Abstract
The purpose of the present study was to assess the hazard potentials of contaminated suspended particulate matter (SPM) sampled during a flood event for floodplain soils using in vitro bioassays and chemical analysis. Sediment-contact tests were performed to evaluate the direct exposure of organisms to native soils and SPM at two different trophic levels. For comparison, acetonic extracts were tested using both contact tests and additionally two cell-based biotests for cytotoxicity and Ah receptor-mediated activity (EROD-Assay). The sediment-contact tests were carried out with the dehydrogenase assay with Arthrobacter globiformis and the fish embryo assay with Danio rerio. The results of this study clearly document that native samples may well be significantly more effective than corresponding extracts in the bacteria contact assay or the fish embryo test. These results question the commonly accepted concept that acetonic extracts are likely to overestimate the toxicity of soil and SPM samples. Likewise, the priority organic compounds analyzed failed to fully explain the toxic potential of the samples. The outcomes of this study revealed the insufficient knowledge regarding the relationship between the different exposure pathways. Finally, there is concern about adverse effects by settling suspended particulate matter and remobilized sediments in frequently inundated floodplain soils due to an increase of the hazard potential, if compared with infrequently inundated floodplain soils. We showed that the settling of SPM and sediments revealed a significant impact on the dioxin-like potencies of riparian soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The European Water Framework Directive as well as other related directives and guidelines established a risk-based management of river basins to achieve a good ecological and chemical status by 2015 at a basin-wide scale (Brack et al. 2009; Hollert et al. 2007; van Gils et al. 2009). In this context, river-basin-wide sediment management was considered as fundamental to reduce risks of particle-bound pollutants to the goods and services of river ecosystems (Heise 2009). The functions of sediments and suspended particulate matter (SPM) in aquatic ecosystems were defined as sinks and secondary sources for organic and inorganic pollutants (Power and Chapman 1992; Schulze et al. 2007).
Besides water pollution due to human activities of urban, industrial, or agricultural origin, floods and droughts were identified as main impacts of climate change on water quality (Delpla et al. 2009). Historically contaminated sediments may remobilize during flood events (Förstner 2004), increase the effective potential of SPM (Hollert et al. 2000, 2003a; Wölz et al. 2008, 2010b), and might be deposited during flood events in populated areas, floodplains, and flood retention areas (Maier et al. 2006; Middelkoop et al. 2010; Schwartz et al. 2006). However, even a disastrous flood event such as the Elbe flood in 2002 does not necessarily cause increased contamination and adverse effects due to sediments but can instead cause a wide distribution of contaminants and thus decrease sediment toxicity due to dilution (Oetken et al. 2005).
Floodplains are important deposits for settling sediments and SPM and hence have an important function for the removal of pollutants from the aquatic system (Ensenbach 1998). However, those deposited contaminated solids might affect terrestrial ecosystems (Klok and Kraak 2008; Tockner et al. 2010), could be remobilized during flood events (Hollert et al. 2000, 2003a; Schüttrumpf et al. 2011; van Gils et al. 2009), or pose a risk to drinking water resources due to leaching of pollutants (Kühlers et al. 2009; Maier et al. 1997; Maier et al. 2006). Therefore, in drinking water protection areas, there may be a conflict of interests between the potential risk of ground-water contamination during flood events and the construction of natural retention areas due to remobilization of particularly bound pollutants under certain conditions (Busche and Hirner 1997; Kedziorek et al. 1998; Maier et al. 2006; Sauvé et al. 2000; Fig. S1 in Electronic supplementary material).
Furthermore, reservoirs of persistent chemicals (e.g., dioxins, polychlorinated biphenyls (PCBs), other persistent organic pollutants, or heavy metals) in soils and sediments are important sources for food and feed contamination (Kamphues et al. 2011; Torres et al. 2013; Weber et al. 2008a; Rice and Ray 1985a, b). Mobilization by flooding (Prantner et al. 2005; Weber et al. 2011; Wölz et al. 2008; Zennegg et al. 2010) but also by construction measures, including sediment dredging (Heinisch et al. 2006) or by landfill mining (Torres et al. 2013), are mechanisms for trigger remobilization and increase of exposure of food and feed. These releases of contaminants from reservoirs into the aquatic chain can lead to increased exposure and subsequent bioaccumulation of contaminants in fish and other aquatic foods (Prantner et al. 2005; Weber et al. 2011; Zennegg et al. 2010). Likewise, the contaminants in sediments transferred to flood plains can contaminate grazing animals both directly and via fodder (Eichbaum et al. 2013; Hembrock-Heeger 2011; Kamphues et al. 2011; Lake et al. 2014; Schulz et al. 2005).
The purpose of the present study was to assess the hazard potentials of contaminated SPM sampled during a flood event for floodplain soils using in vitro bioassays and chemical analysis. Beyond the analysis of organic pollutants, the samples were characterized for their physico-chemical properties as well as the content of trace metals (see Electronic supplementary material). Sediment-contact tests were performed to evaluate the direct exposure of organisms to native soils and SPM at two different trophic levels. For comparison, acetonic extracts were tested using both contact tests and additionally two cell-based biotests. The sediment-contact tests were carried out with the dehydrogenase assay with Arthrobacter globiformis and the fish embryo assay with Danio rerio. The cytotoxicity and the dioxin-like effects were determined with the neutral red and the 7-ethoxyresorufin-O-deethylase (EROD) assay, respectively, using the permanent rainbow trout cell line RTL-W1.
Four hypotheses were tested: (1) Exposure to acetonic extracts in the bioassays overestimates the hazard potential comparing to native soils and SPM exposure. (2) The hazard potential of SPM increases during flood events due to remobilization of contaminated sediments. (3) The settling of contaminated SPM in frequently inundated floodplain soils increases the hazard potential comparing with soils from infrequently inundated areas. (4) The priority compounds detected do not fully explain the effects found in the bioassays.
Material and methods
Chemicals
Certified standard solutions containing the 16 Environmental Protection Agency-polynuclear aromatic hydrocarbons (EPA-PAHs) were purchased from Dr. Ehrenstorfer (Augsburg, Germany). Certified standards of PCBs and hexachlorobenzene (HCB) as well as the internal standards acenaphthene-d10, phenanthrene-D12, chrysene-D12, perylene-d10, PCB 53, and PCB 159 were obtained from Promochem (Wesel, Germany). The analytical standards and acids for the inorganic compounds were purchased from Merck (p.a. or suprapur® grade, Merck, Darmstadt, Germany). Other chemicals were bought from Sigma-Aldrich (Steinheim, Germany)
Sampling sites, collection of soil and SPM samples, and sample preparation
Three different types of samples were investigated, representing several risk scenarios:
-
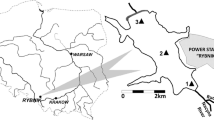
Soil samples from a frequently inundated floodplain riverside of the levee (BT1-BT7; coordinates: UTM 32U, 447586.26E, 5426588.24N); this area is frequently inundated with potential sedimentation of SPM.
-
Soil samples from the land side of the levee, representing an infrequently inundated area (B1-B7; coordinates: UTM 32U, 447700.12E, 5426453.43N).
-
SPM samples from Rhine collected in the fish pass at the upstream location Iffezheim barrage (Rhine km 232) during different water levels representing a potential source of pollutants entering the planned retention basin (S1-S6; coordinates: UTM 32U, 434636.80E, 5409283.84N).
Detailed information could be found in Fig. S2 and Tables S1–S3 in Electronic supplementary material.
The frequently inundated floodplain was flooded from February to October 2001. Therefore, it was possible to analyze the contribution of freshly deposited SPM to the effect potential of the floodplain soil.
The top soil samples (B1, B3–B7, and BT1–BT6; depth, 0–4 cm, recent pollution) were collected using a stainless steel shovel. Coring samples (B2 and BT7; depth, 80–100 cm; reference conditions or potential migration of pollutants to sub ground) were sampled using a corer. The SPM samples were collected using a sedimentation box (Hollert et al. 2000). Samples for biological and chemical analyses were freeze-dried over 48 h (Christ Beta 1–8, Osterode, Germany), sieved at <1.25 mm using a stainless steel mesh, homogenized, and then divided in sub-samples by quartering. All sub-samples were stored at 4 °C for a maximum of 8 weeks.
Soxhlet extraction and silica gel fractionation
The sieved (<1.25 mm) and freeze-dried soil and SPM samples were extracted using Soxhlet extraction with acetone with six cycles per hour over 24 h to obtain the acetone-extractable fraction according to Hollert et al. (2000). Rotary evaporation and nitrogen was used to evaporize solvent nearly to dryness. The residues were reconstituted in 0.5 ml n-hexane and separated into six fractions by column chromatography (2 g silica gel 60, Merck, Darmstadt, Germany) according to polarity (Ricking and Terytze 1999). Mixtures of n-pentane, dichloromethane, and methanol were used as eluents (Bundt et al. 1991; Franke et al. 1998; Heim et al. 2005). Fractions F1 to F4 were evaporized to a final volume of 200 μl using nitrogen and transferred to gas chromatography (GC) vials that were stored at −20 °C until analysis.
Instrumental analysis of organic compounds
GC-mass spectrometry (MS) analysis was carried out on an HP 5890 II GC coupled to an HP MSD 5971 (Agilent, Palo Alto, USA), equipped with a 60 m × 0.25 mm I.D. ×0.25-μm film DB-XLB fused capillary silica column. The MS was operated in electron ionization mode (EI+, 70 eV) with a source temperature of 180 °C scanning from 50 to 550 amu (full-scan mode; scan time, 1.5 s) or single-ion monitoring for quantification. The chromatographic conditions for analysis of EPA-PAHs (PAH) and PCBs according to Ballschmiter and Zell (1980) as well as for full-scan analysis were as follows—300 °C injector temperature, 1 μl splitless injection at oven temperature of 80 °C, then programmed to 300 °C at 4 K/min (1 min isotherm), and finally programmed to 310 °C at 0.8 K/min (35 min isotherm). Carrier gas velocity (helium 5.0, Air Liquide, Böhlen, Germany) was 1 ml/min at constant flow.
For quality assurance, the internal standards PCB 53 and PCB 139 were spiked to fractions F1 and F2; deuterated PAHs (acenaphthene-D10, phenanthrene-D12, chrysene-D12, and perylene-D10) were spiked to fraction F3 and F4 prior to analysis. The recovery values of the internal standards are listed in Table S4 in Electronic supplementary material. The concentrations of the compounds in simultaneously analyzed blanks (n = 2) were below detection limits. The detection limits of the compounds were between 0.5 and 5.5 μg/kg.
Polychlorinated dibenzodioxins and dibenzofurans
The 17 WHO-prioritized polychlorinated dibenzodioxins and dibenzofurans in samples B1, B2, BT1, BT7, and S2 were analyzed in different sample aliquots by Analysen Service GmbH (Berlin, Germany) according to DIN 38414–24 (2000–10).
Bioassays
Neutral red retention assay
Acute cytotoxic effects were determined using the neutral red retention assay (Babich and Borenfreund 1992) using the protocol detailed elsewhere (Heeger et al. 2012; Klee et al. 2004; Wetterauer et al. 2012). Briefly, cells from the CYP1A-expressing cell line RTL-W1 (Bols et al. 1999; Lee et al. 1993) were exposed to serial dilutions of sediment extracts along seven wells in six replicates of a 96-well microtiter plate (TPP, Trasadingen, Switzerland) at a final concentration range of 1.56–100 mg/ml. 3.5-Dichlorophenol (80 mg/l) was used as a positive control. After incubation at 20 °C for 48 h, cells were stained with neutral red (2-methyl-3-amino-7-dimethylamino-phenanzine) for 3 h, and neutral red retention was determined at 540 nm with a reference wavelength of 690 nm using a microtiter plate reader (Tecan, Crailsheim, Germany).
EROD induction assay
The dioxin-like inducing potential of sediment extracts was investigated using the 7-ethoxyresorufin-O-deethylase induction assay (EROD; Behrens et al. 1998) with the protocol detailed in Gustavsson et al. (2007), and Wölz et al. (2011a, b). RTL-W1 cells were seeded into 96-well microtiter plates and exposed to sediment extracts in eight dilution steps with six replicates. As a positive control, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) was serially diluted on two separate rows of each plate. Following incubation at 20 °C for 72 h, EROD induction was terminated by disrupting the cells by shock-freezing in the vapor space of liquid nitrogen. Subsequently, 100 μl of the substrate 7-ethoxyresorufin was added to each well before deethylation was initiated for 10 min with nicotinamide adenine dinucleotide phosphate in phosphate buffer. The reaction was stopped by adding 100 μl of fluorescamine in acetonitrile. The production of resorufin as a metabolite of the substrate was recorded fluorometrically at 544 nm (excitation) and 590 nm (emission) using a microtiter plate reader (Tecan, Crailsheim, Germany). Whole protein was also determined fluorometrically using the fluorescamine method (excitation 355 nm, emission 590 nm; Hollert et al. 2002a; Kennedy and Jones 1994). Fluorescent units were converted to mass of resorufin and protein with the aid of calibration curves.
Fish embryo test with D. rerio
The fish embryo test was performed according to DIN 38415–6 (2001) for 48 h detailed in Braunbeck et al. (2005) and Hollert et al. (2003b). Briefly, the acetone extracts were tested with 6.25 to 200 mg sediment per test using 24-well microtiter plates. The positive contrast was 5 ml of 3.5-dichloroanilin (3.75 mg/l; 3.5-DCA) in bi-distilled water. In each well, one fertilized fish egg in four-cell or eight-cell stage was added. The microtiter plates were incubated for 48 h at 27 °C with a day–night cycle of 14/10 h.
The whole top soil and SPM samples were tested at concentrations of 3, 2.5, 2, 1.5, 1, 0.5, 0.25, and 0.1 g per 5 ml of artificial water (ISO 7346–3 1996), which was fabricated according to DIN 38415–6 (2001). The samples were diluted using quartz powder (grain size W4; Quarzwerke Frechen, Germany), adjusted to 3 g, and homogenized using mortar and pistil. The samples were transferred into six-well plates, and each well was wetted using 5 ml artificial water, which was vented to oxygenize. Five fish eggs were added, and the well plate was closed using a cover. The plates were incubated for 48 h at 27 °C with a day–night cycle of 14/10 h. Three grams of quartz powder spiked with 5 ml of 3.5-DCA (3.75 mgl/l) was used as positive control. The wells were controlled for oxygen after exposure in order to ensure that the effects were due to sediment toxicity and not lack of oxygen (cf. Strecker et al. 2011).
The fish eggs were assessed after 24 hpf for the lethal endpoints lack of somite formation, coagulation of embryos, and non-detachment of tails. After 48 hpf, these endpoints were re-evaluated and additionally for lack of heart function and lack of blood circulation.
Bacteria contact test with A. globiformis
The bacteria contact test was performed according to modified draft DIN 38412–48 (1998) with the protocol detailed in Keiter et al. (2006). The acetone extracts were tested with 400, 200, 100, 50, and 25 mg sediment per test using 24-well microtiter plates. The positive control was 4-nitrophenol in bi-distilled water (250 mg/l). The plates were incubated for 2 h at 30 °C, spiked with 500 μl of the resazurin solution, and incubated for 30–120 min at 30 °C until the absorption of resazurin at 595 nm (OD595) of the test wells (with bacteria) were 70 % of the OD595 of the blank wells (without bacteria) due to metabolic transformation of resazurin to resorufin.
The direct sediment contact test was performed in triplicate with 2 g of each sample using 15 ml polyethylene Falcon-type tubes. Quartz sand (grain-size W4; Merck, Darmstadt, Germany) was used as control sample and spiked with 4 ml of 4-nitrophenol in bi-distilled water (250 mg/l). Three milliliters bi-distilled water was added to samples and controls followed by 2 ml of DSM medium per 2 ml bacteria suspension. The samples and controls were incubated for 2 h at 30 °C using an overhead shaker at 70 rpm.
The OD595 was measured photometrically at 525 nm using a microtiter well plate reader (Tecan, Crailsheim, Germany). The inhibition of bacteria was computed using Eq. 1:
Due to an enhanced positive dehydrogenase activity, some of the samples showed negative inhibition, which was considered as toxic effects as well (Liß and Ahlf 1997; Ulrich et al. 2002).
Data analysis
Concentration–response relationships
Concentration–response relationships were calculated using nonlinear regression with GraphPad Prism® 5 (GraphPad Software Inc., La Jolla, USA). A sigmoid function with variable slope was used to fit the EROD and fish embryo bioassay data, and a three- or four-parameter Hill function adapted from Sigma Plot 11 (Systat Software Inc., Chicago, USA) was used for fitting the bacteria contact test data. If neither of these fitted, a second-order polynomial function was used. The functions were tested for best fit using GraphPad Prism®. If it was not possible to calculate concentration–response curves, the lowest effect concentrations were used.
Bio-TEQ values
Bioassay-derived TCDD equivalents (Bio-TEQs) were calculated by relating the biological EROD activities of the samples to the positive control TCDD using the fixed effect level quantification method (cf. Wölz et al. 2008). Mean TCDD-EC25 and standard deviation values were determined using a sigmoid log-logistic model with GraphPad Prism® 5. Bio-TEQs with concentrations in picogram TCDD per gram of sample equivalent (SEQ) were calculated as per Eq. 2:
Chem-TEQ values
In order to explain the determined Bio-TEQs, chemically derived TEQ values (Chem-TEQs) were calculated using relative potency factors (REP; Bols et al. 1999) as per Eq. 3:
where for a given chemical i, c i is the measured concentration in the sample, and TEF i is the toxic equivalency factor for each compound relative to TCDD. TEFs were derived for RTL-W1 cells according to Bols et al. (1999) and Clemons et al. (1997)—if any cell specific TEFs have been available—and according to World Health Organization (WHO; van den Berg et al. 2006).
Significance testing and multivariate explorative analysis
Data were tested for normal distribution (Kolmogorov–Smirnov test (K-S)) and for variance homogeneity (Bartlett’s test). If data passed K-S and Bartlett’s test, one-way analysis of variance (ANOVA) with Tukey’s posttest was performed to test significances using GraphPad Prism®; otherwise, Kruskal–Wallis ANOVA with Dunn’s posttest was used. Agglomerated hierarchical analysis (cluster analysis) using complete linkage as cluster rule and 1-Pearson rs as distance measure as well as principal compound analysis was performed for multivariate explorative data analysis using Statistica 8.0 (StatSoft Inc., Tusla, USA).
Results
Organic compounds
Figure 1a–c shows the results of organic compounds (PAHs, HCB, and PCBs). The ranges of EPA-PAHs were 188–754 μg/kg in the B samples, 345–3034 μg/kg in the BT samples, and 474–2318 μg/kg in the SPM samples (S; Fig. 1a). HCB concentrations were in the ranges of 0.9–1.8 μg/kg in the B samples, 15–53 μg/kg in the BT samples, and 25–203 μg/kg in the SPM samples (Fig. 1b). PCBs were below detection limits in the B samples and were in the ranges of 8–23 μg/kg in the BT samples and of 3–32 μg/kg in the SPM samples (Fig. 1c).
Box-and-whisker plots (boxes: median, 25th-/75th-percentiles; whiskers: minimum, maximum) of the levels of organic compounds (a PAHs; b HCB; c PCB) in inundated (BT; n = 6) and infrequently inundated (B; n = 6) top soil layers as well as in SPM samples (S; n = 6); analysis was performed using the grain size fraction <1.25 mm; values from borehole samples (B2 and BT7) were not included; *p < 0.05, ***p < 0.001 (Kruskal–Wallis ANOVA with Dunn’s posttest)
The summarized concentrations of the 17 analyzed congeners of PCDD/Fs were 118 pg/g in B1, 2,017 pg/g in BT1, 2,178 pg/g in BT7, and 3,304 pg/g in S2 (Table 1). The composition in B1 was strongly dominated by PCDFs with a content of more than 90 % (Fig. S6 in the Electronic supplementary material). In sample BT1 and the borehole sample BT7, there were still were found about 79 and 58 % of PCDFs, respectively. The SPM sample S2 contained more PCDDs (56 %) than PCDFs (44 %).
Bioassays
Neutral red retention assay
The results from acetonic extracts of the samples tested in the neutral red retention assay with RTL-W1 cells are shown in Fig. 2a. The half-maximum effective concentrations (NR50) were 80–300 mg/ml in landside soils (B), 70–101 mg/ml in floodplain soils (BT), and in the range 81–300 mg/ml in the SPM samples (S). The samples B2 and S6 were not cytotoxic.
Box-and-whisker plots (boxes: median, 25th-/75th-percentiles; whiskers: minimum, maximum) of a cytotoxicity of acetonic extracts tested with the NR-assay with RTL-W1 cells (data given as the half maximum effective concentration NR50), Bio-TEQs (A) and Chem-TEQs (b) derived from EROD assay as well as PAH and PCDD/Fs analyses (data given as picograms per gram; borehole samples (B2 and BT7) were not included); B: infrequently inundated soils; BT: frequently inundated soils; S: suspended particulate matter; *p < 0.05 (ANOVA with Tukey’s posttest or Kruskal–Wallis ANOVA with Dunn’s posttest); data taken from Garke (2003)
EROD induction assay
The EROD induction assay showed dioxin-like effects in acetonic extracts of all samples tested except the borehole sample B2 from the landside soils (Fig. 2b, Garke 2003). The Bio-TEQ values were 77–1,439 pg/g in the B samples, 1,099–6,333 pg/g in the BT samples and 2,534–4,554 pg/g in the S samples. In comparison, the Chem-TEQs values were 29–137 pg/g in the B samples, 59–257 pg/g in the BT samples, and 77–424 pg/g in the S samples (Fig. 2c).
Fish embryo assay with D. rerio
The half-maximum lethal concentration (LC50) of acetonic extracts tested in the fish embryo test with D. rerio reached values 73–129 mg/ml in the landside soils (B), 7–50 mg/ml in the floodplain soils (BT), and 16–21 mg/ml in the SPM samples (S; Fig. 3a; Table S9 in Electronic supplementary material). The levels of LC50 of native samples tested in the fish embryo test were 21–76 mg/ml in the native landside soils (B), 43–61 mg/ml in the floodplain soils (BT), and 99–113 mg/ml in SPM (S) (Fig. 3a; Table S10 in Electronic Supplementary material).
Toxicity of acetonic extracts (solid white) and native samples (solid black) tested in the 48 h fish embryo assay with D. rerio (a) (data given as the half maximum lethal concentration LC50 in mg SEQ per milliliter test medium) and inhibition by acetonic extracts (solid white) and native samples (solid black) tested in the contact assay with A. globiformis (b; data given as the half maximum inhibitory concentration IC50 in mg SEQ per ml test medium); B: landside soils; BT: floodplain soils; S: suspended particulate matter; SEQ: sediment equivalents
The drilling core samples B2 and BT7 showed no effects either. The samples BT3 and BT1 were not toxic in the native sample exposure scenario. S2native, S5native, S6acetonic, and BT7native showed inconsistent concentration–response curves, and effect concentrations could not be derived.
Bacteria contact assay
Acetonic extracts of the samples tested in the bacteria contact assay with A. globiformis resulted in half maximum inhibition concentrations (IC50) from 33 and 272 mg/ml for the landside soils (B), in the range of 71 to 132 mg/ml for the floodplain soils (BT), and between 15 and 242 mg/ml for the SPM samples (S; Fig. 3b; Table S9 in Electronic supplementary material).
Native samples revealed IC50 values of 119–1,235 mg/ml for the landside soils, 42–99 mg/ml for the floodplain soils, and 227–553 mg/ml for the SPM (S; Fig. 3b; Table S10 in Electronic supplementary material).
Discussion
Comparison of the exposure pathways acetonic extract and native sample
Comparing the results from the fish embryo test with native samples and acetonic extracts thereof revealed an inconsistent picture of the different sample groups. While the soils from the infrequently inundated area (B) showed significantly higher effects in the native samples (p < 0.01, Kruskal–Wallis ANOVA with Dunn’s posttest), the soils from frequently inundated area (BT) showed no difference in effects, whereas the SPM samples (S) showed significantly higher effects in the acetonic extracts (Fig. 4a; p < 0.01, Kruskal–Wallis ANOVA with Dunn’s posttest).
Comparison of effects by acetonic extracts (solid white) and native samples (solid black) tested in the 48 h fish embryo test with D. rerio (a) and in the bacteria contact assay with A. globiformis (b); data given as relative effect potentials as means ± standard deviations; B: infrequently inundated soils; BT: frequently inundated soils; S: suspended particulate matter; **p < 0.01 (Kruskal–Wallis ANOVA with Dunn’s posttest)
Elevated effects with D. rerio of the native B soil samples might be explained by the relatively high contents of total organic carbon (Table S1-S3 in Electronic supplementary material) and maybe by dissolved inorganic phosphor, nitrogen, or sulfur species. Höss et al. (2010) showed that these parameters were correlated significantly with effects for fish embryos in a comprehensive investigation into the responses in different sediment contact tests with several sediment samples. The comparison of the values from the contact test with A. globiformis confirmed the higher toxicity of acetonic extracts regarding the SPM samples (Fig. 4b) and comparable effects for the floodplain soils (BT).
The hypothesis that acetonic extracts overestimate the hazard potential relative to sediment contact tests with native samples (Rönnpagel et al. 1995) cannot be supported by our investigation, because there were no clear-cut differences in effect responses (Ulrich et al. 2002). Due to missing data (i.e., no grain size analysis of the SPM samples, analytical values below the detection limits, and a lack of effectivity in different samples), it was impossible to run further statistical analyses such as regression or multivariate analyses. Recent investigations have shown that most adverse effects could not be fully explained even if using a battery of different sediment contact tests and a wide range of physico-chemical parameters to characterize the soil or sediment samples (Höss et al. 2010; Tuikka et al. 2011). Running a meta-analysis was not beneficial, because there are only few studies on the use of raw acetonic extracts for testing in the fish embryo test with D. rerio (Hallare et al. 2005; Wu et al. 2010). However, those studies were not directly comparable because nonlethal and lethal endpoints were used for risk estimation.
Changes in hazard potentials of SPM during a flood event due to sediment remobilization
Figure 5 shows the Bio-TEQs derived from EROD assay as well as the PAH- and PCDD/F-related Chem-TEQs according to Clemons et al. (1997) for the SPM samples in comparison with the discharge during the sampling periods of the SPM. Samples S1 and S2 with Bio-TEQs of 2,387 pg/g and 4,553 pg/g, respectively, were collected during mean to low discharge conditions (Fig. 5a). S3 was collected during increasing discharge with a Bio-TEQ of 3,214 pg/g. S4 represents a higher discharge plateau level with a Bio-TEQ of 2,534 pg/g. Samples S5 and S6 were collected while discharge increased further to almost the two-yearly flood level (3,100 m3/s; LUBW 2011), each with a Bio-TEQ of 3,014 pg/g. The levels of Bio-TEQs in this study were comparable with those found by Wölz et al. (2010b) at the same location. Heimann and co-workers (2011) found dioxin-like activities with Bio-TEQ values between 3,620 and 7,920 pg/g in an EROD induction assay using RTL-W1 cells within sediment extracts from a connected oxbow lake of the Upper Rhine. The shown homogenous profile was caused most likely by extreme floods, which lead to a large input of river sediments and a mixture with remobilized lake sediments.
Discharge at gauging station Maxau (Rhine-km 362.2) (solid line; data provided by Rheinkraftwerk Iffezheim GmbH, Iffezheim Germany), Bio-TEQs (a), Chem-TEQs (b) according to Clemons et al. (1997), PAHs (c) and HCBs in the SPM samples; data are given as picograms per gram; S1, S2, S4, and S5 are shown as sampling periods (grey areas); S5 and S6 are single samples (x); dotted line: average low water discharge 1998–2007; dashed line: mean water discharge 1998–2007 (LUBW 2011)
The pattern of the Chem-TEQs according to Clemons et al. (1997; Fig. 5b) and of the related PAHs (Fig. 5c) was inverse to that of the Bio-TEQs. The HCB pattern followed the Bio-TEQs except of sample S6 (Fig. 5d). However, there was no significant correlation between HCB levels and Bio-TEQs (Pearson r = 0.24, p ≥ 0.05). In contrast, Wölz et al. (2010a, b) revealed a good concordance between HCB content and Bio-TEQs.
Results indicated that (1) PAHs and HCB had a moderate mixture toxicity effect on the Bio-TEQs during steady-state discharge levels (S2 and S4), that (2) the PAHs had a predominant effect potential during moderate discharge peaks (S1), and that (3) HCB was more hazardous than the PAHs at flood-like discharge conditions (S5). The reason for the latter case could be the remobilization of HCB-contaminated sediments due to the flood event. HCB is one of the major Rhine-specific contaminants (IKSR 2011) and is present in contaminated sediments at Iffezheim Barrage (Boettcher and Klose 2003; Hollert et al. 2007).
Increased hazard potential of frequently inundated soils compared with infrequently inundated soils due to settlement of contaminated SPM or sediments
The accumulation of trace elements and organic contaminants in flood plain soils due to settlement of SPM and suspended sediments is a widely known and investigated issue (Baborowski et al. 2007; Fiedler et al. 1996; Förstner 2004; Gocht et al. 2001; Japenga et al. 1990; Malmon et al. 2002; Martin 2009; Martin 1997; Middelkoop 2000; Pies et al. 2007; Umlauf et al. 2005; Witter et al. 1998). Most of the studies have investigated the input and fate of contaminants but not the potential adverse effects of such settlement. However, there are some studies that have shown effects of floodplain soils in vitro and in vivo (e.g., de Jonge et al. 1999; Hamers et al. 2006; Hobbelen et al. 2004; Klok and Kraak 2008; Rader et al. 1997; Schwartz et al. 2006; Wölz et al. 2011a).
In our study, we had the opportunity to investigate the effect potentials of SPM and potentially impacted floodplain soils due to settlement of SPM or sediments during a flood event. We proved the hypothesis that settling SPM and sediments increase the effect potentials of frequently inundated soils compared with non-frequently soils. Figure 6 gives box-and-whisker plots of biotest results to compare the relative effect potentials of the different sample groups.
Box-and-whisker plots (boxes: median, 25th-/75th-percentiles; whiskers: minimum, maximum) of acetonic extracts tested in the neutral assay (a) and EROD (b) assays as well as by acetonic extracts (solid white) and native samples (solid grey) tested in the fish embryo test with zebrafish (D. rerio) (c) and in the bacteria contact assay with A. globiformis (d); data given as relative effect potentials; B: infrequently inundated soils; BT: frequently inundated soils; S: suspended particulate matter; Ac: acetonic extract; Nat: native sample; *p < 0.05 (ANOVA with Tukey’s posttest or Kruskal–Wallis ANOVA with Dunn’s posttest)
The cytotoxic potential (Fig. 6a) of the BT samples was higher and less variable than that of the B samples but not significantly different (p ≥ 0.05, ANOVA with Tukey’s posttest). The cytotoxic effect potentials of the BT samples were significantly higher (p < 0.05; ANOVA with Tukey’s posttest) compared with the S samples (Fig. 6a) and may be caused by inputs of cytotoxic compounds during flooding of the area (Garke 2003).
A significantly increased hazard potential regarding the dioxin-like potential (p < 0.05; ANOVA with Tukey’s posttest) was found (Fig. 6b), revealing the significant impact of flood SPM on the dioxin-like potencies of frequently inundated soils. The dioxin-like potential of the BT and S samples were comparable (Fig. 6b).
The effects in the fish embryo-toxic effects of the acetonic extract of frequently inundated soil (B samples) were present comparing with those of infrequently inundated soils (BT samples; Fig. 6c). An evaluation of the fish embryo test results was difficult due to absence of effects and inconsistent concentration–response relationships for several samples, and thus, no strength deduction is possible.
The bacterial effect potentials (Fig. 6d) in both acetonic extracts and native samples of the BT samples were insignificantly higher than those of the B samples (p ≥ 0.05, ANOVA with Tukey’s posttest). However, the results of the BT samples were more variable. The bacterial effect potentials in the BT samples were above those of the S samples, but there was no significant difference between the groups. Based on the observations in negative and positive controls, all tests were considered valid (details not shown).
The hypothesis that the settlement of contaminated SPM increases the hazard potential of the frequently inundated soils compared with the infrequently inundated is thus supported by the results of this investigation, particularly regarding Ah-receptor-mediated (dioxin) effects. In respect to human exposure to dioxin-like compounds, the most relevant hazard is the contamination of cattle (cows and sheep) grazing on the floodplain (Hembrock-Heeger 2011; Kamphues et al. 2011; Schulz et al. 2005). It has recently been discovered that soil levels of PCB, PCDD, and PCDF below currently regulated pollution levels for soil can be sufficient that cattle reach Dioxin and PCB liver and meat levels above EU food regulations (Weber et al. 2013).
Ecotoxicological effects versus analyzed pollutants
Linking ecotoxicological effects in biotests to the pollutants detected in the samples is often a major problem in the studies regarding the evaluation environmental hazard potentials. Synergistic, antagonistic, or additive mixture toxicity effects as well as varying modes of toxic actions at different effect concentration levels are in contrast to a commonly relatively low number of identified and quantified compounds in the respective samples or sub-fractions thereof (Brack 2003; Brack et al. 2008; Grote et al. 2005; Kammann et al. 2005).
Box-and-whisker plots of Bio-TEQs derived from the EROD assay and the Chem-TEQs derived from the PAH and PCDD/F analyses are shown in Fig. 6. Chem-TEQs explained in average 7.9 ± 5.9 % of the effects expressed by the Bio-TEQs. This result is in concordance with similar previous studies (Heimann et al. 2011; Hilscherova et al. 2001; Hilscherova et al. 2003; Kammann et al. 2005; Schulze et al. 2012; Wölz et al. 2010a), whereas it has to be considered that the contents of PCDD/Fs in this study were at the lower levels of PCDD/Fs analyzed in sediments of the Elbe after the flood in 2002 (3–140 pg/g WHO-TEQ; Stachel et al. 2005), and thus, PCDD/Fs might contribute in a higher ratio to the Chem-TEQ in higher contaminated sites.
However, the analysis of a few priority pollutants such as PAHs, PCBs, and PCDD/Fs is not sufficient to achieve real cause–effect relationships of adverse effects of anthropogenic environmental pollution as the majority of the dioxin-like activity was not explained. Numerous known and unknown chemicals that may cause adverse effects are present in soils and sediments (Brack et al. 2008; Schwarzbauer 1997). Here, the concept of effect-directed analyses (Brack 2003; Hecker and Hollert 2009; Brack et al. 2012, 2013) can be used to identify the contribution of non-priority pollutants to the overall hazard potential (as determined by the bioassays).
Ecotoxicological assessment
The ecotoxicological assessment of environmental samples using different endpoints is usually very complex, and mathematical models are recommended for successful results (Hollert et al. 2002b; Keiter et al. 2009). It was shown that partial order theory (for example used in the Hasse diagram technique) and fuzzy logic theory are appropriate approaches for the risk estimation of complex environmental data (Hollert et al. 2002b; Lerche et al. 2002; Voigt et al. 2006). In order to develop a general classification system, Keiter et al. (2009) processed a huge dataset of effect data of different bioassays (i.e., fish embryo assay with D. rerio, neutral red assay, comet assay, and EROD assay) using fuzzy logic. In the present study, this classification was used to assess the results of the biotests (Table 2). The empirical model was used due to the highest correlation between the biotest results and the fuzzy logic alternative (Keiter et al. 2009).
With respect to cytotoxicity, only two samples were classified as moderately cytotoxic (B5 and BT1). The samples of the frequently inundated area and the SPM samples were categorized strongly toxic in the EROD and the fish embryo assays with native samples, except sample BT6 which was ranked moderately toxic (Table 3). The samples of the landsite soils were moderately toxic in these bioassays. In contrast, the fish embryo assay with acetonic extracts resulted in a nontoxic grouping of the landsite soils (B samples) and two BT samples (BT2 and BT3). One sample was strongly toxic (BT1), and the analyzed SPM samples were classified as moderately toxic (Table 3).
Conclusions
The results of this study clearly document that native samples may well be significantly more effective than corresponding extracts in the bacteria contact assay or the fish embryo test. These results question the commonly accepted concept that acetonic extracts are likely to overestimate the toxicity of soil and SPM samples. Likewise, the priority organic compounds analyzed failed to fully explain the toxic potential of the samples. The outcomes of this study revealed the insufficient knowledge regarding the relationship between the different exposure pathways. Finally, there is concern about adverse effects by settling suspended particulate matter and remobilized sediments in frequently inundated floodplain soils due to an increase of the hazard potential—especially the dioxin-like potential—if compared with infrequently inundated floodplain soils.
References
Babich H, Borenfreund E (1992) Neutral red assay for toxicology in vitro. In: Watson RR (ed) In vitro methods of toxicology. CRC Press, Boca Raton, pp 238–251
Baborowski E, Büttner O, Morgenstern P, Krüger F, Lobe I, Rupp H, von Tümpling W (2007) Spatial and temporal variability of sediment deposition on artificial-lawn traps in a floodplain of the River Elbe. Environ Pollut 148:770–778
Ballschmiter K, Zell M (1980) Analysis of polychlorinated biphenyls (PCB) by glass capillary gas chromatography. Fresenius J Anal Chem 302:20–31
Behrens A, Schirmer K, Bols NC, Segner H (1998) Microassay for rapid measurement of 7-ethoxyresorufin-O-deethylase activity in intact fish hepatocytes. Mar Environ Res 46:369–373
Boettcher G, Klose H (2003) Sedimentationsproblem Iffezheim. In: Bundesanstalt für Gewässerkunde (Hrsg.), Schwebstoffe und Schwebstofftransport in Binnenwasserstraßen. BfG-Veranstaltungen 03/2003. Bundesanstalt für Gewässerkunde, Koblenz, pp. 79–87
Bols NC, Schirmer K, Joyce EM, Dixon DG, Greenberg BM, Whyte JJ (1999) Ability of polycyclic aromatic hydrocarbons to induce 7-ethoxyresorufin-O-deethylase activity in a trout liver cell line. Ecotoxicol Environ Saf 44:118–128
Brack W (2003) Effect-directed analysis: a promising tool for the identification of organic toxicants in complex mixtures? Anal Bioanal Chem 377:397–407
Brack W, Schmitt-Jansen M, Machala M, Brix R, Barcelo D, Schymanski E, Streck G, Schulze T (2008) How to confirm identified toxicants in effect-directed analysis. Anal Bioanal Chem 390:1959–1973
Brack W, Apitz SE, Borchardt D, Brils J, Cardoso AC, Foekema EM, van Gils J, Jansen S, Harris B, Hein M, Heise S, Hellsten S, de Maagd PG-J, Müller D, Panov VE, Posthuma L, Quevauviller P, Verdonschot PFM, von der Ohe PC (2009) Toward a holistic and risk-based management of European river basins. Integr Environ Assess Manag 5:5–10
Brack W, Dulio V, Slobodnik J (2012) The NORMAN Network and its activities on emerging environmental substances with a focus on effect-directed analysis of complex environmental contamination. Environ Sci Eur 24:1–5
Brack W, Govender S, Schulze T, Krauss M, Hu M, Muz M, Hollender J, Schirmer K, Schollee J, Hidasi A, Slobodnik J, Rabova Z, Ait-Aissa S, Sonavane M, Carere M, Lamoree M, Leonards P, Tufi S, Ouyang X, Schriks M, Thomas K, De Almeida AC, Froment J, Hammers-Wirtz M, Ahel M, Koprivica S, Hollert H, Seiler TB, Di Paolo C, Tindall A, Spirhanzlova P (2013) EDA-EMERGE: an FP7 initial training network to equip the next generation of young scientists with the skills to address the complexity of environmental contamination with emerging pollutants. Environ Sci Eur 25:1–7
Braunbeck T, Böttcher M, Hollert H, Kosmehl T, Lammer E, Leist E, Rudolf M, Seitz N (2005) Towards an alternative for the acute fish LC50 test in chemical assessment: the fish embryo toxicity test goes multi-species—an update. ALTEX 22:87–102
Bundt J, Herbel W, Steinhart H, Franke S, Francke W (1991) Structure-type separation of diesel fuels by solid phase extraction and identification of the two- and three-ring aromatics by capillary GC-mass spectrometry. J High Resolut Chromatogr 14:91–98
Busche U, Hirner AV (1997) Mobilisierbarkeit von hydrophoben organischen Schadstoffen in Belasteten Böden und Abfällen. Teil II: Mobilsierbarkeit von PAK, PCB und Phenolen durch reale Wässer. Acta Hydrochim Hydrobiol 25:248–252
Clemons JH, Dixon DG, Bols NC (1997) Derivation of 2,3,7,8-TCDD toxic equivalent factors (TEFs) for selected dioxins, furans and PCB with rainbow trout and rat liver cell lines and the influence of exposure time. Chemosphere 34:1105–1119
de Jonge J, Brils JM, Hendriks AJ, Ma WC (1999) Ecological and ecotoxicological surveys of moderately contaminated floodplain ecosystems in The Netherlands. Aquat Ecosyst Health Manag 2:9–18
Delpla I, Jung AV, Baures E, Clement M, Thomas O (2009) Impacts of climate change on surface water quality in relation to drinking water production. Environ Int 35:1225–1233
DIN 38412–48 (1998) Entwurf Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung-Testverfahren mit Wasserorganismen (Gruppe L)-Teil 48: Arthrobacter globiformis-Kontakttest für kontaminierte Feststoffe (L 48)
DIN 38414–24 (2000–10) German standard methods for the examination of water, waste water and sludge—Sludge and sediments (group S)—part 24: determination of polychlorinated dibenzodioxins (PCDD) and polychlorinated dibenzofurans (PCDF) (S 24)
DIN 38415–6 (2001) Draft German standard methods for the examination of water, waste water and sludge—subanimal testing (group T)—part 6: toxicity to fish; determination of the non-acute-poisonous effect of waste water to fish eggs by dilution limits (T 6)
Eichbaum K, Brinkmann M, Buchinger S, Hecker M, Engwall M, van Bavel B, Reifferscheid G, Hollert H (2013) The dioRAMA project: assessment of dioxin-like activity in sediments and fish (Rutilus rutilus) in support of the ecotoxicological characterization of sediments. J Soils Sediments 13:770–774
Ensenbach U (1998) Embryonic development of fish—a model to assess the toxicity of sediments to vertebrates. Fresenius Environ Bull 7:531–538
Fiedler H, Lau C, Kjeller LO, Rappe C (1996) Patterns and sources of polychlorinated dibenzo-p-dioxins and dibenzofurans found in soil and sediment samples in Southern Mississippi. Chemosphere 32:421–432
Förstner U (2004) Traceability of sediment analysis. TrAC Trends Anal Chem 23:217–236
Franke S, Schwarzbauer J, Francke W (1998) Arylesters of alkylsulfonic acids in sediments. Part III of organic compounds as contaminants of the Elbe River and its tributaries. Fresenius J Anal Chem 360:580–588
Garke V (2003) Optimierung und anpassung eine in vitro bioassays mit RTL-W1- und RTG-2-Zellen zum Nachweis der cytotoxischen und Dioxin-ähnlichen Wirkung von komplexen Umweltproben. Staatsexamensarbeit, Ruprechts-Karls-Universität, Heidelberg, 62 pp
Gocht T, Moldenhauer K-M, Püttmann W (2001) Historical record of polycyclic aromatic hydrocarbons (PAH) and heavy metals in floodplain sediments from the Rhine River (Hessisches Ried, Germany). Appl Geochem 16:1707–1721
Grote M, Brack W, Walter HA, Altenburger R (2005) Confirmation of cause–effect relationships using effect-directed analysis for complex environmental samples. Environ Toxicol Chem 24:1420–1427
Gustavsson L, Hollert H, Joensson S, van Bavel B, Engwall M (2007) Reed beds receiving industrial sludge containing nitroaromatic compounds—effects of outgoing water and bed material extracts in the umu-C genotoxicity assay, DR-CALUX assay and on early life stage development in zebrafish (Danio rerio). Environ Sci Pollut Res 14:202–211
Hallare AV, Kosmehl T, Schulze T, Hollert H, Kohler H-R, Triebskorn R (2005) Assessing contamination levels of Laguna Lake sediments (Philippines) using a contact assay with zebrafish (Danio rerio) embryos. Sci Total Environ 347:254–271
Hamers T, van den Berg JHJ, van Gestel CAM, van Schooten F-J, Murk AJ (2006) Risk assessment of metals and organic pollutants for herbivorous and carnivorous small mammal food chains in a polluted floodplain (Biesbosch, The Netherlands). Environ Pollut 144:581–595
Hecker M, Hollert H (2009) Effect-directed analysis (EDA) in aquatic ecotoxicology: state of the art and future challenges. Environ Sci Pollut Res 16:607–613
Heger S, Bluhm K, Agler MT, Maletz S, Schäffer A, Seiler TB, Angenent LT, Hollert H (2012) Biotests for hazard assessment of biofuel fermentation. Energy Eviron Sci 5:9778–9788
Heim S, Ricking M, Schwarzbauer J, Littke R (2005) Halogenated compounds in a dated sediment core of the Teltow Canal, Berlin: time related sediment contamination. Chemosphere 61:1427–1438
Heimann W, Sylvester M, Seiler T-B, Hollert H, Schulz R (2011) Sediment toxicity in a connected oxbow lake of the Upper Rhine (Germany): EROD induction in fish cells. J Soils Sediments 11:1279–1291
Heinisch E, Kettrup A, Bergheim W, Wenzel S (2006) Persistent chlorinated hydrocarbons (PCHC), source-oriented monitoring in aquatic media 5. Polychlorinated biphenyls (PCBs). Fresenius Environ Bull 15:1344–1362
Heise S (2009) Sediments in river basins. J Soils Sediments 9:393–399
Hembrock-Heeger A (2011) Dioxine und PCB in Böden, Pflanzen, Futter- und Lebensmitteln in Überschwemmungsgebieten in NRW. Fachgespräch. Belastung der terrestrischen Umwelt mit Dioxinen und PCB des Umweltbundesamtes am 13./14.Oktober 2011 in Berlin
Hilscherova K, Kannan K, Kang Y-S, Holoubek I, Machala M, Masunaga S, Nakanishi J, Giesy JP (2001) Characterization of dioxin-like activity of sediments from a Czech river basin. Environ Toxicol Chem 20:2768–2777
Hilscherova K, Kannan K, Nakata H, Hanari N, Yamashita N, Bradley PW, McCabe JM, Taylor AB, Giesy JP (2003) Polychlorinated dibenzo-p-dioxin and dibenzofuran concentration profiles in sediments and flood-plain soils of the Tittabawassee River. Mich Environ Sci Technol 37:468–474
Hobbelen PHF, Koolhaas JE, van Gestel CAM (2004) Risk assessment of heavy metal pollution for detritivores in floodplain soils in the Biesbosch, The Netherlands, taking bioavailability into account. Environ Pollut 129:409–419
Hollert H, Dürr M, Erdinger L, Braunbeck T (2000) Cytotoxicity of settling particulate matter (SPM) and sediments of the Neckar River (Germany) during a winter flood. Environ Toxicol Chem 19:528–534
Hollert H, Dürr M, Olsman H, Halldin K, Van Bavel B, Brack W, Tysklind M, Engwall M, Braunbeck T (2002a) Biological and chemical determination of dioxin-like compounds in sediments by means of a sediment triad approach in the catchment area of the River Neckar. Ecotoxicology 11:323–336
Hollert H, Heise S, Pudenz S, Brüggemann R, Ahlf W, Braunbeck T (2002b) Application of a sediment quality triad and different statistical approaches (Hasse diagrams and fuzzy logic) for the comparative evaluation of small streams. Ecotoxicology 11:311–321
Hollert H, Haag I, Dürr M, Wetterauer B, Holtey-Weber R, Kern U, Westrich B, Färber H, Erdinger L, Braunbeck T (2003a) Untersuchungen zum ökotoxikologischen Schädigungspotenzial und Erosionsrisiko von kontaminierten Sedimenten in staugeregelten Flüssen. Umweltwiss Schadstoff Forsch 15:5–12
Hollert H, Keiter S, König N, Rudolf M, Ulrich M, Braunbeck T (2003b) A new sediment contact assay to assess particle-bound pollutants using zebrafish (Danio rerio) embryos. J Soils Sediments 3:197–207
Hollert H, Heise S, Keiter S, Heininger P, Förstner U (2007) Wasserrahmenrichtlinie-Fortschritte und Defizite. Umweltwiss Schadstoff Forsch 19:58–70
Höss S, Ahlf W, Fahnenstich C, Gilberg D, Hollert H, Melbye K, Meller M, Hammers-Wirtz M, Heininger P, Neumann-Hensel H, Ottermanns R, Ratte HT, Seiler TB, Spira D, Weber J, Feiler U (2010) Variability of sediment-contact tests in freshwater sediments with low-level anthropogenic contamination—determination of toxicity thresholds. Environ Pollut 158:2999–3010
IKSR (2011) Rheinstoffliste 2011. IKSR-Bericht, 189d. Internationale Kommission zum Schutze des Rheines, Koblenz, 13 pp
ISO 7346–3 (1996) Water quality—part 3: flow-through method—determination of the acute lethal toxicity of substances to a freshwater fish [Rachydanio rerio Hamilton-Buchanan (Teleostei, Cyprinidae)]
Japenga J, Zschuppe KH, De Groot AJ, Salomons W (1990) Heavy metals and organic micropollutants in floodplains of the River Waal, a distributary of the River Rhine, 1958–1981. Neth J Agric Sci 38:381–397
Kammann U, Biselli S, Reineke N, Wosniok W, Danischewski D, Hühnerfuss H, Kinder A, Sierts-Herrmann A, Theobald N, Vahl H-H, Vobach M, Westendorf J, Steinhart H (2005) Bioassay-directed fractionation of organic extracts of marine surface sediments from the North and Baltic Sea—part II: results of the biotest battery and development of a biotest index. J Soils Sediments 5:225–232
Kamphues J, Schulz AJ, Gude K, Bruns-Weller E, Severin K, Appuhn H, Taube V (2011) Investigations concerning the use of dioxin exposed grassland along rivers by food producing ruminants with special regard to food safety. Organohalogen Compd 73:2040–2041
Kedziorek MAM, Dupuy A, Bourg ACM, Compère F (1998) Leaching of Cd and Pb from a polluted soil during the percolation of EDTA: laboratory column experiments modeled with a non-equilibrium solubilization step. Environ Sci Technol 32:1609–1614
Keiter S, Rastall A, Kosmehl T, Wurm K, Erdinger L, Braunbeck T, Hollert H (2006) Ecotoxicological assessment of sediment, suspended matter and water samples in the upper Danube River—a pilot study in search for the causes for the decline of fish catches. Environ Sci Pollut Res 13:308–319
Keiter S, Braunbeck T, Heise S, Pudenz S, Manz W, Hollert H (2009) A fuzzy logic-classification of sediments based on data from in vitro biotests. J Soils Sediments 9:168–179
Kennedy SW, Jones SP (1994) Simultaneous measurement of cytochrome P4501A catalytic activity and total protein concentration with a fluorescence plate reader. Anal Biochem 222:217–223
Klee N, Gustavsson L, Kosmehl T, Engwall M, Erdinger L, Braunbeck T, Hollert H (2004) Changes in toxicity and genotoxicity of industrial sewage sludge samples containing nitro- and amino-aromatic compounds following treatment in bioreactors with different oxygen regimes. Environ Sci Pollut Res 11:313–320
Klok C, Kraak MH (2008) Living in highly dynamic polluted river floodplains, do contaminants contribute to population and community effects? Sci Total Environ 406:455–461
Kühlers D, Bethge E, Hillebrand G, Hollert H, Fleig M, Lehmann B, Maier D, Maier M, Mohrlok U, Wölz J (2009) Contaminant transport to public water supply wells via flood water retention areas. Nat Hazards Earth Syst Sci 9:1047–1058
Lake IR, Foxall CD, Fernandes A, Lewis M, White O, Mortimer D, Dowding A, Rose M (2014) The effects of river flooding on dioxin and PCBs in beef. Sci Total Environ 491–492:184–191
Lee LE, Clemons JH, Bechtel DG, Caldwell SJ, Han KB, Pasitschniak-Arts M, Mosser DD, Bols NC (1993) Development and characterization of a rainbow trout liver cell line expressing cytochrome P450-dependent monooxygenase activity. Cell Biol Toxicol 9:279–294
Lerche D, Brüggemann R, Sørensen P, Carlsen L, Nielsen OJ (2002) Comparison of partial order technique with three methods of multi-criteria. J Chem Inf Comput Sci 42:1086–1098
Liß W, Ahlf W (1997) Evidence from whole-sediment, porewater, and elutriate testing in toxicity assessment of contaminated sediments. Ecotoxicol Environ Saf 36:140–147
LUBW (2011) Hochwasservorhersagezentrale Baden-Württemberg, http://www.hvz.badenwuerttemberg.de/ (accessed: 02.06.2011)
Maier D, Maier M, Fleig M (1997) Belastung von Grundwasseranreicherungsflächen mit Schadstoffen durch Schwebstoffeintrag, 2. Deutsch-Niederländischer Workshop Künstliche Grundwasseranreicherung, Schwerte, DVGW-Schriftenreihe Wasser Nr. 90, pp. 17–24
Maier M, Kühlers D, Brauch H-J, Fleig M, Jirka GH, Mohrlok U, Bethge E, Bernhart HH, Lehman B, Hillebrand G, Wölz J, Hollert H (2006) Flood retention and drinking water supply—preventing conflicts of interest. J Soils Sediments 6:113–114
Malmon DV, Dunne T, Reneau SL (2002) Predicting the fate of sediment and pollutants in river floodplains. Environ Sci Technol 36:2026–2032
Martin CW (1997) Heavy metal concentrations in floodplain surface soils, Lahn River. Germany Environ Geol 30:119–125
Martin C (2009) Recent changes in heavy metal storage in flood-plain soils of the Lahn River, central Germany. Environ Geol 58:803–814
Middelkoop H (2000) Heavy-metal pollution of the river Rhine and Meuse floodplains in The Netherlands. Geol Mijnb 79:411–428
Middelkoop H, Erkens G, van der Perk M (2010) The Rhine delta—a record of sediment trapping over time scales from millennia to decades. J Soils Sediments 10:628–639
Oetken M, Stachel B, Pfenninger M, Oehlmann J (2005) Impact of a flood disaster on sediment toxicity in a major river system—the Elbe flood 2002 as a case study. Environ Pollut 134:87–95
Pies C, Yang Y, Hofmann T (2007) Distribution of polycyclic aromatic hydrocarbons (PAHs) in floodplain soils of the Mosel and Saar River. J Soils Sediments 7:216–222
Power EA, Chapman PM (1992) Assessing sediment quality. In: Burton GA (ed) Sediment toxicity assessment. Lewis Publishers, Boca Raton, pp 1–18
Prantner J, Korherr U, Weber R (2005) A groundwater treatment plant for remediation of DDT, halogenated organics, arsenic and mercury at the Lago Maggiore (North Italy). Proceedings of the 8th HCH and Pesticide Conference, Sofia
Rader BR, Nimmo DWR, Chapman PL (1997) Phytotoxicity of floodplain soils contaminated with trace metals along the Clark Fork River, Grant-Kohrs Ranch national historic site, Deer Lodge, Montana. USA Environ Toxicol Chem 16:1422–1432
Rice PM, Ray GJ (1985) Heavy metals in flood plain deposits along the upper Clark Fork River. Proceedings-Clark Fork Symposium, Montana Academy of Sciences, Montana College of Mineral Science and Technology, Butte
Ricking M, Terytze K (1999) Trace metals and organic compounds in sediment samples from the River Danube in Russe and Lake Srebarna (Bulgaria). Environ Geol 37:40–46
Rönnpagel K, Liß W, Ahlf W (1995) Microbial bioassays to assess the toxicity of solid-associated contaminants. Ecotoxicol Environ Saf 31:99–103
Sauvé S, Hendershot W, Allen HE (2000) Solid-solution partitioning of metals in contaminated soils: dependence on pH, total metal burden, and organic matter. Environ Sci Technol 34:1125–1130
Schulz A-J, Wiesmüller T, Appuhn H, Stehr D, Severin K, Landmann D, Kamphues J (2005) Dioxin concentration in milk and tissues of cows and sheep related to feed and soil contamination. Anim Phys Anim Nutr 89:72–78
Schulze T, Ricking M, Schröter-Kermani C, Körner A, Denner H-D, Weinfurtner K, Winkler A, Pekdeger A (2007) The German Environmental Specimen Bank—sampling, processing, and archiving sediment and suspended particulate matter. J Soils Sediments 7:361–367
Schulze T, Seiler T-B, Streck G, Braunbeck T, Hollert H (2012) Comparison of different exhaustive and biomimetic extraction techniques for chemical and biological analysis of polycyclic aromatic compounds in river sediments. J Soils Sediments 12:1419–1434
Schüttrumpf H, Brinkmann M, Cofalla C, Frings RM, Gerbersdorf SU, Hecker M, Hudjetz S, Kammann U, Lennartz G, Roger S, Schaeffer A, Hollert H (2011) A new approach to investigate the interactions between sediment transport and ecotoxicological processes during flood events. Environ Sci Europe 23, 39-Article No.: 39
Schwartz R, Gerth J, Neumann-Hensel H, Bley S, Förstner U (2006) Assessment of high polluted fluvisol in the Spitelwasser floodplain. Based on national guideline value and MNA-criteria. J Soils Sediments 6:145–155
Schwarzbauer J (1997) Screening, identifizierung und quantitative analyse organischer substanzen in sediment und schwebstoff des elbesystems. Universität Hamburg, Hamburg, 282 pp
Stachel B, Jantzen E, Knoth W, Krüger F, Lepom P, Oetken M, Reincke H, Sawal G, Schwartz R, Uhlig S (2005) The Elbe flood in August 2002—organic contaminants in sediment samples taken after the flood event. J Environ Sci Health A Toxic Hazard Subst Environ Eng 40:265–287
Strecker R, Seiler T-B, Hollert H, Braunbeck T (2011) Oxygen requirements of zebrafish (Danio rerio) embryos in embryo toxicity tests with environmental samples. Comp Biochem Physiol C Toxicol Pharmacol 153:318–327
Tockner K, Pusch M, Borchardt D, Lorang MS (2010) Multiple stressors in coupled river-floodplain ecosystems. Freshw Biol 55:135–151
Torres JPM, Leite C, Krauss T, Weber R (2013) Landfill mining from a deposit of the chlorine/organochlorine industry as source of dioxin contamination of animal feed and assessment of the responsible processes. Environ Sci Pollut Res 20:1958–1965
Tuikka AI, Schmitt C, Höss S, Bandow N, von der Ohe PC, de Zwart D, de Deckere E, Streck G, Mothes S, van Hattum B, Kocan A, Brix R, Brack W, Barceló D, Sormunen AJ, Kukkonen JVK (2011) Toxicity assessment of sediments from three European river basins using a sediment contact test battery. Ecotoxicol Environ Saf 74:123–131
Ulrich M, Schulze T, Leist E, Glaß B, Maier M, Maier D, Braunbeck T, Hollert H (2002) Abschätzung des Gefährdungspotenzials für Trinkwasser und Korrelation verschiedener Expositionspfade (Acetonischer Extrakt, Natives Sediment) im Bakterienkontakttest und Fischeitest. Umweltwiss Schadstoff Forsch 14:132–137
Umlauf G, Bidoglio G, Christoph EH, Kampheus J, Krüger F, Landmann D, Schulz AJ, Schwartz R, Severin K, Stachel B, Stehr D (2005) The situation of PCDD/Fs and dioxin-like PCBs after the flooding of River Elbe and Mulde in 2002. Acta Hydrochim Hydrobiol 33:543–554
van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE (2006) The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci 93:223–241
van Gils J, van Hattum B, Westrich B (2009) Exposure modeling on a river basin scale in support of risk assessment for chemicals in European river basins. Integr Environ Assess Manag 5:80–85
Voigt K, Brüggemann R, Pudenz S (2006) A multi-criteria evaluation of environmental databases using the Hasse Diagram Technique (ProRank) software. Environ Model Softw 21:1587–1597
Weber R, Gaus C, Tysklind M, Johnston P, Forter M, Hollert H, Heinisch H, Holoubek I, Lloyd-Smith M, Masunaga S, Moccarelli P, Santillo D, Seike N, Symons R, Torres JPM, Verta M, Varbelow G, Vijgen J, Watson A, Costner P, Wölz J, Wycisk P, Zennegg M (2008a) Dioxin- and POP-contaminated sites—contemporary and future relevance and challenges. Environ Sci Pollut Res 15:363–393
Weber R, Tysklind M, Gaus C (2008b) Dioxin—contemporary and future challenges of historical legacies (editorial, dedicated to Otto Hutzinger). Environ Sci Pollut Res 15:96–100
Weber R, Watson A, Forter M, Oliaei F (2011) Persistent organic pollutants and landfills—a review of past experiences and future challenges. Waste Manag Res 29:107–121
Weber R, Hollert H, Ballschmiter KH, Kamphues J, Blepp M (2013) Relevanz von PCB Quellen für Rindfleisch: Boden–Futter–Punktquellen. Vortrag UBA Fachgespräch “Belastung von Rindfleisch mit PCB aus extensiver Haltung” 5. Februar 2013, BMU, Bonn
Wetterauer B, Ricking M, Otte JC, Hallare AV, Rastall A, Erdinger L, Schwarzbauer J, Braunbeck T, Hollert H (2012) Toxicity, dioxin-like activities, and endocrine effects of DDT metabolites-DDA, DDMU, DDMS, and DDCN. Environ Sci Pollut Res 19:403–415
Witter B, Francke W, Franke S, Knauth H-D, Miehlich G (1998) Distribution and mobility of organic micropollutants in River Elbe floodplains. Chemosphere 37:63–78
Wölz J, Engwall M, Maletz S, Olsman Takner H, van Bavel B, Kammann U, Klempt M, Weber R, Braunbeck T, Hollert H (2008) Changes in toxicity and Ah receptor agonist activity of suspended particulate matter during flood events at the rivers Neckar and Rhine—a mass balance approach using in vitro methods and chemical analysis. Environ Sci Pollut Res 15:536–553
Wölz J, Brack W, Möhlenkamp C, Claus E, Braunbeck T, Hollert H (2010a) Effect-directed analysis of Ah receptor-mediated activities caused by PAHs in suspended particulate matter sampled in flood events. Sci Total Environ 408:3327–3333
Wölz J, Fleig M, Schulze T, Maletz S, Lübcke-von Varel U, Reifferscheid G, Kühlers D, Braunbeck T, Brack W, Hollert H (2010b) Impact of contaminants bound to suspended particulate matter in the context of flood events. J Soils Sediments 10:1174–1185
Wölz J, Grosshans K, Streck G, Schulze T, Rastall A, Erdinger L, Brack W, Fleig M, Kühlers D, Braunbeck T, Hollert H (2011a) Estrogen receptor mediated activity in bankside groundwater, with flood suspended particulate matter and floodplain soil—an approach combining tracer substance, bioassay and target analysis. Chemosphere 85:717–723
Wölz J, Schulze T, Lübcke-von Varel U, Fleig M, Reifferscheid G, Brack W, Kühlers D, Braunbeck T, Hollert H (2011b) Investigation on soil contamination at recently inundated and non-inundated sites. J Soils Sediments 11:82–92
Wu L, Chen L, Hou J, Zhang Y, Zhao J, Gao H (2010) Assessment of sediment quality of Yangtze River estuary using zebrafish (Danio rerio) embryos. Environ Toxicol 25:234–242
Zennegg M, Schmid P, Tremp J (2010) PCB fish contamination in Swiss rivers—tracing of point sources. Organohalogen Compd 72:362–365
Acknowledgments
This study was supported by the Stadtwerke Karlsruhe (Germany) during the project «Ecotoxicological assessment of the Rhine sediments and suspended particulate matter in inundated areas». Volker Garke performed the cytotoxicity and EROD bioassays within his state examination thesis (Garke 2003) and friendly provided the raw data for publication. We are grateful to Mr. Beiser (Stadtwerke Karlsruhe) for technical support during the sampling as well as to Beate Kemink, L. Dunne, H. Johannsen, Dr. Anne Seebach, und Manuela Scholz for technical support of the laboratory analyses. Dr. Emma Schymanski (Eawag, Dübendorf, Switzerland) gave valuable comments to a previous version of the manuscript, which is part of the dissertation thesis of TS (urn:nbn:de:kobv:188-fudissthesis000000094124-8). Supplementary material is provided for download at the Springer website.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1788 kb)
Rights and permissions
About this article
Cite this article
Schulze, T., Ulrich, M., Maier, D. et al. Evaluation of the hazard potentials of river suspended particulate matter and floodplain soils in the Rhine basin using chemical analysis and in vitro bioassays. Environ Sci Pollut Res 22, 14606–14620 (2015). https://doi.org/10.1007/s11356-014-3707-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3707-9