Abstract
Lead (Pb) is one of the most toxic elements on earth. The harmful effects of Pb at higher concentrations were seen on plant vegetation because plants are directly exposed towards it. Indian mustard, a well-known hyperaccumulator plant is the most promising crop for the environment, engaged in a variety of scenarios for ecological cleanup. In the present study, we used ethylene diamine tetraacetic acid (EDTA), a chelating agent that is of remarkable efficiency. The pot experiments were conducted in soil pretreated with 1000 mgkg-1 Pb with different concentrations of EDTA (2-10 mmol). All the growth parameters were reduced significantly in the plants treated with Pb and EDTA, however, a non-significant effect was observed in 5 mmol EDTA compared to Pb alone treatment. Photosynthetic pigments yield, nitrate reductase activity and NPK content were affected negatively; in contrast, superoxide dismutase and catalase activity was increased in Pb and Pb+EDTA treated in both the varieties. The Pb accumulation was elevated significantly by the augmentation of 5 mmol EDTA in both varieties. Accumulation of Pb in the shoot was higher in PM 25 than in P. Vijay, whereas root Pb accumulation showed the opposite, i.e., more Pb in roots of P. Vijay than PM 25. Moreover, The Pb accumulation per plant was observed more in P. Vijay as compared to PM 25. Hence, the present study implies that the augmentation of Pb-polluted soil with EDTA works well while dealing with B. juncea assisted phytoremediation and P. Vijay to be a stronger variety than PM 25. Further, 5 mmol of EDTA was optimum for phytoremediation of the soil polluted with up to 1000 mg Pb kg-1 soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is universally dispersed as a highly hazardous pollutant in the soil environment. The toxicity of Pb adversely affects both flora and fauna. Since plants are immobile, they are easily affected by Pb concentrations (Shukla et al. 2023). Hazardous effects of Pb may affect the morphology, impair the photosynthesis leading to change in plant’s physiology and genetic structure (Cenkci et al. 2010; Ashraf et al. 2017). Furthermore, increased Pb accumulation affects chlorophyll content, protein, nitrate reductase activity (NRA) and leaf area of plants (Kumar et al. 2012; Ashraf et al. 2015; Chen et al. 2017). Pb phytotoxicity involves decreased water potential, alteration in membrane structure and interrupted hormonal electron transport activities of plants (Ashraf et al. 2017; Zulfiqar et al. 2019). These disturbances cause alteration of the internal structure of various cell organelles of leaf tissue, including thylakoids in the chloroplast, integrity of the mitochondrial membrane and stomata at the ultra-structural level (Cay 2023). Phytoremediation is an effective and eco-friendly technique for removing heavy metals (HMs) from soil (Madhav et al. 2023). It involves exploiting the ability of plants, such as grasses, shrubs and trees, to absorb and accumulate metals from soil (Song et al. 2022; Saldarriaga et al. 2023). Natural hyperaccumulator plants not only escape from the elevated amount of Pb but also extract Pb from the contaminated soil. However, limited availability of Pb in soil restricts wild hyper-accumulator plants which removes high amounts of soil-bound Pb, restricts the effectiveness of the phytoremediation process. Indian mustard is one of the most promising environmental crops used in diverse situations for environmental clean-up (Amabogha et al. 2023). Despite significant effort put into creating Pb extraction methods utilising B. juncea, the majority of Pb stays in the soil or roots without supplement modification. B. juncea is unable to uptake Pb alone from contaminated soil (Chaney et al. 2007). The solubility of Pb in the soil is low for many reasons, such as precipitation as carbonates, phosphates, hydroxides, sorption on clays and oxides, complexation with organic matter etc. (McBride 1994). The solubility of Pb can be enhanced by adding chelating agents to the soil. Chelates are high molecular weight compounds used to wrench out various metals, for example, copper, cadmium, zinc, lead, nickel, gold, silver, etc. Chelates form organic metal complexes through desorption of bound metal form water-soluble complexes. Desorption and dissolution of precipitated compounds continues until equilibrium is reached between the metal-organic complex, insoluble metal and free metal fraction. In the last decade, the use of EDTA, a persistent amino-polycarboxylic acid was recommended as a chelating agent to support the phytoextraction processes (Evangelou et al. 2007; Saman et al. 2022). The EDTA has high complexation properties, making it the most widely used chelating agent (Shahid et al. 2014). It can enhance HMs uptake and translocation up to many folds (200 times in some cases). However, its effect on metal dissolution and translocation is mainly acknowledged for immovable and stable metals like Pb (Singh et al. 2010; Shahid et al. 2014; Rathika et al. 2021).
In light of the aforementioned information, the current study sought to increase Pb uptake in both P. Vijay (tolerant) and PM 25 (sensitive) varieties of Indian mustard by treating Pb-contaminated soil with the chelating agent EDTA. Therefore, the precise mechanisms underlying the effects of Pb and EDTA in combination are still unknown and require additional research, particularly into the Pb detoxification pathways in B. juncea plants. We investigated the role of EDTA in augmentation on Pb uptake, morphological, physiological and biochemical changes during both Pb and EDTA interaction in mustard plants. It is hypothesized that increasing the EDTA concentration up to 5 mmol might result in enhanced Pb uptake without imposing any remarkable effect on growth, yield and antioxidant enzymes compared to the plants treated with Pb alone. It would be intriguing to explore this enhanced phytoremediation approach for detoxification of the soil system, for the benefit of the environment.
Materials and methods
Seeds were obtained from Genetics division of the Indian Agriculture Research Institute (IARI), New Delhi, India. Healthy seeds of both varieties were washed four times with sterile distilled water and soaked for 12 hours. Afterwards, soaked seeds were sown in three earthen pots for each treatment. All the pots were kept and maintained in the green-house condition in complete randomized block design. Based on screening of 10 varieties of Indian mustard, P. Vijay was found to be high Pb tolerant whereas, PM 25 was found to be a sensitive variety. Further, 1000 mg Pb kg-1 soil was selected as a maximum dose in the form of lead nitrate (Naaz and Chauhan 2019). In the present study, the combined effect of an optimized dose of Pb with different concentrations (2, 5 and 10 mmol) of EDTA was evaluated on growth, biochemical, accumulation and yield parameters on selected B. juncea. Three kg of garden soil and farmyard manure (6:1) was filled in each earthen pot (23Dx28Wx23H cm). Before sowing, liquid lead nitrate [Pb (NO3)2] was added at a concentration of 1000 mg kg-1 soil. After 30 days of sowing (DAS), pots were irrigated with EDTA solutions (500 ml pot-1). After successful seedling establishment, thinning was done and three plants per pot were maintained. Afterwards, sowing of the seedlings under natural condition was done during the Rabi season and watering along-with weeding was done when required. Sampling was performed at 60 DAS. Before metal amendment, soil samples were taken and measured physicochemical properties. Soil characteristics (before treatment) were analyzed at Quarsi Agriculture Farm, Koil, Aligarh, Krishi Vibhag U.P. Department of Agriculture and Cooperation, Ministry of Agriculture and Farmers' Welfare, Govt. of India except for Pb content (Table 1).
Growth measurements
Whole plants were uprooted carefully from the experimental pots and washed gently in running tap water and blotted. The root and shoot of plants were separated from the point of root emergence. Root and shoot length were measured on a meter scale. The fresh weight of the root and shoot was calculated with the help of an electronic balance. Plant dry weight was recorded after drying the samples under oven at 75 0C till no more weight reduction was detected. The leaf area was calculated with graph paper. Length, fresh & dry weight and leaf area of the plant were expressed in cm, g and cm2 respectively.
Estimation of photosynthetic pigments
The chlorophyll and carotenoid content in fresh leaves was extracted following the method of Mackinney (1941). One gram of finely cut fresh leaves was grind to a fine pulp using a mortar and pestle in 20 ml of 80% acetone. The supernatant was collected after centrifugation at 5000 rpm for 5 min of the mixture. The residue was washed thrice and final volume was maintained up to 100 ml in 80% acetone. The absorbance was recorded at 645 and 663 nm compared to the blank (80% acetone) under spectrophotometer (Shimadzu, Japan, UV-spec 1700). The chlorophyll and carotenoid content in the extract (mg kg-1 tissue) was calculated according to Arnon (1949) with following equation.
A = Absorbance; V = final volume; w = fresh mass
d =1cm (length of light path); V = 100 ml (acetone); w = weight of sample
In vitro nitrate reductase activity assay
The activity of NR enzyme was estimated by following the method of Jaworski (1971) in fresh leaf samples. The fresh leaves were chopped into pieces (about 1 cm2) and 0.2 gm of leaf samples were transferred to the test tube. In each sample containing tube, 0.1M phosphate buffer (pH 7.5, 2.5 ml), 0.2M potassium nitrate solution (0.5 ml) and 5% isopropanol (2.5 ml) were added. The samples were then incubated at 30 ± 2 0C in the dark. After two hour of incubation, 0.4 ml mixture was pooled out in a fresh tube. In this mixture 1% sulphanilamide solution (0.3 ml) and 0.02% NED-HCl (0.3 ml) were added. After that, the tubes were allowed to incubate for 20 minutes for the maximum color development. The volume of the mixture was maintained to final volume of 5 ml with double distilled water (DDW). Absorbance was recorded at 540 nm under spectrophotometer (Shimadzu, Japan, UV-spec 1700) with reference to the blank. The NR enzyme activity was calculated by comparing the absorbance of samples with absorbance of NaNO2 (sodium nitrite) solution with known concentrations. A standard curve was plotted by using graded concentrations of NaNO2 solution. Sulphanilamide solution and NED-HCl solution were also added to the NaNO2 graded tubes for the development of maximum color and the final volume was makeup to 5 ml as test solution. NR activity (nmole NO2 g-1 FW h-1) was estimated on a fresh mass basis.
Antioxidative enzyme activities
The antioxidative enzyme activities were evaluated in fresh leaves (0.5 g). The sample was homogenized with the help of mortar and pestle and augmented with potassium phosphate buffer (5 ml; 100 mM, pH 7.0) under ice-cold conditions. Then, the mixture was centrifuged at 15,000 rpm for 20 min and obtained supernatant was stored at -20 °C to analyze SOD and catalase activity.
In vitro superoxide dismutase assay
In vitro superoxide dismutase (SOD) activity was estimated according to Kono (1978). A 1.3 ml sodium carbonate buffer, NBT (Nitroblue tetrazolium) (500 μl) and Triton X-100 (100 μl) were added in a tube. To this mixture, hydroxylamine hydrochloride (100 μl) was added to start the reduction of NBT to formazon. Further, 70 μl of the enzyme extract was added after 2 min to reduce the NBT reduction by SOD enzyme. The percent inhibition of NBT reduction was recorded as an increase in absorbance at 540 nm under spectrophotometer (Shimadzu, Japan, UV-spec 1700). Hydroxylamine hydrochloride is auto oxidized to nitrite and generates superoxide radicals. This superoxide radical reduces NBT into blue formazon. Accumulation of blue formazon induces an increase absorbance at 540 nm. Superoxide radicals get trapped with the addition of enzyme extract (SOD) and the reduction process of NBT to blue formazon decreases. The present inhibition of NBT reduction was calculated as:
y% of inhibition is produced by 70 μl of the sample.
Hence, 50% inhibition is produced by
The amount of enzyme required to inhibit chromogenic production's absorbance at 540 nm by 50% over the course of one minute in experimental settings is referred to as one unit of SOD activity. SOD activity is expressed as units per milligram of protein.
In vitro catalase assay
The catalase activity was determined as per the method proposed by Aebi (1983). A 1.5 ml phosphate buffer, 1.2 ml of hydrogen peroxide and 300 μl of enzyme extract were mixed in a tube. The decomposition rate of H2O2 was followed by a decrease in absorbance at 240 nm under spectrophotometer (Shimadzu, Japan, UV-spec 1700) in reaction mixture. The amount of enzyme required to release half of the peroxide oxygen from H2O2 was used to measure one unit of enzyme activity, and the following equation can be used to calculate one unit of enzyme activity:
Where, extinction coefficient = 0.036 mM-1cm-1
Estimation of leaf N, P and K contents
After 60 DAS, leaf samples were collected and oven-dried for the estimation of N, P and K content. The dried leaves were powdered with mortar and pestle and sieved through 72 mm mesh. A 100 mg of the powder from each replicates were taken in the borosilicate boiling tube containing 2 ml H2SO4. The content in tubes was heated on a controlled temperature assembly for 2 hours at 80 °C. Nitrate present in the plant material was completely reduced and the content of the tubes turned black. Tubes were allowed to stand for 15 min to reduce the temperature. After cooling, 0.5 ml of 30% H2O2 was added to tubes and heated again until the color of the solution turned light yellow. The tubes were allowed to cool down and after cooling, a few drops of 30% H2O2 were added and heated further. This process was continued to the solution became transparent. The solution was then collected to a 100 ml volumetric flask and the tubes were washed gently with DDW and the wash over was also collected in the flask. The volume was makeup with DDW and the aliquot was used to estimate N, P and K content in the leaves.
Estimation of nitrogen content
Nitrogen content was estimated following the method of Lindner (1944). In 50 ml conical flask, aliquot of 10 ml peroxide digested, 2.5N NaOH and 1 ml of 10% sodium silicate solution was added. Volume was make up to 50 ml with DDW. From this, a 5 ml aliquot was collected in a fresh tube, 0.5 ml of Nessler's reagent and 4.5 ml DDW was added to makeup the final volume up to 10 ml. The tubes were incubated 5 min for the maximum color development. The absorbance was recorded at 525 nm using spectrophotometer (Shimadzu, Japan, UV-spec 1700).
Calculation with standard curve
The ammonium sulphate (50 mg) was dissolved in one liter DDW and from this solution, a gradient of 0.1 to 1.0 mL were taken in ten fresh tubes. The Nessler's reagent (0.5 ml) was added to each tube and the final volume was maintained to 5 ml with DDW. The absorbance was recorded at 525 nm under spectrophotometer. A standard curve was plotted using the absorbance recorded from the graded solutions at 525 nm in reference with different concentration of ammonium sulphate. Concurrently, a blank was also run simultaneously. The nitrogen content of the sample was calculated with reference to standard.
Estimation of phosphorus content
Phosphorus content was determined according to Fiske and Subbarow (1925). A 5 ml of peroxide-digested aliquot was taken in test tube and 1 ml of 2.5% molybdic acid reagent along-with 0.4 ml 1-amino-2-naphthol-4-sulphonic acid was added carefully. After addition of above reagents, the solution turned blue in color. Consequently, the solution was diluted up to 10 ml with DDW and shaken for 5 min. After maximum color development, the absorbance of the solution was recorded at 620 nm using spectrophotometer (Shimadzu, Japan, UV-spec 1700). Simultaneously a blank was also run for comparison of data.
Calculation with standard curve
Quantity of 350 mg KH2PO4 was taken in a conical flask (1000 ml) and dissolved in sufficient amounts of DDW. 10 ml of 10N H2SO4 was added to it and the volume was made up to the mark with DDW. In separate test tubes, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, and 1.0 ml were collected from this solution. One ml of molybdic acid reagent and 0.4 ml 1-amino-2 naphthol-4- sulphonic acid was added to each tube and the final volume was maintained up to 10 ml with DDW. Followed this, tubes were allowed to stand for color development and the absorbance was recorded at 620 nm under spectrophotometer with reference to the blank. A standard curve was plotted using the absorbance of graded KH2PO4 solutions at 620 nm against their respective concentrations. Phosphorus content was estimated by putting the absorbance of the test solution into a standard curve.
Estimation of potassium content
Potassium content was determined by the method proposed by Hald (1946). A digested sample aliquot of 10 ml was taken in a test tube and the readings were observed under flame photometer (C150, AIMIL, India) using a potassium filter in reference to the blank.
Calculation with standard curve for potassium
In 100 ml DDW, potassium chloride (1.91 g) was dissolved and 1 ml was pooled out in a conical flask (1000 ml) and the final volume was maintained up to the mark. The solution prepared was 10 ppm in concentration, and the solution was aliquoted at 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0 and 10 ml in separate test tubes. The final volume of each was maintained up to 10 ml with DDW. Samples were examined under flame photometer against the blank and readings were recorded using galvanometer scale. A standard curve was plotted using galvanometer readings of the graded potassium chloride solutions against their respective concentrations. Potassium content was estimated by putting the galvanometer readings of test solutions in standard curve.
Estimation of Pb concentration and accumulation
Soil Pb concentration was measured by digesting dried soil samples in aqua regia (HNO3: HCl; 3:1; v/v). Tissue-dried root and shoot samples were powdered, weighed and digested in HNO3:HClO4 (3:1; v/v) mixture until colorless (Piper 1966) using a digestion assembly. After maintaining the final volume with deionized water, Pb content was measured using AAS (Atomic Absorption Spectrophotometer:Perkin-Elmer A, Analyst, 300; Norwalk, CT) using an appropriate cathode lamp and standard. Pb concentration (μgg-1) and accumulation (μgplant-1) were calculated using the following formula with a slight modification: (Monni et al. 2000).
Yield attributes
Seeds per siliqua and siliqua per plant were counted after harvesting. After being cleaned and allowed to dry for a few hours, whole seeds were winnowed from the dried samples of each treatment. The weight of the seeds was recorded for 1000 seeds and the total seed yield measurement in terms of g and g plant-1, respectively.
Statistical analysis
Statistical data analysis was achieved using SPSS Software ver.16. Test of significance was carried out by ANOVA (Analysis of Variance) followed by DMRT (Duncan's Multiple Range Test) at 0.05 α level. An error was also calculated with three replicates.
Results
As described in the material and methods, the experiment was conducted to study the effects of EDTA on growth, biochemical, Pb accumulation and yield parameters in both varieties. Plants were treated with three different concentrations of EDTA in combination with 1000 mg Pb Kg-1 soil.
Growth attributes
Shoot length
As evident in Tables 2 and 3, it is clear that EDTA application leads to retarded growth of plants. A linear drop in the values of shoot length in proportion to the EDTA was produced by the plants grown in soil supplemented with varied EDTA levels (2, 5, and 10 mmol EDTA Kg-1 soil) at 30 DAS, more pronouncedly in PM 25 than P. Vijay. The shoot length was decrease significant at all applied doses of Pb supplemented with EDTA as compared to control. However, maximum decline in shoot length was observed at 10 mmol EDTA Kg-1 soil with Pb. The reduction in shoot length of PM 25 was recorded in the range of 37.94-56.28% at 2-10 mmol EDTA Kg-1 soil, and the reduction in shoot length of P. Vijay in the range of 16.21-28.09% at 2-10 mmol EDTA Kg-1 soil (Tables 2 and 3).
Root length
As depicted in Tables 2 and 3, the root length was decreased in plants grown in soil with a concentration of 1000 mg Pb Kg-1 in both varieties (PM 25 and P. Vijay). The values further decreased when subjected to different EDTA levels (2, 5 and 10 mmol EDTA Kg-1 soil). The lowest concentration (2 mmol EDTA Kg-1 soil) proved to be the least toxic of the three EDTA concentrations. Moreover, the highest concentration (10 mmol EDTA Kg-1 soil) caused maximum damage to the plants. The intensity of reduction to root length of PM 25 and P. Vijay caused by EDTA (2-10 mmol EDTA Kg-1 soil) was 42.21-58.61% and 20.43-31.71%, respectively, at 60 DAS. The damage was more prominent in variety PM 25 than in P. Vijay. However, the reduction was found to be significant in reference to control (C1) to the plants treated with EDTA at all levels but significant with control (C2) to the plants treated with 10 mmol EDTA Kg-1 soil only (Tables 2 and 3).
Leaf area
The EDTA administered to the soil induced a significant reduction in the leaf area in both the varieties (PM 25 and P. Vijay) at 60 DAS. The highest concentration of EDTA (10 mmol Kg-1 soil) proved the most toxic. The above tested concentration significantly decreased the leaf area values by 31.87% and 57.57% in P. Vijay and PM 25, respectively, when compared with their respective control plants treated with PbNO3. In contrast, the remaining concentrations of EDTA (2 and 5 mmol Kg-1 soil) also reduced the leaf area of both varieties significantly, with the control without any treatment. The severity of damage was more prominent in PM 25 than in P. Vijay. Data regarding the leaf area of PM 25 and P. Vijay were shown in Tables 2 and 3, respectively.
Root fresh weight
Fresh weight of root was decreased in both the varieties grown in soil in the presence of Pb (Tables 4 and 5). Soil supplemented with EDTA increased the reduction in root fresh weight in both the varieties and therefore the reduction being more prominent in PM 25 than in P. Vijay. The highest EDTA level (10 mmol Kg-1 soil) caused a maximum decrease in fresh root weight both varieties. In terms of percent reduction, it was 36.44-51.01% in PM 25 at 2-10 mmol EDTA Kg-1 soil compared to control and 17.09-29.11% in P. Vijay at 2-10 mmol EDTA Kg-1 soil compared to control at 60 DAS. This reduction due to EDTA doses was significant to control (C1), in PM 25 as well as P. Vijay. However, taking control (C2) into consideration, the reduction was significant in PM 25 and P. Vijay at 5 mmol EDTA Kg-1 soil and 10 mmol EDTA Kg-1 soil respectively (Tables 4 and 5).
Shoot fresh weight
The fresh mass of shoots of PM 25 and P. Vijay decreased in the presence of 1000 mg Pb Kg-1 soil (Tables 4 and 5). Further reduction in fresh shoot weight was observed when EDTA was added to the soil along with Pb. The EDTA administered to the soil combined with Pb caused a significant reduction in the fresh mass of shoot in both the varieties (PM 25 and P. Vijay) at 10 mmol EDTA Kg-1 soil compared to the plants growing in Pb-containing soil alone. Whereas, the reduction in fresh shoots weight of treated plants was significantly different from control (C1). The reduction was recorded in the range of 41.1-55.83% in PM 25 and 13.43-26.61% in P. Vijay at 2-10 mmol EDTA Kg-1 soil over their respective controls. The fresh shoot weight of PM 25 was reduced by EDTA and Pb treatment than that of P. Vijay (Tables 4 and 5).
Root dry weight
Both the varieties showed a significant reduction in root dry mass under EDTA and Pb's influence compared to the control (Tables 6 and 7). The EDTA (2-10 mmol Kg-1 soil) supplemented with Pb resulted in root dry mass reduction in the range of 32.09-47.86% and 11.81-24.24% less in PM25 and P. Vijay respectively, at 60 DAS compared to their respective control plants. The root dry weight of both the varieties was significantly affected by 10 mmol EDTA Kg-1 soil and Pb as compared to control (C2). The variety PM 25 proved more sensitive to EDTA than P. Vijay (Tables 6 and 7).
Shoot dry weight
Shoot dry weight decreased in both varieties while more in PM 25 under 1000 mg Pb Kg-1 soil. The addition of EDTA (2-10 mmol Kg-1 soil) further decreased the dry weight of PM 25 (27.37-41.61%) and P. Vijay (9.68-23.96%). Out of the three tested concentrations, only 10 mmol EDTA Kg-1 soil significantly reduced the dry weight of both varieties compared to Pb alone. The maximum reduction in dry weight was observed in PM 25 with 10 mmol EDTA Kg-1 soil (Tables 6 and 7).
Physio-chemical attributes
Photosynthetic pigments
The plants grown in the soil amended with Pb (1000 mgKg-1 soil) possessed significantly lower values of chlorophyll a, b and total chlorophyll than the stress free control plants of PM 25 and P. Vijay (Tables 8 and 9). Out of the tested EDTA concentrations (2, 5, and 10 mmol Kg-1 soil), 10 mmol EDTA Kg-1 soil was found to be most toxic. EDTA treatment (2-10 mmol Kg-1 soil) decreased the chlorophyll-a (8.6-21.89%), chlorophyll b (20.52-22.05%) and total chlorophyll (12.99-27.40%) in P. Vijay compared with control. Further, EDTA decreased chlorophyll-a value in the range of 21.36-43.78%, chlorophyll-b (57.20-70.45%) and total chlorophyll in the range of 31.59-51.39% in PM 25 compared with the control plants. Photosynthetic pigment decreased significantly at 10 mmol EDTA Kg-1 soil in PM 25 and P. Vijay compared to their respective controls. This declined in chlorophyll contents was more prominent in PM 25 than P. Vijay at 60 DAS. Among chl a, b and total content, chl-b seems most sensitive to EDTA and Pb treatment. Among the tested concentrations, 10 mmol EDTA Kg-1 soil with Pb were most deleterious to the chlorophyll pigments in PM 25 and P. Vijay (Tables 8 and 9).
Unlike the other parameters, the chl ratio (a:b) increased when the plants were treated with Pb in both varieties, PM 25 and P. Vijay (Tables 8 and 9). The chl ratio enhancement was further boosted due to the presence of EDTA in the soil. The increase in ratio was relative to the increase in EDTA level in the soil. The EDTA (2-10 mmol Kg-1 soil) increased the ratio (a:b) by 15.01-23.89% and 85.03-90.39% in P. Vijay and PM 25 respectively, as compared to their respective controls. A significant increase was observed in both the varieties when compared to their control (C1). However, when compared with control (C2), the increase was significant in P. Vijay at 10 mmol EDTA Kg-1 soil, while a non-significant increase was observed in PM 25. The maximum enhancement for the chl ratio (a:b) was observed in PM 25 grown in 10 mmol EDTA Kg-1 soil (Tables 8 and 9).
The data presented in Tables 8 and 9 shows that EDTA with Pb in the soil caused a significant decrease in carotenoid content, both in P. Vijay and PM 25. The reduction becomes more pronounced increased with the increasing concentration of EDTA. Regarding percentage of 2-10 mmol, EDTA Kg-1 soil lowered the carotenoid content by 13.31-27.48% and 35.47-46.14% in P. Vijay and PM 25, respectively. The reduction was found to be significant in terms of control (C1), while significant only at 10 mmol EDTA Kg-1 soil in terms of control (C2) in both the varieties (Tables 8 and 9).
Nitrate reductase activity
Figure 1 shows a decline in NR activity (NRA) in the plants treated with Pb and EDTA. The reduction of NR activity was observed in P. Vijay (10.10%) and PM 25 (30.50%) under Pb concentration. The addition of EDTA at the rate of 2-10 mmol Kg-1 soil further decreased the NR activity in the range of 12.41-21.65% and 33.83-42.83% in P. Vijay and PM 25, respectively. The NR activity was more significantly reduced in 10 mmol EDTA Kg-1 with Pb soil as compare to control in both the varieties. Reduction in NR activity was more prominent in PM 25 than P. Vijay.
Effect of different treatments of Pb and EDTA on nitrate reductase activity (NRA) in the leaf of 60-day-old B. juncea cv. P. Vijay and PM 25. Values represented by bars are means of three replicates. SE was represented as capped bars. Significant difference at p<0.05 was determined by one way ANOVA to compare the effect of varying Pb concentrations on a particular cultivar. Bars bearing same alphabets do not differ significantly (DMRT was applied separately to each cultivar). C1 = Control, C2 = Pb (1000 mg kg-1), E1= EDTA (2 mmol kg-1 soil), E2 = EDTA (5 mmol kg-1 soil), E3 = EDTA (10 mmol kg-1 soil)
Superoxide dismutase activity
The Pb stress led to a considerable rise in SOD activity, which was then further raised by the addition of EDTA as depicted in Fig. 2. The variety P. Vijay showed 47.48% and PM 25 showed 68.18% higher SOD activity as compare to both control (C1 and C2). Addition of EDTA to the Pb-contaminated soil significantly enhances the SOD activity compared to treated and untreated control. It reaches up to 107.63% higher in P. Vijay and up to 129.96% higher in PM 25 at 10 mmol EDTA Kg-1 soil, showing that SOD activity was increased more in PM 25 as compared to P. Vijay.
Effect of different treatments of Pb and EDTA on superoxide dismutase (SOD) activity in the leaf of 60-day-old B. juncea cv. P. Vijay and PM 25. Values represented by bars are means of three replicates. SE was represented as capped bars. Significant difference at p<0.05 was determined by one way ANOVA to compare the effect of varying Pb concentrations on a particular cultivar. Bars bearing same alphabets do not differ significantly (DMRT was applied separately to each cultivar). C1 = Control, C2 = Pb (1000 mg kg-1), E1= EDTA (2 mmol kg-1 soil), E2 = EDTA (5 mmol kg-1 soil), E3 = EDTA (10 mmol kg-1 soil)
Catalase activity
The presence of Pb in the soil significantly increased catalase activity, as shown in Fig. 3. In comparison to the untreated and treated controls for both varieties, the soil amendment containing EDTA considerably increased catalase activity. Under Pb stress, the catalase activity of P. Vijay enhanced by 27.63%. In contrast, addition of EDTA to the Pb-contaminated soil leads to the maximum enhancement in catalase activity by 73.69% at 10 mmol EDTA Kg-1 soil. Similarly, Pb-treated PM 25 showed 13.48% enhancement in catalase activity. The addition of 2-10 mmol EDTA Kg-1 soil led to a maximum elevation (30.47%) of catalase activity in comparison to untreated control. The activity was more significantly enhanced in P. Vijay compared to PM 25.
Effect of different treatments of Pb and EDTA on catalase (CAT) activity in the leaf of 60-day-old B. juncea cv. P. Vijay and PM 25. Values represented by bars are means of three replicates. SE was represented as capped bars. Significant difference at p<0.05 was determined by one way ANOVA to compare the effect of varying Pb concentrations on a particular cultivar. Bars bearing same alphabets do not differ significantly (DMRT was applied separately to each cultivar). C1 = Control, C2 = Pb (1000 mg kg-1), E1= EDTA (2 mmol kg-1 soil), E2 = EDTA (5 mmol kg-1 soil), E3 = EDTA (10 mmol kg-1 soil)
Leaf nitrogen content
The nitrogen content in the leaves of both P. Vijay and PM 25 exhibited a significant reduction when plants treated with Pb (Tables 10 and 11). The nitrogen content found to be reduced in the leaves of both the varieties that received EDTA (2, 5 and 10 mmol Kg-1 soil). A significant reduction was observed in comparison to control (C1) at all the EDTA concentrations, however, the reduction was more in 10 mmol EDTA Kg-1 soil with Pb in both varieties. The EDTA reduced the leaf nitrogen content in the range of 22.93-35.27% in P. Vijay and 37.57-49.56% in PM 25 at 2-10 mmol Kg-1 soil. The result indicates that P. Vijay resists the loss in leaf nitrogen content more efficiently than PM 25.
Leaf phosphorus content
The leaf phosphorus content followed a pattern similar to leaf nitrogen in both varieties (Tables 10 and 11). Soil application of EDTA at the rate of 2, 5 and 10 mmol Kg-1 soil with Pb reduced the leaf phosphorus content in P. Vijay and PM 25. Maximum reduction was observed with 10 mmol EDTA Kg-1 soil and found significant in both the varieties with reference to Pb alone. Leaf phosphorus content was found to be 29.14-46.78% lowered in PM 25 and 19.58-31.82% lowered in P. Vijay when subjected to 2-10 mmol EDTA Kg-1 soil over their respective controls 1 (Tables 10 and 11).
Leaf potassium content
The leaf potassium content in the plants of both varieties (P. Vijay and PM 25) expressed a response similar to leaf nitrogen and phosphorus (Tables 10 and 11). A significant decrease in the leaf potassium content was recorded in the plants supplemented with EDTA (2, 5 and 10 mmol Kg-1 soil) along with Pb. EDTA-treated plants showed a maximum reduction at 10 mmol Kg-1 soils. Plants exhibited a similar trend in the significance of data as leaf nitrogen and phosphorus content. A reduction in potassium content was recorded in the range of 15.92-28.12% in P. Vijay and 17.46-34.57% in PM 25 concerning the untreated control (Tables 10 and 11).
Yield attributes
Seeds per siliqua
Application of Pb reduced the quantity of seeds per siliqua in both cultivars, however P. Vijay revealed a less pronounced decline. The seeds per siliqua continue to decrease when soil was treated with EDTA and Pb. The application of EDTA significantly reduced seeds per siliqua at all the applied doses of EDTA on P. Vijay in comparison to both control. However, in the case of PM 25, doses of EDTA (2, 5 and 10 mmol Kg-1 soil) reduced the number of seeds per siliqua significantly with control (C1) only; concerning with control (C2), number of seeds per siliqua was reduced significantly at 10 mmol EDTA Kg-1 soil. Application of EDTA (2-10 mmol Kg-1 soil) reduced the number of seeds per siliqua by 12.5-18.11% and 15.24-22.46% in P. Vijay and PM 25 respectively, over their respective untreated control (Tables 12 and 13).
Siliqua per plant
The number of siliqua per plant was reduced in both of the 1000 mg Pb Kg-1 treated varieties, however the reduction was only found to be significant in PM 25 when compared to the control. Moreover, the loss in the number of siliqua per plant was significantly declined in the presence of EDTA and Pb in soil. In P. Vijay, siliqua per plant was significantly reduced by applying 2, 5 and 10 mmol EDTA/Kg soil concerning control 1 and 2. The variety PM 25 showed the same response concerning control 1, whereas, with control 2, the reduction was found to be significant only with applying 10 mmol EDTA Kg-1 soil. In terms of percentage, the reduction in siliqua number per plant was declined in the range of 13.18-20.06% and 19.92-31.8% in P. Vijay and PM 25, respectively, over their untreated control, on the application of EDTA (2-10 mmol Kg-1 soil) (Tables 12 and 13).
1000 seeds weight
The reduction in weight of 1000 seeds was observed in Pb-treated plants of both varieties compared to their untreated control and the significant reduction was observed in PM 25 only (Tables 12 and 13). Further, additions of EDTA in Pb treated plants worsen the loss in weight of 1000 seeds. A significant reduction was observed in P. Vijay supplemented with 10 mmol EDTA Kg-1 soil reference to the untreated control, whereas, concerning the treated control, no significant effect was noticed. On the application of 2, 5, and 10 mmol EDTA Kg-1 soil in addition to Pb in PM 25, relative to the untreated control, and on the application of 10 mmol EDTA Kg-1 soil in addition to Pb, relative to the treated control, considerable weight loss in 1000 seeds was detected. When subjected to 2–10 mmol EDTA Kg-1 soil together with Pb, values for 1000 seeds weight were recorded in P. Vijay that were 3.31–14.36% less and in PM 25 that were 5.84–17.53% less than the untreated control (Tables 12 and 13).
Lead accumulation
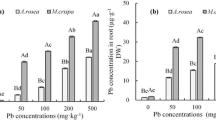
In shoot
In the present experiment, Pb was not detected in untreated control plants of both P. Vijay and PM 25 (Tables 14 and 15). Whereas, 30.03 μg of Pb g-1 in dry weight (DW) of the shoot in P. Vijay and 2529.47 μg of Pb g-1 DW in PM were recorded respectively, when exposed to 1000 mg Pb Kg-1 soil. When EDTA was added together with Pb, the accumulation of Pb in both types increased. In P. Vijay, the highest uptake was noted in plants treated with 10 mmol EDTA Kg-1 soil (90.98 g of Pb g-1 DW of the shoot), while it was comparable to 5 mmol EDTA Kg-1 soil (89.27 g of Pb g-1 DW of the shoot), and the remaining values were statistically significant. Maximum uptake of PM 25 was also observed in plants treated with 10 mmol EDTA Kg-1 soil (95.47 g of Pb g-1 DW of the shoot), while it was comparable to plants treated with 5 mmol EDTA Kg-1 soil (94.77 g of Pb g-1 DW of the shoot). Significant accumulation was observed with the remaining treatments (Tables 14 and 15).
In root
Traces of Pb were detected in roots of untreated control plants in both P. Vijay (0.04 μg of Pb g-1 DW of the root) as well as in PM 25 (0.06 μg of Pb g-1 DW of the root) (Tables 14 and 15). A 59.8 μg of Pb g-1 DW of root in P. Vijay and 50.9 μg of Pb g-1 DW in PM 25 were recorded when exposed to 1000 mg Pb Kg-1 soil. The addition of EDTA along with Pb, enhances the accumulation of Pb in both varieties. In P. Vijay, maximum uptake was found in plants treated with 10 mmol EDTA Kg-1 soil (75.28 μg of Pb g-1 DW of the root) while it was at par with 5 mmol EDTA Kg-1 soil (73.32 μg of Pb g-1 DW of the root), rest of the values were significant with each other. In PM 25, maximum uptake was also noted in plants treated with 10 mmol EDTA Kg-1 soil (66.57 μg of Pb g-1 DW of the root) while it was at par with 5 mmol EDTA Kg-1 soil (64.5 μg of Pb g-1 DW of the root), significant accumulation was achieved with rest of treatments (Tables 14 and 15).
Per plant
It was calculated with the help of the above parameters. The Pb accumulation in the fresh and dry weight of root, shoot was found to be a minimal amount of Pb (0.07 μg plant-1 in P. Vijay and 0.08 μg plant-1 in PM 25) was uptake by the untreated control. Supplementing the soil with Pb increases both varieties' Pb uptake per plant, and amendment of the soil with EDTA and Pb resulted in improved Pb accumulation per plant, significant to the treated and untreated control at each level of EDTA. Maximum Pb uptake per plant was recorded with 5 mmol EDTA Kg-1 soil in P. Vijay (761 μg plant-1) and PM 25 (537.25 μg plant-1). According to above findings, P. Vijay accumulates more lead per plant than PM 25 (Tables 14 and 15).
Discussion
The present study supported our hypothesis under the regime of phytoremediation that the application of EDTA affects the morphological, physiological, biochemical and yield parameters in two varieties of B. juncea under Pb stress. The experiment revealed that EDTA and 1000 mg kg-1 soil Pb further decreased the growth of both varieties. This result was found in agreement with the findings of Grcman et al. (2001). Among the tested EDTA concentrations, 5 mmol EDTA kg-1 soil was the highest dose on which reduction in growth was not significant. Further increase in EDTA concentration started reducing the plant's growth significantly when compared to the plant treated with Pb alone. A similar phytotoxic effect of EDTA concentrations was reported by Bareen and Tahira (2010). Ali et al. (2019) found that an excessive amount of trace metals solubilized by EDTA can cause a reduction in the biomass of the plant. Vassil et al. (1998) suggested that in the plasma membrane, stabilizing divalent cations such as zinc ion (Zn+2), calcium ion (Ca+2), ferrous ion (Fe+2), copper ion (Cu+2) were removed by the uptake and accumulation of chelators like EDTA, and the physiological barrier that controls the uptake of minerals was disrupted; also the biochemistry of cells get affected leading to cell death. Dose-dependent free protonated EDTA (H-EDTA) phytotoxicity was reported in Cynara cardunculusin (Hernandez-Allica et al. 2007). Moreover, the action of EDTA severely affects the plant water content, due to which disruption in osmoregulation arises, thus leaving the plant under stress. Therefore, the effect of high EDTA concentration on the plant's growth was reflected in reduced biomass (Zulfiqar et al. 2019). The results represent that a lower level of EDTA does not diminish the effects of Pb from the soil. These findings are in agreement with Saygideger and Dogan (2004). The photosynthetic pigments were reduced significantly when the plants were treated with EDTA and Pb. This decrease may be attributed to the free protonated EDTA or Pb ions, which cause toxicity. The uncoordinated protonated EDTA tends to bind with essential divalent cations like Fe2+, Zn2+, Cu2+ and Magnesium ion (Mg2+) (Vassil et al. 1998). The above report explains the cause behind the chl degradation in high-concentration EDTA-exposed plants. Addition of EDTA to the Pb-contaminated soil does not significantly affect the plant. However, due the higher concentration of EDTA, carotenoid content decreases significantly compared to Pb stress. This result was found concomitant to Mohammadi et al. (2018) on sunflower against chromium (Cr) and suggested that EDTA significantly enhances the metal mobility to the plants resulting in higher metal uptake, which could cause oxidative damage and decrease in the photosynthetic pigments.
The NR activity did not alter during the addition of EDTA (5 mmol EDTA Kg-1) in soil compared to Pb alone, however, NR activity declined in both varieties under 10 mmol EDTA Kg-1 soil. The NR activity shows that PM 25 is more negatively affected than P. Vijay. NR catalyzes electron transfer by reducing nitrate to nitrite and nitrite to nitric oxide (NO) using NAD(P)H, which also results in superoxide anions as a byproduct. Thus, NR regulation is necessary at transcriptional and post-translational levels. For the inactivation of NR, phosphorylation of NR on a serine residue takes place, which creates a binding site for 14-3-3 proteins. The binding of 14-3-3 to phosphorylated NR in the presence of divalent cations (Mg2+, +EDTA) inactivates NR completely (Kaiser et al. 2002). This may be the possible reason behind lower NR activity in the presence of Pb and EDTA. Studies on the kinetics of primary antioxidative enzymes showed a significant increase under the stress of all the tested Pb and EDTA concentrations in single metal applications and binary combinations. HMs cause oxidative stress due to the production of free radicals and reactive oxygen species (Kumar et al. 2014; Hasanuzzaman et al. 2020). Metallic ions interfere with the electron flow system in PSII, well known for chlorophyll excitation, which leads to reactive oxygen species (ROS) production (Kato and Simizi 1985; Dolui et al. 2022). Plants scavenge ROS with many proficient enzymatic and non-enzymatic antioxidative defense systems, including SOD, CAT, ascorbate peroxidase (APX) and glutathione reductase (GR). SOD is a well-known antioxidative enzyme that has a vital role in the cellular defense mechanism against free radicals. It facilitates the dismutation of superoxide radicals to H2O2 and O2. The data obtained on the SOD enzyme activity of P. Vijay and PM 25 showed an increase in SOD activity under the regime of Pb, followed by EDTA applications. This signifies that these varieties can detoxify the toxicants produced by the accumulated HM ions (Jiang et al. 2019; Huang et al. 2020) in response to exceeding levels of HMs; however, the enhancement in SOD activity was slightly low. On the other hand, producing free superoxide radicals above a threshold limit could hamper the proper functioning of the SOD enzyme (Alam et al. 2020; Shukla et al. 2023). Several other studies on antioxidative enzyme responses to environmental stresses have documented similar observations (Kumar et al. 2014; Saman et al. 2022; Alam et al. 2023a, b). The results of the present study propose that Indian mustard cv. P. Vijay and PM 25 counter balance the increased ROS concentration developed due to Pb stress by regulating antioxidant enzymes' activity in a synchronized manner. In both P. Vijay and PM 25 varieties, CAT enzyme activity amplifies with an increase in Pb and EDTA concentration, which indicates the efficient H2O2- scavenging system in the plants. The coordination in the enhancement of CAT and SOD parallel to the concentration of treatment plays a critical part in the scavenging procedure of O2- and H2O2 (Khan et al. 2019; Huang et al. 2020; Huihui et al. 2020; Rahbari et al. 2020; Rathika et al. 2021). However, the coordination between CAT and SOD amplification was more effective in P. Vijay than in PM 25. Thus, Pb toxicity can be known to elevate ROS production, which can stimulate the antioxidant protection system of the plants. Increased enzymatic actions under Pb stress in the current study for detoxification are all-embracing by many other earlier studies (Zulfiqar et al. 2019; Song et al. 2022): It has been shown that metal-induced oxidative stress leads to ROS manifestation of ROS-activated enzymes (Rahbari et al. 2020). The application of EDTA and 1000 mg Pb kg-1 soil further increases the Pb accumulation of both varieties: P. Vijay and PM 25. Chelators can enhance the availability of trace elements in soil, which helps facilitate the hyperaccumulation process (Hai et al. 2022). Chelator causes the dissolution of adsorbed trace elements and enhances its mobilization so plants can easily absorb and accumulate it (Ebrahimi 2016). The use of EDTA is well known to enhance metals' availability for plant uptake (Wu et al. 2004; Jiang et al. 2019; Li et al. 2020). Augmentation of soil with EDTA can elevate higher total metal uptake. However, a large amount of free protonated EDTA could decrease plant biomass and renders the plant in an even more stressful condition (Turgut et al. 2005). The reductions in yield characteristics due to 1000 mg Pb kg-1 soil were not significantly affected by treatment of 5 mmol EDTA Kg-1 soil. In contrast, the application of 10 mmol EDTA Kg-1 soil significantly lowered yields in both varieties, possibly due to the availability of free protonated EDTA, which is toxic to the plant, as discussed earlier. Hence decreased seed yield could be attributed to suppressing dry matter production in Pb-exposed plants (Wani et al. 2007; Saman et al. 2022).
Conclusion
In the previous study, we performed a screening of B. juncea cv. which helps to select P. Vijay and PM 25 as hyper and hypo accumulators, respectively (Naaz and Chauhan 2019). The present study established that selected varieties were co-cultivated in Pb-contaminated soil with EDTA. In comparison to the control, the morphological, physiological, biochemical changes and yield were adversely affected under Pb stress. The Pb accumulation increased in Pb stress in plant, while more in Pb + EDTA (5 mmol Kg-1 soil). The biochemical characteristics, such as photosynthetic pigments and the enzyme NR activity, were not significantly affected in both the varieties after co-cultivation (Pb) with up to 5 mmol EDTA Kg-1 soil. The antioxidant enzymes were more efficient in the hyper-accumulator than the hypo-accumulator cultivar. Yield components of both the varieties were somewhat affected by co-cultivation compared to untreated control. The results of the current study showed that both varieties considerably increased Pb accumulation when exposed to EDTA (5 mmol Kg-1). Therefore, using EDTA (a chelating agent) may increase the amount of Pb that B. juncea plants accumulate per plant during field studies.
Data availability
All the data generated or analyzed in the present study are included in this manuscript.
References
Aebi HI (1983) Catalase. Methods of enzymatic analysis 673-686
Alam N, Anis M, Javed SB, Alatar AA (2020) Stimulatory effect of copper and zinc sulphate on plant regeneration, glutathione-S-transferase analysis and assessment of antioxidant activities in Mucuna pruriens L.(DC). Plant Cell, Tissue Organ Cult 141:155–166
Alam N, Ahmad A, Ahmad N, Anis M (2023a) Polyamines Mediated In Vitro Morphogenesis in Cotyledonary Node Explants of Mucuna pruriens (L.) DC.: A Natural Source of L-Dopa. J Plant Growth Regul 1-13
Alam N, Siddique W, Mishra MK et al (2023b) Micropropagation of Hoya carnosa, H. kerrii, H. parasitica, and H. longifolia using tray-based floating and stationary hydroponic systems. Sci Hortic 311:111804
Ali SY, Paul M, Bhattacharyya D, Sinha D, Chaudhury S (2019) Evaluation of multiple applications of EDTA and LMWOAs on phytotoxicity and phytoextraction of Zn, Cd, Pb and Cu in soil with Tagetes sp. Indian J Soil Conserv 47(1):45–54
Amabogha ON, Garelick H, Jones H, Purchase D (2023) Combining phytoremediation with bioenergy production: developing a multi-criteria decision matrix for plant species selection. Environ Sci Pollut Res 1-14
Ashraf U, Kanu AS, Mo Z et al (2015) Lead toxicity in rice: effects, mechanisms, and mitigation strategies-a mini review. Environ Sci Pollut Res 22(23):18318–18332
Ashraf U, Kanu AS, Deng Q et al (2017) Lead (Pb) toxicity; physio-biochemical mechanisms, grain yield, quality, and Pb distribution proportions in scented rice. Front Plant Sci 8:259
Bareen EF, Tahira SA (2010) Efficiency of seven different cultivated plant species for phytoextraction of toxic metals from tannery effluent contaminated soil using EDTA. Soil Sedm Contam 19:160–173
Cay S (2023) Assessment of tea saponin and citric acid–assisted phytoextraction of Pb-contaminated soil by Salvia virgata Jacq. Environ Sci Pollut Res 30(17):49771–49778
Cenkci S, Cigerci IH, Yildiz M et al (2010) Lead contamination reduces chlorophyll biosynthesis and genomic template stability in Brassica rapa L. Environ Exp Bot 67:467–473
Chaney RL, Angle JS, Broadhurst CL et al (2007) Improved understanding of hyperaccumulation yields commercial phytoextraction and phytomining technologies. J Environ Qual 36(5):1429–1443
Chen Q, Zhang X, Liu Y et al (2017) Hemin-mediated alleviation of zinc, lead and chromium toxicity is associated with elevated photosynthesis, antioxidative capacity; suppressed metal uptake and oxidative stress in rice seedlings. Plant Growth Regul 81:253–264
Dolui D, Hasanuzzaman M, Saha I, Ghosh A, Adak MK (2022) Amelioration of sodium and arsenic toxicity in Salvinia natans L. with 2, 4-D priming through physiological responses. Environ Sci Pollut Res 1-16
Ebrahimi M (2016) Enhanced phytoremediation capacity of Chenopodium album L. grown on Pb-contaminated soils using EDTA and reduction of leaching risk. Soil Sediment Contam: An Internat J 25:652–667
Evangelou MWH, Ebel M, Schaeffer A (2007) Chetale assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemophere 68(6):989–1003
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66(2):375–400
Grcman H, Velikonja-Bolta S, Vodnik D, Kos B, Lestan D (2001) EDTA enhanced heavy metal phytoextraction: metal accumulation, leaching and toxicity. Plant and Soil 235(1):105–114
Hai NNS, Sanderson P, Qi F et al (2022) Effects of chelates (EDTA, EDDS, NTA) on phytoavailability of heavy metals (As, Cd, Cu, Pb, Zn) using ryegrass (Lolium multiflorum Lam.). Environ Sci Pollut Res 29(28):42102–42116
Hald PM (1946) The flame photometer for the measurement of sodium and potassium in biological materials. J Biol Chem 167(2):499–510
Hasanuzzaman M, Bhuyan MHM, Zulfiqar F et al (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9(8):681
Hernandez-Allica J, Garbisu C, Barrutia O, Becerril JM (2007) EDTA-induced heavy metal accumulation and phytotoxicity in cardoon plants. Environ Exp Bot 60(1):26–32
Huang H, Zhao Y, Fan L, Jin Q, Yang G, Xu Z (2020) Improvement of manganese phytoremediation by Broussonetia papyrifera with two plant growth promoting (PGP) Bacillus species. Chemosphere 260:127614
Huihui Z, Xin L, Zisong X et al (2020) Toxic effects of heavy metals Pb and Cd on mulberry (Morus alba L.) seedling leaves: Photosynthetic function and reactive oxygen species (ROS) metabolism responses. Ecotoxicol Environ Saf 195:110469
Jaworski EG (1971) Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun 43(6):1274–1279
Jiang M, Liu S, Li Y et al (2019) EDTA-facilitated toxic tolerance, absorption and translocation and phytoremediation of lead by dwarf bamboos. Ecotoxicol Environ Saf 170:502–512
Kaiser WM, Weiner H, Kandlbinder A et al (2002) Modulation of nitrate reductase: some new insights, an unusual case and a potentially important side reaction. J Exp Bot 53(370):875–882
Kato M, Simizi AS (1985) Chlorophyll metabolism in higher plants. VI. Involvement of peroxidase in chlorophyll degradation. Plant Cell Physiol 26:1291–1301
Khan AHA, Butt TA, Mirza CR, Yousaf S, Nawaz I, Iqbal M (2019) Combined application of selected heavy metals and EDTA reduced the growth of Petunia hybrida L. Sci Rep 9(1):1–12
Kono Y (1978) Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Bioph 186(1):189–195
Kumar A, Prasad MN, Sytar O (2012) Lead toxicity, defense strategies and associated indicative biomarkers in Talinum triangul are grown hydroponically. Chemosphere 89:1056–1065
Kumar A, Singh RP, Singh PK et al (2014) Selenium ameliorates arsenic induced oxidative stress through modulation of antioxidant enzymes and thiols in rice (Oryza sativa L.). Ecotoxicol 23:1153–1163
Li FL, Qiu Y, Xu X et al (2020) EDTA-enhanced phytoremediation of heavy metals from sludge soil by Italian ryegrass (Lolium perenne L.). Ecotoxicol Environ Saf 191:110185
Lindner RC (1944) Rapid analytical methods for some of the more common inorganic constituents of plant tissues. Plant Physiol 19(1):76
Mackinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140(2):315–322
Madhav S, Mishra R, Kumari A, Srivastav AL et al (2023) A review on sources identification of heavy metals in soil and remediation measures by phytoremediation-induced methods. Int J Environ Sci Technol:1–22
McBride MB (1994) Environmental Chemistry of Soils. Oxford University Press, New York, p 125
Mohammadi H, Hatami M, Feghezadeh K, Ghorbanpour M (2018) Mitigating effect of nano-zerovalent iron, iron sulfate and EDTA against oxidative stress induced by chromium in Helianthus annuus L. Acta Physiol Plant 40(4):69
Monni S, Salemaa M, Millar N (2000) The tolerance of Empetrum nigrum to copper and nickel. Environ Pollut 109(2):221–229
Naaz G, Chauhan KL (2019) Lead tolerance and accumulation potential of Brassica juncea L. Varieties in imitatively contaminated soil. Res J Life Sci Bioinfor Pharma Adn Chem Sci 5:436–447
Piper CS (1966) Soil and plant analysis. Hans Publishers, Bombay
Rahbari A, Fatemi H, Pour BE, Rizwan M, Soltani AA (2020) Lead (Pb)-resistant bacteria inhibit Pb accumulation in dill (Anethum graveolens L.) by improving biochemical, physiological, and antioxidant enzyme response of plants. Environ Sci Pollut Res:1-10
Rathika R, Srinivasan P, Alkahtani J et al (2021) Influence of biochar and EDTA on enhanced phytoremediation of lead contaminated soil by Brassica juncea. Chemosphere 271:129513
Saldarriaga JF, López JE, Díaz-García L, Montoya-Ruiz C (2023) Changes in Lolium perenne L. rhizosphere microbiome during phytoremediation of Cd-and Hg-contaminated soils. Environ Sci Pollut Res 30(17):49498–49511
Saman RU, Shahbaz M, Maqsood MF et al (2022) Foliar Application of Ethylenediamine Tetraacetic Acid (EDTA) Improves the Growth and Yield of Brown Mustard (Brassica juncea) by Modulating Photosynthetic Pigments, Antioxidant Defense, and Osmolyte Production under Lead (Pb) Stress. Plants 12(1):115
Saygideger S, Dogan M (2004) Lead and Cadmium Accumulation and Toxicity in the Presence of EDTA in Lemna minor L. and Ceratophyllum demersum L. Bull Environ Contam Toxicol 73(1):182–189
Shahid M, Austruy A, Echevarria G et al (2014) EDTA-enhanced phytoremediation of heavy metals: a review. Soil Sediment Contam An Internat J 23:389–416
Shukla P, Kidwai M, Narayan S, Shirke PA et al (2023) Phytoremediation potential of Solanum viarum Dunal and functional aspects of their capitate glandular trichomes in lead, cadmium, and zinc detoxification. Environ Sci Pollut Res 30:41878–41899
Singh R, Tripathi RD, Dwivedi S, Kumar A, Trivedi PK, Chakrabarty D (2010) Lead bioaccumulation potential of an aquatic macrophyte Najas indica are related to antioxidant system. Bioresour Technol 101(9):3025–3032
Song P, Xu D, Yue J, Ma Y, Dong S, Feng J (2022) Recent advances in soil remediation technology for heavy metal contaminated sites: A critical review. Sci Total Environ 156417
Turgut C, Pepe MK, Cutright TJ (2005) The effect of EDTA on Helianthus annuus uptake, selectivity, and translocation of heavy metals when grown in Ohio, New Mexico and Colombia soils. Chemosphere 58:1087–1095
Vassil AD, Kapulnik Y, Raskin I, Salt DE (1998) The role of EDTA in lead transport and accumulation by Indian mustard. Plant Physiol 117:447–453
Wani PA, Khan MS, Zaidi A (2007) Effect of metal tolerant plant growth promoting Bradyrhizobium sp.(vigna) on growth, symbiosis, seed yield and metal uptake by greengram plants. Chemosphere 70(1):36–45
Wu LH, Luo YM, Xing XR, Christie P (2004) EDTA-enhanced phytoremediation of heavy metal contaminated soil with Indian mustard and associated potential leaching risk. Agric Ecosyst Environ 102:307–318
Zulfiqar U, Farooq M, Hussain S et al (2019) Lead toxicity in plants: Impacts and remediation. J Environ Manage 250:109557
Acknowledgment
The authors are highly thankful to the Chairman Department of Botany Aligarh Muslim University Aligarh for providing necessary needs during experiments. Authors thank to Professor John Pichtel, Ball State University, USA and Dr. Firoz Ahmad Ansari, AMU, Aligarh, India, for language editing. The author Gul Naaz and Naushad Alam also acknowledge UGC university fellowship.
Author information
Authors and Affiliations
Contributions
Gul Naaz and Naushad Alam: Visualization, Investigation, Data analysis, Writing – original draft. Amit Kumar: Review and revised the manuscript. The paper was carefully read and approved by all of the authors.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for participation
All authors have consent to participate in the manuscript.
Consent for publication
All authors have consent to publish this manuscript.
Conflict of interest
The authors declare submitted article does not have any conflict.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Naaz, G., Alam, N. & Kumar, A. Impact of ethylene diamine tetraacetic acid on physiochemical parameters and yield attribute in two varieties of Brassica juncea under lead stress. Environ Sci Pollut Res 30, 118246–118262 (2023). https://doi.org/10.1007/s11356-023-29204-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-29204-2