Abstract
Various chemicals are known to impact biological systems disrupting normal homeostasis, especially the endocrine system, called endocrine disrupting chemicals. Endocrine disrupting chemicals are universal in the environment, and a low concentration (µg/L to ng/L) severely impacts human health and wildlife due to transgenerational and multigenerational effects. The present review analyzed original research articles (109), review articles (76), short communication (20), article report (1), monograph (1), and scientific reports (2) via PubChem, science direct, National Center for Biotechnology Information, web of science on the source and fate of endocrine disrupting chemicals in the environment which are a prime concern to public health. A thorough investigation of the fate of these contaminants in environmental matrices such as air, water, soil, and biota is prioritized to form a clear link between the exposure and its effect on humans and biota. Environmental guidelines for these chemicals in environmental matrices and their treatment methods are also discussed in the review.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endocrine disrupting chemicals (EDCs) are exogenous in origin and potentially harm human and wildlife health even at a low concentration. The increased demand and consumption have resulted in the global existence and pseudo-persistence of these chemicals in the environment. Chemicals disrupting growth, reproduction, and the equilibrium of the body system include industrial chemicals, plasticizers, pharmaceuticals and personal care products (PPCPs) (Bergman et al. 2012).
The primary source of EDCs contamination in the environment is wastewater effluent, as conventional treatment technologies are ineffective and insufficient in removing the pollutants (Ebele et al. 2020). Concentrations from a few nanograms per litre (ng/L) to 15 µg per litre (µg/L) cause severe acute and chronic toxic effects raising serious environmental health concerns (Kasonga et al. 2021). Scientific studies and research reveal that exposure to endocrine disrupting chemicals can lead to permanent developmental damage, such as diethylstilbestrol (DES), an artificial nonsteroidal estrogen used by medical practitioners to treat menopause-related problems in females, affecting follicular growth, fertilization, and implantation in females, also cause abnormality in reproductive tracts with reduced sperm production causing infertility in males. Other classic examples including organochlorines like dicofol, methoxychlor, and dichlorodiphenyltrichloroethane (DDT) cause thinning of eggshells leading to deformity in the oviducts of female birds. Even the metabolites of dicofol cause additional effects such as the feminization of male alligators, non-formation of vitellogenin in fishes, and neurological disorders in humans. Pharmaceuticals such as ciprofloxacin, sulfamethoxazole, azithromycin, and acetaminophen, with long-term exposure to infants through their mothers, cause DNA methylation defects (Montrose et al. 2018). Further, the in vivo studies on the mixture of EDCs revealed the impact on the physiology of the hypothalamic-pituitary-thyroid axis, growth stress, and immune system in humans (Hamid et al. 2021).
Currently, occasional legislation and policies are being released in countries globally challenging to achieve water security, ensuring better quality and healthier. Waterborne disease outbreaks and the growing number of new pollutants have prompted regulatory agencies to formulate legislation and policies.
Figure 1 represents the roadmap taken up by the European and Non-European countries to combat the occurrence of EDCs in the environment. It could be observed that 205 substances fall under the category of "Substances of Very High Concern" (SVHC), with only 16 of them with the potential to cause endocrine disrupting chemicals. Currently, registration, evaluation, authorization, and restriction of chemicals (REACH) 2006 formed by the European Union (EU) identifies the risks and restricts the usage of the substances. Forty-three substances have been included in Annex XIV of REACH (two recognized as EDCs), indicating the intent to ban their use as soon as their technically and economically appropriate alternatives are available. In the USA, the Food and Drug Administration (FDA), the Environmental Protection Agency (EPA), along with Toxic Substances Control Act (TSCA) identify the risks of the chemicals, controlling and regulating the strict compliance of the laws and legislation (Kassotis et al. 2020). Organizations such as United Nations Environment Programme (UNEP) and World Health Organization (WHO) reported and commissioned the International Panel on Chemical Pollution, identifying the twenty-eight policies to address and evaluate the EDCs and other substantial chemicals globally. Exposure to EDCs was observed in treated drinking water ranging from 0.2 to 5510 ng/L, with a maximum concentration of 280,000.0 ng/L reported in groundwater (wells) in India. WHO drinking water guidelines are inadequate compared to the exposure of the EDCs (Wee and Aris 2018). Table 1 presents the global view of the concentration ranges in diverse environmental matrices such as water and solids. This infers the ubiquitous and consistent presence of EDCs in low concentrations worldwide daily. The twelve countries or regions have regulated drinking water standards for few selected EDCs namely, 17β-estradiol (E2), 17α-ethinylestradiol (EE2), bisphenol a (BPA), 4-n-nonylphenol (NP), di-2-(ethylhexyl) phthalate (DEHP), benzyl butyl phthalate (BBP), di-butyl phthalate (DBP), di-ethyl phthalate (DEP), and di-2-(ethylhexyl) adipate (DEHA). The twelve countries include the EU, USA, UK, Australia, China, Israel, Japan, Korea, New Zealand, Oman, Philippines, Singapore, and UAE. Japan, China and the USA have been successful in regulating six, four, and two EDCs, respectively, according to their drinking water standards, while the other eight countries have only considered DEHP in their national drinking water standards. The EU has regulated only BPA in their drinking water standards (Liu et al. 2021). India follows drinking water standards for pesticides, halogenated compounds, and a few pharmaceuticals and personal care products (Wee and Aris 2018). These indicate that regulations for EDCs in environmental matrices are region-specific and lack universal guidelines without consistent monitoring.
Figure 2 explains the different point and non-point sources of EDCs entering the environment, like volatilization, precipitation, and evapotranspiration. Further, the uptake of EDCs via ingestion and inhalation has resulted in various reproductive and genotoxic effects via physicochemical and biochemical disruptions.
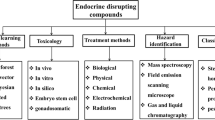
The present review focuses on comprehensively summarizing the current state of knowledge regarding the various classes of EDCs, some of which the EPA has identified as "the priority pollutants" primarily used in production and manufacturing processes. These EDCs also end up in various industrial effluents as a byproduct. Further, the study discusses the sources, fate, and adverse effects of various EDCs on human and biota, jeopardizing public health and the sustainable development goals (SDGs) globally. The review compares various EDC degrading methods and offers corrective actions to reduce EDC emissions into the environment.
Endocrine disrupting chemicals (EDCs)
EDCs are exogenous substances that can affect the endocrine and homeostatic systems by interfering with endogenous hormone synthesis, secretion, transport, metabolism, receptor binding, or elimination. USEPA defines them as “exogenous chemicals or mixture of chemicals that interfere with any aspect of hormone action” (Kasonga et al. 2021). EDCs enter the human body through different routes. The most frequent exposure modes are inhalation, food consumption, and direct contact. These routes suggest that EDCs could enter the food chain and build up in animal tissues and humans. Because most EDCs seem extremely lipophilic and accumulate in adipose tissue, they often have a long half-life. Being at the top of the food chain, humans, large mammals, and top predators may accumulate higher dosages of EDCs due to bioaccumulation and bioamplification. These could form a "cocktail" of effects with unknowable effects.
Figure 3 and Table 2 explain the physicochemical characteristics of EDCs based on the origin, which are broadly divided into five main categories to highlight the prevalence of these target categories in daily use.: natural and synthetic hormones (estradiol, testosterone and ethinylestradiol); industrial (furans-dioxins, perfluoroalkyl, polychlorinated biphenyls (PCBs), heavy metals, and alkylphenols); agricultural (pesticides, phytoestrogens, and fungicides); household (phthalates, polybrominated biphenyls, and bisphenol A); and pharmaceutical and personal care products (parabens, antibiotics, sunscreen blockers).
Phthalates (PAEs)
PAEs are a group of compounds that include alkyl, aryl, and dialkyl esters of phthalic acid (1,2-benzene dicarboxylic acid). They are produced by the reaction of phthalic anhydride with an appropriate alcohol. PAEs lack a covalent bond between them and the polymer and are available in the free and mobile form in the environment (Baloyi et al. 2021). Hence, PAEs enter the environment through several pathways, including losses during manufacturing processes, leaching or volatilization from final products, waste, and treated effluents (with minute concentration) from wastewater treatment plants (Das et al. 2021, Przybylinska and Wyszkowski 2016). Table 3 summarizes the physicochemical properties of PAEs that play a crucial role in determining the fate and transportation in environmental matrices and the effectiveness of the treatment methods for maximum removal efficiency. The presence of PAEs in the air is determined by the Henry constant value and the log Kow parameters. Long-chain PAEs with a higher log Kow value have lower water solubility and vapour pressure and are unlikely to evaporate. In the case of moist soil, PAEs are moderately volatile and are not likely to bioaccumulate except in the case of DEHP. Low molecular weight (LMW) PAEs in a pure state have a high value of the air–water partitioning (log KAW) and hence evaporate more rapidly from water than in the aqueous form. Many PAEs absorb the aerosol particles due to the high value of octanol–air partitioning (log KOA). Biodegradation is more dominant in PAEs than hydrolysis and photolysis, which are proven to have gradual degradation in nature, such as the half-life for BBP is > 100 days, DMP is three years, and DEHP is 2000 years.
Since the 1930s, PAEs have been widely used in synthetic organic chemicals due to increased economic and commercial interests in agriculture, packaging materials, and personal care goods (Heo et al. 2019). Global production of PAEs has increased from 2.7 to 6 million tons per year (approximately) from 2007 to 2017 (Bu et al. 2020). Among various PAEs, compounds such as dibutyl phthalate (DnBP), di-iso-butyl phthalate (DBP), benzyl butyl phthalate (BBP), and di(2-ethyl-hexyl) phthalate (DEHP) are classified under reproductive toxicants (Class 1B). According to the United States Environment Protection Agency (USEPA) 2B and C group, BBP and DEHP are regarded as potential human carcinogens (Lee et al. 2019). The route of exposure in humans and biota is through dermal, ingestion, and inhalation. (Teil et al. 2016) detected 10-100 ng/m3 PAEs concentration in air of surrounding urban area, 74 µg/L of DEHP concentration in leachate, which was 1.7-56 times higher than recommended by the EU limit for surface water, i.e., 1.3 µg/L (Wowkonowicz and Kije 2017) and 4000 ng/L of PAEs in drinking water (Net et al. 2015).
The direct and indirect release of the PAEs into the environment risks human and wildlife species. PAEs mimic the normal homeostasis and estrogenicity (mediated by two estrogen receptors, alpha and beta) and disrupt the hypothalamic-pituitary-gonadal (HPG) axis pathway in vertebrates. A recent review by Mohammadi and Ashari reported the mechanism of toxicity associated with PAEs via mimicking on the nuclear surfaces (such as erythroid 2–related factor 2 (Nrf2), nuclear factor-κB (NF-κB), and phosphatidylinositol-3-kinase (PI3K)/AKT), resulting in the inexpression of genes, leading to alterations in cell hemostasis and toxic effects such as reproductive, cardio, hepato, nephro, teratogenicity, and tumor development (Mohammadi and Ashari 2021). It is evident that PAEs and their metabolites interact and disrupt several endocrine molecular signalling pathways, proving toxic (Kahn et al. 2020; Ribeiro et al. 2020). Agencies like USEPA have established the tolerated daily intake (TDI) (g/kg body weight per day) values for mono-methyl phthalates (3500 g/kg body weight per day) with PAEs (i.e., DEHP, BBP, DNBP, and DIBP) listed as “Substances of Very High Concern.” by the EU.
Parabens
Parabens are p-hydroxybenzoic acid alkyl esters (p-HBA) which are inert, odourless, tasteless, colourless, lipophilic, and stable in a wide pH range (Fransway et al. 2019; Nowak et al. 2020). Parabens have antimicrobial properties and are used in various processed and packaged foods, such as fruit juices, pickles, sauces, soft drinks, in cosmetic products like lotions, deodorants, hair gels, shampoos, creams, and toothpaste. Parabens are ubiquitous in environmental matrices, and the main sources of parabens in the aquatic environment have been identified as municipal wastewater that enters via sewage and industrial discharge, surface runoff via urban drainage, or atmospheric deposition. Moderately soluble in water and hence detected in the dissolved or sorbed in the form of sludge or organic-rich floating particles in WWTPs (Haman et al. 2014). Five WWTPs detected ∑6 parent parabens and ∑5 paraben metabolites in influent, effluent, and sludge samples, ranging from 131–920 ng/L, 16–67 ng/L, and 104–1090 ng/g dry weight (dw) in influent, effluent, and sludge samples, respectively, with a removal efficiency of 80–100%. While ∑5 Paraben metabolites ranged from 4110 to 34,600 ng/L in influent, 2560–3800 ng/L in effluent, and 1220–35,900 ng/g dw in sludge with 28–76% removal efficiency. The study signifies the presence of transformed products or metabolites are more dangerous than the original compounds (Karthikraj et al. 2017). Chen et al. studied 80 indoor dust samples for parabens in the U.S. due to personal care products with a maximum concentration of 140–39,090 ng/g (Chen et al. 2018).
Human and animal exposure to parabens is due to inhalation, ingestion, and dermal contact. The toxicity of parabens is debatable, as a recent review by Fransway et al. mentioned that parabens are transformed into a less toxic compound by the cutaneous carboxyesterases enzyme (Fransway et al. 2019). However, previous scientific evidence does depict the toxicity of parabens. Dobbins et al. studied the lethal concentration (LC50) of Daphnia magna and Pimephales promelas. Paraben concentrations of 0.12–25 mg/L showed negative growth in both species, causing toxicity in their normal body functioning. Other studies, such as Yamamoto et al. (2011), Tersaki et al., Nagar et al. (2020), and Gouukon et al. (2020), all reflect the toxicity of parabens on different species (Gouukon et al. 2020; Nagar et al. 2020). Humans observe several negative impacts on synthesis and metabolism. Parabens' interaction with various steroid-sensitive tissues causes malfunctioning the central nervous system and immune system (Raza et al. 2017). The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor employed as a toxin indicator, the activation of which by xenobiotics in the environment is called a toxicity sensor (Irigaray and Belpomme 2010). Gouukon et al. (2020) studied the AhR agonist activity of mono-brominated parabens mediating similar toxicity by polychlorinated biphenyls (PCBs) due to similar structures, supporting Sasaki and Terasaki's findings (Gouukon et al. 2020).
Bisphenol A
Bisphenol A (BPA), also known as 2,2-(4,4′-dihydroxyphenyl) propane, is an organic synthetic chemical with two phenol moieties linked together by functional groups in construction materials and consumer products, including plastics and resins. Even canned foods and beverages, medical equipment, toys, and thermal receipts contain it (Iribarne-durán et al. 2019; Molina-Molina et al. 2018). The European Chemicals Agency recently designated BPA as a reproductive toxin and a chemical of high concern (ECHA 2017). Many structural counterparts have been introduced to replace BPA, such as bisphenol S (BPS), bisphenol F (BPF), bisphenol AF (BPAF), bisphenol AP (BPAP), bisphenol Z (BPZ), and bisphenol B (BPB) which induce estrogenic and neurobehavioral disruption as BPA (Chen et al. 2016; Rosenfeld 2017; Soto et al. 2017).
The physicochemical properties of bisphenol A and analogues determine the fate and transportation in the environment. Bisphenol A is moderately soluble due to its two non-polar methyl groups. At the same time, its analogues range from low to high as presence of non-polar groups have equal distribution of electron charge between C–C and C–H bonds making then unreactive (i.e., poor polarity), resulting in decreased water solubility (BPP). In contrast, the vice-versa behaviour results in improved water solubility (BPS). Bisphenols tend to partition in soil, vegetation, and aerosols more than gases due to the moderate to high log Kow (Morin et al. 2015). Bisphenols being low volatile (H < 1.01 × 10−2 Pa·m3/mol) do not evaporate in the air. Bisphenol A and its mixture of analogues cause the breaking of the double DNA strands by up-regulation of CYP1A1 and UGT1A1 and CDKN1A and GADD45a genes and down-regulation of GST1A (Hercog et al. 2019). BPA is a known EDC to cause geno- and cytotoxicity in humans. USEPA has set a maximum TDI of 50 µg/kg body weight per day, and the Canadian government has also banned the use of BPA in the import and sale of BPA-containing bottles. Furthermore, the European Union replaced BPA with its equivalents, which are more dominant EDCs than bisphenol A (Pelch et al. 2019; Zhu et al. 2020).
Pharmaceuticals and personal care products
Many pharmaceuticals and personal care products (PPCPs) have been continuously found in aquatic habitats worldwide in recent decades. Antibiotics, analgesics, steroids, antidepressants, disinfectants, scents, and various other compounds are among the many chemicals used daily for various purposes. PPCPs are frequently used (pseudo persistent) to help improve the overall health and well-being of humans and animals, with a rare potential to cause physiological distress at low concentrations, making them dangerous enough to alter the biological processes (Ebele et al. 2020; Sangion and Gramatica 2016).
Anthropogenic activities such as sewage discharge, animal breeding, and leachate can release PPCPs into the environment, causing them to be present in surface water and groundwater at ng/L to mg/L concentrations. Continuous exposure to low, subtoxic doses of certain PPCPs has unforeseen repercussions and unintended impacts on non-target. Recent studies have indicated that drugs are present in all environmental matrices from 71 nations (Beek et al. 2016; Fischer et al. 2021; Patel et al. 2018). The physicochemical features of PPCPs determine their environmental route and movement through the aquatic ecosystem. Pollutants enter surface water by runoff from agriculture, rainfall, and direct waste discharge into water bodies. They then leach into the groundwater, adding to the pollution load in the system (Puri et al. 2022). There were concentrations of ofloxacin, norfloxacin, and azithromycin in Spain that ranged from 10.2 to 367 ng/L, BDL-2 ng/L in the aquifer (karst system), and BDL-68 ng/L in the Danube River, Serbia, respectively (Tijani et al. 2016). In 148 samples, caffeine and other stimulants were found in the urban catchment area of Singapore at a concentration of BDL-16249 ng/L with a detection frequency of 80–83% (Tri et al. 2016). In Lagos, Nigeria, drinking water samples included amoxicillin concentrations of 1614, 238 and 358 ng/L, respectively. With detection rates above 70%, acetaminophen, nicotine, ibuprofen, and codeine are some of the most frequently found drugs (Limousy et al. 2016). E1, E2, and EE2 are estrogenic chemicals found in low concentrations in Northern America and Europe, ranging from 0.1 to 12.5 ng/L, 0 to 5.5 ng/L, and 0.1 to 4.3 ng/L, respectively (Adeel et al. 2017).
PPCPs aim to maximize biological activity at low dosages while targeting specific metabolic, enzymatic, or cell-signalling pathways. The probability that these medications will be pharmacologically active in organisms other than the targets is increased by the evolutionary conservation of these molecular targets in a particular species. The concept of the mode of action (MoA) can be applied to any aquatic biota that unintentionally comes into contact with pharmaceuticals in its natural environment, raising the risk of ecotoxicological effects and what is sometimes referred to as antimicrobial resistance. The antidepressant fluoxetine, which targets the serotonin (5-HT) signalling system, was used to evaluate the MoA conceptual framework. Because 5-HT is a top-tier physiological controller in aquatic species, fluoxetine changes had many adverse effects on essential physiological activities in mussels, including reproduction, metabolism, and motility, at concentrations close to or even below environmental limits (Fabbri et al. 2016; Ford et al. 2018). The compounds diphenhydramine, thioridazine, sertraline, and dextropropoxyphene were shown to have the highest potential for acute toxicity in the populations of algae, invertebrates, and fish. The acute toxicity of analgesic drugs was shown to be relatively insensitive to bacteria, fish, and amphibians but most sensitive to phytoplankton and invertebrates. Another study investigated the sublethal and cytotoxic effects of PPCP, both singly and in combination, by measuring cell viability, oxidative stress, cellular senescence, and activation of CYP1A in the RTG-2 rainbow trout cell line. Cashmeran and galaxolide had EC50 values for the β-galactosidase assay of 40 µg/L, whereas most of the individual studied compounds had EC50 values of 150 µg/L or above. Contrarily, in this assay, the mixture of pharmaceuticals and EDCs had an experimentally observed EC50 value of 32.23 µg/L and a predicted EC50 value of 489.21 µg/L for β-galactosidase. (Fernández et al. 2012). Drinking water or ingesting organisms are two possible sources of human exposure to pharmaceuticals. Some PPCP molecules can persist in the drinking water system for extended periods (Liu et al. 2019). In recent investigations, drugs in urine have been studied, and the health hazards associated with them have been analyzed. The detection of many low-dose antibiotics in the urine of adults (21–75 years old) demonstrated health hazards caused by antibiotic-induced gut microbial disruption (Wang et al. 2018).
Impacts of EDCs
Impact of EDCs on humans
EDCs, when present in nanomolar or micromolar concentrations, activate receptors and change the body's feedback mechanism causing chromosomal instability. Figure 4 demonstrates that the hormones produced activate receptor cells where they bind to the target cell. Further, the EDCs attach to ligands, mimic the normal function, and changes occur in the process, leading to alterations in the transcription of the newly synthesized proteins. EDCs also interact with steroid hormones via distinct pathways. The membrane oestrogen receptor (mER) releases Ca2 + ions along with changes in prolactin secretion, which impairs immunological response and causes cell proliferation. Even receptors such as cAMP, kinases, and cyclases are impacted (Annamalai and Namasivayam 2015).
The in vivo test with 750–1000 mg/kg/day of DEP (0.71–4.6 µg/kg/day permissible limit) for 12–30 days causes testicular & epididymal atrophy, haemorrhagic testis, foetal death, respiratory distress, and pathological changes (Annamalai and Namasivayam 2015). A study by Yawer et al. (2020) reported that EDCs disrupt testicular homeostasis due to the testicular gap junctional intracellular communication (GJIC). Disruption in the early phase of steroidogenesis in prepubertal Leydig cells contributes to the prolonged damage to male reproductive health (Yawer et al. 2020). The effect of EDCs is not limited to the in vivo and in vitro studies but is also observed in infants because of the affected mothers. Since the infant is attached to the mother's umbilical cord, the transportation of compounds from the mother to the infant becomes easy, disrupting DNA-methylation. Compounds such as Bisphenol A (BPA) and phthalates are known for causing these disruptive effects (Montrose et al. 2018).
The severity of effects of EDCs is dependent on variable factors such as the age of exposure, modification of DNA genes, the delay between exposure and onset of symptoms, the concentration of EDCs, and low dose effect. EDCs exposure at a young age has its severe impacts evident in adulthood. Some substances are 100 times more potent in low concentration than in high (low-dose effect), and direct exposure to the same causes DNA mutations in generations (Kabir et al. 2015). Infants are vulnerable to getting affected through the umbilical cord of the mother. BPA and phthalates effects are sex-specific and affect the foetal liver enzymes, which control the β- oxidation process, responsible for the infant’s growth and normal homeostatic development. Due to BPA and phthalates in the first trimester, changes in DNA occur, disrupting the ability of gene expression and bringing toxic-epigenetic changes (Montrose et al. 2018).
Numerous experimental research data have substantiated the disruption in the growth, function, hormone production, action, and metabolism of mammalian endocrine systems due to the increase in the number of EDCs. Additionally, the results inferred from both animal and human model studies support the hypothesis that exposure to EDCs during foetal development and puberty causes an increase in cases of reproductive disorders, endocrine-related cancers, infections, asthma, and possibly obesity and diabetes, as well as behavioural and learning problems like attention-deficit/hyperactivity disorder (ADHD). They can afflict abnormalities up to 5–10% in babies and cause autism spectrum disorders up to 1% among children. Hence, it can be concluded that these complex non-communicable diseases have both a genetic and an environmental component (up to 24%) that contribute to the development of human diseases and disorders following exposure to EDCs (Bergman et al. 2012; Nuttal et al. 2013).
Impact of EDCs on biota
The effects of EDCs on the biota are different in terms of function, growth, reproduction, and metabolism. The unspecified sources, concentration, and biotransformation products of EDCs in the environmental matrices increase toxicity impacting the biota (Miller et al. 2019). Castro-català et al. (2016) studied a series of approaches to detect the ecotoxicological effects in the organism and identified the variables responsible for the toxicity in rivers. The organophosphate chlorpyrifos resulted in toxicity in Daphnia magna and mortality in Chironomus riparius as long-term toxicity (Castro-català et al. 2016). Exposure to EDCs during the critical developmental stages induces changes in both reproductive and non-reproductive organs. A study on the effect of BPA and Vinclozolin (Vz) on the terrestrial isopod Porcellio scaber (common rough woodlouse) was performed by Lemos et al. (2010). The chemicals affect the developmental toxicity in the species by decreasing the overall growth and inducing chronic sensitivity (Lemos et al. 2010). The estrogenic chemicals affect specific genes due to the absence of ER receptors in cells, resulting in ligand-independent gene activation, such as in octopus and sea slugs. Pesticides, namely pyriproxyfen (3–30 nM) and TCDD, lead to increased mortality in Eucypris virens (freshwater ostracod) and reproductive dysfunction in lake trout, respectively (Watanabe et al. 2018).
Wang et al. (2019) studied the endocrine disrupting effect of pesticide thiamethoxam and its metabolite clothianidin on gonads in Eremias argus (farmland lizard). The 28 days of exposure to the pesticide and its metabolite on the male and female farmland lizard led to the endocrine disruption of gonads by the up-regulation of cyp17 & cyp19 genes in testis and hsd17β gene in ovaries resulting in a decrease of testosterone levels with an increase in 17-estradiol concentration in plasma in males and increased levels of testosterone in plasma in females (Wang et al. 2019). EDCs alone and in a mixture creates a cocktail of effects in an intact organism with possible antiandrogen and antithyroid behaviour. The mixture tends to have more toxicity than the individual EDCs. Rainieri et al. (2017) studied the toxic effects of the perfluorinated compounds (PFCs), namely perfluoro-octane sulfonate (PFOS), perfluoro-octanic acid (PFOA), and perfluorononanoic acid (PFNA) in a human macrophage cell line (TLT cells) and zebrafish embryos. Depending on the concentration, the mixture of PFCs showed more acute toxicity than the individual compounds in the zebrafish (Rainieri et al. 2017).
Further, exposure to EDCs has compromised the endocrine system function in wildlife species, such as PCB contamination in the Baltic Sea, and high levels of POP observed in very high rates of reproductive failures and disruption in the thyroid and bone health in grey seals, respectively. Additionally, the antifouling agent tributyltin has disrupted mollusc sexual development worldwide. The most current research on PCB-exposed animals has revealed vital details on exposure levels, early and subclinical impacts, and the clinical neurotoxicity of these chemicals, shedding light on the mechanisms underlying the effects and results of EDCs exposure identical to those in humans. EDCs affect behaviour, fecundity, growth, survival, and immunological function, making vertebrates—especially marine mammals—more vulnerable to infectious illnesses (Bergman et al. 2012; Nuttal et al. 2013).
The data demonstrate that exposure to EDCs significantly impacts trends in wildlife and human health, as shown in Table 4.
Treatment methods
Several contaminants originating from industrial sources observe endocrine activity. Many issues about these compounds remain unanswered, and their long-term persistence makes them a significant hazard for future generations.
In nature, most EDCs are persistent, widespread, lipophilic, and bio-accumulative. They are hydrophobic and do not disintegrate quickly in the environment (Chen et al. 2019). Its widespread presence in the environment, even at low amounts, poses a threat. This section deals with the removal methods for the group of EDCs. Table 5 represents the currently available methods employed in degrading different categories of EDCs with their removal efficiencies obtained for various treatment processes at optimal conditions. While Fig. 5 signifies the mechanism of action involved in the discussed treatment methods for the target categories of EDCs.
Physical treatment
Coagulation/flocculation and adsorption are used to reduce suspended solids, ions, colloid particles, floating materials, oil and grease, colour, heavy metals, and other persistent and non-persistent substances. However, there are some downsides to these processes, including the production of sludge, the release of metal ions, the difficulty of regenerating adsorbents, the absence of suitable low-cost adsorbents, and the poor mineralization of harmful organic molecules into safe byproducts (Gu et al. 2021).
Coagulation/flocculation
In order to destabilize the colloidal dispersion and encourage agglomeration of the individual colloidal particles through charge neutralization, the process is carried out by adding various kinds of coagulants or floc-forming chemical reagents to the wastewater. Sedimentation is then performed after the sedimentation. In order to evaluate the removal of PAEs from landfill leachate, three coagulants—ferric chloride, aluminium sulphate, and polyaluminum chloride—were utilized. The results show that the coagulation-flocculation method simultaneously eliminates compounds with log KOW > 4 and dissolved organic matter with a removal rate better than 50%, while aluminium chloride only removes 30% (Bhatnagar and Sillanpää 2011). The processes are known to be ineffective for removing parabens and bisphenols (Barzegari et al. 2015; Tufail et al. 2020). Pharmaceuticals, namely acetaminophen, atenolol, antipyrine, indomethacin, roxithromycin, sulfamethoxazole, tiamulin, and trimethoprim, were all treated, with only indomethacin removed at better than 40% efficiency. Pollutant-specific parameters, such as the octanol–water partition coefficient (Kow) and the weak acid-hydrolysis coefficient (pKa), would influence the removal efficacy of coagulation-flocculation and sedimentation. The greater pKa value of a contaminant during treatment would help to exchange PPCPs into ionic states, where they may be easily adsorbed onto particles and the flocs generated in coagulation by electrostatic interactions (Vieno et al. 2007).
Higher Kow values are linked to increased hydrophobicity improving target contaminant removal through hydrophobic interactions. Kow had a lower contribution than pKa, to the removal efficiency of combined coagulation-flocculation and sedimentation, which is consistent with previous research that found Kow to be a limited and unstable predictor of the removal of some PPCPs, such as polar compounds, example, tiamulin (Kim et al. 2010).
Adsorption
Adsorbate builds up on a solid or liquid surface due to the surface phenomenon known as adsorption. The adsorbent's available surface area, or active sites, is the most important factor regulating the adsorption process. Two more important factors that determine the adsorption process are temperature and the medium's pH, which regulates the surface charge of adsorbents and the process's thermodynamics. Additionally, the adsorption of an organic molecule is governed by its chemical makeup and polarity. Moreover, the availability of different functional groups affects how much the chemical structure is involved. In the context of the octanol–water system, the partitioning coefficient KOW, a quantitative measure of polarity, establishes a molecule's hydrophobicity (lipophilicity). As a result, the substance's capacity to adsorb depends on the value of Log KOW (Gao et al. 2017).
Ester groups operate as electron acceptors when adsorbing PAEs with long alkyl chains from an aqueous medium, interacting with the adsorbent's electron donor to create the electron–donor–acceptor (EDA) interaction (Ye et al. 2020). Additionally, some functional groups with which the ester group can form hydrogen bonds can help the adsorption process. As a result, depending on the pH, phthalates interact with charged adsorbents through electrostatic attraction or repulsion (Lu et al. 2017; Gao et al. 2017). DEP sorption was studied on activated carbon isolated from the aqueous phase with a surface area of 500 m2/g and a geometric mean size of 70 mm. They studied, among other things, the effects of starting concentration, contact time, carbon dose, and operating pH. The trials showed that DEP was quickly eliminated and gradually decreased over time until equilibrium was established. The author came to the conclusion that the existence of active surface spots was responsible for the high initial removal rate. Additionally, the DEP concentration had an impact on the rate of adsorption. Using single-walled carbon nanotubes (SWCNTs) with outer diameters of 1–2 nm and MWCNTs (MWCNT10: outer diameters of 10–10 nm, MWCNT20: outer diameters of 10–20 nm, and MWCNT40: outer diameters of 10–40 nm) as an adsorbent, they have demonstrated the adsorption of three phthalate compounds (DMP, DEP, and DBP). Additionally, they found that the hydrophobicity of particular DEPs affects adsorption capacity in the following ways: DMP, DEP, and DBP. They also found that when CNTs' external diameter rose, the effectiveness of adsorption was reduced in the following ways: MWCNT10 > MWCNT20 > MWCNT40 SWCNT > MWCNT10 > MWCNT20 > MWCNT40 (Wang et al. 2015).
It has been extensively discussed how to eliminate parabens for maximum adsorption using magnetic nanoparticles. The parabens were eliminated using magnetite (PS/Fe3O4) covered in polystyrene (PS). As the alkyl groups on the paraben grew, PS/sorption Fe3O4's capacity increased. After five reuse cycles, the parabens' adsorption capacities for MeP, EtP, and PrP were 19.5%, 42.4%, and 63.7%, respectively, while their desorption capacities were 42.5%, 77.5%, and 99.7%. Since the parabens' adsorption on PS/Fe3O4 was mediated via electron-donor acceptor interactions, it was pH-independent. For methylparaben and propylparaben, the adsorbent demonstrated high adsorption capacities of 85.9 mg/g and 90.0 mg/g, respectively. The magnetic adsorbent remained constant for up to seven adsorption/desorption cycles before dropping to 87% (Chen et al. 2017). In a different study, a porous N grafted graphene-NiO (NiO@N-G) nanoparticle was utilized as an adsorbent to remove parabens. NiO was chosen as an ingredient in this adsorbent because it may prevent the aggregation of graphene layers by acting as a stabilizing agent. Methyl, ethyl, propyl, and butylparaben were all reduced by the NiO@N-G nanoparticle adsorbent by 39.2%, 56.6%, 69.5%, and 79.3%, respectively. An equilibrium between adsorption and desorption was attained after three hours. The adsorbent was stable for four adsorption/desorption cycles with 100% ethanol, and the desorption efficiency was 50.1%, 81.1%, 99.0%, and 99.9%, respectively. Paraben molecules are adsorbed by interactions through double bonds in parabens (Husein 2019).
As a BPA sorbent, ultrafine powdered activated carbon (SPAC) with a particle size of 1.38 ± 0.03 µm was used to study the breakdown of BPA by oxidized manganese (Mn) species. BPA was strongly sorbed by SPAC, necessitating the use of organic solvents to remove it. No degradation of adsorbed BPA (278.7 ± 0.6 mg BPA g–1 SPAC) was detected with synthetic, solid-MnO2 with a particle size of 15.41 ± 1.35 µm; however, an 89% mass reduction was noticed after the addition of 0.5 mM liquid Mn (III). Results from small-angle neutron scattering indicated that BPA adsorption and breakdown occurred in SPAC pores. The results contradict the widely held belief that contaminant desorption and diffusion out of pore structures are essential steps in degradation by demonstrating that Mn (III) triggered the oxidative transformation of both liquids and adsorbed BPA (Sun et al. 2021).
PPCPs adsorption characteristics differ from chemical to compound and are difficult to anticipate due to interactions with specific functional groups or intricate pH-dependent speciation. Clofibric acid, ibuprofen, naproxen, triclosan, diclofenac, and bisphenol A sorptive and degradation properties in 4 US agricultural soils with varied physicochemical parameters were studied. The Freundlich equation could represent the adsorption of all compounds studied in soils, and their adsorption affinities on soil were as follows: Triclosan > bisphenol A > clofibric acid > naproxen > diclofenac > ibuprofen, according to a batch equilibrium test. According to the retardation factor, triclosan and bisphenol A were easily delayed in soils, while ibuprofen might travel downhill with percolating water (RF). Selected PPCPs had half-lives in soils that ranged from 0.81 to 20.44 days, and they degraded using the kinetics of first-order exponential decay. The amount of organic matter and clay in the soil affected how quickly PPCP degraded. The removal of triclosan, ibuprofen, acetaminophen, carbamazepine, caffeine, prometryn, carbendazim, and 4-Acetylamino-antipyrine by multiwalled nanotubes is efficient. As feeding concentration decreased, the elimination of PPCPs increased. Through hydrogen bonding, multiwalled nanotubes absorbed acetaminophen. Additionally, they found that larger inner diameter carbon nanotubes did not outperform smaller inner diameter carbon nanotubes in the adsorption competition between PPCPs and the fulvic acid in the river (Wan and Wang 2016). Physical treatment alternatives are efficient in removing the set mentioned above of substances, but they do not accomplish total removal, necessitating the development of more effective options.
Chemical treatment or advanced oxidation processes
The shortcomings of conventional wastewater treatment plants, including incomplete organic pollutant breakdown, sludge formation, secondary pollutant generation, and high costs, are eliminated by chemical oxidation or advanced oxidation processes (AOPs). The generation of highly reactive species, notably the hydroxyl radical (·OH), which can degrade organic pollutants in water and cause their mineralization, is necessary for the AOP processes (Zanias et al. 2020).
Ultraviolet irradiation and Photocatalysis
The principal degradation process of PAEs is believed to be the hydrolytic photolysis of the carbon in the and-position of the ester chain. PAEs were photo-degraded using 254 nm UV in a relatively rapid and orderly manner. The photolysis of PAEs were examined, numerous studies have employed artificial irradiation sources such as mercury lamps, Xenon arc lamps, and UV light. However, there is little information on the utilization of natural UV irradiation, such as sunlight (Mailhot et al. 2002; Rastkari et al. 2018). After 28 days of natural UV irradiation, less than 5% BBP was degraded (Gledhill et al. 1980). In the photocatalysis process, the utilization of ROS in the degradation of PAEs is explained below.
Hollow glass microspheres (HGM) and magnetic poly (methyl methacrylate) (mPMMA) were utilized as catalyst support structures by Jiang et al. (2013) to improve filtration and catalyst recovery, respectively. In both experiments, DMP was successfully broken down; however, Jiang et al. reported a 20–60% improvement in removal when Pt was added. Additionally, the PtTiO2/mPMMA composite material was verified to be functional in numerous experimental runs after being recovered (Jiang et al. 2013). Huang et al. (2013) employed TiO2 immobilized on hydrophobic layered double hydroxides to create a support structure that might enhance the procedure and increase substrate adsorption (LDHs). LDHs were shown to have significant adsorption characteristics, combined with photocatalytic degradation via TiO2, for the first time to eliminate DMP (Huang et al. 2013). Another typical technique for degrading parabens is the use of photosensitizers. This method combined benzyl-, n-butyl-, propyl-, ethyl-, and methylparabens with tetrasulfonic acid and aluminium phthalocyanine as photosensitizers, a light source to break them down. Singlet oxygen has been shown to degrade when photosensitizers are applied under light and produce singlet oxygen. In a study, singlet oxygen generated from tetraphenylporphyrin, zinc phthalocyanine, and electrospun nanofibers degraded the parabens butyl and benzyl (Gmurek and Olak-kucharczyk 2015). An oxygen stream was used to facilitate the reaction, and benzylparaben and butylparaben degradation was monitored with a UV diode array detector and high-performance liquid chromatography. It was found that the rate of decay increases as the amount of photosensitizer used for the reaction also does. When exposed to UV light, the parabens may undergo de-esterification, hydroxylation, or breakdown (Gmurek and Olak-kucharczyk 2015; Gryglik and Gmurek 2015). For photocatalytic destruction of BPA, the TiO2 nanoparticles have been examined. Zhao et al. reported a 91.2% treatment efficacy using a titania/titanate (TiO2/TNTs) composite for BPA breakdown. However, several investigations have been devoted to the photocatalytic oxidation of BPA using graphitic carbon nitride (g-C3N4). BPA in surface waters was transformed into chlorinated intermediates using solar photodegradation (Ohore and Zhang 2019). Photolysis occurs when light (natural or artificial) interacts with a molecule, causing photochemical processes that result in the molecule's disintegration into intermediate products and, eventually, complete mineralization. As in case of 17α-ethinylestradiol (EE2), diclofenac, sulfamethoxazole, and iopromide. In dilute solutions of buffered water undergoing photolysis at pH 7.0, only minor removals (0.4–27%) were observed. Alone UV exposure of 2768 mJ/cm2 in 15 min, several macrolide antibiotics (clarithromycin, azithromycin, and erythromycin) was degraded with a low 4–7% efficiency (Hu and Oakes 2015). For usage in a slurry reactor with 385 nm LEDs, Hossaini et al. (2014) created and studied brand-new TiO2-based catalysts doped with N, NS, FeNS, and FeFNS. The outcomes showed that FeFNS-doped TiO2 exhibited the best photocatalytic activity for the mineralization and degradation of diazinon, making it a promising material for use in real applications (Hossaini et al. 2014). Under flow-through UVA LED/TiO2 conditions, the antibiotics sulfamethoxazole and trimethoprim decomposed in Milli Q-water, causing significant chronic toxicity with reduced residual antibacterial action and no acute toxicity (Cai and Hu 2016).
Oxidation using sulfate radicals (SO4.−·) and hydroxyl radical (OH·)
The substrates' chemical characteristics strongly influence \({\text{SO}}_{4}^{ - \cdot }\) and degradation OH· efficiency. In theory, the non-selective actions of the OH· and \({\text{SO}}_{4}^{ - \cdot }\)· radicals on organic and organometallic contaminants in the aqueous medium lead to their total mineralization into CO2, water, and inorganic ions. The four main ways that hydroxyl radicals assault organic pollutants are radical addition, hydrogen abstraction, electron transfer, and radical combination. When interacting with organic molecules, they produce radicals (R· or R·–OH) containing a carbon nucleus. With the aid of oxygen, these carbon-centred radicals can be transformed into organic peroxyl radicals (ROO·). These organic molecules experience chemical degradation and even mineralization due to all of the radicals' further reactions, which form more reactive species like H2O2 and super oxide (O2·) (Qi 2010). Sulfate radicals are highly reactive substances with a limited life period, similar to hydroxyl radicals, although they react differently. Hydroxyl radicals can either contribute to C=C bonds or abstract H from C–H bonds when interacting with organic molecules. On the other hand, sulfate radicals frequently absorb electrons from organic molecules, leading to the creation of organic radical cations. It should be noted that sulphate radicals can also form hydroxyl radicals as follows:
Studies such as Fang et al. (2015) studied the 3 stages mechanism of action by producing OH· radical from biochar suspensions: (a) In biochar suspensions, free radicals (FRs) transfer electrons to O2 to make \({\text{O}}_{{2^{ \cdot } }}^{ - }\) (b) \({\text{O}}_{{2^{ \cdot } }}^{ - }\) receives an electron from FRs or dismutates to form H2O2 (c) H2O2 combines with FRs to form OH· radical via a single-electron transfer process (Fang et al. 2015). Wang et al. (2020) studied that the primary radical in the CuS/PMS system was found to be hydroxyl radical (·OH), which performed better than an alternate ·OH-based oxidation system (CuS/H2O2) for DEP degradation than sulphate radical-based PMS activation procedures. Furthermore, anions such as Cl− and \({\text{NO}}_{3}^{ - }\) have only a minor inhibitory effect on DEP breakdown (Wang et al. 2020).
Biological degradation and membrane technology
The biological degradation process is more or more important than the AOPs. Microbes are accessible to culture, have more removal efficiency and have estrogenic potency for the complex compounds. Consortia of microbes can be more effective and target many xenobiotic compounds under the same conditions, with less toxic intermediates and no more chemicals in the environment.
Many experiments successfully remove and reduce estrogenic potency and its presence in the environment. Maximum studies are conducted with fungi mainly used due to their intracellular enzymes. Compounds like triclosan, 4-nonylphenol, nonylphenol, BPA, and 17-α-ethinylestradiol (EE2) were studied for biodegradation by eight different strains of fungi (Cajthaml and Kr 2009). All eight strains could degrade BPA and nonylphenol entirely within three days of incubation. In EE2, the degradation achieved was 60% with P.magnoliae, and estrogenic potential was reduced entirely. Bacteria like Bacillus sp, Sphingomonas bisphenolicum, Pseudomonas sp, etc., have also been used. Sphingomonas bisphenolicum has been regarded as a novel pathway for BPA degradation (Chouhan et al. 2013). The bacterial strain was isolated for the vegetable field. It was the DNA-DNA hybridized strain that made it unique for the removal. 100 mg/L concentration of BPA was degraded aerobically in 10 days by the bacterial strain. Majorly P450 is responsible for degrading the BPA and coenzymes like NAD+, NADH, NADPH, and NADPH+. The drawback of the strain was that the intermediate of 4,4'-dihydroxy-α-methylstilbene (DHMS) was formed with a similar structure (Chouhan et al. 2013).
Algae Monoraphidium braunii was tested for the complete removal of the BPA. Non-living organic matter (NOM) is added to algae to enhance degradation. The removal efficiency of BPA + NOM addition in aqueous solution concentrations of 2, 4, and 10 mg/L obtained was 39, 48 and 35%. A study showed an increase in removal efficiency in aerobic conditions than in anaerobic conditions (Gattullo et al. 2012).
Due to their high efficiency, ability to conserve space, and simplicity of use, membrane filtration techniques using different types of membranes have much potential for eliminating EDCs. The pollutant that can be retained depends on the membrane's pore size, surface charge, and hydrophobicity, which vary depending on the material used to make it. Pore size, hydrophobicity, functional groups, pKa of membrane materials and pollutants, and the quality of the water treated are a few of the many factors that affect removal. Despite the large pore size of the microfiltration membrane, the combined system successfully removed almost all of the endocrine disruptors from the effluent (Rodriguez et al. 2017). Three membranes were used to study the degradation of DEP and DEHP: the RO-DS3SE reverse osmosis membrane, the NF-DS5DK nanofiltration membrane, and the UF-DSGM ultrafiltration membrane. Under regulated conditions (RO, NF 2.0 MPa, UF 0.3 MPa), all membranes eliminated a sizable portion of PAEs from water, varying from 97.6% to nearly 99.9% (Miriyam et al. 2022). Similar or higher quantities of these compounds were found in ultrafiltration (UF) effluent with 3.1–9.9% removal efficiency compared to paraben concentrations in secondary effluent. Because the UF membrane pores are more significant than the molecules of target compounds and the high pH variations. This could be explained because parabens are challenging to retain by size exclusions (Li et al. 2015).
It could be concluded that EDCs may still be present in final effluents depending on various factors, such as the physicochemical properties (pKa, Kow) of the target pollutants and wastewater characteristics, despite using conventional and advanced oxidation processes. Physical treatments are usually pH-dependent interactions such as electrostatic and speciation specific that can remove EDCs up to 97%. At the same time, chemical or advanced oxidation processes have the potential to deal with the chemical properties of substrates, resulting in the breaking down of the parent molecule more quickly for the effective degradation of EDCs. The drawback of using these processes is forming residual breakdown products that tend to cause chronic toxicity. Hence, biodegradation is critical in efficiently mineralizing the parent and residual degraded products into carbon dioxide and water. In an environmentally and economically sustainable manner, the employment of microorganisms can benefit the degradation of broad classes of EDCs, either as individual compounds or in combination.
Research in the last few years has concentrated chiefly on modelling AOP remediation of wastewater without considering strategies combining them with biological treatments. Based on the current state of the art, emphasis must be laid on the need for combined treatment methods focussing on oxidizing the persistent or non-biodegradable components chemically to yield biodegradable byproducts, which the microbes can further take up to achieve enhanced or complete mineralization. When designing or modelling the combined treatment methods at optimal operating parameters, the focus must be on the effects of waste characteristics, flow rates, reactor volumes, organic and inorganic loads, and reactor configurations to achieve maximum removal degradation efficiency. In addition to modelling, generic measures like chemical oxygen demand (COD) and total organic carbon (TOC) lack specificity in presenting the whole wastewater treatment. Nevertheless, these parameters are essential, and ecotoxicity testing could be used as an alternative to assess mineralization.
Though the aforementioned factors are significant when working on combined treatment processes, a thorough economic evaluation of the proposed process that promotes environmental sustainability should still be carried out before implementation. Additionally, the microbes used in biodegradation must adhere to biosafety norms while prioritizing public health.
Recommendations and future directions
It is evident from data reported in the papers and scientific assessments by WHO and UNEP that these low-concentration substances pose a risk to people and the environment. More than 1,000 compounds have been recognized as endocrine disrupting properties. The UN-IPCC is monitoring the most recent version of 2021 lists 45 chemicals and 77 after the global meeting on July 5, 2018. The USEPA has stated a framework for legal limits and estimated daily dietary intakes (EDI) for a small number of substances, such as BPA and phthalates, among others. Additionally, toxicity tests for the intermediates or byproducts from degradation studies are required. In order to meet the Sustainable Development Goals (SDGs) 2050, water should be safe and clean; the biota above and below should not be in danger. Small levels of substances that are present in groundwater, drinking water, and the air must be treated. Even minute amounts of a substance taken continuously can have physiological, neurological, and endocrinological effects that completely dominate the usual homeostatic regulation systems. Thus, the promise of improved public health welfare is essential. It is recommended to frequently monitor the release of these man-made toxins and investigate these substances posing significant physicochemical properties with sophisticated instruments in quantification and detection. Studies on EDCs in various matrices with potential removal or reduction treatment processes must be investigated for cleaner, economic, and sustainable development.
The emission of EDCs can be controlled by the mechanism of ‘downstream regulations’ that focuses on the inclusion of the harmful chemicals used in production in industrial settings and their presence in final products, as well as in the environment and waste. Though EDCs are addressed in many circumstances under European Union (EU) law, including the Water Framework Directive, Registration, Evaluation and Authorization of Chemicals (REACH), Plant Protection Products Regulation (PPPR), and Cosmetic Regulation, even though there is no consensus on their regulation. Therefore, the government and regulatory authorities can focus on enforcing rigorous compliance with EDCs across diverse industries. However, the fundamental problem is that the wide range of chemicals generated are not evaluated for endocrine disrupting properties, nor are their combination interactions and ensuing consequences studied.
Conclusion
EDCs are ubiquitous and disrupt the hormonal-receptor activity affecting humans' reproductive and non-reproductive organs. Rapid industrialization and the increasing demand for drugs lead to the discharge of excretes and secretions in the environment in metabolites and other unknown conjugates. It is crucial to comprehend the genetic and epigenetic pathways with differing phenotypic responses of vertebrates and invertebrates to provide a unique perspective in toxicological investigations. The experimental set-up is crucial when determining the quantity and length of human exposure to EDCs. The mixture of EDCs causes severe malfunctions even at low concentrations; hence, it is vital to comprehend the genotoxic effect on humans' HPA and thyroid axis. Data availability and a universal approach to regulating the production and the release of chemicals is the way forward. The ensurity of the legislation will significantly impact the environment and vulnerability. Screening of hazardous chemicals can aid research in achieving SDG 2050.
References
Abbasi Y, Mannaerts CM (2020) Exploring the environmental exposure to methoxychlor, α -HCH and endosulfan – sulfate residues in lake Naivasha (Kenya) using a multimedia fate modeling approach. Int J Environ Res Public Health 17:1–18. https://doi.org/10.3390/ijerph17082727
Abderrazik NB, Azman A, R’kiek C, Song W, O'Shea KE (2005) Iron (II)-catalyzed enhancement of ultrasonic-induced degradation of diethylstilbestrol (DES). Catal today 101:369–373. https://doi.org/10.1016/j.cattod.2005.03.012
Adeel M, Song X, Wang Y, Francis D, Yang Y (2017) Environmental impact of estrogens on human, animal and plant life : a critical review. Environ Int 99:107–119. https://doi.org/10.1016/j.envint.2016.12.010
Annamalai J, Namasivayam V (2015) Endocrine disrupting chemicals in the atmosphere : their effects on humans and wildlife. Environ Int 76:78–97. https://doi.org/10.1016/j.envint.2014.12.006
Aslam M, Alam M, Rais S (2013) Detection of atrazine and simazine in ground water of Delhi using high performance liquid chromatography with ultraviolet detector. Curr World Environ 8:323–329. https://doi.org/10.12944/CWE.8.2.21
Baloyi ND, Tekere M, Maphangwa KW (2021) Insights into the prevalence and impacts of phthalate esters in aquatic ecosystems. Front Environ Sci 9:1–19. https://doi.org/10.3389/fenvs.2021.684190
Barzegari Z, Bina B, Pourzamani HR, Ebrahimi A (2015) The combined treatment of bisphenol A (BPA) by coagulation / flocculation (C / F) process and UV irradiation in aqueous solutions. Desalin Water Treat. https://doi.org/10.1080/19443994.2015.1030706
Bedoux G, Roig B, Thomas O, Dupont V, Bot BL (2012) Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ Sci Pollut Res 19:1044–1065. https://doi.org/10.1007/s11356-011-0632-z
Beek TAD, Weber F-A, Bergmann A, Hickmann S, Ebert I, Hein A, Kuster A (2016) Pharmaceuticals in the environment: global occurrences and perspectives. Environ Toxicol Chem 35:823–835. https://doi.org/10.1002/etc.3339
Bergman A, Heindel JJ, Jobling S, Kidd KA, Zoeller RT (2012) State of the science of endocrine disrupting chemicals
Beshaa AT, Gebreyohannes AY, Tufa RA, Bekele DN, Curcio E, Giorno L (2017) Removal of emerging micropollutants by activated sludge process and membrane bioreactors and the effects of micropollutants on membrane fouling: a review. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2017.04.027
Bhatnagar A, Sillanpää M (2011) A review of emerging adsorbents for nitrate removal from water. Chem Eng J 168:493–504. https://doi.org/10.1016/j.cej.2011.01.103
Bu S, Wang Y, Wang H, Wang F, Tan Y (2020) Analysis of global commonly-used phthalates and non-dietary exposure assessment in indoor environment. Build Environ. https://doi.org/10.1016/j.buildenv.2020.106853
Cai Q, Hu J (2016) Decomposition of sulfamethoxazole and trimethoprim by continuous UVA / LED / TiO 2 photocatalysis : decomposition pathways, residual antibacterial activity and toxicity. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2016.06.006
Cajthaml T, Kr C (2009) Biodegradation of endocrine-disrupting compounds by ligninolytic fungi: mechanisms involved in the degradation. Environ Microbiol. https://doi.org/10.1111/1462-2920.12460
Cao X (2010) Phthalate esters in foods : sources, occurrence, and analytical methods. Comp Rev Food Sci Food Saf. https://doi.org/10.1111/j.1541-4337.2009.00093.x
Castro-català ND, Kuzmanovic M, Roig N, Sierra J, Ginebreda A, Barceló D, Pérez S, Petrovic M, Picó Y, Schuhmacher M, Muñoz I (2016) Ecotoxicity of sediments in rivers : Invertebrate community, toxicity bioassays and the toxic unit approach as complementary assessment tools. Sci Total Environ 540:297–306. https://doi.org/10.1016/j.scitotenv.2015.06.071
Celi M, Skrbi BD, Insa S, Zivan J, Petrovi M (2020) Occurrence and assessment of environmental risks of endocrine disrupting compounds in drinking, surface and wastewaters in Serbia. Environ Pollut 262:114–344. https://doi.org/10.1016/j.envpol.2020.114344
Chen D, Kannan K, Tan H, Feng Y, Wu Y, Widelka M (2016) Bisphenol analogues other than BPA : environmental occurrence, human exposure, and toxicity—a review. Environ Sci Technol. https://doi.org/10.1021/acs.est.5b05387
Chen H, Chiou C, Chang S (2017) Comparison of methylparaben, ethylparaben and propylparaben adsorption onto magnetic nanoparticles with phenyl group. Powder Technol. https://doi.org/10.1016/j.powtec.2017.01.060
Chen H, Wang C, Li H, Ma R, Yu Z, Li L, Xiang M, Chen X, Hua X, Yu Y (2019) A review of toxicity induced by persistent organic pollutants (POPs) and endocrine-disrupting chemicals (EDCs) in the nematode Caenorhabditis elegans. J Environ Manage 237:519–525. https://doi.org/10.1016/j.jenvman.2019.02.102
Chen J, Hartmann EM, Wymelenberg KV, Den H, R.U., (2018) Assessment of human exposure to triclocarban, triclosan and fi ve parabens in U. S. indoor dust using dispersive solid phase extraction followed by liquid chromatography tandem mass spectrometry. J Hazard Mater 360:623–630. https://doi.org/10.1016/j.jhazmat.2018.08.014
Choi KJ, Kim SG, Kim CW, Kim SH (2005) Effects of activated carbon types and service life on removal of endocrine disrupting chemicals : amitrol, nonylphenol, and bisphenol-A. Chemosphere 58:1535–1545. https://doi.org/10.1016/j.chemosphere.2004.11.080
Chouhan S, Yadav SK, Prakash J, Singh PS (2013) Effect of Bisphenol A on human health and its degradation by microorganisms : a review. Annu Microbiol. https://doi.org/10.1007/s13213-013-0649-2
Clara M, Windhofer G, Hartl W, Braun K, Simon M, Gans O, Scheffknecht C, Chovanec A (2010) Occurrence of phthalates in surface runoff, untreated and treated wastewater and fate during wastewater treatment. Chemosphere 78:1078–1084. https://doi.org/10.1016/j.chemosphere.2009.12.052
Das MT, Kumar SS, Ghosh P, Shah G, Malyan SK, Bajar S, Thakur IS, Singh L (2021) Remediation strategies for mitigation of phthalate pollution: challenges and future perspectives. J Hazard Mater 409:124496. https://doi.org/10.1016/j.jhazmat.2020.124496
Dhillon GS, Kaur S, Pulicharla R, Brar SK (2015) Triclosan : current status, occurrence, environmental risks and bioaccumulation potential. Environ Res Public Heal 12:5657–5684. https://doi.org/10.3390/ijerph120505657
Ebele AJ, Oluseyi T, Drage DS, Harrad S, Abdallah MA (2020) Occurrence, seasonal variation and human exposure to pharmaceuticals and personal care products in surface water, groundwater and drinking water in Lagos State, Nigeria. Emerg Contam 6:124–132. https://doi.org/10.1016/j.emcon.2020.02.004
ECHA (2017) Substance name: 4, 4 ’ -isopropylidenediphenol (Bisphenol A; BPA) EC Number: 201–245–8 CAS Number: 80–05–7 member state committee support document (bisphenol a, bpa) as a substance of very high concern because of its endocrine disrupting propert
Encarnação T, Pais AACC, Campos MG, Burrows HD (2019) Endocrine disrupting chemicals : impact on human health, wildlife and the environment. Sci Prog 102:3–42. https://doi.org/10.1177/0036850419826802
Esplugas S, Bila DM, Gustavo L, Krause T (2007) Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J Hazard Mater 149:631–642. https://doi.org/10.1016/j.jhazmat.2007.07.073
Fabbri E, Franzellitti S (2016) Human pharmaceuticals in the marine environment : focus on exposure and biological effects in animal species. Environ Toxicol Chem 35:799–812. https://doi.org/10.1002/etc.3131
Fang G, Zhu C, Dionysiou DD, Gao J, Zhou D (2015) Mechanism of hydroxyl radical generation from biochar suspensions : Implications to diethyl phthalate degradation. Bioresour Technol 176:210–217. https://doi.org/10.1016/j.biortech.2014.11.032
Farounbi AI (2020) Occurrence of selected endocrine disrupting compounds in the eastern cape province of South Africa. Environ Sci Pollut Res 27:17268–17279. https://doi.org/10.1007/s11356-020-08082-y
Feijoo G, Moreira MT, Lema JM (2011) Degradation of selected pharmaceutical and personal care products (PPCPs) by white-rot fungi. World J Microb Biotechnol 27:1839–1846. https://doi.org/10.1007/s11274-010-0642-x
Fernández C, Carbonell G, Babín M (2012) Effects of individual and a mixture of pharmaceuticals and personal-care products on cytotoxicity, EROD activity and ROS production in a rainbow trout gonadal cell line (RTG-2). J Appl Toxicol. https://doi.org/10.1002/jat.2752
Fischer AJ, Kerr L, Sultana T, Metcalfe CD (2021) Effects of opioids on reproduction in Japanese medaka. Oryzias Latipes Aquat Toxicol 236:105873. https://doi.org/10.1016/j.aquatox.2021.105873
Ford AT, Hyett B, Cassidy D, Malyon G, Ford AT (2018) The effects of fluoxetine on attachment and righting behaviours in marine (Gibbula unbilicalis) and freshwater (Lymnea stagnalis) gastropods they highlight that since the mode of action of. Ecotoxicology. https://doi.org/10.1007/s10646-018-1919-3
Franka A, Seitz W, Prasse C, Lucke T, Schulz W, Ternes T (2016) Occurrence and fate of amisulpride, sulpiride, and lamotrigine in municipal wastewater treatment plants with biological treatment and ozonation. J Hazard Mater 320:204–215. https://doi.org/10.1016/j.jhazmat.2016.08.022
Fransway AF, Fransway PJ, Belsito DV, Warshaw EM, Sasseville D, Fowler JF, Dekoven JG, Pratt MD, Maibach HI, Taylor JS, Marks JG, Mathias CGT, Deleo VA, Zirwas JM, Zug KA, Atwater AR, Silverberg J, Reeder MJ (2019) Parabens. Am Contact Dermat Soc. https://doi.org/10.1097/DER.0000000000000429
Gao M, Zhang Y, Gong X, Song Z, Guo Z (2017) Removal mechanism of di- n -butyl phthalate and oxytetracycline from aqueous solutions by nano-manganese dioxide modified biochar. Environ Sci Pollut Res
Gangadharan P, Veetil P (2012) Degradation of triclosan under aerobic, anoxic, and anaerobic conditions. Appl Biochem Biotechnol 167:1603–1612. https://doi.org/10.1007/s12010-012-9573-3
Gattullo CE, Bährs H, Steinberg CEW, Loffredo E (2012) Removal of bisphenol A by the freshwater green alga Monoraphidium braunii and the role of natural organic matter. Sci Total Environ 416:501–506. https://doi.org/10.1016/j.scitotenv.2011.11.033
Giang CND, Sebesvari Z, Renaud F, Rosendahl I (2015) Occurrence and dissipation of the antibiotics trimethoprim, and enrofloxacin in the mekong delta. Vietnam Plos One. https://doi.org/10.1371/journal.pone.0131855
Gledhill WE, Kaley RG, Adams WJ, Hicks O, Michael PR, Saeger VW (1980) An environmental safety assessment of butyl benzyl phthalate. Environ Sci Technol 14:301–305. https://doi.org/10.1021/es60163a001
Gmurek M, Olak-kucharczyk M (2015) Influence of dissolved organic matter in natural and simulated water on the photochemical decomposition of butylparaben. J Environ Heal Sci Eng 13:2–8. https://doi.org/10.1186/s40201-015-0185-z
Golden R, Gandy J (2005) A review of the endocrine activity of parabens and implications for potential risks to human health. Crit Rev Toxicol 35:435–458. https://doi.org/10.1080/10408440490920104
Gouukon Y, Yasuda MT, Yasukawa H, Terasaki M (2020) Occurrence and AhR activity of brominated parabens in the Kitakami. Chemosphere 249:126152. https://doi.org/10.1016/j.chemosphere.2020.126152
Gryglik D, Gmurek M (2015) The photosensitized oxidation of mixture of parabens in aqueous solution. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-014-4059-1
Gu X, Qin N, Wei G, Hu Y, Zhang Y, Zhao G (2021) Efficient photocatalytic removal of phthalates easily implemented over a bi-functional 001 TiO 2 surface. Chemosphere 263:1–11. https://doi.org/10.1016/j.chemosphere.2020.128257
Haman C, Dauchy X, Rosin C (2014) Occurrence, fate and behavior of parabens in aquatic environments : a review. Water Res 8:1–11. https://doi.org/10.1016/j.watres.2014.09.030
Hamid N, Junaid M, Pei D (2021) Combined toxicity of endocrine-disrupting chemicals : a review. Ecotoxicol Environ Saf 215:112136. https://doi.org/10.1016/j.ecoenv.2021.112136
Heo H, Choi M-J, Park J, Nam T, Cho J (2019) Anthropogenic occurrence of phthalate esters in beach seawater in the Southeast Coast Region, South Korea. Water 12:1–13. https://doi.org/10.3390/w12010122
Hercog K, Maisanaba S, Filipi M, Sollner-dolenc M, Ka L, Bojana Ž (2019) Genotoxic activity of bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF and their mixtures in human hepatocellular carcinoma (HepG2) cells. Sci Total Environ 687:267–276. https://doi.org/10.1016/j.scitotenv.2019.05.486
Holbrook RD, Love NG, Novak JT (2004) Sorption of 17 -estradiol and 17 r -ethinylestradiol by colloidal organic carbon derived from biological wastewater treatment systems. Environ Sci Technol 38:3322–3329. https://doi.org/10.1021/es035122g
Hossaini H, Moussavi G, Farrokhi M (2014) The investigation of the LED-activated FeFNS- TiO 2 nanocatalyst for photocatalytic degradation and mineralization of organophosphate pesticides in water. Water Res 59:130–144. https://doi.org/10.1016/j.watres.2014.04.009
Hu A, Oakes KD (2015) Removal of pharmaceuticals and personal care products from wastewater. J Environ Manag. https://doi.org/10.1016/j.jenvman.2016.07.049
Huang C, Hong L, Guo W, Liu Q, Shi L, Guo Y (2018) Occurrence and ecological risk assessment of eight endocrine: disrupting chemicals in urban river water and sediments of South China. Arch Environ Contam Toxicol 75:224–235. https://doi.org/10.1007/s00244-018-0527-9
Huang Z, Wu P, Lu Y, Wang X, Zhu N, Dang Z (2013) Enhancement of photocatalytic degradation of dimethyl phthalate with nano-TiO 2 immobilized onto hydrophobic layered double hydroxides : a mechanism study. J Hazard Mater 246–247:70–78. https://doi.org/10.1016/j.jhazmat.2012.12.016
Husein DZ (2019) Facile one-pot synthesis of porous N-doped graphene based NiO composite for parabens removal from wastewater and its reusability. Desalin Water Treat 166:211–221. https://doi.org/10.5004/dwt.2019.24339
Ingham RR, Gesualdi DA, Toth CR, Clotfelter ED (2004) Effects of genistein on growth and development of aquatic vertebrates. Environ Contam Toxicol 72:625–631. https://doi.org/10.1007/s00128-004-0289-0
Iribarne-durán LM, Artacho-cordón F, Peña-caballero M, Molina-molina JM, Jiménez-díaz I, Vela-soria F, Serrano L, Hurtado JA, Fernández MF, Freire C, Olea N (2019) Presence of bisphenol a and parabens in a neonatal intensive care unit : an exploratory study of potential sources of exposure. Environ Heal Perspect. https://doi.org/10.1289/EHP5564
Ismail NAH, Weea SY, Nitty Hirawaty Kamarulzamanc AZA (2019) Occurrence of endocrine disrupting compounds in mariculture sediment of Pulau Kukup, Johar, Malaysia. Mar Pollut Bull. https://doi.org/10.1016/j.marpolbul.2019.110735
Irigaray P, Belpomme D (2010) Basic properties and molecular mechanisms of exogenous chemical carcinogens. Carcinogenesis 31:135–148. https://doi.org/10.1093/carcin/bgp252
Jafari AJ, Abasabad RP, Salehzadeh A (2009) Endocrine disrupting contaminants in water resources and sewage in Hamadan City of Iran. J Environ Heal Sci Eng 6:89–96
Jiang W, Joens JA, Dionysiou DD, Shea KEO (2013) Optimization of photocatalytic performance of TiO 2 coated glass microspheres using response surface methodology and the application for degradation of dimethyl phthalate. "Journal Photochem. Photobiol A Chem 262:7–13. https://doi.org/10.1016/j.jphotochem.2013.04.008
Kabir ER, Rahman MS, Rahman I (2015) A review on endocrine disruptors and their possible impacts on human health. Environ Toxicol Pharmacol 40:241–258. https://doi.org/10.1016/j.etap.2015.06.009
Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L (2020) Endocrine-disrupting chemicals : implications for human health. LANCET Diabetes Endocrinol 8:703–718. https://doi.org/10.1016/S2213-8587(20)30129-7
Karthikraj R, Vasu AK, Balakrishna K, Sinha RK, Kannan K (2017) Occurrence and fate of parabens and their metabolites in five sewage treatment plants in India. Sci Total Environ 593–594:592–598. https://doi.org/10.1016/j.scitotenv.2017.03.173
Kasonga TK, Coetzee MAA, Kamika I, Ngole-jeme VM, Momba MNB (2021) Endocrine-disruptive chemicals as contaminants of emerging concern in wastewater and surface water : a review. J Environ Manage 277:111485. https://doi.org/10.1016/j.jenvman.2020.111485
Kassotis CD, Vandenberg LN, Demeneix BA, Porta M, Slama R, Trasande L (2020) Endocrine-disrupting chemicals 2 Endocrine-disrupting chemicals : economic, regulatory, and policy implications. LANCET Diabetes Endocrinol 8:719–730. https://doi.org/10.1016/S2213-8587(20)30128-5
Kim SH, Shon HK, Ngo HH (2010) Adsorption characteristics of antibiotics trimethoprim on powdered and granular activated carbon. J Ind Eng Chem 16:344–349. https://doi.org/10.1016/j.jiec.2009.09.061
Lauretta R, Sansone A, Sansone M, Romanelli F, Appetecchia M (2019) Endocrine disrupting chemicals : effects on endocrine glands. Front Endocrinol 10:1–7. https://doi.org/10.3389/fendo.2019.00178
Lee Y, Lee J, Choe W, Kim T, Lee J, Kho Y (2019) Distribution of phthalate esters in air, water, sediments, and fish in the Asan Lake of Korea. Environ Int 126:635–643. https://doi.org/10.1016/j.envint.2019.02.059
Lefebvre C (2016) Presence, bioconcentration and fate of galaxolide and tonalide fragrances in the North Saskatchewan River, Edmonton Claudine Lefebvre
Lemos MFL, Gestel CAMV, Soares AMVM (2010) Developmental toxicity of endocrine disrupters bisphenol a and vinclozolin in a terrestrial isopod. Arch Environ Contam Toxicol 59:274–281. https://doi.org/10.1007/s00244-010-9474-9
Leusch FDL et al (2005) Efficacy of an advanced sewage treatment plant in southeast queensland, australia, to remove estrogenic chemicals. Environ Sci Technol 39:5781–5786. https://doi.org/10.1021/es0484303
Li W, Shi Y, Gao L, Liu J, Cai Y (2015) Occurrence, fate and risk assessment of parabens and their chlorinated derivatives in an advanced wastewater treatment plant. J Hazard Mater 300:29–38. https://doi.org/10.1016/j.jhazmat.2015.06.060
Limousy L, Ghouma I, Ouederni A, Jeguirim M (2016) Amoxicillin removal from aqueous solution using activated carbon prepared by chemical activation of olive stone. Environ Sci Pollut Res 24:9993–10004. https://doi.org/10.1007/s11356-016-7404-8
Lin Y, Peng Z, Zhang X (2009) Ozonation of estrone, estradiol, diethylstilbestrol in waters. Desalination 249:235–240. https://doi.org/10.1016/j.desal.2008.06.034
Lind PM, Lind L (2018) Endocrine-disrupting chemicals and risk of diabetes: an evidence-based review. Diabetologia. https://doi.org/10.1007/s00125-018-4621-3
Liu Z-H, Zhi D, Liu Y (2021) Legislation against endocrine-disrupting compounds in drinking water : essential but not enough to ensure water safety. Environ Sci Pollut Res 28:19505–19510. https://doi.org/10.1007/s11356-021-12901-1
Liu M, Yin H, Wu Q (2019) Occurrence and health risk assessment of pharmaceutical and personal care products (PPCPs) in tap water of Shanghai. Ecotoxicol Environ Saf 183:109497. https://doi.org/10.1016/j.ecoenv.2019.109497
Liu Z, Kanjo Y, Mizutani S (2008) Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatment—physical means, biodegradation, and chemical advanced oxidation : a review. Sci Total Environ 407:731–748. https://doi.org/10.1016/j.scitotenv.2008.08.039
Lu J, Wu J, Zhang C, Zhang Y (2020) Possible effect of submarine groundwater discharge on the pollution of coastal water: Occurrence, source, and risks of endocrine disrupting chemicals in coastal groundwater and adjacent seawater influenced by reclaimed water irrigation. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.126323
Lu L, Wang J, Chen B (2017) Adsorption and desorption of phthalic acid esters on graphene oxide and reduced graphene oxide as affected by humic acid *. Environ Pollut. https://doi.org/10.1016/j.envpol.2017.09.078
Lv X, Xiao S, Zhang G, Jiang P, Tang F (2016) Occurrence and removal of phenolic endocrine disrupting chemicals in the water treatment processes. Nat Publ Gr. https://doi.org/10.1038/srep22860
Mailhot G, Sarakh M, Lavedrine B, Caceres J, Malato S (2002) Fe (III) -solar light induced degradation of diethyl phthalate (DEP) in aqueous solutions. Chemosphere 49:525–532. https://doi.org/10.1016/S0045-6535(02)00418-6
Miller TH, Tiong K, Bury ST, Bury SE, Bury NR, Barron LP (2019) Biomonitoring of pesticides, pharmaceuticals and illicit drugs in a freshwater invertebrate to estimate toxic or effect pressure. Environ Int 129:595–606. https://doi.org/10.1016/j.envint.2019.04.038
Miriyam IB, Anbalagan K, Kumar MM (2022) Phthalates removal from wastewater by different methods: a review. Water Sci Technol 85:2581–2600. https://doi.org/10.2166/wst.2022.133
Mizuno H, Hirai ÆH (2009) Removal of estrogenic activity of iso-butylparaben and n-butylparaben by laccase in the presence of 1-hydroxybenzotriazole. Biodegradation 20:533–539. https://doi.org/10.1007/s10532-008-9242-y
Mohammadi H, Ashari S (2021) Mechanistic insight into toxicity of phthalates, the involved receptors, and the role of Nrf2, NF- κ B, and PI3K / AKT signaling pathways. Environ Sci Pollut Res 28:35488–35527. https://doi.org/10.1007/s11356-021-14466-5
Molina-Molina JM, Fernández MF, Peinado FM, Mustieles V, Barouki R, Piccoli C, Olea N, Freire C (2018) Determination of bisphenol A and bisphenol S concentrations and assessment of estrogen- and anti-androgen-like activities in thermal paper receipts from Brazil, France, and Spain. Environ Res. https://doi.org/10.1016/j.envres.2018.12.046
Montrose L, Padmanabhan V, Goodrich JM, Domino SE, Treadwell MC, Meeker JD, Watkins DJ, Dolinoy DC (2018) Maternal levels of endocrine disrupting chemicals in the first trimester of pregnancy are associated with infant cord blood DNA methylation. Epigentics. https://doi.org/10.1080/15592294.2018.1448680
Morin N, Arp HPH, Hale SE (2015) Bisphenol A in solid waste materials, leachate water, and air particles from norwegian waste-handling facilities: presence and partitioning behavior. Environ Sci Technol. https://doi.org/10.1021/acs.est.5b01307
Mukhopadhyay M et al (2020) Plasticizers and bisphenol A in Adyar and Cooum riverine sediments, India : occurrences, sources and risk assessment. Environ Geochem Heal. https://doi.org/10.1007/s10653-020-00516-3
Na S, Jinhua C, Cui M, Khim J (2012) Ultrasonics Sonochemistry Sonophotolytic diethyl phthalate (DEP) degradation with UVC or VUV irradiation. Ultrason Sonochemistry 19:1094–1098. https://doi.org/10.1016/j.ultsonch.2011.10.004
Nagar Y, Singh R, Parveen T (2020) Toxicity assessment of parabens in Caenorhabditis elegans. Chemosphere 246:125730. https://doi.org/10.1016/j.chemosphere.2019.125730
Nagulapally SR, Ahmad A, Henry A, Marchin GL (2009) Occurrence of ciprofloxacin-, vancomycin-resistant bacteria in a municipal wastewater treatment plant. Water Environ Res. https://doi.org/10.2175/106143008X304596
Net S, Sempe R, Delmont A, Paluselli A, Ouddane B (2015) Occurrence, fate, behavior and ecotoxicological state of phthalates in different environmental matrices. Enviornmental Sci Technol 49:4019–4035. https://doi.org/10.1021/es505233b
Nowak K, Jabłońska E, Ratajczak-wrona W (2020) Controversy around parabens: alternative strategies for preservative use in cosmetics and personal care products. Environ Res. https://doi.org/10.1016/j.envres.2020.110488
Nuttal N, Thomas G, Osseiran N (2013) Effects of human exposure to hormone-disrupting chemicals examined in landmark UN report. World Heal Organ 1
Oguz AR, Kankaya E (2013) Determination of selected endocrine disrupting chemicals in Lake Van. Turkey Bull Environ Contam Toxicol 91:283–286. https://doi.org/10.1007/s00128-013-1036-1
Ohore OE, Zhang S (2019) Endocrine disrupting effects of bisphenol A exposure and recent advances on its removal by water treatment systems. A Review. Sci African. https://doi.org/10.1016/j.sciaf.2019.e00135
Patel M, Kumar R, Kishor K, Mlsna T, Pittman CU, Mohan D (2018) Pharmaceuticals of emerging concern in aquatic systems : chemistry, occurrence, effects, and removal methods. Chem Rev. https://doi.org/10.1021/acs.chemrev.8b00299
Peijnenburg WJGM, Struijs J (2006) Occurrence of phthalate esters in the environment of the Netherlands. Ecotoxicol Environ Saf 63:204–215. https://doi.org/10.1016/j.ecoenv.2005.07.023
Pelch K, Wignall JA, Goldstone AE, Ross PK, Blain RB, Shapiro AJ, Holmgren SD, Hsieh J, Svoboda D, Auerbach SS, Parham FM, Masten SA, Walker V, Rooney A, Thayer KA (2019) A scoping review of the health and toxicological activity of bisphenol A (BPA) structural analogues and functional alternatives. Toxicology 424:152235. https://doi.org/10.1016/j.tox.2019.06.006
Pertunia G, Mpupa A, Nqombolo A, Dimpe KM, Nomngongo PN (2020) Recyclable magnetic waste tyre activated carbon-chitosan composite as an effective adsorbent rapid and simultaneous removal of methylparaben and propylparaben from aqueous solution and wastewater. J Water Process Eng 33:101011. https://doi.org/10.1016/j.jwpe.2019.101011
Prasad B, Suresh S (2012) Biodegradation of dimethyl phthalate, diethyl phthalate, dibutyl phthalate and their mixture by Variovorax Sp. Int J Environ Sci Dev 3:283–288. https://doi.org/10.1016/j.apcbee.2012.03.004
Przybylinska PA, Wyszkowski M (2016) Environmental contamination with phthalates and its impact on living organisms. Ecol Chem Eng S 23:347–356. https://doi.org/10.1515/eces-2016-0024
Puri M, Gandhi K, Kumar MS (2022) Distribution of emerging contaminants, and antimicrobial resistance: occurrence, toxicity, risk assessment, and removal. https://doi.org/10.1007/978-981-19-1847-6
Qi Y (2010) Ozonation of water and waste water : a practical guide to understanding ozone and its applications. Int J Environ Stud. https://doi.org/10.1080/00207233.2010.517628
Radwan EK, Ibrahim MBM, Ahmed A, Farouk M (2020) The occurrence and risk assessment of phenolic endocrine-disrupting chemicals in Egypt’s drinking and source water. Environ Sci Pollut Res 27:1776–1788. https://doi.org/10.1007/s11356-019-06887-0
Rainieri S, Conlledo N, Sala M, Barranco A (2017) Toxic effects of perfluorinated compounds at human cellular level and on a model vertebrate. Food Chem Toxicol. https://doi.org/10.1016/j.fct.2017.02.041
Rastkari N, Jeddi MZ, Yunesian M, Ahmadkhaniha R (2018) Effect of sunlight exposure on phthalates migration from plastic containers to packaged juices. J Environ Heal Sci Eng 16:27–33. https://doi.org/10.1007/s40201-018-0292-8
Ratola N, Cincinelli A, Alves A, Katsoyiannis A (2012) Occurrence of organic microcontaminants in the wastewater treatment process. A mini review. J Hazard Mater 239–240:1–18. https://doi.org/10.1016/j.jhazmat.2012.05.040
Raza N, Kim K, Abdullah M, Raza W, Brown RJC (2017) Recent developments in analytical quantitation approaches for parabens in human-associated samples. Trends Anal Chem. https://doi.org/10.1016/j.trac.2017.11.009
Ribeiro CM, Teles B, Beserra S, Silva NG, Lima CL, Roberta P, Rocha S, Coelho MS, Assis FD, Neves R, Amato AA (2020) Exposure to endocrine-: disrupting chemicals and anthropometric measures of obesity : a systematic review and meta- analysis. BMJ Open. https://doi.org/10.1136/bmjopen-2019-033509
Rizzo L, Fiorentino A, Grassi M, Attanasio D, Guida M (2015) Advanced treatment of urban wastewater by sand filtration and graphene adsorption for wastewater reuse : effect on a mixture of pharmaceuticals and toxicity. J Environ Chem Eng 3:122–128. https://doi.org/10.1016/j.jece.2014.11.011
Rodriguez O, Peralta-hernandez JM, Goonetilleke A, Bandala ER (2017) Treatment technologies for emerging contaminants in water: a review. Chem Eng J. https://doi.org/10.1016/j.cej.2017.04.106
Rosenfeld CS (2017) Neuroendocrine disruption in animal models due to exposure to bisphenol A analogues. Front Neuroendocrinol. https://doi.org/10.1016/j.yfrne.2017.08.001
Salgado R, Marques R, Noronha JP, Carvalho G (2012) Assessing the removal of pharmaceuticals and personal care products in a full-scale activated sludge plant. Environ Sci Pollut Res 19:1818–1827. https://doi.org/10.1007/s11356-011-0693-z
Samaras VG, Stasinakis AS, Mamais D, Thomaidis NS, Lekkas TD (2013) Fate of selected pharmaceuticals and synthetic endocrine disrupting compounds during wastewater treatment and sludge anaerobic digestion. J Hazard Mater 244–245:259–267. https://doi.org/10.1016/j.jhazmat.2012.11.039
Sangion A, Gramatica P (2016) Ecotoxicity interspecies QAAR models from Daphnia toxicity of pharmaceuticals and personal care products. SAR QSAR Environ Res. https://doi.org/10.1080/1062936X.2016.1233139
Sarmah AK, Northcott GL, Leusch FDL, Tremblay LA (2006) A survey of endocrine disrupting chemicals (EDCs) in municipal sewage and animal waste effluents in the Waikato region of New Zealand. Sci Total Environ 355:135–144. https://doi.org/10.1016/j.scitotenv.2005.02.027
Shareef A, Angove MJ, Wells JD, Johnson BB (2006) Sorption of bisphenol A, 17 α -ethynylestradiol and estrone to mineral surfaces. J Colloid Interface Sci 297:62–69. https://doi.org/10.1016/j.jcis.2005.10.039
Shen C, Zuo Z (2020) Zebrafish (Danio rerio) as an excellent vertebrate model for the development, reproductive, cardiovascular, and neural and ocular development toxicity study of hazardous chemicals. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-10800-5
Snyder SA, Adham S, Redding AM, Cannon FS, Decarolis J, Oppenheimer J, Wert EC, Yoon Y (2007) Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 202:156–181. https://doi.org/10.1016/j.desal.2005.12.052
Srivastava A, Sharma VP, Tripathi R, Kumar R, Patel DK, Kumar P (2010) Occurrence of phthalic acid esters in Gomti River Sediment, India. Environ Monit Assessment. https://doi.org/10.1007/s10661-009-1182-4
Soo Y, Hye K, Lee R, Won H, Kim J (2010) Biodegradation of bisphenol A and its halogenated analogues by Cunninghamella elegans ATCC36112. Biodegradation 21:989–997. https://doi.org/10.1007/s10532-010-9358-8
Soto AM, Schaeberle C, Maier MS, Sonnenschein C, Ma MV (2017) Evidence of absence: estrogenicity assessment of a new food- contact coating and the bisphenol used in its synthesis. Environ Sci Technol. https://doi.org/10.1021/acs.est.6b04704
Sun Y, Im J, Shobnam N, Fanouraksi SK, He L, Anovitz LM, Erickson PR, Sun H, Zhuang J, Loffler FE (2021) Degradation of adsorbed bisphenol A by soluble Mn(III). Environ Sci Technol 55:13014–13023. https://doi.org/10.1021/acs.est.1c03862
Tamagawa Y, Hirai H, Kawai S, Nishida T (2005) Removal of estrogenic activity of endocrine-disrupting genistein by ligninolytic enzymes from white rot fungi. FEMS Microbiol Lett 244:93–98. https://doi.org/10.1016/j.femsle.2005.01.023
Teil M, Moreau-guigon E, Blanchard M, Alliot F, Mandin C, Moukhtar S, Gasperi J, Cladi M, Chevreuil M (2016) Endocrine disrupting compounds in gaseous and particulate outdoor air phases according to environmental factors. Chemosphere 146:94–104. https://doi.org/10.1016/j.chemosphere.2015.12.015
Tijani JO, Fatoba OO, Babajide OO, Petrik LF (2016) Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants : a review. Environ Chem Lett 14:27–49. https://doi.org/10.1007/s10311-015-0537-z
Tri TM, Anh DH, Hoai PM, Minh NH, Nam VD (2016) Emerging endocrine disrupting chemicals and pharmaceuticals in vietnam : a review of environmental occurrence and fate in aquatic and indoor environments. https://doi.org/10.1021/bk-2016-1244.ch010
Tufail A, Price WE, Mohseni M, Pramanik BK, Hai FI (2020) A critical review of advanced oxidation processes for emerging trace organic contaminant degradation : mechanisms, factors, degradation products, and effluent toxicity. J Water Process Eng. https://doi.org/10.1016/j.jwpe.2020.101778
Vieno NM, Harkki H, Tuhkanen T, Kronberg L (2007) Occurrence of pharmaceuticals in river water and their elimination in a pilot-scale drinking water treatment plant. Environ Sci Technol 41:5077–5084. https://doi.org/10.1021/es062720x
Wan Z, Wang J (2016) Ce-Fe-reduced graphene oxide nanocomposite as an efficient catalyst for sulfamethazine degradation in aqueous solution. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-016-7051-0
Wang H, Yang J, Yu X, Zhao G, Zhao Q, Wang N, Jiang Y, Jiang F, He G, Chen Y, Zhou Z, Jiang Q (2018) Exposure of Adults to Antibiotics in a Shanghai Suburban Area and Health Risk Assessment: a Biomonitoring-Based Study. Environ Sci Technol 52:13942–13950. https://doi.org/10.1021/acs.est.8b03979
Wang I, Karmaus WJJ, Chen S, Holloway JW, Ewart S (2015) Effects of phthalate exposure on asthma may be mediated through alterations in DNA methylation. Clin Epigenetics 7:1–9. https://doi.org/10.1186/s13148-015-0060-x
Wang X, Ding Y, Dionysiou DD, Liu C, Tong Y, Gao J, Fang G, Zhou D (2020) Efficient activation of peroxymonosulfate by copper sul fi de for diethyl phthalate degradation : performance, radical generation and mechanism. Sci Total Environ 749:142387. https://doi.org/10.1016/j.scitotenv.2020.142387
Wang Y, Zhang Y, Zeng T, Li W, Yang L, Guo B (2019) Accumulation and toxicity of thiamethoxam and its metabolite clothianidin to the gonads of Eremias argus. Sci Total Environ 667:586–593. https://doi.org/10.1016/j.scitotenv.2019.02.419
Watanabe H, Oda S, Abe R, Tanaka Y (2018) Chemosphere Comparison of the effects of constant and pulsed exposure with equivalent time-weighted average concentrations of the juvenile hormone analog pyriproxyfen on the reproduction of Daphnia magna. Chemosphere 195:810–816. https://doi.org/10.1016/j.chemosphere.2017.12.124
Wee SY, Aris AZ (2018) Occurrence and public-perceived risk of endocrine disrupting compounds in drinking water. Npj Clean Water. https://doi.org/10.1038/s41545-018-0029-3
Wowkonowicz P, Kije M (2017) Phthalate release in leachate from municipal landfills of central Poland. PLoS. https://doi.org/10.1371/journal.pone.0174986
Xin F, Susiarjo M, Bartolomei MS (2015) Multigenerational and transgenerational effects of endocrine disrupting chemicals: a role for altered epigenetic regulation? Semin Cell Dev Biol. https://doi.org/10.1016/j.semcdb.2015.05.008
Yamamoto H, Tamura I, Hirata Y, Kato J, Kagota K, Katsuki S, Yamamoto A, Kagami Y, Tatarazako N (2011) Aquatic toxicity and ecological risk assessment of seven parabens : individual and additive approach. Sci Total Environ 410–411:102–111. https://doi.org/10.1016/j.scitotenv.2011.09.040
Yang Y, Sik Y, Kim K, Kwon EE, Fai Y (2017) Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water / sewage treatment plants : a review. Sci Total Environ 596–597:303–320. https://doi.org/10.1016/j.scitotenv.2017.04.102
Yawer A, Sychrova E, Laboha P, Raska J, Jambor T, Babica P, S.I., (2020) Endocrine-disrupting chemicals rapidly affect intercellular signaling in Leydig cells. Toxicol Appl Pharmacol. https://doi.org/10.1016/j.taap.2020.115177
Ye H, Zhao B, Zhou Y, Du J, Huang M (2020) Recent Advances in Adsorbents for the Removal of Phthalate esters from Water : material, modification, and application. Chem Eng J. https://doi.org/10.1016/j.cej.2020.128127
Ye X, Guo X, Cui X, Zhang X, Zhang H, Wang MK, Chen S (2012) Occurrence and removal of endocrine-disrupting chemicals in wastewater treatment plants in the Three Gorges Reservoir area, Chongqing, China. J Environ Monit 14:2204–2211. https://doi.org/10.1039/c2em30258f
Zanias A, Frontistis Z, Vakros J, Arvaniti OS, Ribeiro RS (2020) Degradation of methylparaben by sonocatalysis using a Co–Fe magnetic carbon xerogel. Ultrason Sonochem 64:105045. https://doi.org/10.1016/j.ultsonch.2020.105045
Zhang Z, Feng Y, Gao P, Wang Ce, Ren N (2011) Occurrence and removal efficiencies of eight EDCs and estrogenicity in a STP. J Environ Monit 13:1366–1373. https://doi.org/10.1039/c0em00597e
Zhou X, Peng F, Luo Z, Li Y, Li H, Yang Z (2019) Assessment of water contamination and health risk of endocrine. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.135277
Zhu X, Wu G, Xing Y, Wang C, Yuan X, Li B (2020) Evaluation of single and combined toxicity of bisphenol A and its analogues using a highly-sensitive micro-biosensor. J Hazard Mater 381:120908. https://doi.org/10.1016/j.jhazmat.2019.120908
Acknowledgments
Authors are thankful to the Director, CSIR-NEERI, Nagpur, India, for providing encouragement, and kind permission for publishing the article.
Funding
No funding from any sources is involved in producing the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed in formulating the design and the concept of the review paper. MP contributed to conceptualization, writing—original draft, review and editing, data presentation; KG and MSK contributed to conceptualization, review and editing, and supervision.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Editorial responsibility: Lifeng Yin.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Puri, M., Gandhi, K. & Suresh Kumar, M. A global overview of endocrine disrupting chemicals in the environment: occurrence, effects, and treatment methods. Int. J. Environ. Sci. Technol. 20, 12875–12902 (2023). https://doi.org/10.1007/s13762-022-04636-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04636-4