Abstract
Sewage sludges are obtained as a by-product from the process of the wastewater treatment plants. Production is anticipated to increase as asset in environmental organization rises and further municipal wastewater is treated to even advanced standards. The consistent development in the environmental impact of water resources is expected to be answered by a noticeable upsurge in sludge volumes formed. While there are numerous ways of placing sewage sludge, using it in water decontamination can turn it into a resource. Sadly, a large proportion of the sludge produced is not valorized but is excluded together with other residues in waste tips. The necessities of investigating probable innovative routes are obvious for sewage sludge valorization. Considering the account factors such as the existence of volatile components and the detail that sewage sludge is carbonaceous in nature, the sludge may be considered as potentially appropriate for the manufacture of activated carbon which are very useful in mixture separation and liquid purification due to their high adsorption capacity. Discarding of industrial wastewater poses a foremost environmental problem since such effluents comprise various pollutants that are resistant to conventional biological methods and thus difficult to remove. Re-use of that sludge may not only progress the particulate pollutant removal efficiency of a primary sewage treatment, but also ease the burden of water treatment works concerning to sludge treatment and clearance. This chapter aims to throw an insight on production rate, synthesis process, characterization and applications of sludge-based adsorbents (activated carbons).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sludge based activated carbons (SBAC)
- Sludge based adsorbents (SBA)
- Brunauer Emmett Teller (BET) surface area
- Wastewater treatment
1 Introduction
The rising demands from environmental agencies and society towards better environmental quality standards have manifested themselves in private and public service administrators. As low indices of wastewater treatment exist in many developing countries, a future rise in the number of wastewater treatment plants is expected naturally. Therefore, the amount of sludge produced is also expected to rise. The municipal sewage sludge generation has also presently multiplied in congruence with rapid industrialization. Some green agencies in the developing countries now require the procedural definition of the final sludge disposal in the licensing processes. Hence, solid waste management is a burning matter of concern in many countries, tending towards a fast growing aggravation in the future years, as more wastewater treatment plants are implemented.
Wastewater treatment is the process of removing chemical, physical, biological contaminants and other pollutants and produce environmentally safe treated wastewater. The treated water can be further released back into nature. Sludge is a by-product of water, industrial and wastewater treatment operations. It is usually a semi-solid waste or slurry that has to undergo further treatment before being suitable for land application or disposal.
Sewage sludge is obtained from sewage treatment plants and consists of two basic forms, primary and secondary sludge. Biological treatment operations produce sludge which is also known as wastewater biosolids. The watery portion of the sludge is removed from liquid wastewaters containing less solid matter. The primary sludge includes precipitated solids that are generated during the initial treatment in the primary clarifiers. The secondary sludge parted in the secondary cleaners includes the sewage sludge purified from the secondary treatment bioreactors.
Sewage is generated by institutional, residential, industrial and commercial establishments. Above 99% of the sewage constitutes water that is a mixture of industrial and domestic wastes [1]. The difference between sludge and sewage is that sludge is a standard term for solids deducted from suspension in a liquid, while sewage is a suspension of solid waste and water, transported by sewers to be processed or disposed of. Conventional drinking water treatment and several other industrial processes produce sludge as a settled suspension.
Wastewater sludge includes a variety of inorganic and organic compounds. As per the recent researchers the use of sludge as an organic fertilizer in agricultural applications could ideally be a very attractive option [63]. For example, a large portion of insoluble aluminum hydroxides are contained by the aluminium-laden sludge which can be utilized as a coagulant in the primary sewage treatment [2]. However, there are some important drawbacks, which are mainly related to aspects such as appropriate soil availability, sludge quality and difficulties encountered in its monitoring and management.
2 Sludge and Its Production Rate in Wastewater Treatment Plants
The term ‘sludge’ refers to the solid by-products from wastewater/industrial/water treatment. Even though the sludge constitutes hardly 1–2% of the treated water volume, managing it is highly complex because it is frequently undertaken outside the boundaries of the treatment plant and usually costs 20–60% of the total operating costs of the wastewater treatment plant [3]. If the management of sludge generating from wastewater, industrial and water treatment plants is inadequately accomplished it may jeopardize the sanitary and environmental aspects in the treatment systems [4]. It is predicted that an average generation of around 50 g dry matter per inhabitant per day is archetypal for urban sewage plants and a corresponding rise in the quantity produced by industrial sewage plants. With high generation rate, together current and future estimated, the suitable management of sludges which are produced at sewage plants has become a need of the hour [5]. In the operational cost of wastewater treatment plants, the cost of waste sludge disposal is a major factor. Single handedly sludge dewatering constitutes around 40% of the annual operating costs. Although there are numerous ways of sewage sludge disposal, but rational practice of this left-over material can convert it into a resource.
2.1 Stages of Sewage Treatment
The Sewage treatment is mostly categorized into three phases: preliminary, primary and secondary treatment. Additionally, we can include two more stages of treatment for higher degree of separation depending on our needs [1].
2.1.1 Pretreatment or Preliminary Treatment
In preliminary treatment, girt and crude solids (diameter > 20 mm) are separated through screening. The obtained crude materials are not involved in biosolids. A sand or grit channel may be included in a pretreatment in which the velocity of the incoming sewage is adjusted such that it allows stones, sand, broken glass and girt to settle down. These crude solids are separated for the reason that their potential risk of damaging pumps and other equipment’s. The feed in sewage water permits through a bar screen to subtract bulky objects like rags, cans, plastic packets and sticks carried in the sewage stream. These are collected and further incinerated in a landfill. Mesh screens or bar screens of variable sizes can be used for optimizing solid exclusion.
2.1.2 Primary Treatment
Suspended solids, grit and scum can be separated in the primary treatment by using following process of pre-aeration and sedimentation. The floating and settled substances are separated while the residual part may be released or exposed to secondary treatment. Oil and grease from the detached material can occasionally be separated for biodiesel production or saponification. With the help of air pumped via perforated tubes adjacent to the floor of the tanks, the wastewater is aerated making it less dense and causing the coarse solids settle out. As because the air jets are placed in such a way that the water is swirling while moving down the tanks, the suspended particles are inhibited from settling out. Dissolved oxygen is also provided by the air for the bacteria to use afterwards in the process. But for bacterial action to occur in the process, the wastewater in these tanks is not sufficient. The scrapers eliminate the solids from the tank bottom and the water jets washes off the scum. The solids and scum are brought to a common collection point wherein they are merged forming sludge and further forwarded to secondary treatment. In the primary sedimentation stage, sewage gushes through large tanks usually known as primary sedimentation tanks, pre-settling basins or primary clarifiers. Although the sludge settles down in the tanks, but the grease and oils mount to the surface and are skimmed off further. The primary wastewater treatment engages gravity sedimentation of the screened wastewater to separate the distorted solids. A fraction of the suspended waste stream passes through primary operation and discharges concentrated suspension as residue. This residue is also known as primary sludge and is further treated to yield biosolid.
2.1.3 Secondary Treatment
The secondary wastewater treatment is achieved through a biological process in which the biodegradable materials are removed. Microorganisms are used in this process to use up suspended and dissolved matter thereby producing carbondioxide and other byproducts. The density increases when the microorganisms are added. The cleaned water is then separated resulting to a formation of a concentrated suspension called secondary sludge. This whole process takes place at the bottom part of the water tank. It is necessary to separate the microorganisms from the water before releasing it or sending it for tertiary treatment. In secondary treatment the organic content of the sewage which is derived from various sources are considerably reduced. The nutrients required to uphold the microorganism population is supplied through the organic material and the ones within the sludge are converted to carboxylic acids and further to carbondioxide by aerobic fermentation or methane by anaerobic fermentation. The biogas thus obtained is an important source of fuel. The volume of the sludge leaving the digesters reduces up to its half. Mostly the municipal plants treat the settled sewage water through aerobic organic processes. The biota requires both food and oxygen for living efficiently. Inclusion of secondary clarifiers in secondary treatment processes helps in settling down the organic flocculated substances developed inside the bioreactor. Many designs of hybrid treatment plants are produced to treat tough wastes, consume less space and for intermittent flows also.
2.1.4 Tertiary Treatment
Further processing is necessary in case of high quality waste requirement such as discharging them directly to the drinking water source. The tertiary treatment usually yields a solid residue which primarily includes the chemicals added to the raw waste before evacuating and thus it is not regarded as a biosolid. If the whole treatment process is minutely managed throughout then plant operators can control nutrients, solid ingredients and various other components of biosolids. For municipal biosolid production, majority of the material used is through operating primary and secondary effectors simultaneously. The prime reason of tertiary treatment is to improve the effluent quality before discharging it to the environment. There may be requirement of more than one tertiary treatment processes. No matter what, disinfection is always considered the last process and is usually known as effluent polishing.
2.1.5 Fourth Treatment Stag
Particles of chemicals used in small industries, households, pharmaceuticals or pesticides are considered as micro-pollutants and they may not be able to be separated through the usual treatment process like primary, secondary and tertiary treatment. Also if they are not separated it may lead to water pollution. Therefore, a separate treatment named fourth treatment stage is introduced in the sewage treatment process to remove the micro-pollutants. These techniques are not yet functional on a regular basis as they are still very expensive.
2.2 Volume Reduction Processes
The quantity of wastewater treatment plant sludge produced can be expressed in terms of volume (wet basis) or mass (dry basis). The sludge production can be expressed in an easy way in terms of per capita and chemical oxygen demand (COD) bases for mass and volume calculations. The organic sludge is generally made up from the biomass which is formed from the conversion of some of the COD in the biological wastewater treatment. This sludge is actually produced from secondary sludge and thus has suspended solid composition is less than 1% (wt.). However, primary sludges are high concentrated and the combination of both primary and secondary sludge of solid concentrations contains around 3% by weight. As the sludges are naturally in voluminous, treatment processes are named as dewatering, thickening, conditioning and drying. Water removal helps in improving the efficiency of the further treatment processes, reducing the storage and decreasing transportation costs.
2.2.1 Thickening
A concentrated product is produced in the sludge thickening process and that product basically retains the liquid properties. The most common thickening process usually applied to municipal sludges is concentration by simple sedimentation or gravity thickening. The sludge product obtained from gravity thickening. Practice of centrifuges, gravity drainage belts, perforated rotating drums and floatation method are alternative to gravity thickening. The product from gravity sludge thickening frequently comprises around 5–6% solid by weight. Floatation is a process wherein a gas is included in sludge solids which results in making them to float.
2.2.2 Dewatering
In the sludge dewatering process, the product ensures solid properties even though the water content retained in it is still not negligible. The thickened sludge is transported through tank truck but in case of dewatered sludge a dump truck is used. Sand drying beds and at times lagoons are used for dewatering process but removal of moister in thickening step is enabled through gravity drainage and sedimentation. Mostly, dewatering mechanical sludge equipments namely, vacuum filters, belt filter press, centrifuges and filter press are used in big municipal installations. Mechanical method is more efficient than other processes because the solid content of the product sludge by weight ranges from 20–45% which is quite higher.
2.2.3 Conditioning Sludge
Conditioning process does not reduce the moisture content directly, rather changes the physical and chemical properties of the sludge which helps it in water discharging in dewatering process. Without the prior conditioning of the sludge, mechanical dewatering process will not be economical. In chemical conditioning, mostly synthetic organic polymers or inorganic chemicals like ferric chloride and lime are added to the sludge before dewatering. The mass of the solid sludge increases because of the huge dosages of inorganic chemical conditioning. Physical conditioning on the other hand includes freeze–thaw treatment and heat treatment.
2.2.4 Drying
Drying step is usually required if the need for further water removal arises even after dewatering step. Thermal drying in association with indirect or direct driers are generally used to attain almost entire water exclusion from sludges. Also, solar drying can be an option in several locations. The heat generated in biochemical reactions during composting and various other chemical reactions results in partial drying.
2.3 Stabilization Processes
Sludge stabilization is practiced to reduce the problems aroused due to biodegradation of organic substances and is generally performed through chemical and biological treatment methods. The vector attraction reduction provision of the Part 503 Sludge Rule [EPA, 1993a] is concerned in Stabilization processes. Vectors are nothing but organisms that may get fascinated to sludges which are not stabilized and may lead to spread of infectious diseases. While applying sewage sludge to the agricultural lands if we inject it below the surface or into the soil then the vector attraction can be minimized. The sludges can also be stabilized by drying it adequately to obstruct microbial action. Combusting the sludge can also facilitate its stabilization. Various stabilization processes can also inactivate pathogenic organisms and viruses.
2.3.1 Biological Stabilization
In this process, the biological sludges are cut down through biological degradation processes in a exact and well-engineered manner. Methane is yielded as a byproduct when the household wastewater sludge is stabilized biologically in the form of a liquid inside anaerobic digesters. This liquid sludge also can be stabilized biologically in an aerobic digester in presence of oxygen. Composting is an aerobic process which facilitates in biological stabilization of the dewatered sludge. This process takes place in thermophilic temperature of around 55 °C due to heat released during biochemical transformations. Sawdust and Wood chips should be additional to progress friability to encourage aeration. The heat from the same source can be used in case of operating the aerobic digesters thermophilically.
2.3.2 Chemical Stabilization
In this process the sludges not only intend to reduce the biodegradable organic matter quantity and also generating suitable conditions for the inhibition of microbial activity in order to prevent odors. Out of all the chemical stabilization methods available the most common one is raising the pH value of the sludge using cement kiln dust and lime. Liquid or dewatered forms of sludge can be chemically stabilized. During the chemical stabilization of dewatered sludge an exothermic reaction between lime and water results in heating that facilitates in pathogen destruction and water evaporation as well.
3 Preparation of Adsorbents Derived from Sludge
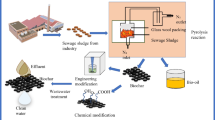
Lately liquid-phase adsorption is popping up as a promising option to remove the non-biodegradable pollutants from water streams. The most common adsorbent for the liquid phase adsorption is activated carbons because of their versatility and effectiveness. Small particle sized adsorbents are preferred in case of solution phase because of their large surface area and results in small diffusion distance. The activated carbon prepared by treating wastewater sludge is a black amorphous can be used to treat pollutants. The wastewater sludges are blended, carbonized, activated and acid or alkaline treated to form activated carbon. The safe eco-friendly sludge based activated carbon has higher absorbability due to its dense pore characteristic with more specific surface area and complex structure. It has wide variety of raw material source, good thermal stability, even chemical properties and can also be recovered and utilized repeatedly.
The activated carbons can be prepared from sludge through various methods like physical activation, chemical activation, physical–chemical activation, direct pyrolysis, microwave activation etc. All these methods are used to produce porous carbonaceous adsorbents.
The alteration and synthesis pattern of sludge-based activated carbon is concisely explained below. To obtain sludge based activated carbon, the activated sludge can be dried and pulverized directly under inert gas. This is called direct pyrolysis and it takes place in the following stages:
-
(i)
The drying is the first stage
-
(ii)
The second stage is the pyrolytic wherein huge amounts of volatile components are melted.
-
(iii)
The last stage is where the remaining material carries on pyrolysing gradually.
Through physical activation, the ground activated sludge can be directly pyrolysed and dried under inert gas protection and further pyrolysed to finally obtain the sludge based activated carbon under various other protective gases like water vapor, carbondioxide and flue gas. The traditional Muffle furnace heating method is often used in physical activation of the sludges.
In chemical activation method, the raw sludge components are either put together with chemical reagents at a ratio or the sludge is dip dried in a solution of chemical reagent as per a definite solid-liquid ratio and further the mixture is pyrolysed to get our desired product.
Physical–chemical activation is the combination of both physical and chemical activation wherein the sludge is mixed with chemical reagent in a certain ratio and further they are pyrolysed under the protection inert gas to yield sludge based activated carbon.
In microwave activation the sludge is pyrolysed and carbonized into activated carbon through microwave heating. Microwave activation has recently drawn consideration since it is easy to control, energy consumption is low, high efficiency, cost effective, less pollution and more feasibility. As the carbon content in these activated carbons are low therefore carbon source materials like wood chip, corn kernel, peanut and hazelnut shells are added to increase the carbon source so that it can exhibit abundant pore structure and high adsorption efficiency [3].
4 Sludge-Based Adsorbents Characterization
The efficiency of sludge based adsorbents in removing the contaminants is decided by their surface and structure chemistry features. The Brunauer Emmett Teller (BET) surface area is the most common analysis to assess the structure of an adsorbent. The Barrette Joynere Halenda (BJH) method helps in calculating the pore size distribution, macropore and mesopore volumes. The t-plot method on the other hand helps in calculating the micropore volume. In the following sections the effects of pyrolysis condition on the structure of the sludge based adsorbents are discussed [4].
4.1 Carbonization
The Table 1 tabulates the pore structures and BET surface areas of sludge-based adsorbents when they undergo only carbonization. The various influences in carbonization that affects the chemical and physical features of sludge-based adsorbents are pyrolysis temperature, dwell time, feedstock type and heating rate. These are discussed below
4.1.1 Pyrolysis Temperature
The temperature of pyrolysis plays an important role in altering the characteristics of sewage sludge and industrial sludge adsorbents. As per our observations, an increase in pyrolysis temperature rises the ash content of these adsorbents but decreases its yield too [6, 7]. At high pyrolysis temperature, devolatilization of the solid hydrocarbons and the integrant gasification of the carbonaceous residues in the adsorbents take place [8]. Pyrolysis temperature also affects the surface acidity or basicity, morphology and surface characteristics changes in sludge based adsorbents. Usually, the adsorbents that are produced at high temperature of around 500 °C are alkaline and those in low temperature are acidic in nature [6]. At high temperature sodium oxide is released from the sludge which increases its alkalinity [9].
Generally, with increase in pyrolysis temperature the pore volume surface area and the BET surface area also increases. But when the temperature is excessively high, destruction of porous structure takes place and its combination of mesopore inhibits further development of porosity [10]. This increase in surface structure on high pyrolysis temperature is resulted due to the increase in the degree of atomization and the rearrangement in nitrogen chemistry [11]. The porosity is increased through carbonization due to the mass loss during the thermal decomposition and evolution of volatile matter [6]. Additionally, the creation of micropore is also boosted when the high moisture content of wet sludge generates a steamy atmosphere at high temperature resulting to partial gasification of the solid char [8]. On the other hand, very high temperature can probably lead to a decrease in surface area because of the porous structure destruction development of deformation, cracks or blockages of micropores in those adsorbents [10]. The optimum carbonization temperatures for increasing the BET surface areas to maximum are reported as 450, 500, 550, 650 and 950 °C.
4.1.2 Dwell Time
Dwell time is the period of time that an element or system remains in a given state. According to the studies, higher pyrolysis temperature results in shorter dwell time. The optimum dwell time estimated at 500 °C, 650 °C and 950 °C are 240, 120 and 60 min respectively [9, 11,12,13]. Also it has been found that at 650 °C with increase of dwell time the micropore volume of sludge based adsorbents remained constant while the surface area and mesopore volume decreased [11]. Some experiments have also deduced that at the same pyrolysis temperature of 650 °C, with increase of dwell time the micropore volume decreases [14].
4.1.3 Heating Rate
According to the studies, higher heating rates improves the product yield and carbon content but decreases the hydrogen content of the sludge based adsorbents. Whereas, lower heating rates of about 3 °C/min increases the BET surface area [9, 15]. It was probably due to larger sample residence time during the pyrolysis processes. As per Table 1, the heating rates are 3, 5, 10, 20 and 40 °C/min.
4.1.4 Type of Feedstock
Researches implies that addition of high carbon content materials like leaf litter, disposal filter cake, waste oil sludge and solid residue of pyrolysed tyres to the sludge can improve their porosities [13, 16, 17]. Due to the oil volatilization and hydroxide formation during pyrolysis, the addition of waste oil sludge in sewage sludge in the mass ratio 1:1 improves the BET surface areas and micropores volume [11]. Also the adsorbent yielded by mixing the sewage sludge and filter cake in the mass ratio 85:15 has higher BET surface area of about 60 m2/g than the regular sludge based adsorbents which has a BET surface area of about 15 m2/g [16].
4.2 Physical Activation
The process of physical activation generally takes place in the following two steps:
The first step is to carbonize the sludges around the temperature between 400–700 °C in presence of inert gas like nitrogen or helium to break down the cross-linkage bonds among carbon atoms. The next step is to activate it with the help of gases like nitrogen, oxygen or air, steam, carbondioxide etc. at a high temperature of about 800–1200 °C. This facilitates in developing the porosity of the sludge based adsorbents further. The most common activator gases are steam and carbondioxide. In the Table 2 a summary of the characteristics of sludge based adsorbents that are activated at various conditions.
4.2.1 Steam
As compared to the activation using carbondioxide, the steam activation at a given temperature, leads to larger adsorption capacity and wiser pore size distribution in the sludge based adsorbents. This method encourages the micropore and mesopore creation [18]. Steam activation mechanism is the collective effect of fixed carbon loss and devolatilization that results from water gas reaction in this case. With increase in activation temperature, the BET surface areas of adsorbents prepared by steam activation also rises up due to the higher rate of diffusion of the water molecule to the inside thereby broadening the pore network [19, 20]. But if the activation temperature crosses 850 °C, BET surface areas decreases as more particles start burning out.
4.2.2 Carbondioxide
The porosity of the sludges can be enhanced with the help of carbondioxide activation. The development of opened micropore and closed opening of micropore takes place by removing the carbon atoms from the interior of the particle through gasification at higher temperatures of about 900–1200 °C and lengthier dwell time [21]. During physical activation, the development of porosity of sludge based adsorbents is restricted if the raw material contains high ash content. It is observed that the acidity of the sludge-based adsorbents prepared through carbondioxide activation declines with an inclination in the activation temperature because of the oxygenated acidic surface group’s degradation [22].
4.3 Chemical Activation
Chemical activation is nothing but activating the sludge based adsorbents by chemical treatment at specified conditions. The factors that affect the chemical activation are activator kinds, activator temperature, activation concentration, addition of binder. The Table 3 describes the pore structure characteristics of sludge based adsorbents by chemical activation.
4.3.1 Types of Activator and Activation Temperature
Activator plays the most important role in influencing the processes of chemical activation [23]. Various activators like sulfuric acid, phosphoric acid, potassium hydroxide, sodium hydroxide, zinc chloride, ferric chloride and potassium carbonate can be used but zinc chloride, sodium hydroxide, potassium hydroxide and phosphoric acid are the most common used ones.
According to the observations tabulated in Table 3, potassium hydroxide has proved to be the most effective activator as it has produced sludge based adsorbents with high BET surface area with a value as high as 1882 m2/g [24]. This process is high energy consuming and the product can be obtained through a two-stage method—carbonizing before impregnating and activating it while activator to solid ratio is maintained as 1:1. Also as per the research conducted, sludge based adsorbent prepared through single stage method with potassium hydroxide maintaining activator to solid ratio as 3:1 yields a value of BET surface areas as 1832 m2/g [25]. The mechanism of potassium hydroxide activation is that an intercalation compound of carbon and potassium oxide is formed which infiltrates inside and at high temperature this potassium oxide reduces to metallic potassium atoms [26]. This results in gasification and emission of steam and carbondioxide which facilitates in pore formation. Also, potassium vapor widens the gap between carbonaceous layers and thus increases the surface area.
Zinc chloride is another such effective activator with yields BET surface area as high as 757m2/g [27]. It helps in dehydrating and forming tar to suppress the activation process and also promotes carbon skeleton aromatization to form pores [28]. As per Table 3, the optimum activation temperatures for zinc chloride depending on the feedstock types are reported as 300, 375, 500 and 750 °C. A washing step can create extra micro and mesoporosity to remove the zinc chloride and zinc oxide entrapped.
Although as per Table 3 the BET surface area of sludge based adsorbents activated through phosphoric acid is merely 290.6 m2/g but the only advantage it has its low cost and activation temperature. During activation processes the effects of phosphoric acid are dehydration, depolymerization, rearrangement of biopolymers constituent, specifically, encouraging the change of aliphatic to aromatic compounds thereby growing the yield of solid phase products.
4.3.2 Concentration of Activator
The optimum value of activator concentration be subject to on its characteristics and the type of feedstock. Usually with the increase of activator concentration, the BET surface areas and adsorption capacities increase but if it exceeds appropriate values the BET surface areas and adsorption capacities start decreasing due to partial destruction of microporosity resulted from hyper-activation [29].The optimum activation concentration for potassium hydroxide, sodium hydroxide and zinc chloride as per research are 1 M, 1.25 M, 2 M respectively [29, 10].
4.3.3 Binder Addition
Although adding binders to the sludge based adsorbents prior to chemical activation produces huge granules but reduces the surface areas of BET [30]. In catalytic wet air oxidation process these hard adsorbents can be used to increase the pollution removal rate [31].Phenolic resin, clay, polyvinyl acetate (PVA), lignosulphonate, humic acid are the most commonly used binders. Clay, phenolic resins and humic acid decreased the micro and macroporosity of sludge based adsorbents [32]. Although humic acid and phenolic resin addition had negligible effect on BET surface areas but in case of clay it decreased to a considerable extent. While sludge based adsorbents formed without binder are friable with a hardness number in the range of 58–71% those produced in a combination of steam activation and polyvinyl acetate binder (5 wt %) yields hardness number of around 92–93% [33].
4.4 Post Treatment
Post treatment, implies to treatments such as acid washing, alkaline washing or distilled water washing which helps in decreasing the ash contents, increasing the BET surface areas and porosity and even removing the extra reaction products and activation agents in case of chemical activation. An appropriate ash-dissolution technique like washing with hydrochloric acid can be applied to reduce this high ash content [33]. Acid Washing is the most widely used one among them as it cut downs the inorganic content of the carbonaceous material by dissolving the basic oxides like aluminium oxide, ferric oxide, calcium oxide etc. from the adsorbent to increase porosity [34].
5 Applications
The sludge based adsorbent has found its demand in various sectors these days. They are explained as follows [3]:
5.1 Organic Matter Removal
In the Table 3 it is shown that the organic matters like phenol, toluene, trinitrotoluene, nitrobenzene, rhodamine B and ibuprofen can be removed with the sludge based adsorbents over physico-chemical adsorption and hydroxyl radical oxidation [5,6,7,8,9,, 36,37,38,39,40]. The sludge based adsorbents prepared through phosphoric acid microwave method was used to remove trinitrotoluene from water and from the previous research it was concluded that it has pretty bulky surface and plentiful extension holes [38]. When more amount of aluminum oxide and iron is added to the sludge based adsorbents then the deletion rate of UV254 and dissolved organic carbon by it are around 85.8% and 59.7% respectively which is almost similar to the commercial ones [41]. When suitable amount of raw materials like sawdust, corn cobs, coconut shell activated by zinc chloride is added to dehydrated sludge the adsorption efficiencies generally increases [42, 43]. In the toluene adsorption experiment it was shown that the equilibrium efficiency of shell sludge based adsorbent is the highest followed by coal adsorbent and sawdust adsorbent. Similarly, for phenol and nitrobenzene removal, sludge based adsorbents with corn cores were used which proved that higher doping proportion of corn cores results in larger micropore volume, BET surface area [44].
These adsorbents prepared from sludge can also act as catalyst or carrier to prepare necessary conditions for new composite photocatalytic material preparation [45, 46]. As per the studies, the sludge based activated carbons mixed with oxides of manganese has decent catalytic activity [47]. The reaction mechanisms like hydroxyl radical reactions and surface reaction were involved in the catalytic ozonation process of oxalic acid mineralization. For increasing the productivity of ozone oxidation of wastewater pollutants, transition metals like iron oxide and manganese were usually doped into the sludge based adsorbents through impregnation method [48]. Due to various modification methods involved, the principles for organic matter removal are at times different from one another. In the catalytic ozone oxidation of rhodamine, the sludge based activated carbon prepared with a mixture of biological and chemical sludge follows the mechanism of hydroxyl radical oxidation [49].
Sludge based adsorbents can also be mixed with Fe3O4 or metal free materials like nitrogen rich urea and these are named as F-SBAC and N-SBAC respectively [50, 51]. The F-SBACs produced hydroxyl radicals to catalyze hydrogen peroxide and it can be prepared at different temperatures like 600, 800 and 1000 °C and based on it the surface area, porous structure and removal rates differed. The chemical microenvironment and microstructure is influenced by N-SBAC and to remove the organic contaminant it can oxidized effectively too. For adsorbing phenol, the sludge based activated carbon followed electron donor receptor reaction mechanism among the aromatic phenolic rings and the adsorbent surface functional groups [52]. It is estimated that either due to the competition between the two composites at the surface adsorption sites of the sludge based activated carbons or due to the space resistance of the co-adsorbent phenol, its adsorption capacity in removing cadmium ions is decreased.
As per the research works, citric acid-zinc chloride mixed with sludge based adsorbent seemed to be a good green technique in char manufacture with good pore structure [53]. The sludge derived char is a hybrid material that contains carbon in elemental form, aromatic organic matter and inorganic ash and this char can also treat various kinds of benzene derivatives in aqueous solution.
Dibenzothiophene (DBT) can also be removed from n-octane by these sludge based adsorbents and its adsorption rate increases with increase oxygen-containing functional groups like carbonyl groups [54]. Out of the activator used in DBT removal, potassium hydroxide enabled the highest adsorption capacity even more than commercial activated carbons.
5.2 Heavy Metal Removal
The sludge based activated carbons helps in removing heavy metal ions by surface precipitation, ion exchange reaction, chemical and physical adsorption [55]. These metal ions tend to create an exchange reaction on the surface of activated carbon. After modifying or adding a reagent to the sludge based activated carbons, its surface can get exposed to special surface groups to strengthen the absorption of heavy metal ions and form products. These special groups develop into ligands with heavy metal ions. The type and stability of these ligands helps in determining adsorption capacity and quantity of the sludge based activated carbon which usually goes for chemical adsorption. The sludge based adsorbents are produced by anaerobic pyrolysis under approximately 900 °C which has higher adsorption capacity than commercial ones to remove metals like lead, zinc, copper and cadmium [55].
In case of high pH, the heavy metals convert to hydroxide and precipitate on the surface of sludge based adsorbents and in case of low pH, surface precipitation is less and many heavy metal ions gets exchanged with calcium ions and further adsorbed on the surface of those adsorbents. As compared to the BET and micropore volume ratio of coir and coal, the sludge based activated carbons have smaller value but the equilibrium adsorption efficiencies of lead (II), cadmium (II), chromium (VI) and copper (II) in case of sludge based adsorbents are quite higher than the commercial ones due to high acid group content. Many a times the comparative study between adsorption effect on copper (II) and lead (II) removal with the help of sludge based activated carbons activated with zinc chloride and that of commercial coal carbon were studied [56]. Although the results implied that the pore volume and BET surface area of sludge based adsorbents activated was lesser than that of the commercial ones but due to the presence of acid functional group on the surface its equivalent adsorbate uptake on the two metals was much higher than the commercial ones.
There are some heavy metal ions that are sedimented on the sludge based activated carbon’s surface and they are being removed through physical adsorption which has increased adsorption capacity [57, 58]. From the research data it has been found that the adsorbent prepared with sludge and bagasse through pyrolysis under 800 °C for 0.5 h and further treatment with 60% nitric acid yielded product with around 806.57 m2/g BET surface area [59]. As per the studies, when the sludge based activated carbon was loaded with nano-titanium oxide using the impregnation sintering method to remove the mercury ion, the performance of adsorption and efficiency of catalysis were high with mercury ion removal rate from 20 mg/L aqueous solution was around 88.5.
5.3 Gas Pollutant Removal
The surface of sludge based activated carbon contains a certain amount of active components whose functional groups are rich which helps to contact with the reaction gas [60]. As per the research data, the sludge based adsorbent contains a large number of micropores, ultramicropore and other nitrogen containing group which helps in low concentration formaldehyde adsorption from air [61]. The optimum adsorption rate of this sludge based activated carbon is around 83% which is nearly equal to that of commercial activated carbon. The studies have shown that the activated carbon from sludge which contains nitric acid iron sludge based catalyst facilitates a maximum 98.3% conversion of gaseous oxides of nitrogen [62]. Similarly, if titanium oxide photocatalyst is used, then photocatalytic degradation of acetone gas yields great results [63]. Also hydrogen sulphite gas was removed efficiently using sludge based activated carbon mixed with an active agent zinc chloride which was further improved with Cerium [64]. Further, phosphoric acid was also mixed with sludge to react chemically and yield mesoporous activated carbons with surface area of about 300 m2/g [65]. The adsorption capacity of sulfur dioxide gas is associated with the average size of micropore and can controlled by the ratio of impregnation that is used mainly to make the activated carbons.
5.4 Others
The sludge-based adsorbents also can be combined with other water treatment processes to reduce the operating cost of various other processes. If we couple these adsorbents with membrane bioreactors for treating waste leachate then the structure and properties of the cake layer on the surface of the membrane can get better resulting in good performance of filtration and water permeability. The merits of this combination process are it reduces membrane fouling, protein and humic acid, increases the duration of membrane operation cycle and decreases the operation cost. During liquefaction of sludge based activated carbon, the energy density and yield of bio-oil at around 350 °C usually increases [66]. It is generally denoted as 350-SBAC. It helped in lowering the risk of copper, lead, cadmium and zinc. On the other hand, the sludge based activated carbon liquefied at temperature around 400 °C favored the risk reduction of strontium more. In terms of the yield of bio-oil, liquefaction is done at 350 °C with SSAC-550 was more suitable.
6 Conclusion
A generous amount of activated sludge is produced from sewage treatment. Conversion of sludge into activated carbon can bring considerable economic value and reduce environmental pollution. As compared to the traditional activated carbon, the cost of production of sludge based activated carbon is lesser because of the availability of its wide range of source. Therefore, there is a great potential value associated with the research and application of sludge based activated carbon and also presently it has obtained a certain achievements. However, some problems still remain to be unsolved and hence require further processing. Firstly, potential release of some hazardous and toxic substances during the synthesis of sludge based activated carbon was noticed. For example, it is possible to release the heavy metals from sludge based activated carbon. The mechanism of conversion of soluble heavy metal into insoluble metal compound is still not clear in the synthesis of sludge based activated carbon. Secondly, the effects of sludge based activated carbon on environment need to be further studied such as the effective reuse and recycle of sludge based activated carbon adsorption materials, the discarding of waste sludge based activated carbon, the leakage of its adsorbed substance in the transfer, the renewal technology and regeneration performance comparison between commercial activated carbon and sludge based activated carbon. Thirdly, profound study is required on the reaction mechanisms of preparation of sludge based activated carbon. Due to the complexity of sludge composition and the influence including factors like pyrolysis equipment and pyrolysis conditions etc., in the synthesis process of activation, the organic matters in activated sludge can initiate chemical reaction as a result of the activation by temperature. In the interim, the additives and chemical activators complicate chemical reaction even more. Therefore, analyzing the variations of the activation mechanism and process of activation can guide in the synthesis, application and variation of sludge based activated carbon at a broader level (Fig. 1).
References
Edris G, Alalayah WA (2017) Sludge production from municipal wastewater treatment in sewage treatment plant. AyhanDemirbas
Chu W (2001) Dye removal from textile dye wastewater using recycled alum sludge
Bian Y, Yuan Q, Zhu G, Ren B, Hursthouse A, Zhang P (2018) Recycling of waste sludge: preparation and application of sludge-based activated carbon
Xu G, Yang X, Spinosa L (2014) Development of sludge-based adsorbents: preparation, characterization, utilization and its feasibility assessment
Otero M, Rozada F, Calvo LF, Garcı´a AI, Mora´n A (2003) Elimination of organic water pollutants using adsorbents obtained from sewage sludge
Hossain MK, Strezov V, Chan KY, Ziolkowski A, Nelson PF (2011) Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J Environ Manag 92(1):223–228
Sanchez (2009) Effect of pyrolysis temperature on the composition of the oils obtained from sewage sludge. Biomass Bioenergy 33(6–7):933–940
Zhang B, Xiong S, Xiao B, Yu D, Jia X (2011) Mechanism of wet sewage sludge pyrolysis in a tubular furnace. Int J Hydrogen Energy 36(1):355–363
Mendez A, Fidalgo JM, Guerrero F, Gasco G (2009) Characterization and pyrolysis behavior of different paper mill waste materials. J Anal Appl Pyrol 86(1), 66–73; Mendez A, Barriga S, Fidalgo JM, Gasco G (2009) Adsorbent materials frompaper industry waste materials and their use in Cu (II) removal from water. J Hazard Mater 165(1–3):736–743
Mahapatra K, Ramteke DS, Paliwal LJ (2012) Production of activated carbon fromsludge of food processing industry under controlled pyrolysis and its applicationfor methylene blue removal. J Anal Appl Pyrolysis 95:79–86
Kante K, Qiu J, Zhao Z, Cheng Y, Bandosz TJ (2008) Development of surfaceporosity and catalytic activity in metal sludge/waste oil derived adsorbents:effect of heat treatment. Chem Eng J 138(1–3):155–165
Yilmaz AE, Boncukcuoglu (2011) Waste utilization: the removal of textile dye (Bomaplex Red CR-L) from aqueous solution onsludge waste from electrocoagulation as adsorbent. Desalination 277(1–3):156–163
Ding R, Zhang P, Seredych M, Bandosz TJ (2012) Removal of antibiotics from water using sewage sludge and waste oil sludge-derived adsorbents. WaterRes 46(13):4081–4090
Seredych M, Bandosz TJ (2007) Sewage sludge as a single precursor for developmentof composite adsorbents/catalysts. Chem Eng J 128(1):59–67
Liu J, Jiang X, Zhou L, Han X, Cui Z (2009) Pyrolysis treatment of oil sludge andmodel-free kinetics analysis. J Hazard Mater 161(2–3):1208–1215
Velghe RC Yperman J (2012) Characterization of adsorbents prepared by pyrolysis of sludge and sludge/disposal filter cake mix
Ren X, Liang B, Liu M, Xu X, Cui M (2012) Effects of pyrolysis temperature, timeand leaf litter and powder coal ash addition on sludge-derived adsorbents fornitrogen oxide. Biores Technol 125:300–304
Ncibi MC, Jeanne-Rose V, Mahjoub B, Jean-Marius C, Lambert J, Ehrhardt JJ, Bercion Y, Seffen M, Gaspard S (2009) Preparation and characterization ofraw chars and physically activated carbons derived from marine Posidoniaoceanica (L.) fibres. J Hazard Mater 165(1–3):240–249
Foo KY, Hameed BH (2009) A short review of activated carbon assisted electrosorptionprocess: an overview, current stage and future prospects. J Hazard Mater 170(2–3):552–559
Xin-hui D, Srinivasakannan C, Jin-hui P, Li-bo Z, Zheng-yong Z (2011) Comparison of activated carbon prepared from Jatropha hull by conventional heating and microwave heating. Biomass Bioenergy 35(9):3920–3926
Jindarom C, Meeyoo V, Kitiyanan B, Rirksomboon T, Rangsunvigit P (2007) Surface characterization and dye adsorptive capacities of char obtained frompyrolysis/gasification of sewage sludge. Chem Eng J 133(1–3):239–246
Hofman M, Pietrzak R (2012) NO2 removal by adsorbents prepared from wastepaper sludge. Chem Eng J 183:278–283
Smith KM, Fowler GD, Pullket S, Graham NJD (2009) Sewage sludge-basedadsorbents: a review of their production, properties and use in water treatmentapplications. Water Res 43(10):2569–2594
Lillo-Rodenas (2008) Further insights into the activation process of sewage sludge based precursors by alkaline hydroxides. Chem Eng J 142(2):168–174
Monsalvo VM, Mohedano AF, Rodriguez JJ (2011) Activated carbons from sewage sludge: application to aqueous-phase adsorption of 4-chlorophenol.Desalination 277(1–3):377–382
Marsh H, Rodríguez-Reinoso F (2006) Activated carbon. Oxford, pp 322–365
Tsai J, Chiang H, Huang G, Chiang H (2008) Adsorption characteristics of acetone, chloroform and acetonitrile on sludge-derived adsorbent, commercial granularactivated carbon and activated carbon fibers. J Hazard Mater 154(1–3):1183–1191
Lin QH, Cheng H, Chen GY (2012) Preparation and characterization of carbonaceousadsorbents from sewage sludge using a pilot-scale microwave heatingequipment. J Anal Appl Pyrolysis 93:113–119
Hwang H, Choi W, Kim T, Kim J, Oh K (2008) The preparation of an adsorbentfrom mixtures of sewage sludge and coal-tar pitch using an alkaline hydroxideactivation agent. J Anal Appl Pyrol 83(2):220–226
Ocampo-Perez R, Rivera-Utrilla J, Gomez-Pacheco C, Sanchez-Polo M, Lopez-Pe~nalver JJ (2012) Kinetic study of tetracycline adsorption on sludge-derivedadsorbents in aqueous phase. Chem Eng J 213:88–96
Stüber F, Smith KM, Mendoza MB, Marques RRN, Fabregat A, Bengoa C, Font J, Fortuny A, Pullket S, Fowler GD, Graham NJD (2011) Sewage sludge based carbons for catalytic wet air oxidation of phenolic compounds in batch and trickle bed reactors. Appl Catal B Environ 110:81–89
Gomez-Pacheco CV, Rivera-Utrilla J, Sanchez-Polo M, Lopez-Pe~nalver JJ (2012) Optimization of the preparation process of biological sludge adsorbents for application in water treatment. J Hazard Mater 217–218:76–84
Lebigue J et al (2010) Application of sludge-based carbonaceous materials in a hybrid water treatment process based on adsorptionand catalytic wet air oxidation
Zou et al (2013) Structure and adsorption properties of sewage sludge-derived carbon with removal of inorganic impurities and high porosity
Xuemin H, Xin S, Quan Y (2013) Adsorption of toluene by fixed sludge-based activated carbon bed. Chin J Environ Eng 7:1085–1090
Ping F, Ruihua S, Juan R (2011) Adsorption of phenol from aqueous solution using activated carbon. Carbon Techniq 30:12–16
Daojing L (2011) Study on sludge activated carbon preparation and its adsorption properties of phenol and nitrobenzene. Beijing Forestry University
Lijun Y, Wenju J (2005) Adsorption of red-water from trinitrotoluene manufacturing by sludge-based adsorbent. Ind Water Wastewater 36:26–28
Jiarong H (2016) The adsorption study of rhodamine-B by activated carbon of activated sludge. J Minnan Normal Univ (Natural Science) 83–87
Xue W, Liqiu Z, Li F (2015) Removal efficiency of ibuprofen and determination of active sites in catalytic ozonation process by modified SCACs. Chin J Environ Eng 9:621–626
Zhihui P, Chaosheng Z, Jiayu T, Qing Z, Guibai L (2014) Application of chemical sludge based adsorbent in wastewater treatment. Water Wastewater Eng 40:142–145
Xin S, Xue-min H, Li C, Quan Y (2012) Preparation of sludge-based activated carbon and adsorptive properties of toluene. Environ Sci Technol 35:32–35
Hongjuan W, Fei Q, Li F, Liqiu Z (2012) Catalytic ozonation of ibuprofen in aqueous solution by activated carbon made from sludge and corn cob. Environ Sci 33:1591–1596
Daojing L, Li F, Liqiu Z (2012) Effects of corncob addition onproperties of sludge activated carbon. Chin J Environ Eng 6:1010–1014
Wen G, Pan Z-H, Ma J, Liu Z-Q, Zhao L, Li J-J (2012) Reuse of sewage sludge as a catalyst in ozonation—efficiency for the removal of oxalic acid and the control of bromated formation. J Hazard Mater 239–240:381–388
Zhuang H, Han H, Hou B, Jia S, Zhao Q (2014) Heterogeneous catalytic ozonation of biologically pretreated Lurgi coal gasification wastewater using sewage sludge based activated carbon supported manganese and ferric oxides as catalysts. Biores Technol 166:178–186
Huang Y, Sun Y, Xu Z, Luo M, Zhu C, Li L (2017) Removal of aqueous oxalic acid by heterogeneous catalytic ozonation with MnOx/sewage sludge-derived activated carbon as catalysts. Sci Total Environ 575:50–57
Haifeng Z (2015) Research on efficiency of advanced treatment of coal gasification wastewater by catalytic ozonation integrated with biological process. Harbin Institute of Technology
Yangyang Y, Xueqiang L, Danyu X, Tao Z, Yan S, Ang Y (2015) The catalytic ozonation of sludge-based composite activated carbon for the degradation of Rh B in aqueous solution. Ind Water Treatment 35:56–59
Gu L, Zhu N, Zhou P (2012) Preparation of sludge derivedmagnetic porous carbon and their application in Fenton likedegradation of 1-diazo-2-naphthol-4-sulfonic acid. Biores Technol 118:638–642
Sun H, Peng X, Zhang S et al (2017) Activation of peroxymonosulfate by nitrogen-functionalized sludge carbon for efficient degradation of organic pollutants in water. Bioresour Technol 241:244–251
Gupta A, Garg A (2015) Primary sewage sludge-derived activated carbon: characterization and application in waste water treatment. Clean Technol Environ Policy 17(6):1619–1631
Kong L, Xiong Y, Sun L et al (2014) Sorption performanceand mechanism of a sludge-derived char as porous carbonbasedhybrid adsorbent for benzene derivatives in aqueoussolution. J Hazard Mater 274:205–211
Nunthaprechachan T, Pengpanich S, Hunsom M (2013) Adsorptive desulfurization of dibenzothiophene by sewagesludge-derived activated carbon. Chem Eng J 228:263–271
Tan C, Rong H, Hongtao W, Wenjing L, Yuancheng Z, Zeyu Z (2014) Adsorption of heavy metals by biochar derived from municipal sewage sludge. J Tsinghua Univ 54:1062–1067
Weiwei Y, Li F, Liqiu Z (2014) Preparation of columnarsludge-based activated carbon and its application in pollutantsremoval. Acta Sci Circum 34:385–391
Ziyan F (2014) Technological research of adsorption of heavy metals by modified activated carbon. Tsinghua University
Zaini MAA, Zakaria M, Alias N et al (2014) Removal of heavymetals onto KOH-activated ash-rich sludge adsorbent. Energy Procedia 61:2572–2575
Tao HC, Zhang HR, Li JB, Ding WY (2015) Biomass based activated carbon obtained from sludge and sugarcane bagasse for removing lead ion from wastewater. Bioresour Technol 192:611–617
Yifan Z, Kangsheng B (2017) Study on the adsorption characteristicof formaldehyde in the air by modified PSAC. AnHuiChem Ind 43:33–37
Qingbo W, Caiting L, Zhihong C, Wei Z, Hongliang G (2010) Application of sewage sludge based activated carbon inform aldehyde adsorption. China Environ Sci 30:727–732
Tao L (2007) Preparation and properties of nitrogen oxide catalyst derived from sewage sludge. Hunan University
Yanjing Z (2015) Study on preparation of TiO2/sludge activated carbon and its photocatalytic purification of acetone gas. Hebei University of Science & Technology
Wei J, Linhuan Z, Fen L, Bo Y, Anxi J (2016) Study on preparationand performance of cerium modified active carbonadsorbent from sewage sludge. Mater Rev 30:411–414
Boualem T, Debab A, Martínez de Yuso A, Izquierdo MT (2014) Activated carbons obtained from sewage sludgeby chemical activation: gas-phase environmental applications. J Environ Manage 140:145–151
Zhai Y, Chen H, Xu B et al (2014) Influence of sewage sludgebasedactivated carbon and temperature on the liquefactionof sewage sludge: yield and composition of bio-oil, immobilizationand risk assessment of heavy metals. Bioresour Technol 159:72–79
Al-Malack MH, Dauda M (2017) Competitive adsorption ofcadmium and phenol on activated carbon produced from municipal sludge. J Environ Chem Eng 5(3):2718–2729
Hanfeng B (2013) Performance and mechanism of four kinds of heavy metals removal from water by prepared sludge-based activated carbon. Beijing Forestry University
Qing L, Xueying R, Jiali L (2012) The Preparation of titanium dioxide photocatalyst loaded on the modified products of municipal sewage sludge. Guangdong Chem Ind 39:59–60
Dezhi L, Guangzhi W, Xin L, Ping W (2015) Study on membrane fouling properties in treatment of landfill leachate by SBAC/MBR process. China Water Wastewater 31:21–26
Razali M, Zhao YQ, Bruen M (2006) Effectiveness of a drinking water treatment sludge in removing different phosphorus species from aqueous solution
Book named 3—Municipal wastewater and sludge treatment
Andreoli CV, von Sperling M, Fernandes Book named biological wastewater treatment series, vol 6. Sludge treatment and disposal
Acknowledgements
The authors gratefully acknowledge the editors Prof. Eric Lichtfouse and Mr. Ali Khadir for granting this wonderful opportunity of contributing a manuscript in this highly esteemed edition “Inorganic-Organic Composites for Water and Wastewater Treatment”. The authors would also like to thank the entire Department of Chemical Engineering, Sathyabama Institute of Science and Technology for facilitating all the necessary help and support throughout this journey. And lastly the authors would like to thank the authors of referred papers for providing the valuable information in them.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Shome, S., Venkatesan, D., Aravind Kumar, J. (2022). Role of Water/Wastewater/Industrial Treatment Plants Sludge in Pollutant Removal. In: Lichtfouse, E., Muthu, S.S., Khadir, A. (eds) Inorganic-Organic Composites for Water and Wastewater Treatment. Environmental Footprints and Eco-design of Products and Processes. Springer, Singapore. https://doi.org/10.1007/978-981-16-5916-4_9
Download citation
DOI: https://doi.org/10.1007/978-981-16-5916-4_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5915-7
Online ISBN: 978-981-16-5916-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)