Abstract
Chemical oxidation as a pretreatment step coupled with bioremediation for petroleum-contaminated soil may pose serious impacts on indigenous microorganisms and the available nutrients. Petroleum-contaminated soil were treated by hydrogen peroxide (H2O2) at initial concentrations of 105 mM (HH), 21 mM (HL), and 105 mM in three equal amounts (HT) without adding any external catalyst. The contents of total petroleum hydrocarbons (TPH) and dissolved nutrients (total organic compounds, nitrogen, and phosphate), and the indigenous bacteria community succession (analyzed by high-throughput sequencing of 16S rDNA) were investigated over 50 days. Compared to the control treatment without H2O2 addition, H2O2 treatments for the petroleum-contaminated soil significantly promoted the TPH removal especially in the first 4 days and impacted the contents of dissolved nutrients. Both of chemical oxidation and nutrients contributed to microbial community structure changes in alpha diversity. Although the soil microbial community structure had undergone significant changes after different chemical oxidation pretreatments, Firmicutes, Proteobacteria, Gemmatimonadetes, and Actinobacteria were the main bacterial phyla. Compared with adding H2O2 at one time, H2O2 added in stepwise was beneficial to indigenous bacterial diversity recovery and TPH removal. H2O2 oxidation treatments showed a great influence on the microbial community structures in the start-up stage, while recovery time rather than the oxidation treatments presented greater effects on the composition of the microbial community structure with the incubation time extended. Therefore, adding H2O2 as pretreatment for petroleum-contaminated soil showed little effect on the structure of soil indigenous microbial community from a long-term scale, and was conducive to the continuous removal of TPH by indigenous microorganisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil contaminated with petroleum oil and its derivatives is a major concern worldwide, especially in oilfields in China. In the process of oil extraction, transportation, refining, and use, soil pollution problems caused by leakage or improper transportation are becoming increasingly serious (Liu et al. 2018). Petroleum pollutants would change the structure and properties of the soil, disrupt the soil ecological balance, and affect the composition and structure of soil microbial communities (Lim et al. 2016).
In situ bioremediation (ISB) is one of the primary methods for the treatment of oil-contaminated soil due to the relatively low costs and no risks of causing secondary pollution (Sutton et al. 2014b). When using microbial technology to remediate the soil, the removal efficiency of petroleum hydrocarbons is closely related to soil microbial activity, the number of degrading bacteria, soil microbial community structure, and the soil flora structure (Shahi et al. 2016). However, biological-based remediation technologies require long time spans and are limited by various subsurface conditions and contaminant types. In most cases, indigenous bacteria are difficult to degrade the petroleum hydrocarbons efficiently in oil-contaminated soil (French et al. 2020).
In situ chemical oxidation (ISCO) can rapidly degrade organic pollutants by adding strong oxidants to the organic polluted soil. ISCO could improve biodegradability by reducing the toxicity and concentration of pollutants (Lu et al. 2010), and by increasing the availability of pollutants (Biache et al. 2015). Therefore, applying ISCO in petroleum-contaminated soil could reduce the biotoxicity of petroleum hydrocarbons and increase bioavailability, and thus promote the ISB process. However, the oxidative components produced during ISCO could inactivate microorganisms, and affect the structure of microbial communities (Martinez-Pascual et al. 2015). In addition, ISCO would change soil pH and the availability of nutrients to impact the growth and metabolic activity of microorganisms (Xu et al. 2016, Medina et al. 2018). The increase of mobilized nutrients can improve the biodegradation of total petroleum hydrocarbons (TPH) (Sutton et al. 2014a). Moreover, the recovery period of indigenous bacteria required 30 to 60 days after ISCO treatment (Pardo et al. 2014).
H2O2 as an oxidant for ISCO not only can oxidatively decompose hydrocarbon pollutants, but also may enhance the activity of microorganisms by releasing dissolved oxygen to promote the growth and reproduction of microorganism. When treating diesel-contaminated soil with H2O2 (1–5%, w/w) pre-oxidation and ISB, the cost was reduced by 27–40% compared with ISCO alone (Chen et al. 2016). Fenton pre-oxidation with low concentrations of H2O2 at stepwise addition can significantly reduce the damage to indigenous bacteria (Xu et al. 2011, 2016). However, previous studies did not consider the effect of stepwise addition of oxidant on indigenous bacterial community structure. Moreover, little attention has been paid to the responses of the soil indigenous bacteria to petroleum contamination and further oxidation treatment. Besides, a plenty of iron oxides in soil can be used as the activator of H2O2 rather than the addition of FeSO4 or other catalysts into soil.
This study aims to evaluate the effects of adding H2O2 at different dosages and stepwise addition as peroxidation process into a simulated newly contaminated soil with one-time exposure to crude oil on the removal of petroleum hydrocarbons and the succession of indigenous bacterial community in 50 days of incubation. The mobilized nutrients like dissolved total organic compound (DOC), dissolved total nitrogen (TN), and dissolved total phosphorus (TP) were determined. The bacterial diversity and the relationships among the bacterial community and soil environmental factors were studied with a metagenomic analysis of bacterial communities via high-throughput sequencing of the 16S rDNA gene.
Materials and methods
Soil sample contaminated with crude oil
The simulated oil spill-contaminated soil was prepared by artificially mixing crude oil with fresh soil from a woodland surface soil (0–20 cm) near Southwest Petroleum University (in Chengdu, Sichuan, China). Briefly, soil after air dried, grounded, and sieved through a 0.5-mm mesh was spiked with petroleum crude oil (from an oil well in Liaohe, Liaoning, China) dissolved in carbon tetrachloride, and the carbon tetrachloride was allowed to evaporate, providing a uniform TPH concentration determined as 4957.77 ± 63.83 mg/kg. The physico-chemical properties of the soil samples were summarized previously (Yang et al. 2019) with available Fe, available Mn, \({\mathrm{NH}}_{4}^{+}\)-N, and available P at 694, 9.9, 7.42, and 17.9 mg \(\cdot\) kg−1, respectively, and organic matter at 30.0 g \(\cdot\) kg−1.
Chemicals
Liquid hydrogen peroxide (30% by weight), magnesium silicate, potassium iodide, anhydrous sodium sulfate, and potassium persulfate were all of analytical grade and purchased from Chengdu Kelong Chemical Reagent Factory. Carbon tetrachloride (guaranteed reagent) was purchased from Tianjin Aoran Fine Chemical Research Institute. All solutions were prepared in ultrapure water (resistivity > 18.2 MΩ·cm).
Experimental design
Experiments were conducted in 150-mL conical flasks. The abovementioned petroleum-contaminated soil (5 g), oxidant (5 mL), and ultrapure water (5 mL) were added sequentially. Flasks were placed on a shaking water bath with slow speed in dark at 25 °C. Plastic wrap was used to seal the flasks to reduce moisture loss. Samples were extracted on days 0, 2, 4, 10, 30, and 50. The concentration of residual oxidants, pH, DOC, TN, and TP in solution, and the content of TPH and the microbial community structure in soil were analyzed. All experiments were conducted in triplicate.
H2O2 dosages and stepwise addition treatments were as follows: (a) 5 mL of ultrapure water in one step, termed control check treatment (CK), (b) 5 mL of H2O2 (42 mM) in one step, termed low concentration H2O2 (HL), (c) 5 mL of H2O2 (210 mM) in one step, termed high concentration H2O2 (HH), and (d) 5 mL of H2O2 (210 mM) in three equal amounts at 0, 24, and 48 h of the reaction (HT).
Extraction and analysis
Solid–liquid separation
The petroleum-contaminated soil slurry in the flask was transferred to a 50-mL centrifuge tube. Rinsing the flask twice with 10 mL of ultrapure water and transferring the solutions to the centrifuge tube were to ensure that the soil sample was transferred as completely as possible. The samples were shaken and mixed, then placed in a centrifuge (LC-LX-H185C, LICHEN, Shanghai, China) at 6000 rpm for 10 min. The soil sample after centrifugation was mixed with another 10 mL of ultrapure water and then centrifuged, and the above steps were repeated twice. All the supernatants were collected and filtered through a 0.45-µm membrane into colorimetric tube. The volume was adjusted to 50 mL for measurement of pH, DOC, TN, and TP.
Nutrients extraction and analysis
DOC was determined on a TOC-VCPH total organic carbon analyzer. TN was determined by the National Standard of the People’s Republic of China (GB/T 11,894–1989 Basic potassium persulfate digestion UV spectrophotometry). TP was determined by the National Standard of the People’s Republic of China (GB/T11893-89 Water quality total phosphorus molybdenum antimony spectrophotometry and potassium persulfate digestion chromatography). pH was measured by using a pH meter (PHS-3C, Rex Electric Chemical, Shanghai, China).
TPH extraction and analysis
The slurry sample in the centrifuge tube was transferred to a crucible, and the centrifuge tube was washed twice with 10 mL of pure water. The cleaning solution was transferred to the crucible, dried at 50 ℃, and ground to determine the TPH content. Carbon tetrachloride was used as the extractant. Ten milliliters of carbon tetrachloride was added with 1 g of petroleum-contaminated dry soil (quantitatively weighed) in a centrifuge tube. The mixture was ultrasonically washed for 15 min, and then centrifuged at 6000 rpm for 10 min. The supernatant solution was collected. The above extracting steps were repeated by two times. All the collected supernatant solutions were filtered through a sand core funnel, and then passed through anhydrous sodium sulfate (drying in a pre-baking oven at 105 ℃ for 2 h) to remove water. The final volume of the extracted oil solution was adjusted to 50 mL. TPH (3-mL extracts) was analyzed using infrared oil analyzer (determination of concentration of petroleum products in soil by non-disperse infrared spectrometry).
DNA extraction and high-throughput sequencing
Total DNA was extracted from 1-g aliquots of soil from each microcosm after treatment, by means of the E.Z.N.A.™ Soil DNA Isolation Kit (Omega Bio-tek, Inc., Norcross, GA, USA) according to the manufacturer’s instructions. For bacterial community structure analysis, the 16S rDNA V3-V4 region was amplified by specific degenerate primers (341F: 5′-CCTAYGGGRBGCASCAG-3′; 806R: 5′-GGACTACNNGGGTATCTAA T-3′) with unique barcodes. High-throughput sequencing was performed using the Illumina Hiseq platform Novo gene (Novo gene Bioinformation Technology. Beijing, China, https://en.novogene.com/). Sequences with ≥ 97% similarity were assigned to the same OTUs. The low abundance OTUs were eliminated from the OTU table if they did not have a total of at least 2 counts across all the samples in the experiment. The microbial diversity in the sample and the abundance of different microorganisms were based on OTU analysis.
Data analysis
All OTUs were analyzed for abundance and diversity. Alpha diversity analysis was evaluated using Shannon and ACE indexes. Intergroup differences in alpha diversity were analyzed by a nonparametric test. For beta diversity, QIIME (Version 1.9.1) was used to calculate weighted pair group method with arithmetic mean (WPGMA). Intergroup differences in beta diversity were analyzed by a nonparametric test. Tukey’s and Wilcox’s tests were chosen for analysis between more than two groups.
The mean and standard deviations of triplicate independent experiments were calculated. The mean values were compared by a parametric one-way ANOVA test. P < 0.05 indicates the significant difference. Parts of the statistical analyses and graphing were performed using Origin 2018 (Origin Lab Corporation, USA).
Results and discussion
Effect of different oxidation treatments on TPH removal rate
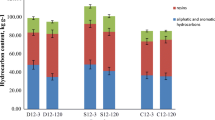
After 50 days of reaction, the TPH removal efficiencies were 7.68%, 43.15%, 50.00%, and 53.50% in the CK, HL, HH, and HT treatments, respectively (Fig. 1a). Compared to the control treatment, preoxidation significantly promoted TPH removal (Fig. 1b), which may partly be attributed to the improvement of the biodegradability of petroleum after oxidation (Gong 2012; Margesin et al. 2007; Palmroth et al. 2006). HT treatment with adding H2O2 at 105 mM in three stepwise additions caused TPH removal at about 2500 mg/kg after 50 days, which showed higher efficiency of unit oxidant than the reported work with TPH removal at 3840 mg/kg after 80 days by adding H2O2 at 900 mM in four stepwise additions (Xu et al. 2016).
Effects of different H2O2 treatments on (a) contents of TPH in soils, (b) TPH degradation rate in different incubation periods, and (c) concentrations of residual H2O2. CK, HL, HH, and HT represent the treatments without addition of H2O2, and with H2O2 at 21 mM, 105 mM, and 105 mM in three equal amounts, respectively. Different lowercase letters indicate significant differences among various treatments in the same incubation period (P < 0.05)
In the three different oxidation treatments, TPH was rapidly degraded in the first 4 days of the reaction, while the TPH removal rate changed gently from 4 to 50 days. TPH in the CK group changed little during the entire incubation process. The change of TPH degradation rates on days 0 to 4 and 4 to 50 under the four treatments were shown in Fig. 1b. After 4 days of reaction, the TPH removal rate in HT treatment (40.39%) increased significantly (P < 0.05), which was 7.23% and 4.34% higher than those in HL (33.16%) and HH (36.05%) treatments, respectively. Since significant iron and other minerals were present in the soil (Yang et al. 2019), including 694 mg \(\cdot\) kg−1 of available Fe and 9.9 mg \(\cdot\) kg−1 of available Mn, H2O2 might be catalyzed/activated by these substances to produce hydroxyl radicals (Liang et al. 2008; Watts and Dilly 1996), thereby enhancing the removal of TPH. However, during the 4th to 50th days of the reaction, the increase in TPH degradation rate in the three oxidation treatments was minuscule, being 9.99% (HL), 13.95% (HH), and 13.11% (HT), respectively. This should be because more degradable components of TPH may be rapidly removed during the early stage. Moreover, adsorption of petroleum hydrocarbon onto organic matters and micropores in soil with time may also cause difficulty in TPH removal (Yen et al. 2011).
Figure 1c shows the change in the residual amount of oxidant in the reaction system under different treatments. In the one-time dosing of H2O2 treatment (HL, HH), no matter the initial oxidant concentration was high or low, H2O2 was consumed on the second day of the reaction. In the three-dose treatment (HT), H2O2 was not detected on the fourth day of the reaction. The results showed that adding H2O2 in portions could increase its residence time in the reaction system, and thus enhance the TPH oxidation removal rate to some extent (Fig. 1a).
The refractory components of petroleum hydrocarbons after preoxidation treatment were more susceptible to microbial degradation (Xu et al. 2016). In this study, the rapid consumption of H2O2 in the first 4 days could minimize the continuous inhibitory effect on the subsequent indigenous microbial remediation process in soil. Moreover, a stepwise addition of H2O2 at relative low concentration improved the continuous oxidation to TPH in soil. Therefore, the increase of TPH removal within 4 to 50 days after preoxidation should be mainly attributed to the role of indigenous microbes rather than chemical oxidation.
Nutrient components after different oxidation treatments
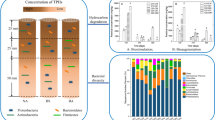
The determined DOC, TN, TP, and pH for soil slurry samples at different incubation time were present in Fig. 2 and Table 1. As shown in Fig. 2a, although DOC contents varied in different treatments, the overall trends were consistent. During the first 4 days of the oxidation reaction, DOC contents increased in all treatments, especially in the HT group, which indicated that H2O2 oxidation would promote organic compounds dissolving from soil. From the 4th to the 50th day of the reaction, the DOC content gradually decreased, which may be attributed to the consumption of organic compounds by indigenous bacteria. The contents of TN and TP in the pre-oxidation treatments decreased in the initial 4 days of the reaction, and then gradually increased with the incubation time extended (Fig. 2b and c). TN and TP contents in HT treatment were significantly higher than those in other treatments (P < 0.05) after 50 days of incubation (Table 1). The pH values decreased in the first 2 or 4 days in HH and HT treatments due to relative higher oxidative intensity, but then gradually increased to close to other treatments. The pH values in HL, HH, and HT treatments were very close from 10 to 50 days of incubation, indicating that different oxidation treatments showed little influence on soil pH from a long-term scale. The relationship between microbial community structure and the contents of dissolved nutrients and pH was further analyzed in “Relationship between microbial community structure and environmental characteristics” part.
Effect of oxidants on petroleum-contaminated soil microbial diversity
Grouping samples under different treatments and incubation time resulted in 20 sample groups, which shared the same top 10 predominant phyla and genera with relative abundances over 1% of total sequences across all samples (Fig. 3). The predominant species in the bacterial communities were largely consistent among the three different oxidation treatments and the CK treatment.
Relative abundance of (a) the dominant phyla and (b) the dominant genus at different incubation stages in soil under various H2O2 treatments. CK, HL, HH, and HT represent the treatments without addition of H2O2, and with H2O2 at 21 mM, 105 mM, and 105 mM in three equal amounts, respectively, and the numbers indicate the incubation time in days
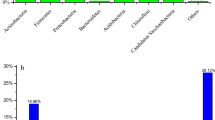
The bacterial phyla with high relative abundance were Firmicutes, Proteobacteria, Gemmatimonadetes, and Actinobacteria, which were the ubiquitous microbial mycobacteria in petroleum-contaminated soil (Bao et al. 2017; Khan et al. 2018; Liu et al. 2019). At the initial stage of the incubation, oxidation would inhibit the growth of bacteria in the soil, but the abundance of Firmicutes increased, indicating that Firmicutes were more resistant to oxidative stress. After 50 days of incubation, the abundance of Firmicutes decreased sharply from 77.65, 94.66, and 69.75 to 7.42%, 11.50%, and 14.76% in the HL, HH, HT treatments with the oxidative stress weakened. However, Proteobacteria became the main dominant phylum with the increase of incubation time, and the relative abundance increasing from 8.41, 2.64, and 17.44 to 64.36%, 38.25%, and 29.57%, respectively, in the HL, HH, HT treatments after 50 days. The Firmicutes and Proteobacteria, which can metabolize and grow with petroleum hydrocarbon as carbon source (Santos et al. 2010; Vinas et al. 2005), may have the main contribution to TPH degradation during 4 to 50 days of incubation (Fig. 1).
The bacterial genera with high relative abundance were Bacillia, Massilia, Paenibacillus, Cavicella, and Gemmatimonas. In the early stage of the incubation, the relative abundances of Bacillia were the highest in all the treatments. With the progress of the incubation, the relative abundance of Cavicella and Gemmatimonas gradually increased. The change of relative abundance at the genus level with incubation time indicated that Bacillia was more resistant to high concentrations of oxidants, while Cavicella and Gemmatimonas preferred low concentration or no oxidants conditions. And unidentified-prevotellaceae and unidentified-clostridiales were bacteria whose genera could not be identified.
Shannon index and ACE index were used to analyze Alpha diversity of soil bacterial community (Fig. 4). ACE index represents the abundance of microbial community. The larger the Shannon index value indicates the higher diversity in species richness and evenness of microorganism. Shannon and ACE indices of all treatments showed an increasing trend with the extension of incubation time (Fig. 4b and d). The ACE indices within 30 days of incubation in oxidation treatments were significantly lower than those without H2O2 addition (Fig. 4c), which indicated that adding H2O2 to petroleum-contaminated soil inhibited the increase of indigenous bacteria abundance to some extent. The ACE indices in HH treatments were significantly lower than those in HL and HT treatments during 50 days of incubation (Fig. 4c), which further indicated that adding H2O2 at high concentration had the greatest inhibition on the increase of indigenous bacteria abundance among oxidation treatments to petroleum-contaminated soil. However, Shannon and ACE indices became the highest in HT treatment after 50 days of incubation (Fig. 4a and c), indicating that adding H2O2 in stepwise was conducive to a higher microbial community diversity from a long-term scale. The above results showed that microbial community diversity was inhibited by the H2O2 addition but would recover after a relatively long time while H2O2 was consumed, which is consistent with the results from previous studies (Jung et al. 2005; Sahl and Munakata-Marr 2006).
Changes of soil bacterial community diversity indicated by (a) and (b) Shannon diversity indexes, and by (c) and (d) ACE diversity indexes based on either different treatments on the same incubation stage or different incubation stages under the same treatment. CK, HL, HH, and HT represent the treatments without addition of H2O2, and with H2O2 at 21 mM, 105 mM, and 105 mM in three equal amounts, respectively. Different lowercase letters indicate significant differences among different treatments on the same incubation stage or different incubation stages under the same treatment (P < 0.05)
The WPGMA was used to estimate the differences, which was clustering the trees based on the phylogenetic data of the group by constructing a cluster analysis perspective (Highton 1993). The WPGMA clustering analysis showed that the oxidant treatments at different incubation time fell into two main groups (Fig. 5). Cluster I contained treatments in the first 10 days with relative abundance of Firmicutes more than 0.6 due to the addition H2O2. Microbial community structures with the same oxidation treatments (like HH2 and HH4; HL4 and HL10) in the first 10 days tended to be closer, indicating that oxidation treatments had a great influence on the microbial community structures in the start-up stage. Cluster II was presented by treatments in 30 days and 50 days with relative abundance of Firmicutes less than 0.25 as the incubation time increased. Microbial community structures at the same incubation time (like HT30, HL30, and HH30) with different oxidation treatments showed high similarity, indicating that the extended incubation time presented greater effects on the composition of the microbial community structure than the oxidation treatments. Additionally, HT10 and CK10 were assigned to cluster II, indicating that adding H2O2 in stepwise (HT) or without H2O2 addition (CK) were conducive to the recovery of microorganism community.
WPGMA dendrogram of the bacterial community structure based on weighted UniFrac distance. Left side of the diagram is the structure of clustering tree. Right side is the relative abundance of different phylum. CK, HL, HH, and HT represent the treatments without addition of H2O2, and with H2O2 at 21 mM, 105 mM, and 105 mM in three equal amounts, respectively, and the numbers indicate the incubation time in days
Relationship between microbial community structure and environmental characteristics
Chemical oxidation would change the physicochemical properties of soil and the structure of microbial communities. Redundancy analysis (RDA) can reveal the microbial community structure related to environmental characteristics. Six kinds of parameters (including oxidant residue (OX), TPH, pH, TN, DOC, and TP) were chosen for RDA. The dbRDA1 and dbRDA2 accounted for 57.1% and 17.6% of the total variations, respectively (Fig. 6). DOC, TN, TP, and TPH were the key parameters contributing to the microbial community structure change. OX and pH showed small effects on the microbial community structure since OX was consumed quickly in the start-up period, and the pH value maintained around 5 to 7 during the whole incubation period. The soil microbial community structure was separated based on different incubation time (2–10 days and 30–50 days) and different oxidation treatments along with dbRDA1 and dbRDA2, respectively. The separations via dbRDA1 were associated with TN, OX, and DOC in relation to different incubation time. The separations via dbRDA2 were mainly associated with TP, pH, and TPH in relation to different oxidation treatments, especially treatments with adding H2O2 or not.
Redundancy analysis (RDA) of MiSeq data (symbols) and environmental characteristics (arrows) under different H2O2 treatments at different incubation stages. CK, HL, HH, and HT represent the treatments without addition of H2O2, and with H2O2 at 21 mM, 105 mM, and 105 mM in three equal amounts, respectively, and the numbers indicate the incubation time in days
Conclusions
All the H2O2 treatments for the petroleum-contaminated soil significantly promoted the TPH removal both in the first 4 days attributed to H2O2 oxidation and in the following 4th to 50th days attributed to the degradation by indigenous microbes. H2O2 oxidation pretreatment showed great influences on the contents of dissolved nutrients (DOC, TN, and TP). Firmicutes, Proteobacteria, Gemmatimonadetes, and Actinobacteria were the main bacterial phyla although their relative abundances changed during the 50 days of incubation. Microbial community diversity was inhibited by H2O2 addition but recovered with incubation time extended. The different H2O2 oxidation treatments had a great influence on the microbial community structures in the start-up stage. With the incubation time extended, recovery time rather than the oxidation treatments presented greater effects on the composition of the microbial community structure. Adding H2O2 in stepwise was conducive to a higher microbial community diversity from a long-term scale. Therefore, applying H2O2, especially at stepwise, as pretreatment combined with soil indigenous microorganisms could be a promising remediation method for petroleum-contaminated soil.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bao Y-J, Xu Z, Li Y, Yao Z, Sun J, Song H (2017) High-throughput metagenomic analysis of petroleum-contaminated soil microbiome reveals the versatility in xenobiotic aromatics metabolism. J Environ Sci 56:25–35. https://doi.org/10.1016/j.jes.2016.08.022
Biache C, Lorgeoux C, Andriatsihoarana S, Colombano S, Faure P (2015) Effect of pre-heating on the chemical oxidation efficiency: implications for the PAH availability measurement in contaminated soils. J Hazard Mater 286:55–63. https://doi.org/10.1016/j.jhazmat.2014.12.041
Chen K-F, Chang Y-C, Chiou W-T (2016) Remediation of diesel-contaminated soil using in situ chemical oxidation (ISCO) and the effects of common oxidants on the indigenous microbial community: a comparison study. J Chem Technol Biotechnol 91:1877–1888. https://doi.org/10.1002/jctb.4781
French KE, Zhou Z, Terry N (2020) Horizontal ‘gene drives’ harness indigenous bacteria for bioremediation. Sci Rep 10:15091. https://doi.org/10.1038/s41598-020-72138-9
Gong X-B (2012) Remediation of weathered petroleum oil-contaminated soil using a combination of biostimulation and modified Fenton oxidation. Int Biodeterior Biodegrad 70:89–95. https://doi.org/10.1016/j.ibiod.2012.02.004
Highton R (1993) The relationship between the number of loci and the statistical support for the topology of UPGMA trees obtained from genetic distance data. Mol Phylogenet Evol 2:337–343. https://doi.org/10.1006/mpev.1993.1033
Jung H, Ahn Y, Choi H, Kim IS (2005) Effects of in-situ ozonation on indigenous microorganisms in diesel contaminated soil: survival and regrowth. Chemosphere 61:923–932. https://doi.org/10.1016/j.chemosphere.2005.03.038
Khan MAI et al (2018) Microbial diversity changes with rhizosphere and hydrocarbons in contrasting soils. Ecotoxicol Environ Saf 156:434–442. https://doi.org/10.1016/j.ecoenv.2018.03.006
Liang C, Huang C-F, Chen Y-J (2008) Potential for activated persulfate degradation of BTEX contamination. Water Res 42:4091–4100. https://doi.org/10.1016/j.watres.2008.06.022
Lim MW, Von Lau E, Poh PE (2016) A comprehensive guide of remediation technologies for oil contaminated soil—present works and future directions. Mar Pollut Bull 109:14–45. https://doi.org/10.1016/j.marpolbul.2016.04.023
Liu L et al (2018) Crude oil removal from aqueous solution using raw and carbonized Xanthoceras sorbifolia shells. Environ Sci Pollut Res 25:29325–29334. https://doi.org/10.1007/s11356-018-2895-0
Liu Q, Tang J, Liu X, Song B, Zhen M, Ashbolt NJ (2019) Vertical response of microbial community and degrading genes to petroleum hydrocarbon contamination in saline alkaline soil. J Environ Sci 81:80–92. https://doi.org/10.1016/j.jes.2019.02.001
Lu M, Zhang Z, Qiao W, Wei X, Guan Y, Ma Q, Guan Y (2010) Remediation of petroleum-contaminated soil after composting by sequential treatment with Fenton-like oxidation and biodegradation. Bioresour Technol 101:2106–2113. https://doi.org/10.1016/j.biortech.2009.11.002
Margesin R, Hammerle M, Tscherko D (2007) Microbial activity and community composition during bioremediation of diesel-oil-contaminated soil: effects of hydrocarbon concentration, fertilizers, and incubation time. Microb Ecol 53:259–269. https://doi.org/10.1007/s00248-006-9136-7
Martinez-Pascual E, Grotenhuis T, Solanas AM, Vinas M (2015) Coupling chemical oxidation and biostimulation: effects on the natural attenuation capacity and resilience of the native microbial community in alkylbenzene-polluted soil. J Hazard Mater 300:135–143. https://doi.org/10.1016/j.jhazmat.2015.06.061
Medina R, David Gara PM, Jose Fernandez-Gonzalez A, Alejandra Rosso J, Teresa Del Panno M (2018) Remediation of a soil chronically contaminated with hydrocarbons through persulfate oxidation and bioremediation. Sci Total Environ 618:518–530. https://doi.org/10.1016/j.scitotenv.2017.10.326
Palmroth MRT, Langwaldt JH, Aunola TA, Goi A, Puhakka JA, Tuhkanen TA (2006) Treatment of PAH-contaminated soil by combination of Fenton’s reaction and biodegradation. J Chem Technol Biotechnol 81:598–607. https://doi.org/10.1002/jctb.1476
Pardo F, Rosas JM, Santos A, Romero A (2014) Remediation of a biodiesel blend-contaminated soil by using a modified Fenton process. Environ Sci Pollut Res 21:12198–12207. https://doi.org/10.1007/s11356-014-2997-2
Sahl J, Munakata-Marr J (2006) The effects of in situ chemical oxidation on microbiological processes: a review. Remediat J 16:57–70. https://doi.org/10.1002/rem.20091
Santos HF, Cury JC, Carmo FL, Rosado AS, Peixoto RS (2010) 18S rDNA Sequences from microeukaryotes reveal oil indicators in mangrove sediment. PLoS ONE. https://doi.org/10.1371/journal.pone.0012437
Shahi A, Aydin S, Ince B, Ince O (2016) Reconstruction of bacterial community structure and variation for enhanced petroleum hydrocarbons degradation through biostimulation of oil contaminated soil. Chem Eng J 306:60–66. https://doi.org/10.1016/j.cej.2016.07.016
Sutton NB, Grotenhuis T, Rijnaarts HHM (2014a) Impact of organic carbon and nutrients mobilized during chemical oxidation on subsequent bioremediation of a diesel-contaminated soil. Chemosphere 97:64–70. https://doi.org/10.1016/j.chemosphere.2013.11.005
Sutton NB et al (2014b) Recovery of microbial diversity and activity during bioremediation following chemical oxidation of diesel contaminated soils. Appl Microbiol Biotechnol 98:2751–2764. https://doi.org/10.1007/s00253-013-5256-4
Vinas M, Sabate J, Guasp C, Lalucat J, Solanas AM (2005) Culture-dependent and -independent approaches establish the complexity of a PAH-degrading microbial consortium. Can J Microbiol 51:897–909. https://doi.org/10.1139/w05-090
Watts RJ, Dilly SE (1996) Evaluation of iron catalysts for the Fenton-like remediation of diesel-contaminated soils. J Hazard Mater 51:209–224. https://doi.org/10.1016/S0304-3894(96)01827-4
Yang B, Chen K, Li Q, Liu Y (2019) Persistence and effectiveness of oxidants during the treatment of petroleum-contaminated soil by chemical oxidation with Na2S2O8 and H2O2. J Agro-Environ Sci 38:2377–2385 (in Chinese)
Xu J, Deng X, Cui Y, Kong F (2016) Impact of chemical oxidation on indigenous bacteria and mobilization of nutrients and subsequent bioremediation of crude oil-contaminated soil. J Hazard Mater 320:160–168. https://doi.org/10.1016/j.jhazmat.2016.08.028
Xu J, Xin L, Huang T, Chang K (2011) Enhanced bioremediation of oil contaminated soil by graded modified Fenton oxidation. J Environ Sci 23:1873–1879. https://doi.org/10.1016/S1001-0742(10)60654-7
Yen C-H, Chen K-F, Kao C-M, Liang S-H, Chen T-Y (2011) Application of persulfate to remediate petroleum hydrocarbon-contaminated soil: feasibility and comparison with common oxidants. J Hazard Mater 186:2097–2102. https://doi.org/10.1016/j.jhazmat.2010.12.129
Funding
This research was partially supported by the National Natural Science Foundation of China (21707111), the Opening Project of Oil & Gas Field Applied Chemistry Key Laboratory of Sichuan Province (YQKF202119), Sichuan Science and Technology Support Project (2020JDTD0018), Qilian Mountains Eco-environment Research Center in Gansu Province (QLS202004), and China Postdoctoral Science Foundation (2021M692263).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Bing Yang: conceptualization, methodology, writing—review and editing. Mi Zhou, Yuan Meng, Keming Chen, and Xiangfu Huang: material preparation, data collection and analysis, writing—original draft and editing. Jie Xu: validation, writing—review and editing. Yucheng Liu: validation, supervision. Lingli Li and Lili Ma: software, validation, writing—review. Mingyan Chen: methodology, supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
The authors have agreed to authorship, read and approved the manuscript, and given consent to submit the manuscript.
Consent for publication
The authors have given consent for subsequent publication of the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Kitae Baek
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, B., Zhou, M., Meng, Y. et al. Hydrocarbons removal and microbial community succession in petroleum-contaminated soil under hydrogen peroxide treatment. Environ Sci Pollut Res 30, 27081–27091 (2023). https://doi.org/10.1007/s11356-022-23875-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23875-z