Abstract
Endocrine disrupting chemicals (EDCs) are increasingly concerned substance endangering human health and environment. However, there is no unified standard for identifying chemicals as EDCs, which is also controversial internationally. In this review, the procedures for EDC identification in different organizations/countries were described. Importantly, three aspects to be considered in identifying chemical substances as EDCs were summarized, which were mechanistic data, animal experiments, and epidemiological information. The relationships between them were also discussed. To elaborate more clearly on these three aspects of evidence, scientific data on some chemicals including bisphenol A, 1,2-dibromo-4-(1,2 dibromoethyl) cyclohexane and perchlorate were collected and evaluated. Altogether, the above three chemicals were assessed for interfering with hormones and elaborated their health hazards from macroscopic to microscopic. This review is helpful for standardizing the identification procedure of EDCs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endocrine disrupting chemicals (EDCs) are exogenous chemicals that can cause adverse health outcomes by affecting the endocrine system (IPCS 2002). EDCs are widely found in daily products and can be ingested by humans through soil, water, food, and air (Azzouz and Ballesteros 2012; Salgueiro-González et al. 2015; Wee and Aris 2017), thereby affecting various systems and organs in humans, including the reproductive, the metabolic, and the nervous systems (Fig. 1). The health risks posed by EDCs to humans, such as reproductive dysfunction, cognitive deficits, and obesity, have been recognized as a major public health issue (Åke Bergman et al. 2012; Kahn et al. 2020; La Merrill et al. 2020). Hence, it is urgent to control and manage EDCs. As the first step, the identification of EDCs cannot be ignored.
The increasing number of emerging substances with EDC properties cannot be identified as EDCs since there is no unified international standard for EDC identification. For example, parabens have estrogenic and anti-androgen properties, which may have potential adverse effects on reproductive development (Golden et al. 2005; Nowak et al. 2018; Sun et al. 2022). However, there is no definite standard to identify them as EDCs (Miao et al. 2023). As emerging environmental pollutants, microcystins can affect the reproductive system of a variety of organisms (Chen et al. 2016; Chen et al. 2021; Xu et al. 2021; Xu et al. 2022). The endocrine disrupting effects of microcystins have been extensively studied; whether they are classified as EDCs is controversial (Zhang et al. 2022). Although different criteria have been used to identify EDCs, there are still limitations in assessing a substance as EDCs in practice. First, there is no completely identical definition of EDCs (Andersson et al. 2018; EPA 2014; Health 2015; OECD 2018b; OEHHA 2011; Safety 2002). Secondly, the sensitivity of different species to endocrine disruptors is varied, as does the exposure period (Browne et al. 2020; Zgheib et al. 2021). For these two reasons, the conclusion on the possibility of identifying a substance as EDC was different in various countries or regions. Therefore, it is necessary to summarize the standards of international agencies and different countries, extracting the commonalities from them as strong evidence to identify EDCs.
In this review, the identification standards and relevant literatures in different countries and organizations were collected. The identification methods of EDCs were summarized and evaluated objectively. The detailed information about EDC identification was collated and analyzed, to rationalize the evidence for identifying an endocrine-disrupting property of a substance from experimental and population epidemiological evidence. In order to elaborate on the three lines of evidence necessary to identify EDCs, evidences of bisphenol A (BPA), 1,2-dibromo-4-(1,2 dibromoethyl) cyclohexane (TBECH), and perchlorate were evaluated to identify their endocrine disrupting effects. Their identification processes were also described with a view to finding the general identification procedure of EDCs. This review provided a clear overview of gathering evidence to evaluate a substance being assessed as an EDC and presented its interference process on hormones, which will help humans to understand the typical characteristics of EDCs in the environment and provide guidance for the prevention and control of the threat of EDCs to humans.
Different standards for EDC identification around the world
To establish a complete standard for the identification of EDCs, strengthen the identification of EDCs, and effectively promote the standardization of endocrine identification research, many countries have issued corresponding standards. In the European Union, the European Chemicals Agency (ECHA) and the European Food Safety Authority (EFSA) jointly drafted the identification standard for EDCs, which are matched with a series of scientific procedures (Niklas Andersson et al. 2018). In the United States, chemicals interfering with hormonal actions have identifiable key characteristics (KCs) that can be used to identify EDCs (La Merrill et al. 2020). In other countries, relevant identification measures of EDCs have also been taken respectively. In China, the standard related to the identification of EDCs is the industry standard “Evaluation Method of Pesticide Endocrine Disruptors” issued in 2015, which is mainly used to assess whether pesticides have endocrine disrupting effects (China 2015). As one of the Organisation for Economic Cooperation and Development (OECD) member countries, Japan has made efforts to develop the tests for identifying EDCs (Health 2015). France will assess about 300 plant protective substances and 100 biocidal substances for their endocrine disrupting properties, in conformity to the regulations and methods set out in the joint EFSA/ECHA guidance document issued by the European Union, as appropriate (ANSES 2021). Regulators use a variety of methods to assess the evidence for the inherent hazards of EDCs; they vary widely in the way they analyze, collect, and interpret the scientific evidence (Abass et al. 2016; Rudén, 2006). In this section, the identification criteria of the EU and the US were introduced and summarized in detail, considering that the identification standards have high international recognition of EDC and are widely used to evaluate the endocrine disrupting characteristics of chemicals.
Strategy for identifying EDCs in the European Union
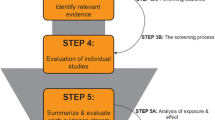
EDCs are defined as exogenous substance or mixture that alter function(s) of the endocrine system and consequently cause adverse health effects in an intact organism, or its progeny, or (sub)populations (EC 1998), the same as the WHO’s. A supporting guidance document for identifying EDCs was drafted by the ECHA and the EFSA jointly on June 7, 2018 (Niklas Andersson et al. 2018). The guidance points out strategy to assess whether a substance meets the criteria of EDCs. The evaluation strategies mainly include five parts (Fig. 2). According to its identification criterion of EDCs, European Chemicals Agency (An agency of the European Union) adds substances with endocrine disrupting properties to Candidate List of substances of very high concern for Authorisation (ECHA 2011).
The definition and the five steps of assessment strategy of endocrine disrupting chemicals in European Union. First, all relevant information includes all available relevant scientific data (in vivo studies or adequately validated alternative test systems predictive of adverse effects in humans or animals; as well as in vivo, in vitro, or, if applicable, in silico studies informing about endocrine modes of action), scientific data generated in accordance with internationally agreed study protocols and other scientific data selected applying a systematic review methodology. Second, the assembling of lines of evidence should take into consideration all the available evidence (positive and negative). Relevant and reliable parameters should be assembled to determine whether and how they contribute to the lines of evidence for adversity and/or endocrine activity. Third, the initial analysis of the evidence comprises an assessment whether either EATS-mediated adversity or EATS endocrine activity has been ‘sufficiently’ investigated. This will allow to stop the EDC assessment in case no EATS-mediated adversity or endocrine activity that have been observed or to decide whether further data need to be generated. Last, in line with the criteria, the conclusions should answer the problem: Is there a biologically plausible link between endocrine activity and observed adverse effect(s)?
It is known from the above standards and guidelines that they not only have a very clear definition of EDCs but also describe how to gather and evaluate all relevant evidences of chemicals need to identify, then carry out a mode of action (MoA) analysis. A MoA can be described as a series of biological events of a substance, which result in the specific adverse effect in animals and human. Apply a weight of evidence (WoE) (OECD 2018a) approach, in order to establish whether the EDC criteria are fulfilled. In fact, as early as 2013, the European Union issued relevant standards for the identification of EDCs. Due to the irrationality of the proposed experimental method (Dietrich et al. 2013), it has caused debate among experts in different fields (Autrup et al. 2015; Bergman et al. 2013; Zoeller et al. 2014). In 2018, after extensive communication in multi-disciplinary fields, scientific screening procedures have been implemented, which assess whether plant protection products and biocidal products have endocrine disrupting properties (Niklas Andersson et al. 2018). In terms of the identification criteria of EDCs, the OECD developed available standardized test guidelines for in vivo and in vitro testing (OECD 2018a), which the European Union has adopted. In addition, there is broad scientific agreement on the interpretation of the effects observed on the investigated parameters (ECHA/EFSA 2018). Therefore, Boberg et al. used this criterion to assess endocrine disruption of butylparaben (Boberg et al. 2020). The adverse health effects of potential EDCs caused by estrogenic, androgenic, thyroidal, and steroidogenic (EATS) modalities mainly are addressed by this guidance document. However, EDCs not only exert endocrine disrupting properties through the above four modalities, such as insulin. Therefore, in order to comprehensively assess the endocrine disrupting effects of emerging pollutants, future deeper studies are needed complement the non-EATS modalities in the testing strategies.
Guidelines for identifying EDCs in the United States
The EDCs were defined by the United States Environmental Protection Agency (US-EPA) as exogenous substances that disrupt the production, release, transport, metabolism, binding, action, or elimination of the natural hormones in the body responsible for the maintenance of homeostasis and the regulation of developmental processes (Diamanti-Kandarakis et al. 2009; Gerald Ankley et al. 1998). The journal Nature Review Endocrinology published a consensus statement written by 15 scientists from the United States on November 12, 2019. This consensus evaluated the potential threat of EDCs to human health. In this paper, the experts argued that chemicals that interfere with hormonal actions have identifiable 10 key characteristics (KCs) that can be used to identify EDCs (La Merrill et al. 2020). These characteristics include interactions with hormone receptors, changes in hormone receptors and receptor cells, and alterations in the hormone itself (Vandenberg et al. 2020). The KC approach eliminates the need for scientists and regulators to demonstrate every molecular mechanism of the adverse outcomes observed in animals or humans exposed to potential EDCs, reducing the tedious task of investigators regarding potential EDCs. The approach precisely meets the common characteristics of EDCs defined in different organizations and countries. Muñoz et al. adopted this consensus when assessing whether glyphosate is an EDC (Muñoz et al. 2021). In fact, in 1998 the U.S. EPA released the Endocrine Disruptor Screening Program (U.S.EPA 1998). The level of biological complexity from molecular interactions to populations is represented by the Tier 1 and Tier 2 screens and tests, to screen and test chemical substances for their endocrine disrupting properties, which consistent with the Adverse Outcomes Pathway (AOP) (Fig. 3) (Browne et al. 2017). An AOP is a conceptual framework designed to enhance the utility of path-based data in assessing hazards to different levels of organisms, human health, and the environment (Ankley et al. 2010). An AOP can be described as the occurrence of a series of adverse outcome events. EDCs trigger some reversible or irreversible perturbations of normal biology through molecular interactions (e.g., binding to receptors and altering receptor expression). Furthermore, they continuously increase at the level of biological tissues, affecting cell and organ function. This is followed by impacts on human health or on the survival, growth, or reproduction of wildlife. Based on various evidences of BPA, Viguié et al. adequately demonstrate that that BPA is an EDC using the AOP method (Viguié et al. 2018).
Concept map of key features of endocrine disrupting chemicals’ adverse outcome pathway. EDCs can cause molecular initiation events such as interactions with multiple hormone receptors. Molecular initiation events lead to adverse outcomes at the cellular level including changes in hormone levels and changes in cell fate. Edema, deformities, and so on appear at the organ level. Biologically, it causes developmental abnormalities, embryo death, increased infertility, and changes in parental behavior. Eventually, the population prevalence increases
Evidence for assessing the endocrine disrupting properties of emerging pollutants: based on commonalities of the EU and US standards for EDC identification
From the above, many countries have been aware of the harm of EDCs for a long time and taken measures to reduce the impact on human beings and other organisms by formulating corresponding identification standards. Accurate definition of EDCs is a premise for identifying EDCs. There are several standards of defining EDCs worldwide currently. Although there are some differences in the definition of EDCs in different organizations and countries, they all contain two common characteristics: they affect the endocrine function, and they have adverse effects on health. Given that the European Union and the United States have the most well-developed and far-reaching EDC identification procedures (Kassotis et al. 2020), this review is aimed at assessing the endocrine disrupting characteristics of emerging environmental pollutants based on their commonalities. The EU is designed to collect all evidence of chemicals that disrupting endocrine and conduct weight analysis to determine whether they are EDCs. Mechanism data are mainly derived from the testing system of OECD. In contrast, in the USA, the identification procedure is to propose 10 KCs based on mechanistic data and to identify them in combination with adverse effects at different biological levels. Despite the fact that there are differences between the EU and the USA in the identification criteria for EDCs, there are some similarities between them: (1) the definition of EDCs is very clear; (2) EDCs are identified based on their risk/hazard profile; and (3) determine the adverse effect of a chemical in the whole organism (animal and human) and the mechanism of action of the chemical responsible for the adverse effect. The evidence presented in this review suggests that the procedure for determining a chemical as an EDC needs to consider three aspects: (1) in vivo and in vitro experiment data show that the adverse endocrine outcome is caused by this substance due to its KCs; (2) animal experiments have shown that the substance can cause pathological changes in animals; and (3) the substance can cause adverse outcomes in the human body; that is, epidemiological studies have shown that a statistical correlation with human diseases (Fig. 4). It is beneficial to identify emerging environmental contaminants as EDCs by sharing toxicological, epidemiological, mechanistic and other EDC information in international collaborative databases.
Evidence of identifying a chemical as an EDC. Three aspects of evidence on chemicals should be evaluated when identifying EDCs. Epidemiological studies provide direct links between EDCs and diseases of the reproductive system, nervous system, and endocrine system in human. Animal experiments show that EDCs can cause adverse endocrine outcomes in different animals including model organisms and wild organisms. As mechanistic data, 10 KCs reveal the causes of adverse outcomes induced by EDCs in humans and animals at the molecular level
Mechanistic data as an integral part of the identification of EDCs: molecular initiation events
Mechanism is the organization and integration of collected evidence for endocrine disruption across data streams, possibly from molecular epidemiological studies, in vivo and in vitro testing in experimental animal models, high-throughput testing, and in silico modelling (La Merrill et al. 2020). The EU criterion is to identify EDCs on the basis of the collection of relevant data, followed by an analysis of a series of molecular initiating events that contribute to adverse outcomes. The ten key characteristics of EDCs freeinvestigators from the intricacies between “molecular initiating events” and specific modes of action or pathways of adverse outcomes (La Merrill et al. 2020).
The mechanism data involved in EDC identification procedures of the EU and US are dependent on various test methods. In 2012, the OECD originally published the Guidelines for Standardized Tests for the Assessment of Endocrine Disrupting Chemicals aimed at determining the endocrine mechanism of chemicals, which detailed the test system for the identification of EDCs. This guidance document discusses in detail both in vitro mechanical screening and in vivo screening and testing, covering endpoints relevant to humans or vertebrate wildlife, and for non-mammalian wildlife screening and testing, test species are fish, amphibians, birds, molluscs animals, crustaceans and insects (OECD 2018b). These assays provide a wealth of mechanistic data to identify a chemical as an endocrine disruptor.
In vivo experiments can identify potential biomarkers of EDCs. For example, there are changes in mRNA and protein levels of vitellogenin (VTG), changes in circulating hormone levels, and histopathological measurements (Browne et al. 2017). It can be directly observed that there are effects of chemicals on specific tissues or cell types by in vitro methods. Because the in vitro studies are more controlled in operation and contain fewer confounding factors, they are suitable for identifying the specific mechanism of action and specific molecular targets of EDCs. However, with the development of experimental methods, moving away from experimental animal toxicity testing is more and more pressured, alternative model organism testing methods and in silico modelling continue to emerge (Rybacka et al. 2015; Schneider et al. 2019). In silico approach can predict that specific chemical structures may cause “endocrine disruption,” mainly including ligand-based and structure-based methods, so as to predict the potential EDC activity of a given chemical structure. Among the ligand-based methods, the calculation of molecular descriptors is the simplest, which treats the molecule as a whole and calculates a value for the entire molecule (Schneider et al. 2019). Estrogen receptors including α and β are the most widely researched targets of endocrine disruption (Shanle and Xu 2011). Similarly, other steroid hormone receptors have been targeted for model development (Chen et al. 2018). Structure-based approaches, also known as target-based approaches, use information from the 3D structure of a protein target to screen endocrine disruptors. Docking procedures are most widely used in virtual screening activities, based on sampling the conformational space of a given ligand in the binding pouch of the target molecule, followed by postural evaluation by scoring functions (Klebe 2006), which has been applied to the prediction of endocrine disruption in androgen receptors and other nuclear receptors. Using environmental chemicals from the Tox21 (toxicity testing in the twenty-first century) database, Jeong et al. used estrogen receptors and androgen receptors and their homology models in C. elegans to identify potential endocrine-disrupting chemicals through molecular docking simulations, demonstrating that C. elegans has the potential to serve as an alternative model for EDCs screening environmental chemicals (Jeong et al. 2019). In silico predictions of EDC properties, Jaladanki et al. proposed a molecular docking-based virtual screening method for the prediction of potential EDC binding to nuclear receptors (Jaladanki et al. 2021).
In line with the above experiments, it is found that there are an increasing number of approaches to explore the mechanism of EDCs. As an emerging class of toxic chemicals, EDCs will be actually identified by their mechanism of action, rather than by their chemical structure or specific type of use (Schneider et al. 2019). Therefore, mechanistic data is the initiating events that have adverse effects on humans and other organisms and an indispensable part of the identification of EDCs.
Adverse effects in different species need to be assessed for the identification of EDCs
EDCs can affect a variety of organisms (Bernanke and Köhler 2009; Chen et al. 2019; Patisaul et al. 2018; Segner 2009). The field of EDC originated in large part from the study of wildlife species; classical toxicology tests are essential to study them in detail. Classical toxicology relies heavily on rodent models, especially rat, and mouse models, but species diversity remains a central element of on-going EDC research (Guillette and Gunderson 2001). Extensive studies of terrestrial and aquatic species are also required. However, classical EDC animal models such as sheep, quail, mini pigs, dogs, rabbits, and non-human primates are rarely used for EDC studies due to numerous factors (Patisaul et al. 2018). For decades, research groups have used Daphnia magna as an EDC screening model (Dang et al. 2012; Kang et al. 2014). The endocrine systems in daphnia are quite different from vertebrates’ “EATS” systems; therefore, daphnia may not be able to serve as an alternative for vertebrate EDCs testing. Wild species including fish, birds, crocodiles, and other reptiles remain sentinels for the health of their vital organisms and ecosystems (Guillette and Gunderson 2001).
A growing diversity of vertebrate models, including transgenic mouse and rat lines, zebrafish Danio rerio, and monogamous rodents, has been used to assess endocrine disruptors (Patisaul et al. 2018). For example, BPA has been extensively studied as a typical EDC. At first, BPA was found to have adverse effects on wild animals. For further study, the researchers observed the phenotype of rats exposed to BPA and found that it can cause vaginal lesions in female rats (Ahmed et al. 2014). For aquatic studies, zebrafish exposed to BPA resulted in pathological changes in testicular tissue (Forner-Piquer et al. 2020) and follicular atresia in the ovary (Giommi et al. 2021; Molina et al. 2021). For birds, exposure to BPA resulted in decreased uterine tubular gland density and mucosal thickness in hens (Yigit and Daglioglu 2010) and resulted in malformed Müllerian ducts (embryosalpinx) in female quail embryos and feminization of the left testis (ovotestis) in male chicken embryos (Berg et al. 2001).
We can see that endocrine system function and health have been compromised in a variety of organisms, including rodents, fish, and birds, and their organ systems have different degrees of pathological changes. While mechanisms of action can provide an efficient way to identify potential EDCs, their endocrine disrupting effect on the whole animal cannot be presented. More importantly, a single independent surrogate model is less accurate in reflecting the overall toxicity of an EDC in an in vivo organism (Fabian et al. 2019). Toxicological experiments are indispensable. Toxicology can play a predictive role by providing alerts about the potential effects of chemicals on humans. The basic assumption is that limiting exposure to chemicals to levels well below those that would have adverse effects on animals will prevent harmful consequences for humans (Adami et al. 2011). Therefore, the outcomes from different organism exposures are necessary to identify EDCs.
Population epidemiology provides direct evidence for the identification of EDCs: the relationship between EDC exposures and human health outcomes
Currently, growing evidence have shown that EDC exposures are associated with endocrine-related diseases, such as male reproductive health (Hauser et al. 2015), female reproductive health (Gallo et al. 2016), and birth outcomes (Hu et al. 2021; Raghavan et al. 2018; Spinder et al. 2021), neurodevelopment (Ramírez et al. 2022), obesity and metabolism (Legler et al. 2015; Zamora et al. 2021), and immune dysfunction (Casas and Gascon 2020; Clayton et al. 2011). These exposure outcomes are inseparable from epidemiological studies.
Epidemiology is “the study of the occurrence and distribution of health-related events, states, and processes in specific populations, including the study of the determinants that influence these processes, and the application of this knowledge to the control of related health problems” (Porta 2016). Epidemiology plays an important role in exploring causes, preventing and controlling diseases, formulating strategies and measures for disease prevention and control, and evaluating the effect of prevention and control, which has an irreplaceable effect on improving the health of the population. The epidemiological study of EDCs is the study of disease phenomena and health status in human exposed to EDCs. That is, it starts from the population and always focuses on the population health effects, not only considering the individual disease problem, but also considering how endocrine disruptors are reflected at the organ and molecular level. Epidemiological studies provide key information of the relationship between EDC exposures and human health effects (Ho et al. 2022). It can provide a direct link between the adverse outcomes and EDCs. There are certain advantages to conduct the research on EDCs directly in humans over animal studies, since it eliminates the need for interspecies extrapolation and allows the study of realistic pathways, admixtures, and exposure durations relevant to humans.
Generally, it is difficult to fully simulate actual human exposure to EDCs in animal studies due to the complex exposures and personal or behavioral factors encountered in real life (Ho et al. 2022). Therefore, animal experiments are no substitute for epidemiological studies. Although the European Union, the United States Environmental Protection Agency, etc. have issued a number of documents on EDC screening and identification, most of the evidence for identifying which chemical can be regarded as EDC mainly comes from in vitro and in vivo studies (ECHA/EFSA 2018; U.S.EPA 1998); more and better evidence is needed to demonstrate the effects of exposure to EDCs on human health. Epidemiological studies are a logical and necessary complement to in vitro and in vivo experimental studies of EDCs to characterize the nature and extent of risk to human EDCs (Lee and Jacobs 2015). Traditionally, epidemiology and toxicology often work in parallel and complement each other. The epidemiological studies can direct present risks of human disease associated with exposure to EDCs and other research efforts; whether in animal models, in vitro or in silico studies further deepen our understanding of potential toxicological mechanism of EDCs (Terry et al. 2019). It can be seen that the three aspects of evidence complement each other, fully revealing that chemicals interfere with endocrine function and health.
The evidence on endocrine disruption of BPA was reviewed based on the procedure in this review
Estrogenic disruptors are considered a type of important chemicals that induce biological responses consistent with the effects of endogenous estrogens (Korach 1993; Li et al. 2012a). Among them, BPA, one of the classic chemicals with estrogenic activity, has been widely used in industrial production since first synthesized in 1891 (Meng et al. 2019). At present, BPA is still an industrial component, widely used in the synthesis of polycarbonate plastic epoxy resin and other polymer materials, and is almost ubiquitous in urban life.
Mechanistic data of BPA about endocrine disruption
Plentiful scientific papers on the mechanism of BPA have been published. These data has revealed the molecular mechanisms underlying the phenotypic effects of BPA in humans and animals, which offer molecular initiating events of BPA.
BPA activates nuclear receptors (Andersen et al. 1999; Li et al. 2012b), membrane receptors (Watson et al. 2007), and G-protein-coupled receptors (Thomas and Dong 2006) in a variety of species.
BPA affects the expression of estrogen receptors. It can increase the expression of ER mRNA in specific regions of the brain in mice exposed during gestation (Rebuli et al. 2014).
BPA alters the signal transduction of estrogen-responsive cells. BPA-induced proliferation of Sertoli TM4 cells is mediated by the induction of ERK phosphorylation. In the human testicular seminoma cell line (JKT-1), BPA activates cAMP-dependent and cGMP-dependent protein kinase pathways to phosphorylate CREB (cAMP-response element binding protein) (Bouskine et al. 2009).
BPA causes epigenetic modification of hormone-associated cells. BPA affects promoter-specific methylation in brain, prostate, and human breast cancer cells (Bhan et al. 2014; Wang et al. 2016; Yaoi et al. 2008). The ER-binding region of the long non-coding RNA HOTAIR promoter is enriched by trimethylation on H3K4 and H3K4-specific methyltransferases in human breast cancer cells (Bhan et al. 2014). In mouse prostate, neonatal exposure to BPA activates the histone methyltransferase MLL1 to persistently increase H3K4 trimethylation at genes associated with prostate cancer (Wang et al. 2016).
BPA affects hormone synthesis. BPA inhibits steroidogenesis in the rat testis (Akingbemi et al. 2004). BPA reduces cytochrome p450 aromatase levels and the expression of other steroidogenic regulatory proteins (Mahalingam et al. 2017).
BPA alters hormone distribution or circulating hormone levels. Drinking water exposure of pregnant Sprague–Dawley rats to BPA, the serum estradiol level of the offspring increased (Wu et al. 2020).
BPA alters fate of hormone-producing or hormone-responsive cells. Developmental exposure to BPA alters the differentiation of mammary epithelial cells and increases the number of alveolar buds (structures that eventually produce milk in lactating females) in the mammary gland (Markey et al. 2001; Vandenberg et al. 2008). BPA also increases the proliferation index in the mammary gland pancreas and uterine endothelial cells (Bosquiazzo et al. 2010; Moral et al. 2008).
Animal experiments of BPA in endocrine disruption
Animal studies have shown the pathological effects of BPA on the female reproductive system and male reproductive system. However, is the evidence that BPA causes reproductive system disorders credible? That is, the study used standardized methods and clearly described experimental procedures and results (SCHEER—Scientific Committee on Health, Revision 2018). In the quality assessment of individual toxicity studies of chemicals, the European Chemicals Agency, the United States Food and Drug Administration (FDA), and the OECD have agreed on the use of the Klimisch method (Vandenberg et al. 2016). Experts need to make a clear quality assessment of the research in terms of validation/validity, reliability, and adequacy and ensure that the assessment results are understandable and convincing. When evaluating animal studies, consider the following: the strain, sex, and age of the tested animals; the origin and purity of TBECH; post-exposure changes of experimental animals (including clinical features, organ tissue changes and hematological changes); presentation of control data; description of test conditions; and route and dose of administration (Klimisch et al. 1997). To evaluate the reproductive system hazards of BPA in animals, the evidence of the effects of BPA on various animals was collected and sorted out, and the results are shown in Table 1.
Epidemiological evidence for BPA endocrine characteristics
There are a lot of epidemiological studies on the endocrine disrupting effects of BPA, mainly focusing on the relationship between BPA and female reproductive system diseases, male reproductive dysfunction, obesity, and so on (La Merrill et al. 2020). Among them, epidemiological studies confirmed that the reproductive system is an important target organ of BPA (Ma et al. 2019).
In the female reproductive system, the effect of BPA on female hormones is related to the thickness of the endometrial wall. The relationship between changes in endometrial wall thickness and BPA levels with age was observed, and it was found that endometrial thickness was positively correlated with urinary BPA level in young women and gradually thickened with the increase of BPA concentration, while endometrial thickness was negatively correlated with urinary BPA concentration in older women (Mínguez-Alarcón et al. 2015). In addition, the number of cases of polycystic ovary syndrome (PCOS) is increasing year by year, and the incidence is higher in adolescent and women of reproductive age, of which the incidence is 5%–10 in women of reproductive age. In population epidemiological studies, elevated BPA concentrations have been observed in adolescent and adult women with PCOS and are positively associated with hyperandrogenism, which suggests a potential role of BPA in the pathophysiology of PCOS (Palioura and Diamanti-Kandarakis 2015). In pregnant women, adverse pregnancy outcomes are also strongly associated with BPA exposure, such as miscarriage and preterm delivery. Cantonwine et al. found that women who gave birth at 37 weeks or less had higher urinary BPA concentrations than women who gave birth after 37 weeks (Cantonwine et al. 2010). In addition, spontaneous preterm birth (PTB) and preterm premature rupture of membranes (pPROM) have also been reported to be associated with BPA levels in adverse pregnancy outcomes; Shen et al. concluded that BPA exposure may be associated with the risk of recurrent abortion (RM) (Shen et al. 2015). Furthermore, Behnia et al. found a positive correlation between BPA concentration and the risk of PTB or pPROM (Behnia et al. 2016).
For the male reproductive system, BPA can affect the quality and function of sperm by altering the levels of related hormones in the body, thus harming fertility. In a cohort study, Mustiels et al. found that BPA was significantly associated with higher serum total testosterone (TT) levels (Mustieles et al. 2018). Furthermore, Ferguson et al. showed that BPA actually decreased serum testosterone (T) concentration and increased estradiol (E2) concentration (Ferguson et al. 2014).
Based on the above review of the evidence on the endocrine disrupting effects of BPA, it can have adverse effects on animals and humans (Fig. 5).
The evidence on endocrine disruption of TBECH was reviewed based on the procedure in this review
Exogenous substances that interfere with androgens are called androgen disruptors. Since male sexual differentiation is entirely androgen dependent (Williams-Ashman 1965), it is highly susceptible to androgen-disruptors. Epidemiological studies and animal experiments have found that exposure to androgen disrupting chemicals is connected with diseases of the reproductive system in both sexes, including reduced sperm counts, increased infertility, testicular dysgenesis syndrome, and testicular and prostate cancers (Luccio-Camelo and Prins 2011). More than a dozen substances, such as TBECH and phthalates, are considered chemicals that disrupt androgen. TBECH is one of the few androgen receptor agonists (Kuang et al. 2014; Luccio-Camelo and Prins 2011). By collecting and analyzing evidence for TBECH, the endocrine disrupting properties of TBECH are assessed and procedures for the chemical to be identified as an EDC are presented.
TBECH, also known as 1,2-dibromo-4-(1,2-dibromoethyl) cyclohexane (DBE-DBCH), is manufactured by Albermarle as Saytex BCL-462 (Nguyen et al. 2017). As a common class of brominated flame retardants, it is widely used in household products and industrial products, including electrical fabrics and furniture, in order to improve the fire performance, so as to have a great impact on human living environment (Brown et al. 2014). The presence of four isomers of TBECH has been detected in a variety of environmental substrates and organisms, including soil, water species, indoor dust, fish, baby feces, and even Arctic environments, due to its extensive production and use (Marteinson et al. 2020). Recently, a large number of experimental studies have shown that TBECH has endocrine disrupting effect, hepatotoxicity, and reproductive toxicity (Wang et al. 2020), and among them, the environmental endocrine disrupting characteristics have gradually become the focus of research.
Mechanistic data of TBECH about endocrine disruption
TBECH interacts with or activates hormone receptors. TBECH activates the human androgen receptor (Khalaf et al. 2009; Larsson et al. 2006) and Zebrafish androgen receptors (Pradhan et al. 2013). TBECH can activate androgen receptors and interact with and alter thyroid and estrogen receptors in chickens (Asnake et al. 2014). TBECH induces AR-mediated physiological responses in LNCaP cells, suggesting that by acting as a partial agonist (Wong et al. 2016).
TBECH alters hormone receptor expression. Prostate-specific antigen (PSA) activity in LNCaP cells and HepG2 cells was determined by enzyme-linked immunosorbent assay. All the TBECH dienantiomers could induce the expression of PSA in LNCaP cells (Khalaf et al. 2009). Effects of transcriptional activation of mutant (ARW741C and ART877A) cells exposed to androgen-derived brominated flame retardant TBECH revealed that TBECH induced the expression of androgen receptor target genes, thereby altering the expression of hormone receptors (Kharlyngdoh et al. 2016).
TBECH alters hormone synthesis. γ- and δ-TBECH altered the transcription of androgen-responsive genes and steroidogenic genes in prostate epithelial cells (Kharlyngdoh et al. 2018). In addition, TBECH was altered in genes involved in the regulation of steroid biosynthesis, steroid metabolism, and prostate epithelial morphogenesis (Bereketoglu et al. 2021). In addition to the hormone-related receptors, EDCs act on enzymes involved in steroidogenesis and the metabolism of hormones (Sifakis et al. 2017). Phthalates, for example, are a specific class of plasticizer that exert anti-androgenic effects by inhibiting the synthesis of testosterone in Leydig cells as a result of direct inhibition of CYP17 (Foster 2005). Thiophosphates are a class of organophosphorus pesticides that inhibit P450 enzymes involved in the metabolism of estrone and testosterone in the liver, namely, CYP3A4 and CYP1A2 (Usmani et al. 2006; Usmani et al. 2003). After exposure to DBE-DBCH, liver mRNA levels of two phase I metabolic enzymes, CYP2H1 and CYP3A37, were significantly increased by fourfold and eightfold, respectively, and CYP3A37 was also significantly induced based on PCR arrays (Crump et al. 2014). Therefore, TBECH can cause changes in the corresponding enzymes, resulting in changes in hormone synthesis.
TBECH alters hormone distribution or circulating hormone levels. Juvenile brown trout exposed to high doses of TBECH significantly reduced total plasma thyroxine (Park et al. 2011). However, there are also experiments showing occasional differences in circulating plasma E2, T, and 11-KT levels after TBECH treatment, but no clear time trend or dose response (Gemmill et al. 2011). TBECH altered the transcript levels of androgen-responsive genes in human cervical cancer (HeLa), ductal breast cancer (T-47D), and prostate cancer (LNCaP) cells (Kharlyngdoh et al. 2016). β-and t-TBECH exposure could affect the expression of one or more of 4 genes involved in the thyroid hormone pathway (Porter et al. 2014).
Animal experiments of TBECH in endocrine disruption
Mice and rats, the most commonly used animal models, have been studied in vivo, which have shown that TBECH can damage the reproductive and nervous system of mice and rats, which strongly proves that TBECH plays an endocrine disrupting effect in animals. Because TBECH is widely distributed in environmental media, and it is present in municipal sewage (Ruan et al. 2019), urban watershed (Wang and Kelly 2017), and seawater and sediments (Liu et al. 2021b; Ruan et al. 2018a; Ruan et al. 2018b), which can affect aquatic organisms. Experiments in zebrafish, amphibians, and others have been carried out. The above experimental animals were all exposed to laboratory conditions. What effects will exposure to TBECH in the natural environment have on animals? Study finds TBECH in ring-billed gulls in highly industrialized stretch of St. Lawrence River downstream of Montreal (Gentes et al. 2012). In addition, herring gulls (Larus argentatus) are from seven colonies of five Laurentian Lakes (Gauthier et al. 2009). It was found that falcones, American kestrels (Falco sparverius), and chicken were all disrupted by TBECH. So TBECH can have adverse effects on a wide variety of animals, at both laboratory and natural environment exposure levels. The evidence of health effects of TBECH on multiple organisms should be to analyze whether it is reliable. Based on the above evaluation criteria for animal studies, we collected and sorted out the evidence of the effects of TBECH on various animals, and the results are shown in Table 2. These studies have clearly described the above content, and it is reasonable to assume that TBECH is reliable in causing adverse health outcomes of animals based on the evidence.
Epidemiological evidence for TBECH endocrine characteristics
The study on a cohort of 61 adults in Oslo shown that dietary exposure was the most important route of TBECH exposure, which was an important part of all exposure routes through multivariate linear regression analysis (Tay et al. 2019). In addition, researchers recruited 60 mothers who gave birth to a healthy child at Uppsala University Hospital in 2009 and 2010, and according to the results, dietary exposure was also found to be the main route of exposure to TBECH (Sahlström et al. 2014). Unfortunately, no epidemiological studies have been conducted on the association between TBECH and related diseases. Although humans may be exposed to TBECH through indoor dust and air, the trend of human exposure is unknown.
These data indicate a lack of evidence for TBECH in population epidemiology. Animal models are abundant in the study of TBECH, including rats, birds, and fish. So based on these studies, some mechanism data are obtained. It is lacking of the evidence for the health effects of TBECH in the population. But it is well known that humans are contaminated by hundreds of man-made chemicals, which makes it extremely difficult to prove conclusively that a chemical is causing harm to human health. Even for chemicals that have been well studied over the past few decades, such as BPA and some phthalates, the evidence for harm to human health has only recently come to light (Sarink et al. 2021). If TBECH is not classified as an EDC due to lack of epidemiological evidence, greater harm to organisms may be caused. It is an important issue how the “missing” evidence for adverse effects on human health could be obtained. The International Program on Chemical Safety first developed a systematic approach to drawing conclusions about human correlation (causation) (Sonich-Mullin et al. 2001); later, it was substantially expanded with the development of the noncancer effect framework (Seed et al. 2005). If there is sufficient evidence in animal studies to establish MoA and it is effective in humans, combined with pharmacokinetics and kinetic characteristics, then the effects seen in animals may also be seen in humans, and the potential effects of a chemical on humans are possible (Julien et al. 2009). More research on that is urgently needed in order to reveal the effects of TBECH in human health.
Through reviewing the evidence of TBECH in endocrine disruption, TBECH can cause adverse outcomes to organisms by interfering with the formation of receptors and hormones (Fig. 6).
The evidence on endocrine disruption of perchlorate was reviewed based on the procedure in this review
Studies have confirmed that a variety of environmental pollutants, including phenolic compounds, brominated flame retardants, pesticides, and perchlorates, can affect the normal function of the thyroid gland, such as inhibiting the synthesis and secretion of TH and inhibiting the absorption of iodine by the thyroid gland (Xu et al. 2017). Perchlorate is a typical substance that interferes with thyroid hormones because perchlorate-induced natrium-iodide symporter (NIS) interference is a well-recognized thyroid disrupting mechanism (Lisco et al. 2020).
Mechanistic data of perchlorate about endocrine disruption
Perchlorates reduce thyroid hormone levels in humans and other animals by limiting the amount of iodine used to synthesize these hormones (La Merrill et al. 2020). Perchlorate inhibits thyroid hormone synthesis and competitively interferes with iodine accumulation in the thyroid gland. This works through the sodium–iodide symporters, which is an effective competitive inhibitor of iodide uptake in human rodents and other vertebrates (Dohán et al. 2007). The sodium–iodide symporters are usually present in the thyroid gut placenta, lactation breast, and choroid plexus membrane and thus plays a role in transporting iodide ion (Zoeller 2006).
Animal experiments of perchlorate in endocrine disruption
In terms of animal experimental evidence, York et al. found that low doses of perchlorate can reduce serum T4 levels in pregnant rats and their young rats (York et al. 2005). In addition, Gilbert and Sui also found that exposure to perchlorate during the development of adult hippocampi can impair synaptic function and irreversible damage to the response to synaptic transmission (Gilbert and Sui 2008). The same as TBECH, the evidences of the effects of perchlorate on various animals were collected and sorted out (Table 3).
Epidemiological evidence for perchlorate endocrine characteristics
A number of population epidemiological studies have demonstrated that urine determination of perchlorate (a biomarker of perchlorate exposure in pregnant women) is associated with reduced maternal thyroid hormone levels (La Merrill et al. 2020). Studies evaluated the relationship between perchlorate exposure and circulating thyroid hormone levels in neonates, since neonates are particularly sensitive to inhibition of thyroid hormone synthesis (van den Hove et al. 1999). Among the five studies in which thyroid hormone levels were measured within a day of birth, there was consistent evidence. Newborn babies from communities that have been exposed to perchlorate have lower T4 levels, higher TSH levels, and prevalence of thyroid disease than those from unexposed communities. Furthermore, in assessing the potential effects of low environmental exposure to perchlorate on thyroid function, urinary perchlorate concentrations were approximately twice as high in the exposed population as in the general population, and both free thyroid hormone and thyrotropic hormone levels were altered. It is concluded that perchlorate exposure may affect the production of thyroid hormones during pregnancy (Steinmaus et al. 2016).
As shown in Fig. 7, perchlorate causes thyroid dysfunction in animals and humans by specifically interfering with thyroid hormone synthesis.
Conclusion and outlook
This review objectively described the identification procedures of EDCs in the European Union and United States. Identification of EDCs is different in various organizations and countries. Here, the reasonableness of the chemical being identified as an EDC was evaluated by collecting information on the toxic mechanisms, experimental animal effects, and epidemiological evidence. Next, based on this information, the identification procedures of EDCs for three types of chemicals (BPA, TBECH, and perchlorate) were resolved. Through reviewing the relevant evidence, BPA, TBECH, and perchlorate have sufficient mechanisms of endocrine disruption. They can cause adverse health effects in animals, mainly manifested in changes in organ morphology, pathological tissues, hormone levels in blood, endocrine behavior, and other aspects. Therefore, they can be referred to as EDCs. In contrast to BPA and perchlorate, the health effects of TBECH on humans are unclear. Although there are ways to extrapolate toxicology findings to the population, epidemiological studies are urgently needed to be conducted.
Regarding the hormonal interference of chemicals, it was shown that BPA can interfere with insulin levels, which may potentially contribute to the development of insulin resistance (Lee et al. 2013). In fact, many emerging chemicals in the environment do not interfere with just one hormone; they often have effects on multiple hormones at the same time. TBECH not only interferes with androgens but also affects thyroid function. A study has shown that plasma thyroid hormone levels decreased and thyroid epithelial cell height increased after exposure to β-TBECH (Park et al. 2011). The above shows that TBECH can interfere with endocrine function through other pathways, which strengthens the rationality of TBECH as an EDC.
It is worth noting that with the development of science and technology, computers and artificial intelligence have been more and more widely used. Recently, French experts identified unvalidated methods for chemical characterization of EDCs through artificial intelligence screening literature and database exploration. They used an updated version of the AOP-helpFinder text mining approach to screen abstracts of articles referenced in PubMed automatically, combining exploring manually. Therefore, 226 unique non-validated methods were identified (Zgheib et al. 2021). Consequently, the application of new techniques can be introduced in the establishment of criteria for the identification of EDCs. Mechanism data is important in identifying EDC properties in chemicals. The current rapid development of novel in vitro and in silico methods is promising to fill information gaps on action mechanisms of EDCs. It is helpful to improve confidence in identifying EDCs. However, there is a need to ensure the reliability and regulatory relevance of such methods, which requires joint efforts and collaboration among method developers, researchers, and regulatory agencies. Due to the differences in endocrine signaling across animal species, an in-depth study of the effects of chemicals on the endocrine systems of various species is required. Given the “3Rs” principle, reduction, refinement and replacement (Russell et al. 1959), fewer model organisms should be tested in vivo, but rather utilizing in vitro screening, cross-species extrapolation and read-across approaches to reduce the needs for animal tests. In addition, there are species differences in the toxicokinetic and biotransformation of EDCs considering the differences in endocrine signaling between animals and humans (Testai et al. 2013). Hence, if a chemical possesses the evidence of adverse outcomes after exposure in experimental animals, the relevance of observed effects to the human then needs to be addressed. Finally, interdisciplinary efforts combining knowledge from wildlife, laboratory animals, in vitro, in silico, and human studies are needed to provide a more comprehensive approach for EDC identification. Identifying potential EDCs requires the integration of mechanistic information, results on the effects on animals, human, and the rational linking of the results. Different related standards of EDCs should be developed and integrated to form a unified global standard, which will be conducive to the management and control of EDCs, so as to protect the health of other organisms and humans.
Data availability
Not applicable.
References
Abass K et al (2016) New approaches in human health risk assessment. Int J Circumpolar Health 75:33845
Abdel-Maksoud FM et al (2019) Prenatal exposures to bisphenol A and di (2-ethylhexyl) phthalate disrupted seminiferous tubular development in growing male rats. Reprod Toxicol 88:85–90
Adami HO et al (2011) Toxicology and epidemiology: improving the science with a framework for combining toxicological and epidemiological evidence to establish causal inference. Toxicol Sci 122:223–234
Ahmed RA et al (2014) Effect of prenatal exposure to bisphenol a on the vagina of albino rats: immunohistochemical and ultrastructural study. Folia Morphol (warsz) 73:399–408
Ahsan N et al (2018) Comparative effects of Bisphenol S and Bisphenol A on the development of female reproductive system in rats; a neonatal exposure study. Chemosphere 197:336–343
Åke Bergman JJH, Jobling S, Kidd KA Thomas Zoeller R (2012) State of the science of endocrine disrupting chemicals 2012. United Nations Environment Programme and World Health Organization
Akingbemi BT et al (2004) Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology 145:592–603
An BS et al (2013) Effects of estrogen and estrogenic compounds, 4-tert-octylphenol, and bisphenol A on the uterine contraction and contraction-associated proteins in rats. Mol Cell Endocrinol 375:27–34
Andersen HR et al (1999) Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environ Health Perspect 107(Suppl 1):89–108
Andersson NAM, Barmaz AD, Grignard S, Kienzler E, Lepper A, Lostia P, Munn AM, Parra Morte S, Pellizzato JM, Tarazona F, Terron J, Van der Linden AS (2018) Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. Efsa J 16:e05311
Gentles A et al (2005) Evaluation of adult quail and egg production following exposure to perchlorate-treated water. Environ Toxicol Chem 24:1930–1934
Ankley GT et al (2010) Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29:730–741
Ankley G, Francis E, Gray E, Kavlock R, McMaster S, Reese D, Sayles G, Sergeant A, Vallero D (1998) Research plan for endocrine disruptors. Office of Research and Development US Environmental Protection Agency Research Plan for Endocrine Disruptors
ANSES (2021) ANSES's work and involvement in the area of endocrine disruptors. https://www.anses.fr/en/content/ansess-work-and-involvement-area-endocrine-disruptors#regulatory. Accessed 27 May 2021
Asnake S et al (2014) 1,2-Dibromo-4-(1,2 dibromoethyl) cyclohexane (TBECH)-mediated steroid hormone receptor activation and gene regulation in chicken LMH cells. Environ Toxicol Chem 33:891–899
Autrup H et al (2015) Principles of pharmacology and toxicology also govern effects of chemicals on the endocrine system. Toxicol Sci 146:11–15
Azzouz A, Ballesteros E (2012) Combined microwave-assisted extraction and continuous solid-phase extraction prior to gas chromatography-mass spectrometry determination of pharmaceuticals, personal care products and hormones in soils, sediments and sludge. Sci Total Environ 419:208–215
Behnia F et al (2016) High bisphenol A (BPA) concentration in the maternal, but not fetal, compartment increases the risk of spontaneous preterm delivery. J Matern Fetal Neonatal Med 29:3583–3589
Bereketoglu C et al (2021) The brominated flame retardants TBECH and DPTE alter prostate growth, histology and gene expression patterns in the mouse. Reprod Toxicol 102:43–55
Berg C et al (2001) Effects of bisphenol A and tetrabromobisphenol A on sex organ development in quail and chicken embryos. Environ Toxicol Chem 20:2836–2840
Bergman Å et al (2013) Science and policy on endocrine disrupters must not be mixed: a reply to a “common sense” intervention by toxicology journal editors. Environ Health 12:69
Bernanke J, Köhler HR (2009) The impact of environmental chemicals on wildlife vertebrates. Rev Environ Contam Toxicol 198:1–47
Bhan A et al (2014) Bisphenol-A and diethylstilbestrol exposure induces the expression of breast cancer associated long noncoding RNA HOTAIR in vitro and in vivo. J Steroid Biochem Mol Biol 141:160–170
Boberg J et al (2020) Using assessment criteria for pesticides to evaluate the endocrine disrupting potential of non-pesticide chemicals: case butylparaben. Environ Int 144:105996
Bosquiazzo VL et al (2010) Effects of neonatal exposure to bisphenol A on steroid regulation of vascular endothelial growth factor expression and endothelial cell proliferation in the adult rat uterus. Biol Reprod 82:86–95
Bouskine A et al (2009) Low doses of bisphenol A promote human seminoma cell proliferation by activating PKA and PKG via a membrane G-protein-coupled estrogen receptor. Environ Health Perspect 117:1053–1058
Bradford CM et al (2005) Perchlorate affects thyroid function in eastern mosquitofish (Gambusia holbrooki) at environmentally relevant concentrations. Environ Sci Technol 39:5190–5195
Brown FR et al (2014) Levels of non-polybrominated diphenyl ether brominated flame retardants in residential house dust samples and fire station dust samples in California. Environ Res 135:9–14
Browne P et al (2017) Application of adverse outcome pathways to U.S. EPA’s endocrine disruptor screening program. Environ Health Perspect. 125:096001
Browne P et al (2020) OECD approaches and considerations for regulatory evaluation of endocrine disruptors. Mol Cell Endocrinol 504:110675
Cantonwine D et al (2010) Bisphenol a exposure in Mexico City and risk of prematurity: a pilot nested case control study. Environ Health 9:62
Casas M, Gascon M (2020) Prenatal exposure to endocrine-disrupting chemicals and asthma and allergic diseases. J Investig Allergol Clin Immunol 30:215–228
Chen H et al (2019) A review of toxicity induced by persistent organic pollutants (POPs) and endocrine-disrupting chemicals (EDCs) in the nematode Caenorhabditis elegans. J Environ Manage 237:519–525
Chen L et al (2016) A review of reproductive toxicity of microcystins. J Hazard Mater 301:381–399
Chen L et al (2021) Effects of acute exposure to microcystins on hypothalamic-pituitary-adrenal (HPA), -gonad (HPG) and -thyroid (HPT) axes of female rats. Sci Total Environ 778:145196
Chen Q et al (2018) Activation of steroid hormone receptors: shed light on the in silico evaluation of endocrine disrupting chemicals. Sci Total Environ 631–632:27–39
China, The Ministry of Agriculture of the People's Republic of China (2015) Evaluation method of pesticide endocrine disrupting effects (NY/T 2873-2015)
Clayton EM et al (2011) The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003–2006. Environ Health Perspect 119:390–396
Crane HM et al (2005) Effects of ammonium perchlorate on thyroid function in developing fathead minnows. Pimephales Promelas Environ Health Perspect 113:396–401
Crump D et al (2014) 1,2-Dibromo-4-(1,2-dibromoethyl)-cyclohexane and tris(methylphenyl) phosphate cause significant effects on development, mRNA expression, and circulating bile acid concentrations in chicken embryos. Toxicol Appl Pharmacol 277:279–287
Curran IH et al (2017) Toxicologic effects of 28-day dietary exposure to the flame retardant 1,2-dibromo-4-(1,2-dibromoethyl)-cyclohexane (TBECH) in F344 rats. Toxicology 377:1–13
Currier HA et al (2013) An assessment of in ovo toxicity of the flame retardant 1,2-dibromo-4-(1,2-dibromoethyl) cyclohexane (TBECH) in the zebra finch. Bull Environ Contam Toxicol 91:455–459
Dang Z et al (2012) Evaluation of the Daphnia magna reproduction test for detecting endocrine disruptors. Chemosphere 88:514–523
Diamanti-Kandarakis E et al (2009) Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr Rev 30:293–342
Dietrich DR et al (2013) Scientifically unfounded precaution drives European Commission’s recommendations on EDC regulation, while defying common sense, well-established science and risk assessment principles. Toxicol in Vitro 27:2110–2114
Dohán O et al (2007) The Na+/I symporter (NIS) mediates electroneutral active transport of the environmental pollutant perchlorate. Proc Natl Acad Sci U S A 104:20250–20255
EC (1998) What are endocrine disruptors? European Commission, https://ec.europa.eu/environment/chemicals/endocrine/definitions/endodis_en.htm. Accessed 21 Jul 2022
ECHA (2011) Candidate List of substances of very high concern for Authorisation. https://echa.europa.eu/regulations/reach/understanding-reach. Accessed 9 Jan 2021
ECHA/EFSA (2018) Guidance for the identification of endocrine disruptors in the context of regulations (EU) No 528/2012 and (EC) No 1107/2009, 16(6):5311
EPA (2014) Framework for human health risk assessment to inform decision making. https://www.epa.gov/risk/framework-human-health-risk-assessment-inform-decision-making. Accessed 21 Jul 2022
Fabian E et al (2019) In vitro-to-in vivo extrapolation (IVIVE) by PBTK modeling for animal-free risk assessment approaches of potential endocrine-disrupting compounds. Arch Toxicol 93:401–416
Ferguson KK et al (2014) Prenatal and peripubertal phthalates and bisphenol A in relation to sex hormones and puberty in boys. Reprod Toxicol 47:70–76
Forner-Piquer I et al (2020) Effects of BPA on zebrafish gonads: focus on the endocannabinoid system. Environ Pollut 264:114710
Foster PMD (2005) Mode of action: Impaired fetal leydig cell function - effects on male reproductive development produced by certain phthalate esters. Crit Rev Toxicol 35:713–719
Gallo MV et al (2016) Endocrine disrupting chemicals and ovulation: is there a relationship? Environ Res 151:410–418
Gámez JM et al (2015) Exposure to a low dose of bisphenol A impairs pituitary-ovarian axis in prepubertal rats: effects on early folliculogenesis. Environ Toxicol Pharmacol 39:9–15
Gardell AM et al (2017) Exogenous iodide ameliorates perchlorate-induced thyroid phenotypes in threespine stickleback. Gen Comp Endocrinol 243:60–69
Gauthier LT et al (2009) Temporal trends and spatial distribution of non-polybrominated diphenyl ether flame retardants in the eggs of colonial populations of Great Lakes herring gulls. Environ Sci Technol 43:312–317
Gemmill B et al (2011) Toxicokinetics of tetrabromoethylcyclohexane (TBECH) in juvenile brown trout (Salmo trutta) and effects on plasma sex hormones. Aquat Toxicol 101:309–317
Gentes ML et al (2012) Novel flame retardants in urban-feeding ring-billed gulls from the St. Lawrence River. Canada Environ Sci Technol 46:9735–9744
Gilbert ME, Sui L (2008) Developmental exposure to perchlorate alters synaptic transmission in hippocampus of the adult rat. Environ Health Perspect 116:752–760
Giommi C, Habibi HR, Candelma M, Carnevali O, Maradonna F (2021) Probiotic administration mitigates bisphenol A reproductive toxicity in zebrafish. Int J Mol Sci 22(17). https://doi.org/10.3390/ijms22179314
Golden R et al (2005) A review of the endocrine activity of parabens and implications for potential risks to human health. Crit Rev Toxicol 35:435–458
Guillette LJ Jr, Gunderson MP (2001) Alterations in development of reproductive and endocrine systems of wildlife populations exposed to endocrine-disrupting contaminants. Reproduction 122:857–864
Hatef A et al (2012) Adverse effects of bisphenol A on reproductive physiology in male goldfish at environmentally relevant concentrations. Ecotoxicol Environ Saf 76:56–62
Hauser R et al (2015) Male reproductive disorders, diseases, and costs of exposure to endocrine-disrupting chemicals in the European Union. J Clin Endocrinol Metab 100:1267–1277
Health, Japan Ministry of Health (2015) The Endocrine Disruptor Page. Japan Ministry of Health, http://www.nihs.go.jp/edc/english/actions/index.htm. Accessed 1 Apr 2022
Ho V et al (2022) Endocrine disruptors: challenges and future directions in epidemiologic research. Environ Res 204:111969
Hu JMY et al (2021) Prenatal exposure to endocrine disrupting chemical mixtures and infant birth weight: a Bayesian analysis using kernel machine regression. Environ Res 195:110749
IPCS (2002) Global Assessment of the State-of-the-Science of Endocrine Disruptors. . In: B. S. WHO/PCS/EDC/02.2. Eds. Damstra T, Bergman A, Kavlock R, and Van Der Kraak G. Geneva, Switzerland: World Health Organization. , (Ed.). IPCS (International Programme on Chemical Safety). http://www.who.int/ipcs/publications/new_issues/endocrine_disruptors/en/. Accessed 25 Mar 2022
Isanhart JP et al (2005) Effects of perchlorate exposure on resting metabolism, peak metabolism, and thyroid function in the prairie vole (Microtus ochrogaster). Environ Toxicol Chem 24:678–684
Jaladanki CK et al (2021) Virtual screening of potentially endocrine-disrupting chemicals against nuclear receptors and its application to identify PPARγ-bound fatty acids. Arch Toxicol 95:355–374
Jeong J, Kim H, Choi J (2019) In silico molecular docking and in vivo validation with Caenorhabditis elegans to discover molecular initiating events in adverse Outcome pathway framework: Case study on endocrine-disrupting chemicals with estrogen and androgen receptors. Int J Mol Sci 20(5). https://doi.org/10.3390/ijms20051209
Julien E et al (2009) The key events dose-response framework: a cross-disciplinary mode-of-action based approach to examining dose-response and thresholds. Crit Rev Food Sci Nutr 49:682–689
Kahn LG et al (2020) Endocrine-disrupting chemicals: implications for human health. Lancet Diabetes Endocrinol 8:703–718
Kang Y et al (2014) Daphnia magna may serve as a powerful tool in screening endocrine disruption chemicals (EDCs). Environ Sci Technol 48:881–882
Kassotis CD et al (2020) Endocrine-disrupting chemicals: economic, regulatory, and policy implications. Lancet Diabetes Endocrinol 8:719–730
Khalaf H et al (2009) Diastereomers of the brominated flame retardant 1,2-dibromo-4-(1,2 dibromoethyl)cyclohexane induce androgen receptor activation in the hepg2 hepatocellular carcinoma cell line and the lncap prostate cancer cell line. Environ Health Perspect 117:1853–1859
Kharlyngdoh JB et al (2016) TBECH, 1,2-dibromo-4-(1,2 dibromoethyl) cyclohexane, alters androgen receptor regulation in response to mutations associated with prostate cancer. Toxicol Appl Pharmacol 307:91–101
Kharlyngdoh JB et al (2018) Androgen receptor modulation following combination exposure to brominated flame-retardants. Sci Rep 8:4843
Klebe G (2006) Virtual ligand screening: strategies, perspectives and limitations. Drug Discov Today 11:580–594
Klimisch HJ et al (1997) A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul Toxicol Pharmacol 25:1–5
Korach KS (1993) Editorial: surprising places of estrogenic activity. Endocrinology 132:2277–2278
Kuang Y et al (2014) Interference of the endocrine disrupting chemicals on androgen receptor function. Journal of Chongqing Normal University. Nat Sci Edition 31:16–22
La Merrill MA et al (2020) Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat Rev Endocrinol 16:45–57
Lahnsteiner F et al (2005) Effect of bisphenol A on maturation and quality of semen and eggs in the brown trout. Salmo Trutta F Fario Aquat Toxicol 75:213–224
Larsson A et al (2006) Identification of the brominated flame retardant 1,2-dibromo-4-(1,2-dibromoethyl)cyclohexane as an androgen agonist. J Med Chem 49:7366–7372
Lee DH, Jacobs DR Jr (2015) Methodological issues in human studies of endocrine disrupting chemicals. Rev Endocr Metab Disord 16:289–297
Lee HA et al (2013) Effect of urinary bisphenolA on androgenic hormones and insulin resistance in preadolescent girls: a pilot study from the Ewha Birth & Growth Cohort. Int J Environ Res Public Health 10:5737–5749
Legler J et al (2015) Obesity, diabetes, and associated costs of exposure to endocrine-disrupting chemicals in the European Union. J Clin Endocrinol Metab 100:1278–1288
Li Y et al (2012a) Differential estrogenic actions of endocrine-disrupting chemicals Bisphenol A, Bisphenol AF, and zearalenone through estrogen receptor α and β in Vitro. Environ Health Perspect 120:1029–1035
Lisco G, De Tullio A, Giagulli VA, De Pergola G, Triggiani V (2020) Interference on iodine uptake and human thyroid function by perchlorate-contaminated water and food. Nutrients 12(6). https://doi.org/10.3390/nu12061669
Liu PY et al (2017) Tetrabromoethylcyclohexane affects gonadal differentiation and development in the frog Pelophylax nigromaculatus. Aquat Toxicol 192:40–47
Liu R et al (2022) Effects of bisphenol A on reproductive toxicity and gut microbiota dysbiosis in male rats. Ecotoxicol Environ Saf 239:113623
Liu X et al (2021a) Chronic exposure of BPA impairs male germ cell proliferation and induces lower sperm quality in male mice. Chemosphere 262:127880
Liu Y et al (2021b) Brominated flame retardants (BFRs) in marine food webs from Bohai Sea. China Sci Total Environ 772:145036
Liu Y et al (2021c) Bioaccumulation and reproductive toxicity of bisphenol A in male-pregnant seahorse (Hippocampus erectus) at environmentally relevant concentrations. Sci Total Environ 753:141805
Luccio-Camelo DC, Prins GS (2011) Disruption of androgen receptor signaling in males by environmental chemicals. J Steroid Biochem Mol Biol 127:74–82
Ma Y et al (2019) The adverse health effects of bisphenol A and related toxicity mechanisms. Environ Res 176:108575
Mahalingam S et al (2017) The effects of in utero bisphenol A exposure on ovarian follicle numbers and steroidogenesis in the F1 and F2 generations of mice. Reprod Toxicol 74:150–157
Markey CM et al (2001) In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol Reprod 65:1215–1223
Marteinson SC et al (2020) A review of 1,2-dibromo-4-(1,2-dibromoethyl)cyclohexane in the environment and assessment of its persistence, bioaccumulation and toxicity. Environ Res 195:110497
Marteinson SC, Fernie KJ (2019) Is the current-use flame retardant, DBE-DBCH, a potential obesogen? Effects on body mass, fat content and associated behaviors in American kestrels. Ecotoxicol Environ Saf 169:770–777
Marteinson SC et al (2015) Exposure to the androgenic brominated flame retardant 1,2-dibromo-4-(1,2-dibromoethyl)-cyclohexane alters reproductive and aggressive behaviors in birds. Environ Toxicol Chem 34:2395–2402
Marteinson SC et al (2012) The flame retardant β-1,2-dibromo-4-(1,2-dibromoethyl)cyclohexane: fate, fertility, and reproductive success in American kestrels (Falco sparverius). Environ Sci Technol 46:8440–8447
Marteinson SC et al (2017) Disruption of thyroxine and sex hormones by 1,2-dibromo-4-(1,2-dibromoethyl)cyclohexane (DBE-DBCH) in American kestrels (Falco sparverius) and associations with reproductive and behavioral changes. Environ Res 154:389–397
McNabb FM et al (2004a) Does thyroid function in developing birds adapt to sustained ammonium perchlorate exposure? Toxicol Sci 82:106–113
McNabb FM et al (2004b) Ammonium perchlorate effects on thyroid function and growth in bobwhite quail chicks. Environ Toxicol Chem 23:997–1003
Meng Z et al (2019) Effects of perinatal exposure to BPA, BPF and BPAF on liver function in male mouse offspring involving in oxidative damage and metabolic disorder. Environ Pollut 247:935–943
Miao Y et al (2023) Within-day variability, predictors, and risk assessments of exposure to parabens among Chinese adult men. Environ Res 218:115026
Mínguez-Alarcón L et al (2015) Urinary bisphenol A concentrations and association with in vitro fertilization outcomes among women from a fertility clinic. Hum Reprod 30:2120–2128
Molina A et al (2018) Hypothalamic-pituitary-ovarian axis perturbation in the basis of bisphenol A (BPA) reproductive toxicity in female zebrafish (Danio rerio). Ecotoxicol Environ Saf 156:116–124
Molina AM et al (2021) Proteomic profile of the effects of low-dose bisphenol A on zebrafish ovaries. Food Chem Toxicol 156:112435
Moral R et al (2008) Effect of prenatal exposure to the endocrine disruptor bisphenol A on mammary gland morphology and gene expression signature. J Endocrinol 196:101–112
Mukhi S, Patiño R (2007) Effects of prolonged exposure to perchlorate on thyroid and reproductive function in zebrafish. Toxicol Sci 96:246–254
Muñoz JP et al (2021) Glyphosate and the key characteristics of an endocrine disruptor: a review. Chemosphere 270:128619
Mustieles V et al (2018) Bisphenol A and reproductive hormones and cortisol in peripubertal boys: The INMA-Granada cohort. Sci Total Environ 618:1046–1053
Newbold RR et al (2007) Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol 24:253–258
Nguyen KH et al (2017) Biotransformation of the flame retardant 1,2-dibromo-4-(1,2-dibromoethyl)cyclohexane (TBECH) in Vitro by Human Liver Microsomes. Environ Sci Technol 51:10511–10518
Niklas Andersson MA, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Morte JMP, Pellizzato F, Tarazona J, Terron A, Van der Linden S (2018) Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. European Food Safety Authority. https://doi.org/10.2903/j.efsa.2018.5311
Nowak K et al (2018) Parabens and their effects on the endocrine system. Mol Cell Endocrinol 474:238–251
OECD (2018a) Revised guidance document 150 on standardised test guidelines for evaluating chemicals for endocrine disruption. OECD Series on Testing and Assessment. OECD Publishing. https://doi.org/10.1787/9789264304741-en. Paris
OECD (2018b) Revised guidance document 150 on standardised test guidelines for evaluating chemicals for endocrine disruption. OECD Series on Testing and Assessment. https://doi.org/10.1787/9789264304741-en
OEHHA (2011) Green chemistry hazard traits, section 69403.4 endocrine toxicity, California Code of Regulations, Division 4.5, Title 22, Chapter 54. https://oehha.ca.gov/media/downloads/risk-assessment/gcisor121710.pdf. Accessed 1 Apr 2022
Palioura E, Diamanti-Kandarakis E (2015) Polycystic ovary syndrome (PCOS) and endocrine disrupting chemicals (EDCs). Rev Endocr Metab Disord 16:365–371
Park BJ et al (2011) Thyroid axis disruption in juvenile brown trout (Salmo trutta) exposed to the flame retardant β-tetrabromoethylcyclohexane (β-TBECH) via the diet. Environ Sci Technol 45:7923–7927
Patiño R et al (2003) Effects of ammonium perchlorate on the reproductive performance and thyroid follicle histology of zebrafish. Environ Toxicol Chem 22:1115–1121
Patisaul HB et al (2018) Animal models of endocrine disruption. Best Pract Res Clin Endocrinol Metab 32:283–297
Porta M (2016) A Dictionary of Epidemiology, 6th edn. Oxford University Press, Oxford, UK
Porter E et al (2014) Use of an avian hepatocyte assay and the avian Toxchip Polymerse chain reaction array for testing prioritization of 16 organic flame retardants. Environ Toxicol Chem 33:573–582
Pradhan A et al (2013) The brominated flame retardant TBECH activates the zebrafish (Danio rerio) androgen receptor, alters gene transcription and causes developmental disturbances. Aquat Toxicol 142–143:63–72
Raghavan R et al (2018) Pharmacologic and environmental endocrine disruptors in the pathogenesis of hypospadias: a review. Curr Environ Health Rep 5:499–511
Ramírez V et al (2022) Role of endocrine disrupting chemicals in children’s neurodevelopment. Environ Res 203:111890
Rebuli ME et al (2014) Investigation of the effects of subchronic low dose oral exposure to bisphenol A (BPA) and ethinyl estradiol (EE) on estrogen receptor expression in the juvenile and adult female rat hypothalamus. Toxicol Sci 140:190–203
Ruan Y et al (2018a) Temporal changes and stereoisomeric compositions of 1,2,5,6,9,10-hexabromocyclododecane and 1,2-dibromo-4-(1,2-dibromoethyl)cyclohexane in marine mammals from the South China Sea. Environ Sci Technol 52:2517–2526
Ruan Y et al (2019) Stereoisomer-specific occurrence, distribution, and fate of chiral brominated flame retardants in different wastewater treatment systems in Hong Kong. J Hazard Mater 374:211–218
Ruan Y et al (2018b) Stereoisomer-specific trophodynamics of the chiral brominated flame retardants HBCD and TBECH in a marine food web, with implications for human exposure. Environ Sci Technol 52:8183–8193
Rudén C (2006) What influences a health risk assessment? Toxicol Lett 167:201–204
Russell WMS, Burch RL, Hume CW (1959) The principles of humane experimental technique. Methuen, London, Vol. 238, Chapter: 5 replacement
Rybacka A et al (2015) Identifying potential endocrine disruptors among industrial chemicals and their metabolites–development and evaluation of in silico tools. Chemosphere 139:372–378
Safety, International Programme on Chemical Safety (2002) Global assessment of the state of the science of endocrine disruptors, an assessment prepared by an expert group on behalf of the World Health Organization, the International Labour Organization, and the United Nations Environment Programme. WHO/PCS/EDC/02.2
Sahlström LM et al (2014) Brominated flame retardants in matched serum samples from Swedish first-time mothers and their toddlers. Environ Sci Technol 48:7584–7592
Salgueiro-González N et al (2015) Analysis and occurrence of endocrine-disrupting chemicals in airborne particles. TrAC - Trends Anal Chem 66:45–52
Sarink D et al (2021) BPA, parabens, and phthalates in relation to endometrial cancer risk: a case-control study nested in the multiethnic cohort. Environ Health Perspect 129:57702
SCHEER-Scientific Committee on Health, Environmental and Emerging Risks (SCHEER) (2018) Memorandum on weight of evidence and uncertainties
Schmidt F et al (2012) Effects of the anti-thyroidal compound potassium-perchlorate on the thyroid system of the zebrafish. Aquat Toxicol 109:47–58
Schneider M et al (2019) In silico predictions of endocrine disruptors properties. Endocrinology 160:2709–2716
Seed J et al (2005) Overview: using mode of action and life stage information to evaluate the human relevance of animal toxicity data. Crit Rev Toxicol 35:664–672
Segner H (2009) Zebrafish (Danio rerio) as a model organism for investigating endocrine disruption. Comp Biochem Physiol C Toxicol Pharmacol 149:187–195
Serrano-Nascimento C et al (2018) Evaluation of hypothalamus-pituitary-thyroid axis function by chronic perchlorate exposure in male rats. Environ Toxicol 33:209–219
Seyoum A et al (2021) Sublethal effects of DBE-DBCH diastereomers on physiology, behavior, and gene expression of Daphnia magna. Environ Pollut 284:117091
Shanle EK, Xu W (2011) Endocrine disrupting chemicals targeting estrogen receptor signaling: identification and mechanisms of action. Chem Res Toxicol 24:6–19
Shen Y et al (2015) Higher urinary bisphenol A concentration is associated with unexplained recurrent miscarriage risk: evidence from a case-control study in eastern China. PLoS ONE 10:e0127886
Sifakis S et al (2017) Human exposure to endocrine disrupting chemicals: effects on the male and female reproductive systems. Environ Toxicol Pharmacol 51:56–70
Siglin JC et al (2000) A 90-day drinking water toxicity study in rats of the environmental contaminant ammonium perchlorate. Toxicol Sci 57:61–74
Sonich-Mullin C et al (2001) IPCS conceptual framework for evaluating a mode of action for chemical carcinogenesis. Regul Toxicol Pharmacol 34:146–152
Spinder N et al (2021) Maternal occupational exposure to endocrine-disrupting chemicals and urogenital anomalies in the offspring. Hum Reprod 37:142–151
Steinmaus C et al (2016) Thyroid hormones and moderate exposure to perchlorate during pregnancy in women in Southern California. Environ Health Perspect 124:861–867
Stojak BL et al (2019) Acute β-tetrabromoethylcyclohexane (β-TBECH) treatment inhibits the electrical activity of rat Purkinje neurons. Chemosphere 231:301–307
Sun J, Fang R, Wang H, Xu DX, Yang J, Huang X, Cozzolino D, Fang M, Huang Y (2022) A review of environmental metabolism disrupting chemicals and effect biomarkers associating disease risks: Where exposomics meets metabolomics. Environ Int 158. https://doi.org/10.1016/j.envint.2021.106941
Tay JH et al (2019) Serum concentrations of legacy and emerging halogenated flame retardants in a Norwegian cohort: relationship to external exposure. Environ Res 178:108731
Terry MB et al (2019) Environmental exposures during windows of susceptibility for breast cancer: a framework for prevention research. Breast Cancer Res 21:96
Testai E et al (2013) A plea for risk assessment of endocrine disrupting chemicals. Toxicology 314:51–59
Thomas P, Dong J (2006) Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol 102:175–179
Thuett KA et al (2002) Effects of in utero and lactational ammonium perchlorate exposure on thyroid gland histology and thyroid and sex hormones in developing deer mice (peromyscus maniculatus) through postnatal day 21. J Toxicol Environ Health A 65:2119–2130
U.S.EPA (1998) Endocrine Disruptor Screening Program (EDSP) 1998 Federal Register Notices. https://www.epa.gov/endocrine-disruption/endocrine-disruptor-screening-program-edsp-1998-federal-register-notices. Accessed 25 Jul 2022
Usmani KA et al (2006) Inhibition of the human liver microsomal and human cytochrome P450 1A2 and 3A4 metabolism of estradiol by deployment-related and other chemicals. Drug Metab Dispos 34:1606–1614
Usmani KA et al (2003) Inhibition and activation of the human liver microsomal and human cytochrome P450 3A4 metabolism of testosterone by deployment-related chemicals. Drug Metab Dispos 31:384–391
van den Hove MF et al (1999) Hormone synthesis and storage in the thyroid of human preterm and term newborns: effect of thyroxine treatment. Biochimie 81:563–570
Vandenberg LN et al (2016) A proposed framework for the systematic review and integrated assessment (SYRINA) of endocrine disrupting chemicals. Environ Health 15:74
Vandenberg LN et al (2008) Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reprod Toxicol 26:210–219
Vandenberg LN et al (2020) Agrochemicals with estrogenic endocrine disrupting properties: lessons learned? Mol Cell Endocrinol 518:110860
Viguié C et al (2018) Evidence-based adverse outcome pathway approach for the identification of BPA as en endocrine disruptor in relation to its effect on the estrous cycle. Mol Cell Endocrinol 475:10–28
Wang Q, Kelly BC (2017) Occurrence and distribution of halogenated flame retardants in an urban watershed: comparison to polychlorinated biphenyls and organochlorine pesticides. Environ Pollut 231:252–261
Wang Q et al (2016) Reprogramming of the epigenome by MLL1 links early-life environmental exposures to prostate cancer risk. Mol Endocrinol 30:856–871
Wang X et al (2020) Tetrabromoethylcyclohexane (TBECH) exhibits immunotoxicity in murine macrophages. Environ Toxicol 35:159–166
Watson CS et al (2007) Xenoestrogens are potent activators of nongenomic estrogenic responses. Steroids 72:124–134
Wee SY, Aris AZ (2017) Endocrine disrupting compounds in drinking water supply system and human health risk implication. Environ Int 106:207–233
Williams-Ashman HG (1965) Androgenic control of nucleic acid and protein synthesis in male accessory genital organs. J Cell Physiol. 66. Suppl 1:111–124
Wisniewski P et al (2015) Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic-pituitary-testicular axis. Toxicology 329:1–9
Wong LI et al (2016) The effects of the organic flame-retardant 1,2-dibromo-4-(1,2-dibromoethyl) cyclohexane (TBECH) on androgen signaling in human prostate cancer cell lines. J Biochem Mol Toxicol 30:239–242
Wu D et al (2020) Impairment of learning and memory induced by perinatal exposure to BPA is associated with ERα-mediated alterations of synaptic plasticity and PKC/ERK/CREB signaling pathway in offspring rats. Brain Res Bull 161:43–54
Xu D et al (2021) Association between semen microcystin levels and reproductive quality: a cross-sectional study in Jiangsu and Anhui Provinces, China. Environ Health Perspect 129:127702
Xu G et al (2022) Male reproductive toxicity induced by microcystin-leucine-arginine (MC-LR). Toxicon 210:78–88
Xu X et al (2017) Body burden of heavy metals among HIV high risk population in USA. Environ Pollut 220:1121–1126
Yaoi T et al (2008) Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochem Biophys Res Commun 376:563–567
Yigit F, Daglioglu S (2010) Histological changes in the uterus of the hens after embryonic exposure to bisphenol A and diethylstilbestrol. Protoplasma 247:57–63
York RG et al (2005) Refining the effects observed in a developmental neurobehavioral study of ammonium perchlorate administered orally in drinking water to rats. I. Thyroid and reproductive effects. Int J Toxicol 24:403–418
Zamora AN et al (2021) Exposure to phenols, phthalates, and parabens and development of metabolic syndrome among Mexican women in midlife. Front Public Health 9:620769
Zgheib E et al (2021) Identification of non-validated endocrine disrupting chemical characterization methods by screening of the literature using artificial intelligence and by database exploration. Environ Int 154:106574
Zhang S et al (2022) A new identity of microcystins: environmental endocrine disruptors? An evidence-based review. Sci Total Environ 851:158262
Zoeller RT (2006) Collision of basic and applied approaches to risk assessment of thyroid toxicants. Ann N Y Acad Sci 1076:168–190
Zoeller RT et al (2014) A path forward in the debate over health impacts of endocrine disrupting chemicals. Environ Health 13:118
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 82273594, 82073512, and 81773384).
Author information
Authors and Affiliations
Contributions
Huizhen Zhang: conceptualization, writing—review and editing, supervision, and funding acquisition. Xing Guo: conceptualization, methodology, software, writing—original draft preparation, and visualization. Bing Liu: conceptualization, methodology, software, writing—original draft preparation, and visualization. Haohao Liu: software and writing—review and editing. Xingde Du: methodology, writing—review and editing, and visualization. Xinghai Chen: writing—review. Wenjun Wang: writing—review. Shumeng Yuan: software. Bingyu Zhang: software. Yongshui Wang: software. Hongxiang Guo: conceptualization and writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, X., Liu, B., Liu, H. et al. Research advances in identification procedures of endocrine disrupting chemicals. Environ Sci Pollut Res 30, 83113–83137 (2023). https://doi.org/10.1007/s11356-023-27755-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27755-y