on their life/feeding strategies and specific sensitivity. The results from the test with D. rerio embryos and the L-YES suggest potential sublethal and long-term effects of sediments, which were not apparent for D. magna. Besides having a different sensitivity to specific toxicants, different organisms may be exposed via different pathways owing to their biological and ecological properties. Contaminants can be bioavailable through ingestion or direct contact with the sediments by bacteria, aquatic plants, benthic invertebrates, and fish. Biomagnification along aquatic food webs may spread these effects further to other groups. Such risks cannot be addressed by risk assessments using single-species tests. New strategies in sediment risk assessment should therefore include mesocosm studies or field experiments (Solomon and Sibley 2002).
4.3 Possible underestimation of sublethal and cellular effects
Both organism biotests (i.e., sediment contact test with D. rerio embryos and the pore water immobilization test with D. magna neonates) consistently indicated an absence of acute toxic effects to whole organisms. However, our results provided sufficient evidence for sublethal and potentially chronic effects. The absence of blood circuit and the lack of pigmentation are retardations in embryo development. This is not necessarily lethal for the embryo, as it has been observed that an embryo may overcome this retardation within 168 h (König 2002; Ulrich 2002). However, retardations suggest direct detrimental effects and can also imply indirect impacts due to a prolonged embryonic phase in which the embryo is vulnerable to external risks (e.g., predation and wave action). A study by Vincze et al. (2014) with sediments from the Neckar River considered the observed low pigmentation after 72 h as a developmental failure. Altered pigmentation can also be associated with vision impairments (Goldsmith and Harris 2003). It is known that certain chemicals such as bromophenols or heavy metals can cause the observed effects (Kammann et al. 2006; Jezierska et al. 2009), but a conclusion is not possible without chemical analysis of the sediment samples. Effects were also measured on the cellular level. Virtually all samples caused estrogenic effects in the L-YES assay. Even though a human estrogen receptor is used in the test, the results indicate that estrogenic effects on fish are possible. The neutral red retention assay revealed acute cytotoxic effects at the cell level. Metabolic processes may also play a role in cytotoxicity as some toxicants need bioactivation. RTL-W1 cells are able to bioactivate substances through cytochrome P450-dependent monooxygenases (Behrens et al. 2001).
4.4 Comparison with other European water bodies
In comparison with other anthropogenically influenced European rivers, the acute cytotoxicity of the Karlskopf was in the same range as the Danube and Rhine Rivers (Keiter et al. 2006, Kosmehl et al. 2007), while Schulze et al. (2015) detected less cytotoxicity in sediments from floodplains and suspended particulate matter from the Rhine (Fig. 4). Furthermore, Hollert et al. (2000) found similar NR50 values for the Neckar using gonad cells from rainbow trout.
Our EEQ values compare to EEQs from sediments of the Upper Danube River, UK estuaries, and the Rotterdam harbor (0.03–1.3, 0.20–13 and 5.9–10.5 ng EEQ/g SEQ, respectively) (Legler et al. 2002; Thomas et al. 2004; Grund et al. 2011). In a mesocosm study, Janssen et al. (1997) demonstrated that sediments from the Rotterdam harbor caused premature induction of vitellogenin synthesis in female flounders. It is known that Rhine River fish suffer from estrogenic effects, such as altered genital development and induction of vitellogenin synthesis (Allner 2003; Pawlowski et al. 2003). Numerous studies give evidence for the role that estrogenic substances play in the decline of fish populations (Länge et al. 2001; Metcalfe et al. 2001; Kidd et al. 2007).
Chemical analysis of surface sediments of harbors and barrages close to the quarry pond Karlskopf revealed high levels of compounds with estrogenic effects, such as polychlorinated biphenyls (PCBs), hexachlorobenzene (HCB), and benzo[a]pyrene, in an earlier study (IKSR 2009a). Fish from the Rhine River showed bioaccumulation of certain endocrine disruptors, such as PCBs and HCB (IKSR 2002; LUA LR-P 2006). Considering the results from this study, it is possible that the fish fauna in the quarry pond Karlskopf suffers from estrogenic effects caused by contaminated sediments. Heimann et al. (2011) performed a chemical analysis of priority dioxin-like compounds in the sediments of the quarry pond Karlskopf and revealed low to moderate contaminations which could not explain the measured dioxin-like effects in the biotest. Non-priority pollutants possibly play an important role for the toxicity of the sediments (Brack et al. 2007; Heimann et al. 2011).
Several studies examining the embryotoxicity and teratogenicity of the Upper Rhine and its suspended particulate matter, oxbow waters, harbors, or barrages to fish found a higher mortality rate (König 2002; Ulrich 2002; Seiler et al. 2006). However, these studies used higher concentrations and freeze-dried sediments or acetonic extracts in the fish egg assay, while in the present study, we used a more realistic scenario and examined native sediments. Native sediments of a Rhine River oxbow lake did not cause mortality in a study by Höss et al. (2010), while a study by Feiler et al. (2013) found a mortality of 20 % in one of three sampling sites of the Rhine River, but none in the other two sites.
4.5 Spatial toxicity patterns in the pond
The present study showed a cytotoxic effect of acetonic sediment extracts increasing with distance to the main river channel in the Karlskopf quarry pond. The same pattern could be observed in the sediment contact test with D. rerio embryos. A possible explanation could be that the sediments at the back part of the Karlskopf pond are predominantly fine-grained (<60 μm) (Heimann et al. 2011). Smaller particles settle slowly and hence can reach sections farther from the main river channel (Asselman and Middelkoop 1995). Finer particles provide a greater surface area and thus more binding sides for pollutants; additionally, finer or lighter particles are more likely of organic nature, potentially carrying heavier loads of pollutants (Kukkonen and Landrum 1996; Duong et al. 2009). A grain size analysis of the sediment samples from the Karlskopf by Heimann et al. (2011), however, did not find differences among the samples. Unfortunately, >90 % of the sediment material in the samples was finer than the minimum grain size determined (60 μm), and thus, no differences could have been detected even if they existed. For clarification, it would be necessary to investigate the grain size of the Karlskopf sediments to a greater detail for clarification. For estrogenic effects measured in the L-YES and for dioxin-like effects investigated by Heimann et al. (2011), no spatial trend could be observed. With these ambiguous results, there is no clear answer to the question of longitudinal patterns of contamination.
5 Conclusions
Results from the present study show that sediments of the quarry pond Karlskopf could affect fish embryo development and give evidence that the estrogenic, cytotoxic, and teratogenic effects we found are not addressed sufficiently by regulatory risk assessment protocols. A recent European technical report on aquatic monitoring tools indicates the importance of bioassays such as the SCA and the L-YES for the quality assessment of surface waters (Wernersson et al. 2015). In general, it would be important for the protection of the fish fauna of the Rhine River if the regulatory risk assessment incorporated sublethal and chronic effects to fish. Chemical analysis and bioassays with invertebrates may underestimate the risk of sediments for the ichthyofauna.
Bioassays alone cannot answer the questions about sediment effects on the ecosystem, the contamination source, and the origin of the substances that cause toxicity, but they may inform about the potential effects and areas of highest concern. To complete the effect assessment of Karlskopf sediments on fish, it would be useful to examine estrogenic effects directly in fish from this pond. Also, additional bioassays (e.g., prolonged fish embryo toxicity test, genotoxicity tests, yeast-based androgen screen (YAS)) or, further, more sensitive estrogenicity tests (e.g., ER-CALUX) would provide further insights into the toxicological risks associated with these sediments. Effect-directed analysis (Brack et al. 2007; Hecker and Hollert 2009) could help in identifying contaminants of special concern. Over the last few decades, the water quality in the Rhine River has greatly improved, which calls for a shift of the attention to sediments and non-priority pollutants which are not commonly monitored. Impacts on the Rhine River aquatic biota may be especially high during dredging activities and flood events resulting in remobilization of sediments. Climate change scenarios may alter the frequency of flood events and further enhance the risk of sediment remobilization (Wölz et al. 2009). This perceived importance of contaminated sediments to ecotoxicological risk also needs to be reflected in regulatory standards for sediment evaluation.
References
Admiraal W, Van der Velde G, Smit H, Cazemier WG (1993) The rivers Rhine and Meuse in the Netherlands: present state and signs of ecological recovery. Hydrobiologia 265:97–128
Ahlf W (1995) Biotests an Sedimenten. In: Steinberg C, Bernhardt H, Klappner H (eds) Handbuch Angewandte Limnologie Teil Aquatische Ökotoxikologie. ecomed Verlag, Landsberg, Germany, pp 1–43
Ahlf W, Hollert H, Neumann-Hensel H, Ricking M (2002) A guidance for the assessment and evaluation of sediment quality: a German approach based on ecotoxicological and chemical measurements. J Soils Sediments 2:37–42
Allner B (2003) Freilanduntersuchungen zur Geschlechterverteilung einheimischer Fischpopulationen. Gobio GmbH, Frankfurt, Germany
Asselman NEM, Middelkoop H (1995) Floodplain sedimentation: quantities, patterns and processes. Earth Surface Process Landforms 20:481–499
Babich H, Borenfreund E (1992) Neutral red assay for toxicology in vitro. In: Watson RR (ed) In vitro methods of toxicology. CRC - Press, Boca Raton, USA, pp 238–251
Behrens A, Schirmer K, Bols NC, Segner H (2001) Polycyclic aromatic hydrocarbons as inducers of cytochrome P4501A enzyme activity in the rainbow trout liver cell line, RTL-W1, and in primary cultures of rainbow trout hepatocytes. Environ Toxicol Chem 20:632–643
BfG (2000) Handlungsanweisung für den Umgang mit Baggergut im Binnenland (HABAB-WSV). Koblenz, Germany, p 35
BfG (2004) Untersuchung und Beurteilung von Sedimentablagerungen im Lampertheimer Altrhein. Altrhein-km 0,0 bis 2,1; Altrhein-Mündung bei Rhein-km 440,3. Koblenz, Germany
BfG (2005) BfG 1474: Ergebnisse aus dem begleitenden Untersuchungsprogramm für die Umlagerung von Baggergut in die fließende Welle unterhalb der Staustufe Iffezheim/Rhein. Koblenz, Germany
BfG (2007) Standardarbeitsanweisung. SOP 1–04. BfG, Koblenz, Germany, p 5
Brack W, Klamer HJC, Lopez de Alda MJ, Barceló D (2007) Effect-directed analysis of key toxicants in European River Basins. A review. Environ Sci Pollut Res 14:30–38
Braunbeck T, Böttcher M, Hollert H, Kosmehl T, Lammer E, Leist E, Rudolf M, Seitz N (2005) Towards an alternative for the acute fish LC50 test in chemical assessment: the fish embryo toxicity test goes multi-species—an update. ALTEX 22:87–102
Braunbeck T, Brauns A, Keiter S, Hollert H, Schwartz P (2009) Fischpopulationen unter Stress–das Beispiel des Unteren Neckars. Umweltwissenschaften und Schadstoff-Forschung 21:197–211
Brian JV, Harris CA, Scholze M, Backhaus T, Booy P, Lamoree M, Pojana G, Jonkers N, Runnalls T, Bonfà A, Marcomini A, Sumpter JP (2005) Accurate prediction of the response of freshwater fish to a mixture of estrogenic chemicals. Environ Health Perspect 113:721
Brühne M, Scharbert A (2003) Die Erschließung des Bienener Altrheins für die Rheinfischfauna http://www.nz-kleve.de/fileadmin/dokumente/fachartikel/Br%C3%BChne_Scharbert_Dornicker-Schleuse_gesch%C3%BCtzt.pdf Accessed 26 May 2015:1–15
Burton GA (1991) Assessing the toxicity of freshwater sediments. Environ Toxicol Chem 10:1585–1627
Charles GD, Gennings C, Zacharewski TR, Gollapudi BB, Carney EW (2002) An approach for assessing estrogen receptor-mediated interactions in mixtures of three chemicals: a pilot study. Toxicol Sci 68:349
de Zwart D, Slooff W (1993) The pT value as an environmental toxicity indicator. Sci Total Environ Suppl 1993:1203–1210
DIN (2001) Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung: Suborganismische Testverfahren (Gruppe T) Teil 6: Giftigkeit gegenüber Fischen: Bestimmung der nicht akut giftigen Wirkung von Abwasser auf die Entwicklung von Fischeiern über Verdünnungsstufen (T 6). DIN 38415–6. Beuth Verlag, Berlin, Germany
Duong CN, Schlenk D, Chang NI, Kim SD (2009) The effect of particle size on the bioavailability of estrogenic chemicals from sediments. Chemosphere 76:395–401
Feiler U, Höss S, Ahlf W, Gilberg D, Hammers-Wirtz M, Hollert H, Meller M, Neumann-Hensel H, Ottermanns R, Seiler TB, Spira D, Heiniger P (2013) Sediment contact tests as a tool for the assessment of sediment quality in German waters. Environ Toxicol Chem 32:144–155
Goldsmith P, Harris WA (2003) The zebrafish as a tool for understanding the biology of visual disorders. Seminars Cell Develop Biol 14:11–18
Grund S, Higley E, Schönenberger R, Suter MJ, Giesy JP, Braunbeck T, Hecker M, Hollert H (2011) The endocrine disrupting potential of sediments from the Upper Danube River (Germany) as revealed by in vitro bioassays and chemical analysis. Environ Sci Pollut Res 18:446–460
Hallare AV, Seiler T-B, Hollert H (2011) The versatile, changing, and advancing roles of fish in sediment toxicity assessment—a review. J Soils Sediments 11:141–173
Harkey GA, Landrum PF, Klaine SJ (1994) Comparison of whole‐sediment, elutriate and pore‐water exposures for use in assessing sediment‐associated organic contaminants in bioassays. Environ Toxicol Chem 13:1315–1329
Hayes TB, Case P, Chui S, Chung D, Haeffele C, Haston K, Lee M, Mai PV, Marjuoa Y, Parker J, Tsui M (2006) Pesticide mixtures, endocrine disruption, and amphibian declines: are we underestimating the impact? Environ Health Perspect 114:40–50
Hecker M, Hollert H (2009) Effect-directed analysis (EDA) in aquatic ecotoxicology: state of the art and future challenges. Environ Sci Pollut Res 16:607–613
Heger S, Blum K, Agler MT, Maletz S, Schäffer A, Seiler T-B, Angenent LT, Hollert H (2012) Biotests for hazard assessment of biofuel fermentation. Energy Environ Sci 5:9778–9788
Heimann W, Sylvester M, Seiler TB, Hollert H, Schulz R (2011) Sediment toxicity in a connected oxbow lake of the Upper Rhine (Germany): EROD induction in fish cells. J Soils Sediments 11:1279–1291
Heise S, Förstner U (2006) Risks from historical contaminated sediments in the Rhine basin, the interactions between sediments and water. Springer, Heidelberg, pp 261–272
Hollert H, Durr M, Erdinger L, Braunbeck T (2000) Cytotoxicity of settling particulate matter and sediments of the Neckar River (Germany) during a winter flood. Environ Toxicol Chem 19:528–534
Hollert H, Keiter S, König N, Rudolf M, Ulrich M, Braunbeck T (2003) A new sediment contact assay to assess particle-bound pollutants using zebrafish (Danio rerio) embryos. J Soils Sediments 3:197–207
Höss S, Ahlf W, Fahnenstich C, Gilberg D, Hollert H, Melbye K, Meller M, Hammers-Wirtz M, Heininger P, Neumann-Hensel H, Ottermanns R, Ratte HT, Seiler TB, Spira D, Weber J, Feiler U (2010) Variability of sediment-contact tests in freshwater sediments with low-level anthropogenic contamination—determination of toxicity thresholds. Environ Pollut 158:2999–3010
Huberman E, Sachs L, Yang SK, Gelboin V (1976) Identification of mutagenic metabolites of benzo (a) pyrene in mammalian cells. Proc Natl Acad Sci USA 73:607
Hünemörder KF (2004) Die Frühgeschichte der globalen Umweltkrise und die Formierung der deutschen Umweltpolitik (1959–1973) vol 53. Historische Mitteilungen - Beihefte. Franz Steiner Verlag, Stuttgart, Germany
IKSR (2002) Kontamination von Rheinfischen 2000. Berichte der Internationalen Kommision zum Schutz des Rheins (IKSR) 124:76
IKSR (2003) Rhein Bestandsaufnahme der Emissionen prioritärer Stoffe 2000. Berichte der Internationalen Kommision zum Schutz des Rheins (IKSR) 134:77
IKSR (2007) Qualitätskomponente Fische – Monitoring Rheinfischfauna. Berichte der Internationalen Kommision zum Schutz des Rheins (IKSR) 173:89
IKSR (2009a) Geschichte - Bilanz des Aktionsprogramms (1986–2000).1
IKSR (2009b) Sedimentmanagementplan Rhein. Berichte der Internationalen Kommision zum Schutz des Rheins (IKSR) 175:101
Janssen PAH, Lambert JGD, Vethaak A, Goos HJT (1997) Environmental pollution caused elevated concentrations of oestradiol and vitellogenin in the female flounder, Platichthys flesus (L). Aquatic Toxicol 39:195–214
Jezierska B, Lugowska K, Witeska M (2009) The effects of heavy metals on embryonic development of fish (a review). Fish Physiol Biochem 35:625–640
Kammann U, Vobach M, Wosniok W (2006) Toxic effects of brominated indoles and phenols on zebrafish embryos. Arch Environ Contamin Toxicol 51:97–102
Kay AL, Jones RG, Reynard NS (2006) RCM rainfall for UK flood frequency estimation. II. Climate change results. J Hydrol 318:163–172
Keiter S, Rastall A, Kosmehl T, Wurm K, Erdinger L, Braunbeck T, Hollert H (2006) Ecotoxicological assessment of sediment, suspended matter and water samples in the upper Danube River—a pilot study in search for the causes for the decline of fish catches. Environ Sci Pollut Res 13:308–319
Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick RW (2007) Collapse of a fish population after exposure to a synthetic estrogen. Proc Natl Acad Sci U S A 104:8897–8901
Klee N, Gustavsson L, Kosmehl T, Engwall M, Erdinger L, Braunbeck T, Hollert H (2004) Changes in toxicity and genotoxicity of industrial sewage sludge samples containing nitro- and amino-aromatic compounds following treatment in bioreactors with different oxygen regimes. Environ Sci Pollut Res 11:313–320
König N (2002) Der Zebrabärbling (Danio rerio) in Fischeitest und Comet-Assay - Embryotoxikologische Untersuchung von Rheinsedimenten, Diploma thesis. Ruprecht-Karls-Universität Heidelberg, Germany, p 64
Kosmehl T, Krebs F, Manz W, Braunbeck T, Hollert H (2007) Differentiation between bioavailable and total hazard potential of sediment-induced DNA fragmentation as measured by the comet assay with zebrafish embryos. J Soils Sediments 7:377–387
Krebs F (1999) The pT-Method as a Hazard Assessment Scheme for sediments and dredged material. In: Blaise C, Férard J-F (eds) Small-scale freshwater toxicity investigations, vol 2. Springer Netherlands, Dordrecht, Germany, pp 115–137
Kukkonen J, Landrum PF (1996) Distribution of organic carbon and organic xenobiotics among different particle-size fractions in sediments. Chemosphere 32:1063–1076
Länge R, Hutchinson TH, Croudace CP, Siegmund F, Schweinfurth H, Hampe P, Panter GH, Sumpter JP (2001) Effects of the synthetic estrogen 17 alpha-ethinylestradiol on the life-cycle of the fathead minnow (Pimephales promelas). Environ Toxicol Chem 20:1216–1227
Legler J, Dennekamp M, Vethaak AD, Brouwer A, Koeman JH, van der Burg B, Murk AJ (2002) Detection of estrogenic activity in sediment-associated compounds using in vitro reporter gene assays. Sci Total Environ 293:69–83
Leppänen M (1995) The role of feeding behaviour in bioaccumulation of organic chemicals in benthic organisms. Annales Zoologici Fennici 32:247–255
Lopez de Alda MJ, Barceló D (2001) Use of solid-phase extraction in various of its modalities for sample preparation in the determination of estrogens and progestogens in sediment and water. J Chromatography A 938:145–153
LUA LR-P (2006) Fischmonitoring Rheinland · Pfalz. Untersuchungsergebnisse aus den Jahren 1999, 2000, 2001 und 2003. Landesuntersuchungsamt Rheinland-Pfalz, Abteilung Lebensmittelchemie, Koblenz, Germany
Lund BO, Bergman A, Brandt I (1988) Metabolic activation and toxicity of a DDT-metabolite, 3-methylsulphonyl-DDE, in the adrenal zona fasciculata in mice. Chem Biol Interact 65:25–40
LUWG, Otto A, Weibel U (1999) Entwicklung der Rhein-Auengewässer - Teil 2 - Entwicklungsplan. Mainz, Germany
Metcalfe CD, Metcalfe TL, Kirparissis Y, Koenig BG, Khan C, Hughes RJ, Croley TR, March RE, Potter T (2001) Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by in vivo assays with Japanese medaka (Oryzias latipes). Environ Toxicol Chem 20:297–308
Molls F (1999) New insights into the migration and habitat use by bream and white bream in the floodplain of the River Rhine. J Fish Biol 55
MUFV R (2006) VSG 6816–403 „Karlskopf und Leimersheimer Altrhein“. Natura 2000 - Steckbriefe. Ministerium für Umwelt, Forsten und Verbraucherschutz Rheinland Pfalz, Germany
Nagel R (2002) DarT: the embryo test with the Zebrafish Danio rerio a general model in ecotoxicology and toxicology. ALTEX 19:38–48
OECD (2004) OECD guideline for testing of chemicals. Daphnia sp. acute immobilisation test (202). OECD Publishing, Paris. doi: http://dx.doi.org/10.1787/9789264069947-en
Pawlowski S, Ternes T, Bonerz M, Kluczka T, van der Burg B, Nau H, Erdinger L, Braunbeck T (2003) Combined in situ and in vitro assessment of the estrogenic activity of sewage and surface water samples. Toxicol Sci 75:57–65
Rocha PS, Luvizotto GL, Kosmehl T, Boettcher M, Storch V, Braunbeck T, Hollert H (2009) Sediment genotoxicity in the Tietê River (São Paulo, Brazil): in vitro comet assay versus in situ micronucleus assay studies. Ecotox Environ Safe 72:1842–1848
Routledge EJ, Sumpter JP (1996) Estrogenic activity of surfactants and some of their degradation products assessed using a recombinant yeast screen. Environ Toxicol Chem 15:241–248
Schmitt C, Oetken M, Dittberner O, Wagner M, Oehlmann J (2008) Endocrine modulation and toxic effects of two commonly used UV screens on the aquatic invertebrates Potamopyrgus antipodarum and Lumbriculus variegatus. Environ Pollut 152:322–329
Schultis T, Metzger JW (2004) Determination of estrogenic activity by LYES-assay (yeast estrogen screen-assay assisted by enzymatic digestion with lyticase). Chemosphere 57:1649–1655
Schulze T, Ulrich M, Maier D, Maier M, Terytze K, Braunbeck T, Hollert H (2015) Evaluation of the hazard potentials of river suspended particulate matter and floodplain soils in the Rhine basin using chemical analysis and in vitro bioassays. Environ Sci Pollut Res 22:14606–14620
Seiler T-B, Rastall AC, Leist E, Erdinger L, Braunbeck T, Hollert H (2006) Membrane dialysis extraction (MDE): a novel approach for extracting toxicologically relevant hydrophobic organic compounds from soils and sediments for assessment in biotests. J Soils Sediments 6:20–29
Seiler T-B, Schulze T, Hollert H (2008) The risk of altering soil and sediment samples upon extract preparation for analytical and bio-analytical investigations—a review. Analytical Bioanalytical Chem 390:1975–1985
Solomon KR, Sibley P (2002) New concepts in ecological risk assessment: where do we go from here? Marine Pollut Bull 44:279–285
Solomon S, Qin D, Manning M, Chen Z, Marrquis M, Averyt KB, Tignor M, Miller HL (2007) Working group I contribution to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK; New York, NY, USA
Strecker R, Seiler TB, Hollert H, Braunbeck T (2011) Oxygen requirements of zebrafish (Danio rerio) embryos in embryo toxicity tests with environmental samples. Comparative Biochem Physiol Part C: Toxicol Pharmacol 153:318–327
Thomas KV, Balaam J, Hurst M, Nedyalkova Z, Mekenyan O (2004) Potency and characterization of estrogen-receptor agonists in United Kingdom estuarine sediments. Environ Toxicol Chem 23:471–479
Tittizer T, Krebs F (1996) Ökosystemforschung: Der Rhein und seine Auen - eine Bilanz. Springer, Berlin, Heidelberg, Germany
UBA (2007) Ökologische Neuorientierung der Bundeswasserstraßenbewirtschaftung (UBA Texte 40/07). Umweltbundesamt, Dessau, Germany
Ulrich M (2002) Gefährdung von Trinkwasser durch partikulär gebundene Schadstoffe in den Rheinauen: Vergleich nativer und extrahierter Proben in zwei in vitro Biotests Diploma thesis. Ruprecht-Karls-Universität Heidelberg, Germany, p 73
USEPA (1996) Method 3660B—sulfur cleanup. US EPA Methods, US Government Printing Office, Washington, pp 1–6
Van den Brink FWB, Van Katwijk MM, Van der Velde G (1994) Impact of hydrology on phyto- and zooplankton community composition in floodplain lakes along the Lower Rhine and Meuse. J Plankton Res 16:351
Vincze K, Graf K, Scheil V, Köhler H-R, Triebskorn R (2014) Embryotoxic and proteotoxic effects of water and sediment from the Neckar River (Southern Germany) to zebrafish (Danio rerio) embryos. Environ Sci Eur 26:1–13
Wagner M, Oehlmann J (2009) Endocrine disruptors in bottled mineral water: total estrogenic burden and migration from plastic bottles. Environ Sci Pollut Res 16:278–286
Wernersson A-S, Carere M, Maggi C, Tusil P, Soldan P, James A, Sanchez W, Dulio V, Broeg K, Reifferscheid G, Buchinger S, Maas H, Van Der Grinten E, O’Toole S, Ausili A, Manfra L, Marziali L, Polesello S, Lacchetti I, Mancini L, Lilja K, Linderoth M, Lundeberg T, Fjällborg B, Porsbring T, Larsson DJK, Bengtsson-Palme J, Förlin L, Kienle C, Kunz P, Vermeirssen E, Werner I, Robinson CD, Lyons B, Katsiadaki I, Whalley C, den Haan K, Messiaen M, Clayton H, Lettieri T, Negrão Carvalho R, Gawlik BM, Hollert H, Di Paolo C, Brack W, Kammann U, Kase R (2015) The European technical report on aquatic effect-based monitoring tools under the water framework directive. Environ Sci Eur 27:1–11
Wölz J, Cofalla C, Hudjetz S, Roger S, Brinkmann M, Schmidt B, Schäffer A, Kammann U, Lennartz G, Hecker M, Schüttrumpf H, Hollert H (2009) In search for the ecological and toxicological relevance of sediment re-mobilisation and transport during flood events. J Soils Sediments 9:1–5
Acknowledgments
The present study was embedded into a project by the University Koblenz-Landau investigating the anthropogenic impact on fish populations in the Rhine River. We received funding by the Structural and Approval Directorate South (SGD Süd) and Ministry for Environment, Forestry and Consumer Protection Rhineland-Palatinate, Germany. The authors would like to thank Dr. N.C. Bols and Dr. L. Lee (University of Waterloo, Canada) for providing RTL-W1 cells and Dr. J. Sumpter for providing yeast cells. Furthermore, we thank Dr. F. Sylvester for helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Arnold V. Hallare
Rights and permissions
About this article
Cite this article
Schulze-Sylvester, M., Heimann, W., Maletz, S. et al. Are sediments a risk? An ecotoxicological assessment of sediments from a quarry pond of the Upper Rhine River. J Soils Sediments 16, 1069–1080 (2016). https://doi.org/10.1007/s11368-015-1309-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1309-x