Abstract
Due to its wide industrial application, chromium (Cr) is known to be a critical environmental pollutant. Contamination of water and agricultural soil by Cr inhibits crop productivity and their physiological and biochemical processes. The objective of the current work was to investigate the effects of appropriate reducing agents such as EDTA, iron sulfate (Fe2+), and zerovalent nano iron (Fe0 nanoparticles) on growth and physiology of sunflower plants under Cr(VI) stress. Results showed that the Cr uptake increased by increasing the amount of EDTA, leading to a significant reduction in morphological and physiological parameters except for MDA and H2O2 contents. Treatment with Fe0 nanoparticles and Fe2+ reduced Cr concentration in root and shoot, increased root and shoot dry weight, plastid pigments (chlorophyll and carotenoids) and proline contents; however, the level of MDA and H2O2 decreased significantly. All parameters were affected by Fe2+ during the first week of sampling; however, Fe0 nanoparticles affected all traits until the end of the third sampling stage. A statistically significant and positive correlation was found between root Cr concentration and MDA and H2O2 seedlings treated with EDTA, Fe2+, and Fe0 grown under Cr stress. From the result of this study, it can be concluded that sunflower has the potential for accumulation of Cr as a heavy metal, and treatment with Fe0 nanoparticles to prevent Cr uptake is more effective than other employed treatments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
By rapid industrial development, mining, and metallurgical and chemical fertilizers containing heavy metals, soil contamination by persistent toxic metals has become one of the major environmental concerns (Motasharrezadeh 2008). Accumulation of heavy metals such as chromium (Cr), cadmium (Cd), and nickel (Ni) in agricultural lands is continuously increasing mainly through anthropogenic activities, and their concentrations can reach to the threatened levels in human food/body. Increasing heavy metal concentrations in the environment resulted in their absorption by plant roots and transportation to shoots. These cause a disruption in plant metabolism, and subsequently limit the growth (Frossard 1993).
Cr is the seventh most mineral on the earth’s crust and one of the most important environmental pollutants released into the soil during industry-wide processes (Gomesa et al. 2017; Panda and Choudhury 2005). Chromium oxide exists in nature in different forms; however, among all Cr oxidation states, Cr3+ (III) and Cr6+ (VI) are the most stable in both terrestrial and aquatic environments (Barnhart 1997). Cr(VI) is more toxic than Cr capacity (III) being a cancer-causing agent for humans and animals (Cohen et al. 1993; Zayed et al. 1998). In contrast, Cr(III) is less mobile and toxic that mainly binds soil organic matter and the aquatic environment (Bishnoi et al. 1993; Barton et al. 2000). Although, some plants grow better at low Cr concentrations, high concentration is toxic and fatal for most plants (Davies et al. 2002). Heavy metals reduce root and shoot growth (Shanker et al. 2005). The major cause of this decline can be attributed to declined root growth and consequently lower translocation of water and nutrients to the aerial parts of plants. Several parameters such as yield, heavy metal content in different plant tissues, acquisition and utilization of elements by the plant, tolerance index and transfer factor, the metal contents in shoots and bioacummulation factor were reported to be indicators of heavy metal (nickel) effects in different plant species (Antonkiewicz et al. 2016a, b). In addition, the transport of heavy metals such as Cr to shoot has a direct impact on cellular metabolism and reduces plant height. Cr detrimentally affects plant physiological and biochemical processes such as photosynthesis, hormone functions, water relations, metabolic pathways, and mineral nutrition (Shanker et al. 2005). Due to structural similarities with specific essential elements, Cr considerably affects the plant mineral nutrition (Shanker et al. 2005). Metabolic changes are also caused by Cr in plants. These changes occur through the direct effect of metal on enzymes, metabolites, or its ability in producing reactive oxygen species (ROS), causing oxidative stress. Oxidative stress leads to structural and functional damage in plant cells (Shanker et al. 2005). Cr also affects the activity of catalase, peroxidase, cytochrome oxidase, nitrate reductase (Ghani 2011), and the accumulation of metal ions by the plant species affected ionic homeostasis within cells (Yadav 2010).
Several strategies have been considered to mitigate the effects of heavy metals in plants, including: (1) phytoremediation (Baker et al. 2000; Luo et al. 2005; Kirkham 2006; Antonkiewicz et al. 2016a, b); (2) using chelating agents such as EDTA (Wu et al. 2004); (3) exogenous application of Fe0 nanoparticles (Contin et al. 2007, 2008; Kumpiene et al. 2008; Qian et al. 2009). Chemical chelators such as acids, chelators used in glasshouse and field play an important role in uptake of toxic heavy metals (Huang et al. 1997; Blaylock et al. 1997). One strategy to achieve a great efficiency in removing pollutants is to facilitate heavy metals from the soil by artificial chelating agents such as EDTA. The effectiveness of the absorption process of heavy metals from soil by plants is dependent on the availability of the desired metal to plant (Lin et al. 2000). If access to the metal for adequate absorption by the plant is not at an acceptable level, chelating agents may be added to the soil for releasing and mobility of metals (Römkens et al. 2002). It is known that chelating agents such as EDTA, EGTA, etc., can improve mobility of the heavy metals in contaminated soils and subsequently increase their accumulation within plant tissues (Lelie et al. 2001). These chelating agents have a high affinity with various metal cations and easily chelate-metal complexes are transported through plant root to shoot (Wu et al. 2004). Chelating agents simultaneously increase the absorption and transport of heavy metals and on the other hand, by forming complexes with metal ions decrease the adverse effects of free metal cations in plant photosynthetic parts (Tandy et al. 2006).
Nanotechnology as an interdisciplinary science can be widely used in the agricultural science by increasing crop production, reducing pesticides and fertilizers application, and contributing to long-term storage of agricultural products, and perhaps improving processes related to agricultural practices (Moraru et al. 2003). Zero-valent iron materials have been successfully used as reactive agents to remove the contaminants from water and soil (Kumpiene et al. 2008). Scientific literature suggests the use of zero-valent iron nanoparticles as a new generation of technology, can properly clean up the environment compared to traditional methods (Zhang 2003). Its small size (less than 100 nm) and large surface area (50–100 m2 g−1), strong reduction power, non-toxicity, and high reactivity and mobility (Meyer et al. 2005) impart unique characteristics that may be efficient for remedial approaches and contaminant reduction (Kumpiene 2005; Liu and Zhao 2007a, b; Xu and Zhao 2007).
Sunflower (Helianthus annuus L.) is one of the terrestrial target key plant species that has great phytoextraction potential because it grows quickly, produces high amounts of biomass and is capable of accumulating heavy metals from contaminated soils in their tissues and organs (Zavoda et al. 2001; Chen and Cutright 2002).
We hypothesized that different reducing and chelating agents as well as engineered nanoparticles have various effects on morphological and physiological traits of sunflower during growth cycle under Cr contaminated soil. Therefore, the objective of this experiment was to evaluate the effects of appropriate reducing agents such as EDTA, iron sulfate (Fe2+), and zerovalent nano iron (Fe0 nanoparticles) on growth and physiological functions of sunflower plants exposed to oxidative stress induced by Cr(VI).
Materials and methods
Plant materials, experimental set up and treatments
Sunflower (Helianthus annuus L.) seedlings were exposed to Cr-induced stress and reducing agents such as EDTA, iron sulfate, and Fe0 nanoparticles in a factorial experiment based on Randomized Complete Block Design (RCBD) in three replications. Seeds of H. annuus L. were provided from Esfahan Pakan Bazr Co., Iran. Then seeds were surface disinfected by soaking in a 1% sodium hypochlorite (NaOCl) solution for 5 min, and washed three times with sterile deionized water accordingly. Nine seeds were directly grown in each plastic pot filled with soil (4 kg) including a mixture of silt (56%), sand (32%) and clay (12%). The pH and electric conductivity (EC) of soil were 7.1 and 1.52 ds m−1, respectively. Total N, P and K concentrations were 0.094, 9.3, and 5.8 mg kg−1, respectively. At the later stage of growth and before applying any treatment, established plants were finally thinned out to five uniform size plant per pot (i.e., four weaker plants were removed). The plants were grown in a greenhouse under 16/8 h day/night photoperiod at 30/18 °C day/night temperature. The relative humidity and light levels inside the greenhouse were considered as 70–80% and 270 μmol m−2 s−1, respectively. During the first 2 weeks, plants were irrigated only with tap water and then in the third week were irrigated with solutions containing different concentrations of Cr, and sampling was carried out three times, 1, 2, 3 weeks after starting of Cr irrigation. Treatments comprised of different concentrations of Cr (0, 50, 100, 150 and 200 ppm), EDTA (0, 0.5 and 2%), iron (II) sulfate (Fe2+; 0, 0.5 and 2.5%), NPs of Fe0 powders (0, 0.5 and 2%), and sampling stages (three levels corresponding to growth stage of BBCH 32–33 during three consecutive weeks). The BBCH (Biologische Bundesanstalt, Bundessortenamt and CHemical industry)-scale is a system for a uniform coding of phenologically similar development stages of crop species (Meier 2001). Here, three separate experiments were set up with aforesaid treatments, each performed in triplicate (n = 3). Six pots (each containing five plants after final thinning) were considered for each experimental unite. On the other hand 18 pots for 3 replications (i.e., 90 plants for each treatment) were used. The first experiment was relevant for understanding the effects of different concentrations of Cr and EDTA on morpho-physiological and biochemical attributes of sunflower at three sampling stages (i.e., during 6 weeks of growing time: started fourth week after planting and continued for three consecutive weeks). Second experiment was conducted using three factors, Cr concentrations (factor a), Fe2+ (factor b) and sampling stages (factor c). The third experiment was performed using two factors, Fe0 concentrations (factor a) and sampling stages (factor b) under Cr stress at 150 ppm.
Nano-zerovalent iron properties
Nano Fe0 was obtained from NANOSANY Company (Mashhad, Iran). The supplied material was further characterized by qualification procedures. The specific surface area (SSA) and purity of nano Fe0 were 8–14 m2 g−1, and > 99%, respectively. The size of Fe0 NPs were investigated by scanning electron microscopy (SEM, Hitachi S-4160, Tokyo, Japan), and calculated to be 35–45 nm (Fig. 1a). XRD technique (XPert PRO MPD, PANalytical) was used to characterize the crystal features of nano Fe0 particles in the range of 2θ = 30°–120°. The instrument was operated with a voltage of 40 kV and current of 40 mA using Cu Kα (λ = 0.15,406 nm) radiation with β-Ni filter (Fig. 1b).
Preparation of samples and measurement procedures
Determination of root and shoot dry weight
Samples from each treatment were taken at 32–33 BBCH after applying treatments and plant morphological traits including root and shoot dry weights were recorded using analytical scale, with precision of 0.0001 g. A part of the samples was oven-dried at 70 °C for 48 h and then used for determination of Cr content in root and shoot tissues.
Estimation of Cr concentration in plant tissues
Cr concentration in roots and shoots of the seedlings was estimated using atomic absorption spectrometry (AA-7000 Shimadzu, JAPAN) following digestion with a volume of 5 mL concentrated HNO3 (69%) and H2O2 (30%). Then, concentrated HNO3 solution was added to 0.25 g of dried and finely grounded plant materials (roots and whole shoots separately) in 75-mL digestion mixture tubes and permitted to sit for the minimum overnight (5 plants were sampled per each replication). The digestion tube samples were kept in a heating block port (Tucker 1974), and the temperature was slowly raised to 60 °C in 15 min, followed by addition of H2O2 (3 mL), and then the samples were digested for 3 h at 150 °C. After digestion process, the test solution was cooled to room temperature, diluted, and filtered before further analysis.
Calculation of total chlorophyll and carotenoid contents
Photosynthetic pigment contents were determined using Lichtenthaler and Wellburn (1983) methods. Chlorophyll and carotenoid extracts were prepared from fresh leaf sample (0.5 g) by grinding in a mortar and pestle together with acetone (0.5 mL, 80% V/V). The absorbance was recorded at 645 and 663 nm (for total chlorophyll), and 470, 645 and 663 nm (for carotenoids) in a UV–Vis spectrophotometer. Measurements were made on the first young fully expended leaf from growing point of three plants in each replicate. The same sampling procedure was applied for examined traits such as photosynthetic pigments, MDA, H2O2 and proline contents. Photosynthetic pigment contents were estimated using following equations:
Determination of malondialdehyde (MDA)
The MDA (ultimate product of lipid peroxidation) content was determined according to Heath and Packer (1968) method. Briefly, fresh leaves (0.5 g) were homogenized in 5 mL of 0.1% (w/v) TCA solution and centrifuged at 12,000×g for 15 min. Thereafter, 2 mL of supernatant was mixed with 2 mL of 0.6% (w/v) TBA. The reactions were heated at 95 °C for 30 min, then cooled in an ice water bath and centrifuged at 4000×g for 15 min. Finally, the absorbance of supernatant was noted at 532 nm wave length. The MDA content was estimated in terms of its extinction coefficient (155 mM−1 cm−1).
Measurement of hydrogen peroxide (H2O2)
The method of Velikova et al. (2000) was used for the measurement of H2O2 content in the leaves of H. annuus L. seedlings. Briefly, fresh leaf sample (500 mg) was homogenized with 5 mL of 0.1% (w/v) TCA. The homogenate was centrifuged at 12,000×g for 15 min, and 0.5 mL of the supernatant was added to 0.5 mL of 10 mM potassium phosphate buffer (pH 7.0) and 1 mL of 1 M potassium iodide solution. Thereafter, the absorbance of the supernatant was recorded at 390 nm in a UV–Vis spectrophotometer (PG Instrument Ltd., T80 Series). The H2O2 content was determined based on a standard calibration curve made by different doses of H2O2.
Measurement of proline content
Proline content was measured using acid-ninhydrin according to the method described by Bates et al. (1973). Fresh leaf sample (500 mg) was ground in a mortar with sulfosalicylic acid (10 mL, 3%), and primarily clarified via centrifugation, and then the obtained supernatant (2 mL) was added to 2 mL of acid-ninhydrin reaction and glacial acetic acid. The samples were oven-heated approximately at 100 °C for 1 h, and then extracted with toluene (4 mL) by vigorous shaking at room temperature using a vortex machine for 20 s, and the absorbance was finally noted at 520 nm. The proline content was expressed as µmol g−1 fresh weight (FW).
Statistical analysis
The data were analyzed by two-way analysis of variance (ANOVA) using SAS statistical analysis software version 6. Three different factorial experiments were carried out in a Randomized Complete Block Design (RCBD) with three replications. Correlations among the studied traits were calculated using the Pearson correlation coefficients by SPSS version 16 software (SPSS Inc., Chicago, United States). Duncan’s Multiple Range Test (DMRT, p < 0.01) was used for analysis of means (ANOM).
Results and discussion
Growth parameters
Data analysis showed that the effects of different concentrations of Cr and EDTA, and sampling stages on root and shoot dry weights were statistically significant at 1% probability level (Table 1). Increasing the concentration of Cr in media leads to reduction in the root and shoot dry weights, especially in the first week of sampling for root dry weight (Table 4). It has been reported that the presence of Cr in the terrestrial ecosystems and external environment may lead to changes in the pattern of plant growth and development (Kocik and Ilavsky 1994; Shanker et al. 2005; Sundaramoorthy et al. 2010). Decreasing of root growth upon exposure to Cr treatments has been attributed to inhibition of root cell division/and or root elongation as well as the extension of root cell cycle (Lodeiro et al. 2008). The reduction in shoot growth might be due to decreased root growth and consequently lower nutrients and water transport to the upper parts of the plant. Furthermore, the direct transport of Cr to aerial parts can also directly affect cellular metabolism and reduce shoot growth (Hanus and Tomas 1993). According to Panda and Patra (2000), Cr application (1 µM) increased the root length in wheat seedlings; however, higher Cr concentrations significantly decreased root length.
Sunflower plants showed various responses to addition of EDTA (Table 4). Application of 0.5% EDTA was more effective to mitigate stress-induced at the highest concentration of Cr (200 ppm) compared to the respective control. Chen and Cutright (2001) revealed that the shoot and root dry weight decreased by 50 and 60% in plants treated with EDTA compared to plants without EDTA, respectively (Chen and Cutright 2001).
Based on the results obtained, Cr and Fe2+ concentrations, and sampling stages affected root and shoot dry weights (Table 5). The Cr-induced reduction in root and shoot dry weights observed in sunflower plants, however, it’s affected by Fe2+ during the first week of the sampling stages, resulting in an increase in mentioned traits (Table 5). Results exhibited that Fe2+ could not affect growth parameters until the end of sampling.
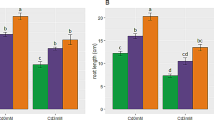
Addition of 2% Fe0 nanoparticles had the least amount of Cr absorbed by the roots and shoots of sunflower plant under Cr stress (150 ppm) (Fig. 3), explaining why the result showed a significant increase in shoot and root dry weight (Fig. 2). Soil stabilization with Fe0 nanoparticles showed that different concentrations of Fe0 nanoparticles had a significant effect on root and shoot dry weights under Cr stress. According to Kumpiene et al. (2006), treatment with Zerovalent iron (Fe0) decreased Cr concentrations in leachates (up to 45%), in soil pore water sample, and in plant aerial parts (Kumpiene et al. 2006). Other studies show that appropriate concentration of TiO2 nanoparticles increases plant photosynthesis, nitrogen metabolism, and plant growth (Hong et al. 2005; Zhang 2003). A negative but significant correlation between root dry weight and root Cr content (r0.01 = − 0.264, r0.01 = − 0.221, and r0.05 = − 0.394) in sunflower seedlings treated with EDTA, Fe2+, and Fe0, respectively, grown under Cr stress (Table 6). Similarly, there was a negative correlation between shoot dry weight and shoot Cr content (r0.01 = − 0.229, r0.01 = − 0.372, and r0.01 = − 0.615) under reference treatments.
Chromium (Cr) accumulation
Results revealed that the application of 2.5% EDTA increased Cr uptake, and transport it into the root and shoot parts (Table 4), consequently reduced root and shoot dry weights in comparison to 0.5% EDTA treatment. EDTA can affect the solubility and mobility of metals in soil (Gupta et al. 2008). It has been reported that chelating agents including ethylene diamine triacetic acid (EDTA) can be applied to improve metal mobility, resulting in enhancing phytoextraction process (Huang et al. 1997; Elless and Blaylock 2000; Chen and Cutright 2001). For example, EDTA (1.0 g/kg) was reported to be the most efficient chelating agent/or chelator, increasing Pb concentration of shoot tissues in pea and maize plants (Huang et al. 1997).
The results indicated that root Cr content differed significantly (p < 0.01) under Cr stress and Fe2+ at different stages of sampling (Table 2). Treatment with Fe2+ partly reduced the concentration of Cr in root and shoots under Cr stress at the 2nd sampling. Plants showed maximum accumulation of Cr in the shoots at zero percent of Fe2+ in all stages of sampling, but application of Fe2+ caused a reduction in Cr uptake until 2nd sampling (Table 5).
Mean comparison of root and shoot Cr content in sunflower plants treated with different levels of Fe0 grown at 150 ppm Cr stress during three sampling stages were presented in Fig. 3. The maximum Cr content in root (0.3 mg/g DW) and shoot (0.9 mg/g DW) tissues of the seedlings were observed at 2% Fe0 nanoparticles under Cr stress (150 ppm) at the first stage of sampling (Fig. 3).
It has been reported that zero-valent nano iron can be coupled with trace metals (e.g., Pt, Pd, Ag), exhibiting significantly enhanced reaction. Nano iron is environmentally benign, and eventually, is mainly transformed into Fe3O4 and Fe2O3, which are abundant elements on earth crust (GeoNano Environ. Tech. Inc. 2007). A positive but significant correlation was observed between root Cr concentration and MDA (r0.01 = 0.298, r0.01 = 0.504, and r0.01 = 0.545) and H2O2 (r0.01 = 0.463, r0.01 = 0.731, and r0.01 = 0.690) in sunflower seedlings treated with EDTA, Fe2+, and Fe0, respectively, grown under Cr stress (Table 6). Similarly, a positive correlation was observed between shoot Cr concentration and H2O2 content (r0.01 = − 0.385, r0.01 = − 0.713, and r0.01 = − 0.867) under experimental treatments.
Photosynthetic pigments
ANOVA results showed significant (p < 0.05) differences in photosynthetic pigments (total chlorophyll and carotenoids) content under the interactions of treatments (Tables 1, 2, 3). Exposure to higher concentrations of Cr significantly reduced the content of total chlorophyll and carotenoids (Tables 4 and 5). However, treatment with 50 ppm Cr led to a significant increase in carotenoids contents at the first time of sampling. Reduction in the content of photosynthetic pigments under Cr stress can also be related to oxidative damage and inhibition of the synthesis of chlorophyll pigments. Cr prevents electron transfer, inactivates the Calvin cycle enzymes, decreases carbon dioxide reduction, and disrupts the structure of chloroplasts (Davies et al. 2002). High concentrations of Cr in the roots inhibit iron absorption, and reduce chlorophyll biosynthesis (Connell and Al-Hamdani 2001). Reduction of iron concentrations in the presence of Cr in cauliflower, beans, wheat, beets plants, and chlorella have also been reported (Dube et al. 2003; Pandey and Sharma 2003).
In Cr stress condition, plants exposed to 0.5–2.5% EDTA triggered a decrease in photosynthetic pigment contents compared to the optimal condition and the other experimental treatment groups (Tables 4, 5). Stressed plants with Cr decreased their total chlorophyll and carotenoids contents over control plant (Table 4).
Treatments with Fe2+ affected plastid pigments (total chlorophyll and carotenoids) contents under Cr stress at different stages of sampling (Table 2). Applying of Fe2+ protected or stimulated their biosynthesis total chlorophyll and carotenoid contents in 50 mM concentration of Cr stress at the 2nd sampling (Table 5), but at higher levels of Cr treatment (100–200 mM), Fe2+ application caused a decrease in plastid pigments contents under different stages of sampling (Table 5).
In the present study, application of Fe0 nanoparticles had positive effects on physiological performance of sunflower plants through improvement of photosynthetic pigment contents under Cr stress (Fig. 4). Maximum content of total chlorophyll (1.8 mg/g FW) and carotenoids (0.9 mg/g FW) were obtained in plants treated with 2% Fe0 nanoparticles at the end of third sampling stages (Fig. 4). It has been reported that the chlorophyll a and total chlorophyll contents as well as quantum efficiency were increased in leaves of nanosilver treated Brassica juncea seedlings (Priyadarshini et al. 2012). Improved chlorophyll and carotenoid contents of plants following different nanomaterials application were previously reported (Racuciu and Creanga 2007; Hatami and Ghorbanpour 2014; Hatami et al. 2017). The adverse impacts of ROS are prevented by the action of lipid soluble antioxidants located in plant cell membranes such as vitamins and carotenoids (Desikan et al. 2004); carotenoids can also efficiently quench the 1O2 produced by the stress conditions. In current study, a negative but significant correlation was observed between carotenoid and H2O2 contents (r0.01 = 0.310, r0.01 = 0.469, and r0.01 = 0.742) in seedlinollowing equationsgs treated with EDTA, Fe2+, and Fe0, respectively, grown under Cr stress (Table 6).
Malondialdehyde (MDA) and hydrogen peroxide (H2O2)
The MDA and H2O2 contents were significantly (p < 0.01) influenced by employed treatments (Tables 1, 2, 3). The lowest and the highest values of MDA were obtained for plants exposed to Fe0 nanoparticles (Fig. 5) and EDTA-treated plants (Table 4) in control and Cr stress conditions, respectively. The same trend as variations of MDA was observed for H2O2 accumulation under employed treatments. The level of H2O2 in plants treated with Fe0 nanoparticles was much lower than that of others, and subsequently peroxidation of lipid was less considerable in cell membrane of such plants (Fig. 5). A common characteristic of different environmental biotic and abiotic stresses is over production of ROS such as superoxide anion, hydroxyl radical (·OH), hydrogen peroxide (H2O2), and organic free radicals as well as peroxides, leading to direct peroxidation of cell continuants such as proteins, lipids, and DNA, and disruption of cell membranes, and finally cell collapse which may be along with programmed or suicidal cell death (Sairam and Saxena 2000; Panda and Choudhury 2005). Other results also indicate that high concentrations of copper increase lipid peroxidation in the roots of rice plants (Chen et al. 2000). It was found that with increasing the rate of EDTA application, absorption of Cr increased, and led to Cr induced oxidative stress, hence, significant increase of MDA and H2O2 contents in the sunflower plants.
H2O2 and MDA levels decreased in treated plants with Fe2+ under Cr stress until 2nd sampling (Table 5). However, application of Fe2+ caused no significant change in H2O2 and MDA contents in 3rd sampling under high levels of Cr stress.
The application of 2% Fe0 nanoparticles significantly reduced MDA and H2O2 content under 150 ppm Cr stress. Application of Fe0 nanoparticles may alleviate plasma membrane damage from oxidative stress triggered by Cr stress conditions. Here, a positive and significant correlation was observed between MDA and H2O2 contents (r0.01 = 0.691, r0.01 = 0.698, and r0.01 = 0.902) in seedlings treated with EDTA, Fe2+, and Fe0, respectively, grown under Cr stress (Table 6).
Proline contents
Proline content of sunflower plants were significantly (p < 0.01) influenced by the employed treatments (Tables 1, 2, 3). Increasing the concentration of Cr by 100 ppm enhanced the proline content, but concentrations of 150–200 ppm Cr reduced the proline contents (Table 4). Some environmental perturbations such as salinity, drought, and accumulation of heavy metals change plant osmotic status. There are many reports of heavy metals in plants, which cause osmotic imbalance. Plants use different adaptive mechanisms against osmotic stress caused by heavy metals. A group of plants that have a higher resistance to osmotic balance are able to the synthesis of a number of osmotic protective metabolites such as proline, glycine betaine and reduced carbohydrates (Ghosh and Singh 2005; Sinha et al. 2005).
Fe2+-treated plants showed a significant change in proline content under Cr stress at the different stages of sampling (Table 5). Application of Fe2+ caused an increase in proline content up to 100 ppm Cr stress, and thereafter decreased. Application of Fe2+ could not increase proline content at the high levels of Cr stress until the end of samplings. Cr stress, similar to other environmental stresses, causes oxidative cell injuries to plants through excessive production of ROS such as H2O2, OH−, and O2−. Plants synthesize different types of enzymatic (e.g. superoxide dismutase, ascorbate peroxidase, glutathione reductase, catalase) and non-enzymatic (e.g. ascorbic acid, glutathione, a-tocopherol, carotenoids and phenolics) antioxidants involved in scavenging toxic oxygen derivatives and limit oxidative injuries (Arora et al. 2002; Grene 2002).
In Cr stressed-plants, exposure to both levels of Fe0 nanoparticles (0.5 and 2.5%) could not significantly change proline content compared to normal conditions. However, the proline content of Cr-stressed plants increased (1.8 fold) at the third sampling time compared to the other stages under different Fe0 nanoparticles concentrations (Fig. 6). A positive and significant correlation was observed between proline and biomass (r0.01 = 0.439, r0.01 = 0.545, and r0.01 = 0.883) in seedlings treated with EDTA, Fe2+, and Fe0, respectively, grown under Cr stress (Table 6).
Conclusions
Our results suggest that the Cr toxicity induced oxidative stress and growth inhibition in sunflower plants. According to the current study, MDA and H2O2 contents in sunflower plants were significantly increased upon exposure to Cr and EDTA. However, engineered nanoscale materials application may reduce or mitigate the generation of H2O2 level via the improved efficiency of cell redox homeostasis, and or upregulation of H2O2 metabolizing enzymes. The mechanism of protection against Cr toxicity in plants is principally associated with the decrease in its concentration in below-and above-ground plant parts. Our results suggest that the application of Fe0 nanoparticles to sunflower plants under Cr stress activate protective mechanisms that may improve growth of root and shoot, and increase the photosynthetic pigments, and mitigate oxidative stress induced by Cr in sunflower plants. Therefore, Fe0 nanoparticles application is recommended in order to mitigate the adverse effects of Cr stress, especially in the case of food crops. Future research needs to pay particular attention to the impacts of Fe0 nanoparticles on Cr toxicity in industrial crops under field conditions.
Authors contribution
Conceived and designed the experiments: Hamid Mohammadi. Performed the experiments: Khatoon Feghezadeh. Statistical data analysis and interpretation: Mehrnaz Hatami. Writing, revising and approving the final manuscript: Mansour Ghorbanpour.
References
Antonkiewicz J, Jasiewicz C, Koncewicz-Baran M, Sendor R (2016a) Nickel bioaccumulation by the chosen plant species. Acta Physiol Plant 38(40):11
Antonkiewicz J, Kołodziej B, Bielińska E (2016b) The use of reed grass and giant miscanthus in the phytoremediation of municipal sewage sludge. Environ Sci Pollut Res 23(10):9505–9517
Arora A, Sairam R, Srivastava G (2002) Oxidative stress and antioxidative system in plants. Curr Sci Bangalore 82:1227–1238
Baker AJ, McGrath S, Reeves RD, Smith J, Terry N, Bañuelos G (2000) A review of the ecology and physiology of a biological resource for phytoremediation of metal polluted soils. In: Terry N, Banuelos G, Vangronsveld J (eds) Phytoremediation of contaminated soil and water. CRC Press, Boca Raton, pp 85–107
Barnhart J (1997) Chromium chemistry and implications for environmental fate and toxicity. Soil Sediment Contam 6:561–568
Barton LL, Johnson GV, O’Nan AG, Wagener BM (2000) Inhibition of ferric chelate reductase in alfalfa roots by cobalt, nickel, chromium, and copper. J Plant Nutr 23:1833–1845
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bishnoi N, Chugh L, Sawhney S (1993) Effect of chromium on photosynthesis, respiration and nitrogen fixation in pea (Pisum sativum L.) seedlings. J Plant Physiol 142:25–30
Blaylock MJ, Salt DE, Dushenkov S, Zakharova O, Gussman C, Kapulnik Y, Ensley BD, Raskin I (1997) Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ Sci Technol 31:860–865
Chen H, Cutright T (2001) EDTA and HEDTA effects on Cd, Cr, and Ni uptake by Helianthus annuus. Chemosphere 45:21–28
Chen H, Cutright TJ (2002) The interactive effects of chelator, fertilizer and rhizobacteria for enhancing phytoremediation of heavy metal contaminated soil. J Soils Sedim 2:203–210
Chen LM, Lin CC, Kao CH (2000) Copper toxicity in rice seedlings: changes in antioxidative enzyme activities, H2O2 level, and cell wall peroxidase activity in roots. Bot Bull Acad Sin 41:99–103
Cohen MD, Kargacin B, Klein CB, Costa M (1993) Mechanisms of chromium carcinogenicity and toxicity. Crit Rev Toxicol 23:255–281
Connell SL, Al-Hamdani SH (2001) Selected physiological responses of kudzu to different chromium concentrations. Can J Plant Sci 81:53–58
Contin M, Mondini C, Leita L, De Nobili M (2007) Enhanced soil toxic metal fixation in iron (hydr) oxides by redox cycles. Geoderma 140:164–175
Contin M, Mondini C, Leita L, Zaccheo P, Crippa L, De Nobili M (2008) Immobilisation of soil toxic metals by repeated additions of Fe(II) sulphate solution. Geoderma 147:133–140
Davies F, Puryear J, Newton R, Egilla J, Saraiva Grossi J (2002) Mycorrhizal fungi increase chromium uptake by sunflower plants: influence on tissue mineral concentration, growth, and gas exchange. J Plant Nutr 25:2389–2407
Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ (2004) ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J Exp Bot 55:205–212
Dube B, Tewari K, Chatterjee J, Chatterjee C (2003) Excess chromium alters uptake and translocation of certain nutrients in citrullus. Chemosphere 53:1147–1153
Elless M, Blaylock M (2000) Amendment optimization to enhance lead extractability from contaminated soils for phytoremediation. Int J Phytoremediation 2:75–89
Frossard R (1993) Contaminant uptake by plants. Soil monitoring. Springer, pp 157–178
GeoNano Environ. Tech. Inc. (2007) gnet.myweb.hinet.net/company2(E).html. Accessed 27 Jul 2009
Ghani A (2011) Effect of chromium toxicity on growth, chlorophyll and some mineral nutrients of Brassica juncea L. Egypt Acad J Biol Sci 2:9–15
Ghosh M, Singh S (2005) Comparative uptake and phytoextraction study of soil induced chromium by accumulator and high biomass weed species. App Ecol Environ Res 3:67–79
Gomesa MAC, Hauser-Davisb RA, Suzukia MS, Vitoriaa AP (2017) Plant chromium uptake and transport, physiological effects and recent advances in molecular investigations. Ecotoxicol Environ Saf 140:55–64
Grene R (2002) Oxidative stress and acclimation mechanisms in plants. The Arabidopsis Book, e0036
Gupta DK, Srivastava A, Singh V (2008) EDTA enhances lead uptake and facilitates phytoremediation by vetiver grass. J Environ Biol 29:903–906
Hanus J, Tomas J (1993) An investigation of chromium content and its uptake from soil in white mustard. Acta Fytotech 48:39–47
Hatami M, Ghorbanpour M (2014) Defense enzyme activities and biochemical variations of Pelargonium zonale in response to nanosilver application and dark storage. Turk J Biol 38:130–139
Hatami M, Hadian J, Ghorbanpour M (2017) Mechanisms underlying toxicity and stimulatory role of single-walled carbon nanotubes in Hyoscyamus niger during drought stress simulated by polyethylene glycol. J Hazard Mater 324:306–320
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hong F, Zhou J, Liu C, Yang F, Wu C, Zheng L, Yang P (2005) Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol Trace Elem Res 105:269–279
Huang JW, Chen J, Berti WR, Cunningham SD (1997) Phytoremediation of lead-contaminated soils: role of synthetic chelates in lead phytoextraction. Environ Sci Technol 31:800–805
Kirkham M (2006) Cadmium in plants on polluted soils: effects of soil factors, hyperaccumulation, and amendments. Geoderma 137:19–32
Kocik K, Ilavsky J (1994) Effect of Sr and Cr on the quantity and quality of the biomass of field crops. Production and utilization of agricultural and forest biomass for energy: proceedings of a seminar held at Zvolen, Slovakia, pp 168–178
Kumpiene J (2005) Assessment of trace element stabilization in soil. Sci Technol 30:248–251
Kumpiene J, Ore S, Renella G, Mench M, Lagerkvist A, Maurice C (2006) Assessment of zerovalent iron for stabilization of chromium, copper, and arsenic in soil. Environ Pollut 144:62–69
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—a review. Waste Manage 28:215–225
Lelie DVD, Schwitzguébel JP, Glass DJ, Vangronsveld J, Baker A (2001) Assessing phytoremediation’s progress in the United States and Europe. Environ Sci Technol 35:446–452
Lin ZQ, Schemenauer RS, Cervinka V, Zayed A, Lee A, Terry N (2000) Selenium volatilization from a soil plant system for the rem ediation of contaminated water and soil in the San Joaquin Valley. J Environ Qual 29:1048–1056
Lichtenthaler H, Wellburn A (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Liu R, Zhao D (2007a) In situ immobilization of Cu (II) in soils using a new class of iron phosphate nanoparticles. Chemosphere 68:1867–1876
Liu R, Zhao D (2007b) Reducing leachability and bioaccessibility of lead in soils using a new class of stabilized iron phosphate nanoparticles. Water Res 41:2491–2502
Lodeiro P, Fuentes A, Herrero R, de Vicente MES (2008) CrIII binding by surface polymers in natural biomass: the role of carboxylic groups. Environ Chem 5:355–365
Luo C, Shen Z, Li X (2005) Enhanced phytoextraction of Cu, Pb, Zn and Cd with EDTA and EDDS. Chemosphere 59:1–11
Meier U (2001) Growth stages of mono-and dicotyledonous plants. BBCH monograph. Federal Biological Research Centre for Agriculture and Forestry. 2 edn. https://doi.org/10.5073/bbch0515
Meyer D, Bhattacharyya D, Bachas L, Ritchie S (2005) Membrane-based nanostructured metals for reductive degradation of hazardous organics. ACS symposium series. Oxford University Press, pp 256–261
Moraru CI, Panchapakesan CP, Huang Q, Takhistov P, Sean L, Kokini JL (2003) Nanotechnology: a new frontier in food science. Food Technol 57:24–29
Motasharrezadeh B (2008) Studying the possibility of increasing the efficiency of phytoremediation of soil contaminated with heavy metals by biological agents. Ph.D thesis in Soil Science, Tehran University, Karaj, Iran.
Panda S, Choudhury S (2005) Chromium stress in plants. Braz J Plant Physiol 17:95–102
Panda S, Patra H (2000) Nitrate and ammonium ions effect on the chromium toxicity in developing wheat seedlings. Proc Natl Acad Sci India Sec B Biol Sci 70:75–80
Pandey N, Sharma CP (2003) Chromium interference in iron nutrition and water relations of cabbage. Environ Exp Bot 49:195–200
Priyadarshini S, Deepesh B, Zaidi MGH, Pardha Saradhi P, Khanna PK, Arora S (2012) Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl Biochem Biotech 167:2225–2233
Qian G, Chen W, Lim TT, Chui P (2009) In-situ stabilization of Pb, Zn, Cu, Cd and Ni in the multi-contaminated sediments with ferrihydrite and apatite composite additives. J Hazard Mater 170:1093–1100
Racuciu M, Creanga DE (2007) TMA-OH coated magnetic nanoparticles internalized in vegetal tissue. Rom J Phys 52:395–402
Römkens P, Bouwman L, Japenga J, Draaisma C (2002) Potentials and drawbacks of chelate-enhanced phytoremediation of soils. Environ Pollut 116:109–121
Sairam R, Saxena D (2000) Oxidative stress and antioxidants in wheat genotypes: possible mechanism of water stress tolerance. J Agron Crop Sci 184:55–61
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753
Sinha S, Saxena R, Singh S (2005) Chromium induced lipid peroxidation in the plants of Pistia stratiotes L.: role of antioxidants and antioxidant enzymes. Chemosphere 58:595–604
Sundaramoorthy P, Chidambaram A, Ganesh KS, Unnikannan P, Baskaran L (2010) Chromium stress in paddy:(i) nutrient status of paddy under chromium stress; (ii) phytoremediation of chromium by aquatic and terrestrial weeds. C R Biol 333:597–607
Tandy S, Schulin R, Nowack B (2006) The influence of EDDS on the uptake of heavy metals in hydroponically grown sunflowers. Chemosphere 62:1454–1463
Tucker M (1974) A modified heating block foe plant tissue digestion 1. Commun Soil Sci Plant Anal 5:539–546
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Wu L, Luo Y, Xing X, Christie P (2004) EDTA-enhanced phytoremediation of heavy metal contaminated soil with Indian mustard and associated potential leaching risk. Agric Ecosyst Environ 102:307–318
Xu Y, Zhao D (2007) Reductive immobilization of chromate in water and soil using stabilized iron nanoparticles. Water Res 41:2101–2108
Yadav S (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179
Zavoda J, Cutright T, Szpak J, Fallon E (2001) Uptake, selectivity and inhibition of hydroponic treatment of contaminants. J Environ Eng 127:502–508
Zayed A, Lytle CM, Qian JH, Terry N (1998) Chromium accumulation, translocation and chemical speciation in vegetable crops. Planta 206:293–299
Zhang WX (2003) Nanoscale iron particles for environmental remediation: an overview. J Nanopart Res 5:323–332
Acknowledgements
This study was financially supported by Deputy of Research and Technology of Azarbaijan Shahid Madani University (217/D/10965), Tabriz, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Krolicka.
Rights and permissions
About this article
Cite this article
Mohammadi, H., Hatami, M., Feghezadeh, K. et al. Mitigating effect of nano-zerovalent iron, iron sulfate and EDTA against oxidative stress induced by chromium in Helianthus annuus L.. Acta Physiol Plant 40, 69 (2018). https://doi.org/10.1007/s11738-018-2647-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2647-2