Abstract
With the rapid development of urbanization, the number of urban sewage treatment plants is increasing, wastewater treatment volume is gradually becoming large, and correspondingly, the sludge production capacity has a rapid growth. As a new method of sludge disposal, sludge carbonization is characterized by low energy consumption, simple products, and wide resource utilization prospects, which is of great help to solve problems of current sludge disposal in China. The residual sludge from sewage plant was used as raw material in this study in order to investigate the physical and chemical properties of sludge charcoal after high temperature carbonization and explore the enhancement in the removal of pollutants including CODcr, NH3-N, TN, and TP during sewage treatment with the used sludge charcoal. The results show that the optimal dosing amount of sludge charcoal was 2 g.L−1 when it was added into SBR equipment at one time, while the optimal dosing amount is 0.06 g.L−1 when it was added into SBR equipment with each influent process. The enhanced removal effect of pollutants in sewage treatment process mainly depended on the physical adsorption and intensified bio-degradation of sludge charcoal, and activated sludge and sludge charcoal were synergistic in water treatment. The removal effect of pollutants is strengthened in the physical adsorption—bio-degradation—sludge charcoal reproduction—re-adsorption system. These suggested that sludge charcoal could be promising for the enhancement of pollutant removal in sewage through activated sludge process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The process of urbanization in China has been accelerating in recent years. To ensure the health of natural environment and people’s living environment, there was increasing in the number of urban sewage treatment plants and the amount of sewage treatment capacity (Y. Wang et al. 28). The sludge production capacity showed a sharp growing trend correspondingly. Municipal sludge carries pathogenic microorganisms (J. Werther et al. 30), parasite eggs (A. Figura et al. 6), refractory heavy metals, persistent organic substances (P. Oleszczuk et al. 22), and other substances that are subject to make pollution to water and the atmosphere, thus damaging ecological environment and human health. Municipal sludge also contains a wide variety of heavy metals, mainly Cu, Pb, Zn, Ni, Cr, Hg, and Cd (T. Chen et al. 3), which is characterized by easy migration, easy enrichment, and high hazard (X. Wang et al. 26). These substances may cause great harm to the ecological environment and human health if improperly disposed of. On the other hand, sludge has potential utilization value, and it contains considerable organic matter, nitrogen, phosphorus, potassium, and various trace elements, and the feasibility of energy and resource utilization exists (Y. Li et al. 16). Therefore, appropriate treatment and disposal of sludge has become an important research topic in recent years.
Traditional sludge disposal methods mainly include landfill, composting, incineration, and land use (R. Fu et al. 7). These methods have gradually exposed different problems in practical application. The sanitary condition of sludge landfill is poor with big odor and lots of mosquitoes and flies (Y. Zhu et al. 39), putting pressure on the environment. Meanwhile, sludge landfill covers a large area. The site selection is difficult and the geographical location is remote (S. P. McGrath et al. 21), which makes the sludge operation cost too high. Sludge composting method is characterized by a large area, long processing time, and much production of odor. Heavy metals in sludge will cause soil pollution, hindering the realization of sludge harmlessness (S. Wang et al. 27). Sludge incineration will produce toxic gases such as NOx, SOx, HCl, and dioxins (H. Li et al. 17), causing secondary pollution to the environment. Sludge land use includes landscaping, soil improvement, and agricultural utilization (J. Yu et al. 36), being characterized by low energy consumption and operating cost, contributing to investment saving. Sludge contains large amount of nitrogen, phosphorus, and many other trace elements that are necessary to plants’ growth, so it has the possibility to be used as grow fertilizer (D. Fytili et al. 8). But excessive application of sludge will lead to heavy metal infiltration of groundwater.
The basic principle of sludge carbonization is to pyrolysis organic matter in the sludge by heating up in anoxic or anaerobic conditions to generate combustible volatile gas composed mainly of hydrocarbons (S. Li et al. 18). The energy released by the process of combustion is used to dry the sludge (J. A. Conesa et al. 4), so that water in sludge is released and the carbon content in excess sludge is retained to the maximum extent. Sludge carbonization can convert surplus sludge into valuable products and concentrate heavy metals in it (Z. Jin et al. 12) and realize the heavy metal immobilization of sludge. The weight and volume of sludge can be reduced by sludge carbonization. The organic components in the sludge are converted into usable energy, the resource utilization value of its products are improved, and the heat energy of sludge is recovered at the same time, achieving the purpose of energy saving (W. A. N. Liguo et al. 19). Compared with traditional sludge treatment methods, sludge carbonization effectively reduces the harm of heavy metals, viruses, and bacteria. It has no secondary pollution and is more energy saving, which can realize thorough treatment of sludge, so that the investment cost will be reduced, the product utilization value is higher, and the product is more widely used (X. Yang et al. 35; D. Xiao et al. 33; L. Xiang et al. 32).
In order to make full use of carbonaceous organics and recover the energy in the sludge, so as to realize resource utilization of sludge, many scholars have conducted further research on the sludge carbonization products in recent years. Research showed that the product from high-temperature carbonization of municipal sludge was characterized by a large specific surface area, rich internal pore structure, and strong adsorption capacity after activation and modification (J. M. Dias et al. 5), which could be used for the removal of organics and heavy metals in wastewater and the treatment of waste gas. In addition, sludge charcoal could also be used to improve the effect of activated sludge process in wastewater treatment. He et al. (11) comprehensively analyzed the adsorption effect of sludge-activated carbon on pollutants in wastewater and found that sludge-activated carbon prepared via chemical activation had strong metal ion adsorption capacity. It had broad prospects as dye adsorbent since its adsorption capacity of dye was higher than that of commercial activated carbon. Its surface chemical properties could improve the adsorption capacity of phenol and phenolic compounds and other pollutants such as COD, chroma and phosphate. Zhang et al. (38) comprehensively introduced recent research status of preparation methods of sludge-based activated carbon: (1) sludge-activated carbon had a significant effect on metal ion adsorption; (2) the sludge-activated carbon was feasible to adsorb dyes; (3) in recent years, the treatment of wastewater that adsorbed antibiotics and phenols with sludge-activated carbon has attracted much attention. Racek et al. (24) reviewed the derived biochar from sludge pyrolysis. Unlike activated carbon, the production of sludge-derived biochar did not require high temperature and additional activation process, so the production cost was low, and it had abundant raw materials, making it a potential low-cost new adsorbent. Gopinath et al. (10) reviewed effects of sludge-derived biochar on pollutant removal through adsorption and catalysis. In summary, sludge carbonization products had a certain adsorption capacity for pollutants in sewage, so it was feasible to add sludge charcoal to the activated sludge process of sewage treatment to improve the removal effect of pollutants. In this paper, the strengthening effect of sludge charcoal on activated sludge process in sewage treatment was explored through experiments. The experiment was divided into two stages. In the first stage, the sludge charcoal was added into the activated sludge SBR reactor in a certain proportion at one time, that is, excessive sludge charcoal was added only in the initial stage of the entire experimental cycle. Then, detect changes in the removal rates of CODcr, NH3-N, TN, and TP in the effluent. In the second stage, the sludge charcoal was added into the activated sludge SBR reactor in a certain proportion respectively, that is, a certain amount of sludge charcoal was added in each influent stage of the entire experimental cycle. Then, detect changes in the removal rates of CODcr, NH3-N, TN, and TP in the effluent and analyze the causing factors.
Materials and methods
Characteristics of sludge charcoal
The used sludge charcoal in this research was prepared referring to the methodology in the literature “From pollutant to solution of wastewater pollution: Synthesis of activated carbon from textile sludge for dye adsorption”: sludge samples were first dried in an oven at 105 ℃ for 24 h to a constant weight and then carbonized in a furnace with nitrogen flow at a heating rate of 5 ℃.min−1 to 650 ℃. After holding for 30 min, the product was rinsed with 10 wt% solution of HCl to eliminate excess dehydrating agents and soluble ash, then rinsed the carbonization with distilled water and dried in an oven at 80 ℃ for 24 h to obtain sludge charcoal (S. Wong et al. 31). Scanning electron microscope was used to observe the surface characteristics of sludge charcoal powder samples. SEM images (Fig. 1) present its rough surface and abundant internal pore structure, indicating that it had the potential to absorb organic pollutants in wastewater. The specific surface area and porosity of sludge charcoal powder sample were detected. The measurement results are shown in Table 1.
The physical and chemical properties of sludge charcoal were slightly inferior to commercially available activated carbon, but sludge charcoal could solve the disposal problem of residual sludge to a certain extent and could realize the reuse of sludge waste. Therefore, sludge charcoal has high research value. The internal pore of sludge charcoal was mainly mesopore, with an average adsorption pore size of 3.7 nm, equaling to the size of the highly carcinogenic gas dioxins (B. Krizanec et al. 14), thus possessing a high adsorption removal rate of it.
Sludge charcoal was mainly composed of C, H, O, N, and S elements, respectively, accounting for 29%, 1.97%, 8.45%, 1.12%, and 0.24%, resulting from determination, which was closely related to the functional groups on the sludge charcoal.
The analysis of metal elements in sludge charcoal powder is shown in Table 2. It can be seen that the content of Al, Ca, and Fe elements were much higher than that of other metal elements. The reason may be that Fe(OH)3 and Al(OH)3 colloid formed by hydrolysis of aluminum salt and iron salt added in sewage could enlarge sludge floc, thus promoting its settlement (C. S. Lee et al. 15), and at the same time adsorb impurities in water to achieve the effect of water purification, so that the generated sludge contained rich Al and Fe elements.
Fourier infrared spectrometer was used to determine the functional groups contained in sludge charcoal. The measurement range was 500–4000 cm−1. Multiple absorption peaks can be seen in the FTIR spectrum (Fig. 2).
Among them, the absorption peak between 3650 and 3200 cm−1 belonged to the stretching vibration of hydrogen–oxygen bonds in hydroxyl groups (-OH); the absorption peak between 2935 and 2915 cm−1 belonged to the anti-symmetric stretching vibration in methylenes (-CH2-); the absorption peak at 1650 cm−1 belonged to the stretching vibration of carbonyl groups (-C = O); the absorption peak at 1541 cm−1 belonged to the stretching vibration of carbon–carbon double bonds in benzene ring; the absorption peak at 1404.70 cm−1 belonged to the anti-symmetric stretching vibration in methyl groups (-CH3); the absorption peak at 1061.04 cm−1 belonged to the Si–O-Si stretching vibration in quartz. The absorption peaks had larger amplitudes at 3378.45 cm−1, 1650 cm−1, and 1061.04 cm−1, indicating that there were more hydroxyl, carbonyl, and Si–O-Si functional groups on the surface of sludge charcoal (A.-L. Ren et al. 25; W. Zhang et al. 37; R. E. N. Ailing et al. 1).
Experiment preparation and methods
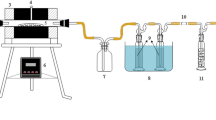
In the experiment, a simulated SBR reactor was used as the activated sludge reaction system, with a total of 5 systems. Operation parameters of each reactor were controlled under same conditions. The operation cycle T of each reactor was 8 h. Treatment processes included water intake, aeration, precipitation, and drainage.
The sludge charcoal powder and water were put into a branched conical flask, then pressurize and vacuum the conical flask from the branch pipe for 2–3 min. Residual gas in the internal pores of charcoal was replaced into liquid through vacuum treatment, which could improve the contact efficiency between the surface of charcoal and liquid, thus enhancing the adsorption capacity of sludge charcoal material in the liquid phase. Then, the sludge charcoal liquid was obtained.
The initial MLVSS/MLSS of the experimental activated sludge was 0.55. The experimental water was prepared artificially. Glucose, ammonium chloride, and potassium dihydrogen phosphate were used as carbon, nitrogen, and phosphorus sources. At the same time, trace element nutrient solution was prepared to meet the needs of microbial growth. The experimental activated sludge was put into a 100-L organic glass container and diluted with water for 24 h. Then, domestic sewage was prepared according to the ratio of C:N:P = 100:5:1, and an appropriate amount of nutrient solution was added for domestication and culture of activated sludge. After running 1 week continuously, the activated sludge changed from light black to yellow brown. Its MLVSS/MLSS increased from 0.55 to 0.69, and it could be used for experiment when free bacteria and bell worms could be found in microscopic examination.
The research was divided into two stages, considered as two different adding methods that sludge charcoal was added into activated sludge SBR test equipment (1) at one time; (2) with each influent process, and they had different adding ratios of sludge carbonization liquid. In the first method, sludge charcoal was added at the ratio of 0.5 g.L−1, 1 g.L−1, 2 g.L−1, and 3 g.L−1 at one time, and in the second method, sludge charcoal was added at the ratio of 0 g.L−1, 0.02 g.L−1, 0.04 g.L−1, 0.06 g.L−1, and 0.08 g.L−1 with each influent process.
Put activated sludge into the SBR reactor, and add the carbonization in different proportions as control experiment. Each stage ran for 20 days. Considering the addition of sludge charcoal decreases as the water comes out in the second method and affects the experiment, the experiment was designed as an independent experiment which means the SBR reactors were disposable after each cycle of the treatment process. And in the next cycle, original volume of sludge charcoal liquid would be added along with new volume of sludge charcoal liquid into new SBR reactors to realize the layering effect of sludge charcoal in relatively ideally situation. The simulated SBR reactors were laboratory size. Then, detect the concentration of pollutants in the influent and effluent and analyze the removal effect of pollutants by comparing each experiment group. The SBR reactor adopted instantaneous water intake. The running time of stirring, aeration, and sedimentation was respectively 2 h, 5 h, and 1 h. Then, it used instantaneous drainage afterwards.
Analysis methods
By comparing the change of pollutant index concentration in and out of the water of each SBR reactor, the enhancement effect of different adding methods and adding ratios of sludge charcoal liquid on biological treatment of activated sludge was analyzed. The pollutant index involved and its determination method are shown in Table 3.
Results
Results in the first stage
The sludge charcoal liquid was added to the SBR reactor at the ratio of 0 g.L−1, 0.5 g.L−1, 1 g.L−1, 2 g.L−1, and 3 g.L−1 according to the treated water volume for continuous cycle of intermittent cultivation. The aeration volume of the reactor was controlled by gas flowmeter, and the duration of cultivation and monitoring cycle was 20 days. The composition and concentration of simulated domestic wastewater are shown in Table 4.
Analysis on the promotion of removal effect of COD cr
Changes of the CODcr concentration in influent and effluent from the SBR reactor and the CODcr removal efficiency are shown in Fig. 3.
During the experiment, the influent CODcr concentration in the reactor was between 413.90 and 506.27 mg.L−1. The effluent CODcr concentration of each experimental device was 15.70–34.33 mg.L−1, 10.81–25.90 mg.L−1, 11.84–28.09 mg.L−1, 12.15–24.72 mg.L−1, and 12.10–31.79 mg.L−1. The average CODcr removal rate was respectively 94.75%, 95.98%, 96.00%, 95.83%, and 95.37%. Compared with the control group, the removal efficiency of CODcr was increased by 1.23%, 1.25%, 1.08%, and 0.62% respectively when the amount of sludge charcoal powder added in the reactor was separately 0.5 g.L−1, 1 g.L−1, 2 g.L−1, and 3 g.L−1.
In general, the activated sludge system after adding sludge charcoal had no obvious improvement on the removal of CODcr in effluent. The reason may be that the carbon source used in domestic sewage was glucose, which was easy to be rapidly degraded by microorganisms in activated sludge. As a result, the original removal rate of CODcr was high, and the promotion of removing CODcr via activated sludge method was inconspicuous. In addition, with the increasing amount of charcoal material added, the removal rate of CODcr decreased on the contrary, since the addition amount of charcoal powder led to high sludge concentration, in which the sludge was easy to settle, and the aeration effect was not good. And when the amount of sludge charcoal exceeded, there would be interaction between the charcoal, leading to desorption phenomenon (F. A. N. Xiaodan et al. 34). Consequently, the activity of activated sludge became lower, and the removal rate of CODcr was reduced.
Analysis on the promotion of removal effect of NH 3 -N
Changes of the NH3-N concentration in influent and effluent from the SBR reactor and the NH3-N removal efficiency are shown in Fig. 4.
During the experiment, the influent NH3-N concentration in the reactor was between 24.25 and 41.25 mg.L−1. The effluent NH3-N concentration of each experimental device was 5.60–13.60 mg.L−1, 2.90–10.70 mg.L−1, 2.79–11.00 mg.L−1, 3.50–10.80 mg.L−1, and 2.70–11.30 mg.L−1. The average NH3-N removal rate was respectively 68.92%, 76.67%, 77.11%, 76.97%, and 77.50%.
By comparing the removal effects of NH3-N with different amount of sludge charcoal powder, it can be seen that compared with the operating device without adding charcoal powder, those adding charcoal had different degrees of improvement effect on the reduction of NH3-N concentration in effluent. When the amount of sludge charcoal was respectively 0.5 g.L−1, 1 g.L−1, 2 g.L−1, and 3 g.L−1, the NH3-N removal efficiency increased separately at the rate of 7.75%, 8.19%, 8.05%, and 8.58%. With the increase of sludge charcoal dosage, the enhancement of removal effect of NH3-N showed a trend of rising and then flattening. In addition, it can also be seen from the figure that in the early stage of the reaction, the addition of charcoal had a relatively obvious improvement effect on the removal of NH3-N in sewage, but when the reaction continued to proceed, the magnitude of the enhancement gradually decreased. It may be presumed that sludge charcoal had certain adsorption effect, so the removal of NH3-N in the early stage of the reaction mainly depended on the adsorption effect of sludge charcoal. As the reaction proceeded, sludge charcoal gradually presented adsorption saturation; thus, the removal of pollutants decreased correspondingly.
Analysis on the promotion of removal effect of TN
Changes of the TN concentration in influent and effluent from the SBR reactor and the TN removal efficiency are shown in Fig. 5.
During the experiment, the influent TN concentration in the reactor was between 29.85 and 51.40 mg.L−1. The effluent TN concentration of each experimental device was 10.07–23.26 mg.L−1, 6.74–20.34 mg.L−1, 7.46–21.29 mg.L−1, 6.74–20.80 mg.L−1, and 6.13–19.57 mg.L−1. The average TN removal rate was respectively 59.02%, 66.90%, 68.13%, 68.85%, and 67.33%.
It can be seen that compared with the operating device without adding charcoal powder, those adding charcoal powder had a great improvement effect on the reduction of TN concentration in effluent. When the amount of charcoal was respectively 0.5 g.L−1, 1 g.L−1, 2 g.L−1, and 3 g.L−1, the TN removal efficiency increased separately at the rate of 7.88%, 9.11%, 9.83%, and 8.31%. With the increase of the adding proportion of charcoal, the removal effect of TN showed a trend of first increasing and then decreasing. In terms of the removal effect of TN, the optimal dosing amount of charcoal was 2 g.L−1.
The overall removal rate of TN was lower than that of NH3-N, since NH3-N was included in TN which also contains organic nitrogen, nitrite, and nitrate nitrogen. But the increase of removal rate of TN was higher than that of NH3-N in general. It may be influenced by the original removal rate. Given that the original removal rate of NH3-N was higher, it had less upside and would quickly reach the adsorption saturation. As a result, the increase of removal rate of TN showed a larger number.
Analysis on the promotion of removal effect of TP
Changes of the TP concentration in influent and effluent from the SBR reactor and the TP removal efficiency are shown in Fig. 6.
During the experiment, the influent TP concentration in the reactor was between 1.80 and 5.40 mg.L−1. The effluent TP concentration of each experimental device was 0.80–3.20 mg.L−1, 0.45–2.85 mg.L−1, 0.55–2.70 mg.L−1, 0.50–2.68 mg.L−1, and 0.60–2.75 mg.L−1. The average TP removal rate was respectively 55.01%, 64.52%, 65.74%, 66.00%, and 62.63%.
The results show that compared with the operating device without adding charcoal powder, those adding charcoal powder had a great improvement effect on the reduction of TP concentration in effluent. When the amount of charcoal was respectively 0.5 g.L−1, 1 g.L−1, 2 g.L−1, and 3 g.L−1, the TP removal efficiency increased separately at the rate of 9.51%, 10.73%, 10.99%, and 7.62%. With the increase of the adding proportion of charcoal, the removal effect of TP showed a trend of first increasing and then decreasing. When the dosing amount was 2 g.L−1, it had the highest improvement of removal effect.
Results in the second stage
The sludge charcoal liquid was added to the SBR reactor at the ratio of 0 g.L−1, 0.02 g.L−1, 0.04 g.L−1, 0.06 g.L−1, and 0.08 g.L−1 according to the treated water volume with each influent process. The units operated continuously for 20 days, and the domestic wastewater treatment period was 8 h. The composition and concentration of simulated domestic wastewater are shown in Table 5.
Analysis on the promotion of removal effect of COD cr
Changes of the CODcr concentration in influent and effluent from the SBR reactor and the CODcr removal efficiency are shown in Fig. 7.
During the experiment, the influent CODcr concentration in the reactor was between 342.72 and 478.94 mg.L−1, averaging at 398.88 mg.L−1. The average effluent CODcr concentration of each experimental device was 13.98 mg.L−1, 11.80 mg.L−1, 11.19 mg.L−1, 10.65 mg.L−1, and 11.23 mg.L−1. The average CODcr removal rate was respectively 96.48%, 97.02%, 97.17%, 97.30%, and 97.17%. Compared with the control group that had no charcoal powder in it, the removal efficiency of CODcr increased by 0.54%, 0.69%, 0.82%, and 0.69% respectively when the amount of sludge charcoal powder added in the reactor was separately 0.02 g.L−1, 0.04 g.L−1, 0.06 g.L−1, and 0.08 g.L−1.
With the increasing amount of charcoal powder added in, the removal rate of CODcr showed a trend of first rising and then falling. It could be analyzed that the result in the second stage of the experiment was almost same as that in the first stage, that is, the activated sludge system after adding sludge charcoal had no obvious improvement on the removal of CODcr in effluent. It came to the inference that the carbon source used in domestic sewage was glucose, which was easy to be rapidly degraded by microorganisms in activated sludge, so the original removal rate of CODcr was high and the increase of treating CODcr through activated sludge method was unobvious.
Analysis on the promotion of removal effect of NH 3 -N
Changes of the NH3-N concentration in influent and effluent from the SBR reactor and the NH3-N removal efficiency are shown in Fig. 8.
During the experiment, the influent NH3-N concentration in the reactor was between 28.95 and 39.97 mg.L−1, averaging at 33.01 mg.L−1. The average effluent NH3-N concentration of each experimental device was 8.39 mg.L−1, 7.31 mg.L−1, 6.79 mg.L−1, 6.56 mg.L−1, and 6.68 mg.L−1. The average NH3-N removal rate was respectively 74.71%, 78.02%, 79.65%, 80.30%, and 79.96%. Compared with the control group that had no sludge charcoal powder in it, the removal efficiency of NH3-N increased by 3.31%, 4.95%, 5.59%, and 5.25% respectively when the amount of sludge charcoal powder added in the reactor was separately 0.02 g.L−1, 0.04 g.L−1, 0.06 g.L−1, and 0.08 g.L−1. As can be seen, the removal rate of NH3-N raised up at first and then went down with growing amount of sludge charcoal added to the wastewater, and the optimal dosing amount was 0.06 g.L−1.
Analysis on the promotion of removal effect of TN
Changes of the TN concentration in influent and effluent from the SBR reactor and the TN removal efficiency are shown in Fig. 9.
The influent TN concentration in the whole reaction was between 34.34 and 42.9 mg.L−1, averaging at 38.59 mg.L−1. The average effluent TN concentration of each experimental device was 13.94 mg.L−1, 12.00 mg.L−1, 11.13 mg.L−1, 10.53 mg.L−1, and 10.89 mg.L−1. The average TN removal rate was respectively 63.92%, 67.00%, 71.25%, 72.80%, and 71.90%. It can be seen that compared with the operating device without adding charcoal powder, those adding charcoal powder had a great improvement effect on the reduction of TN concentration in effluent. The removal efficiency increased by 3.08%, 7.33%, 8.88%, and 7.98% respectively when the amount of sludge charcoal powder added in the reactor was separately 0.02 g.L−1, 0.04 g.L−1, 0.06 g.L−1, and 0.08 g.L−1. The removal rate of TN showed a trend of first rising and then decreasing with growing amount of sludge charcoal added to the wastewater, and the optimal dosing amount was 0.06 g.L−1. And it can be seen in the figure that, at first, the addition of charcoal showed unobvious improvement in removing TN. As the reaction proceeded, the removal efficiency raised up and maintained at a steady state. The data above showed the similar trend as those in the first stage, that is, the overall removal rate of TN was lower than that of NH3-N, and the increase of removal rate of TN was higher than that of NH3-N in general.
Analysis on the promotion of removal effect of TP
Changes of the TP concentration in influent and effluent from the SBR reactor and the TP removal efficiency are shown in Fig. 10.
The influent TP concentration in the whole reaction was between 3.11 and 4.2 mg.L−1, averaging at 3.58 mg.L−1. The average effluent TP concentration of each experimental device was 1.27 mg.L−1, 1.16 mg.L−1, 1.12 mg.L−1, 1.11 mg.L−1, and 1.13 mg.L−1. The average TP removal rate was respectively 64.70%, 67.43%, 68.80%, 69.09%, and 68.37%. It can be seen that compared with the operating device without adding charcoal powder, those adding charcoal powder had a great enhancement effect on the reduction of TP concentration in effluent. The removal efficiency increased by 2.73%, 4.1%, 4.39%, and 3.67% respectively when the amount of sludge charcoal powder added in the reactor was separately 0.02 g.L−1, 0.04 g.L−1, 0.06 g.L−1, and 0.08 g.L−1. The removal rate of TP showed a trend of first rising and then decreasing with growing amount of sludge charcoal added to the wastewater, and the optimal dosing amount was 0.06 g.L−1.
The general increase of removal rate of TP was lower than that of NH3-N and TN. The reason may be that the carbon source used in domestic sewage was glucose. Kargi et al. (13) found that when glucose was used alone as the carbon source, phosphorus release and uptake rates were the lowest. Pijuan et al. (23) found that under anaerobic conditions, when acetic acid, propionic acid, butyric acid, and glucose were used as carbon sources, PHA was synthesized with the lowest efficiency using glucose as carbon source. And different from the first stage, the initial amount of sludge charcoal added to the sewage was smaller. As a result, the final increase removal rate of TP was lower than that of NH3-N and TN.
Comparison of the treatment effect between the two adding forms
By comparing the promotion effect of removing pollutants in activated sludge method between the two adding forms, it can be seen that when sludge charcoal was added into SBR reactors at certain ratio at one time, the enhancement in treatment effect of CODcr, NH3-N, TN, and TP was superior to that when charcoal powder was added into reactors with each influent process. The experiment showed that when sludge charcoal was added through the first method, the effluent water quality improved in the preceding period, and in the later period, the promotion effect of removing pollutants decreased. When sludge charcoal was added through the second method, the enhancement of treatment effect was unobvious, but with the extension of the reaction period, it rose and then maintained at a steady state. The difference may be attributed to the physical adsorption of pollutants by charcoal compounds. When adopting the first adding form, sludge charcoal had large initial amount, intensifying its adsorption capacity of pollutants in sewage, so the effluent had good water quality in the preceding period. With the reaction proceeding, sludge charcoal gradually achieved saturation, which slowed down the removal efficiency of pollutants. Compared with the initial stage, the enhancement effect in removing the pollutants decreased. When adopting the second adding form, sludge charcoal had little initial amount, and the adsorption effect was not obvious. With the reaction period extending, the accumulated amount of charcoal addition grew, the adsorption capacity rose, and with certain ratio of charcoal added into each influent process, the improvement effect in removing pollutants could maintain at a steady state. Meanwhile, the promotion of removing rate was inconspicuous in the first adding method when the amount of charcoal increased could be attributed to that the adding amount exceeded the optimal adding amount. On the other hand, the large adding amount in initial period would lead to high concentration of activated sludge, making it easy to settle and produce dead space during the aeration, thus reducing oxygen transmission efficiency. Comprehensively consider with data, the optimal dosing amount was 2 g.L−1. When adopting the second adding form, the experiment showed that the promotion of removing rate had a trend of first rising and then declining with the increase of charcoal dosing amount, indicating the existence of optimal amount. Since the adding volume was small, the sludge sedimentation phenomenon caused by the increasing concentration of activated sludge would not exist. The experiment data showed that its optimal dosing amount was 0.06 g.L−1.
Discussion
It can be seen from the experimental results that the addition of sludge charcoal could enhance the removal effect of pollutants from sewage in activated sludge process. It can be analyzed that, firstly, the sludge charcoal had a large specific surface area and porous structure. The sludge charcoal powder and liquid were mixed the vacuum liquefaction equipment, in which the contact efficiency between the surface of charcoal and liquid in internal pores was raised up, thus improving the adsorption capacity of sludge charcoal. By analyzing samples in the second method that sludge charcoal was added at the ratio of 0 g.L−1, 0.02 g.L−1, 0.04 g.L−1, 0.06 g.L−1, and 0.08 g.L−1 with each influent process, the activated sludge concentration MLSS and SV30 were measured every 3 days during the experiment period, and the sludge volume index SVI = SV30/MLSS was obtained, which could reflect the sludge settling property. It was found that after a period of time, the control group began to show an increasing trend of SVI, while the SVI of the experimental group remained stable. It was observed that the microstructure of activated sludge floc after adding different proportions of charcoal, indicating that adding sludge carbonization to activated sludge could help form a compact floc structure with sludge charcoal powder as the core, so that the sludge volume got larger and the structure became compact, which could achieve stability rapidly, so as to improve the settlement performance of activated sludge. Therefore, the enhancement of the removal effect of pollutants in activated sludge process is partly due to the enhanced physical adsorption of sludge charcoal.
In addition, the Fourier infrared spectroscopy (FTIR) analysis results showed the increase of hydroxyl groups along with other functional groups that promoted the formation of ionic bonds with polar organic substances and enhanced the adsorption capacity (Lixiaona et al. 20). The oxygen-containing functional groups in sludge charcoal increased the overall hydrophilicity of the activated sludge, along with the surface polarity, resulting in the increase of affinity for organic substances, so as to improve the removal effect of organic pollutants through activated sludge process in sewage.
Moreover, sludge charcoal was easy to be attached by microorganisms. Considering the activated sludge concentration as the indicator to represent the biomass in each reactor, in order to avoid the impact of the quality of sludge charcoal on the sludge concentration, the corresponding quality of added charcoal should be subtracted when determining the sludge concentration in the experimental group. We calculated the biomass of the reactor via this method, and found that the biomass in the reactor after adding sludge charcoal was higher than that in the control group, indicating that it was easy to be attached by microorganisms. Their growth on its surface could increase the density of Zoogloea. Therefore, at the initial stage of influent, it could absorb some of pollutants and reduce the impact of high concentration of pollutants in influent on microbial system in activated sludge system, thus improving the adaptability of activated sludge system to adverse environment. In the later stage of the reaction, the adsorbed organic matter could provide nutrients for the growth of microorganisms, so that the activated sludge system could maintain a high biomass. In other words, the addition of sludge charcoal powder would affect the abundance ratio of activated sludge microbial colonies to a certain extent, promote the removal and degradation ability of activated sludge to macromolecular organic matter and toxic substances, and effectively prevent sludge swelling. Therefore, the enhancement of the removal effect of pollutants in activated sludge process is also partly attributed to the enhanced biochemical reactions of microorganisms in activated sludge species.
It can be concluded from the above that the enhancement of the removal effect of pollutants in activated sludge process by adding sludge charcoal mainly came from physical adsorption and enhanced bio-degradation. At the same time, the microorganisms had a biological regeneration effect on sludge charcoal, so that adsorption sites in the charcoal could be partially reserved, and new pollutants could be adsorbed. In the physical adsorption—bio-degradation—sludge charcoal reproduction—re-adsorption system, the removal effect of pollutants was strengthened, so activated sludge and sludge charcoal are synergistic in water treatment, which complement and promote each other to improve the effect of sewage treatment. The results are consistent with the findings of previous studies (M. Chen et al. 2; K. George et al. 9; G. Wang et al. 29).
Conclusion
The sludge charcoal prepared from municipal sludge was similar to activated carbon, with rough surface and rich internal pore structure, which had good adsorption and removal effect on toxic gas dioxins. It was a by-product of sewage treatment, with large yield and low preparation cost, which could not only improve the utilization value of sludge, but also solve the problem of treatment and disposal of residual sludge to a certain extent, owning great significance to realize the resource utilization of sludge.
The experimental results of adding sludge charcoal to the activated sludge process of wastewater treatment to investigate its enhancement effect on pollutant removal show that the addition of sludge charcoal helped to improve the efficiency of the activated sludge method in removing pollutants, and its resource utilization was feasible. In the first stage of the experiment, when sludge charcoal was added into SBR reactors at certain ratio at one time, the enhancement of removal efficiency of pollutants was obvious at the beginning and then showed a trend of declining. The optimal dosing amount in the first adding method was 2 g.L−1. In the second stage of the experiment, when sludge charcoal was added into SBR reactors at certain ratio with each influent process, the enhancement of removal efficiency of pollutants was inconspicuous at the beginning. With the period extending, it started to rise and finally maintained at a steady state. The optimal dosing amount was 0.06 g.L−1.
The improved removal effect of pollutants in wastewater treatment process could be attributed to the physical adsorption and enhanced bio-degradation of sludge charcoal. Sludge charcoal and activated sludge are synergistic in water treatment, which complement and promote each other to improve the effect of sewage treatment.
The utilization of sludge charcoal indicates a sustainable future of sewage treatment, along with sludge treatment and disposal. The experiment fully shows the feasibility of sludge disposal production being used in sewage treatment for investment saving and wastes reusing, and it is promising to be put in large-scale application.
References
Ailing REN, Qishan W, Bin GUO (2007) Preparation structure and absorption characterization of sludge activated carbon[J]. J Harbin InstTechnol 39(6):993–996
Chen MX, Wang JS, Wang YH (2000) The combination of carbon adsorption with activated sludge for the decolorization of wool-dyeing wastewater[J]. Journal of Environmental Science and Health Part a-Toxic/Hazardous Substances & Environmental Engineering 35(10):1789–1795
Chen T, Huang Q, Gao D et al (2003) Heavy metal concentrations and their decreasing trends in sewage sludges of China[J]. Acta Sci Circumst 23(5):561–569
Conesa JA, Marcilla A, Moral R et al (1998) Evolution of gases in the primary pyrolysis of different sewage sludges[J]. Thermochim Acta 313(1):63–73
Dias JM, Alvim-Ferraz MCM, Almeida MF et al (2007) Waste materials for activated carbon preparation and its use in aqueous-phase treatment: a review[J]. J Environ Manag 85(4):833–846
Figura A, Cencek T, Zbikowska E (2022) Parasitic threat in commercial organic fertilizers[J]. Parasitol Res 121(3):945–949
Fu R, Yang H, Gan M (2004) Sludge disposal in Chinese urban wastewater treatment plant: present status and future[J]. Enuivon Sci Technol 27(5):108–110
Fytili D, Zabaniotou A (2008) Utilization of sewage sludge in EU application of old and new methods - a review[J]. Renew Sustain Energy Rev 12(1):116–140
Nesseris GK, Stasinakis AS (2012) Investigation of municipal and olive mill wastewater co-treatment in activated sludge-powdered activated carbon (AS-PAC) systems[J]. J Chem Technol Biotechnol 87(4):540–545
Gopinath A, Divyapriya G, Srivastava V et al (2021) Conversion of sewage sludge into biochar: a potential resource in water and wastewater treatment[J]. Environ Res 194
He Y, Liao X, Liao L (2014) Preparation of sewage sludge-based activated carbon and its application research in wastewater treatment[J]. Mater Rev 28(4A):90–94
Jin Z, Zhang G, Wang K (2012) Research advance in resource recovery treatment of sewage sludge by pyrolysis[J]. Chem Ind Eng Prog 31(1):1–9
Kargi F, Ahmet U, Baskaya HS (2005) Phosphate uptake and release rates with different carbon sources in biological nutrient removal using a SBR[J]. J Environ Manag 76(1):71–75
Krizanec B, Le Marechal AM (2006) Dioxins and dioxin-like persistent organic pollutants in textiles and chemicals in the textile sector[J]. Croat Chem Acta 79(2):177–118
Lee CS, Robinson J, Chong MF (2014) A review on application of flocculants in wastewater treatment[J]. Process Saf Environ Prot 92(6):489–508
Li Y, Chen T, Luo W et al (2003) Contents of organic matter and major nutrients and the ecological effect related to land application of sewage sludge in China[J]. Acta Ecol Sin 23(11):2464–2474
Li H, Wu X, Jiang L et al (2014) Progress in study on the incineration technology of municipal sewage sludge[J]. Environ Eng 32(6):88–92
Li S, Li C, Shao Z (2022) Microwave pyrolysis of sludge: a review[J]. Sustain Environ Res 32(1)
W a N L, Yu T, Lijun Z et al (2011) Status and Progress on high temperature pyrolysis of sewage sludge[J]. Environ Sci Technol 34(6):109–114
Lixiaona SY, Jia M et al (2017) A review of researches on biochar adsorbing organic contaminants and its mechanism[J]. Acta Pedol Sin 54(6):1313–1325
Mcgrath SP, Chaudri AM, Giller KE (1995) Long-term effects of metals in sewage sludge on soils, microorganisms and plants[J]. J Ind Microbiol 14(2):94–104
Oleszczuk P (2007) Persistent organic pollutants in soil and sewage sludge-amended soil part II. Fate contaminants in soil[J]. Ecological Chemistry and Engineering-Chemia I Inzynieria Ekologiczna 14(S2):185–198
Pijuan M, Casas C, Baeza JA (2009) Polyhydroxyalkanoate synthesis using different carbon sources by two enhanced biological phosphorus removal microbial communities[J]. Process Biochem 44(1):97–105
Racek J, Sevcik J, Chorazy T et al (2020) Biochar - recovery material from pyrolysis of sewage sludge: a review[J]. Waste Biomass Valori 11(7):3677–3709
Ren A-L, Wang Q-S, Guo B (2006) Structure characterization and surface fractal analysis of sludge activated carbon[J]. Acta Chim Sin 64(10):1068–1072
Wang X, Zhou Q (2004) The ecological process, effect and remediation of heavy metals contaminated soil[J]. Ecol Sci 23(3):278–281
Wang S, Liu X, Zheng Q et al (2012) Analysis on sewage sludge characteristics and its feasibility for landscaping in Xi'an City[J]. China Water & Wastewater 28(23):134–137
Wang Y, Yan L, Li J et al (2016) A review of technology for small sewage treatment: the Chinese perspective[J]. Oxid Commun 39(1):275–284
Wang G, Qiu G, Wei J et al (2023) Activated carbon enhanced traditional activated sludge process for chemical explosion accident wastewater treatment[J]. Environ Res 225
Werther J, Ogada T (1999) Sewage sludge combustion[J]. Prog Energy Combust Sci 25(1):55–116
Wong S, Yac’cob N, a N, Ngadi N, et al (2018) From pollutant to solution of wastewater pollution: synthesis of activated carbon from textile sludge for dye adsorption[J]. Chin J Chem Eng 26(4):870–878
Xiang L, Li H, Wang Y et al (2023) Energy utilization assessment of municipal sewage sludge based on SWOTFAHP analysis[J]. Water 15(2)
Xiao D, Li H, Wang Y et al (2023) Distribution characteristics of typical heavy metals in sludge from wastewater plants in Jiangsu Province (China) and their potential risks[J]. Water 15(2)
Xiaodan F, Xiangkai Z, Hongying Y (2007) Preparation and decolorization properties of activated carbon from sewage sludge[J]. Chem Ind Eng Prog 26(12):1804–1807
Yang X, Li H, Wang Y et al (2023) Predicting higher heating value of sewage Sludges via artificial neural network based on proximate and ultimate analyses[J]. Water 15(4)
Yu J, Tian N, Wang K et al (2007) Analysis and discussion of sludge disposal and treatment of sewage treatment plants in China[J]. Chin J Environ Eng 1(1):82–86
Zhang W, Yang L, Jiang H et al (2014) Characterization of sludge-based activated carbon and its adsorption properties on Cr(VI)[J]. Chin J Environ Eng 8(4):1439–1446
Zhang J, Shao J, Huang H et al (2017) Review on the preparation of activated carbon from sludge and its adsorption characteristics[J]. Chem Ind Eng Prog 36(10):3876–3886
Zhu Y, Zhao Y, Li H (2010) Analysis on landfill method and Technology of Sludge from municipal wastewater treatment plant[J]. China Water & Wastewater 26(20):12–15
Acknowledgements
The support of sewage treatment plants for providing raw sewage sludge for the study is greatly appreciated.
Author information
Authors and Affiliations
Contributions
H.L. contributed to the conceptualization, data curation, formal analysis, methodology, software, and writing—original; Y.S. contributed to the conceptualization, project administration, supervision, and writing—review and editing; Y.W. and X.Z. contributed to the investigation, resources, software, and validation. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Zhihong Xu
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Shi, Y., Wang, Y. et al. A research on the strengthening effect of sludge charcoal on activated sludge process in sewage treatment. Environ Sci Pollut Res 31, 5289–5303 (2024). https://doi.org/10.1007/s11356-023-31213-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31213-0