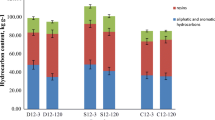
Abstract
Oil spills during extraction and use are seriously threatening human health. Hence, effective bioremediation is a prerequisite for environmental and human well-being. In this study, bioremediation was performed by natural attenuation (NA), bioaugmentation (BA), and biostimulation (BS) microcosmic experiments. Gas chromatography and high-throughput sequencing were used to explore the changes in hydrocarbon degradation and bacterial community structure in different layers of contaminated soil, respectively. The results showed that the deep soil contained mainly C17‒C21 fractions. In 300 days, the degradation rates of soil total petroleum hydrocarbons in surface, middle, and deep soils were 99.87%, 96.04%, and 71.63% in the BA group; 99.75%, 95.73%, and 99.84% in the BS group; and 8.55%, 8.64%, and 9.84% in the NA group, respectively. Most of the petroleum hydrocarbon fractions were completely degraded by the end of remediation experiments. There were noticeable changes in the structure and relative abundance of soil bacteria in the different treatments compared to the original soil. Proteobacteria was the dominant phylum in all soil samples and Pseudomonas became the dominant genus in the BA group. In the BA and BS group, the germination rate of mung bean seeds was > 72.5% in all cases, which was six times higher than that of the NA group. Thus, bioremediation reduced the toxicity of contaminated soil in a shorter period of time than NA, and this study could act as a reference for treatment of contaminated soil in oil recovery areas.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Oil spills frequently occur during extraction, transportation, and use of petroleum products (Wu et al., 2017). Toxic organic compounds in petroleum have negative impacts on human health and ecosystems (Shaoping et al., 2021). Total petroleum hydrocarbons (TPHs) are persistent, hydrophobic, and slightly bioavailable, making them difficult to degrade naturally (Varjani et al., 2017). Spilled petroleum is adsorbed by soil particles or trapped in soil micropores (Gautam et al., 2020); low-molecular-weight alkanes readily enter the atmosphere or soil gas phase, whereas high-molecular-weight hydrocarbons are more strongly adsorbed by soil (Kwon et al., 2016). Some alkanes can infiltrate and pollute underground soil and groundwater, threatening drinking water resources (Ma & You, 2016). Therefore, remediation of petroleum-contaminated soils is critical to human well-being.
Among the various petroleum hydrocarbon remediation methods, traditional physical and chemical remediations are being eliminated due to their high cost and low efficiency. Bioremediation with its advantages of low cost, high efficiency, and environmental friendliness has gradually replaced physical and chemical remediation methods as a research hotspot (Varjani and Upasani et al., 2017; Wu et al., 2017). Bioremediation relies on microorganisms with degradation capabilities. Biostimulation (BS) and bioaugmentation (BA) are the most commonly used bioremediation techniques. These two remediation techniques are used to achieve biodegradation by increasing the population of petroleum hydrocarbon–degrading bacteria to a certain level (Hoang et al., 2021; Wei et al., 2021). BS involves the promotion of hydrocarbon degradation by increasing the metabolic activity of native microorganisms through nutrient supplementation (e.g., nitrogen and phosphorus) that are essential for microbial growth (Andrea Silva-Castro et al., 2016; Shahi et al., 2016a). BA promotes hydrocarbon degradation by combining endogenous or exogenous petroleum hydrocarbon–degrading bacteria to increase the number of degrading bacteria (Ruffini Castiglione et al., 2016).
When oil enters the soil, the relative abundance of petroleum hydrocarbon–degrading bacteria increases dramatically and they become dominant (Geng et al., 2022; Shaoping et al., 2021). In polluted soil remediation, the phyla Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria become the dominant phylum in the soil (Deng et al., 2020; Yu et al., 2019). Proteobacteria and Bacteroidetes have high adaptability in soils with high TPH concentrations (Geng et al., 2022; Singha & Pandey, 2021). Xu et al., (2018) reported that Pseudomonas sp., Alcanivorax sp., and Bacillus sp. can degrade petroleum hydrocarbons. The main alkane-degrading bacteria in Dagang Oilfield were Proteobacteria phylum and Pseudomonas sp. (Feng et al., 2019; Liu et al., 2019). Therefore, analyzing the changes in soil microbial community structure during bioremediation will deepen our understanding of oilfield soil remediation.
Previous studies on oil-contaminated soil remediation were mostly limited to soils at 0–20 cm depth, and less attention was paid to the infiltration of petroleum hydrocarbons and the deep remediation mechanism (Wolińska et al., 2016; Wu et al., 2021). In this study, contaminated soils at different depths were collected from Dagang Oilfield and indoor microcosmic experiments were established. Efficient degradation strains were isolated from the oil-contaminated soil, and the bioremediation effects of petroleum hydrocarbon–contaminated soils at different depths and the changes of microbial community structure in soil were studied. The removed hydrocarbons were quantified to assess the performances of BS and BA. Then, phytotoxicity testing using mung bean seeds was carried out to verify the pollutant remediation effect.
2 Materials and Methods
2.1 Soil for Bioremediation Research
Soil samples were collected from the oil production area of Dagang Oilfield, which is located in southeastern Tianjin, China (38° 39′‒38° 44′N, 117° 20′‒117° 32′E). Soil samples were collected longitudinally with a soil sampler by the five-point sampling method. The sampling depths were 0‒10, 10‒20, and 20‒40 cm, which were numbered GN1, GN2, and GN3, respectively. From the surface layer to the deep layer, the soil color changed from black to gray-black to gray-yellow. GN1 was a heavily petroleum-contaminated soil, and a strong petroleum odor was present even at a depth of 40 cm below the surface. The collected samples were sealed in a metal container, stored in an ice box at 4 °C, and transported to the laboratory. The physicochemical characteristics of this contaminated soil are shown in Table 1.
2.2 Isolation and Identification of Petroleum Hydrocarbon–Degrading Bacteria
Soil samples (2 g) were suspended in 50 mL of sterile mineral salt medium (MSM). The MSM culture medium contained 0.5 g/L KH2PO4, 0.5 g/L K2HPO4, 2.5 g/L NH4NO3, 1.0 g/L NaSO4, 1.0 g/L MgSO4·2H2O, and 1 mL of trace element solution (1.0 g/L FeSO4, 0.1 g/L NaSeO4·2H2O, 0.05 g/L ZnCl2, 0.01 g/L NiCl2·6H2O, 0.015 g/L CuSO4·5H2O, and 0.0016 g/L H2MoO4). The medium contained 1% crude oil as the sole carbon and energy source. For microbe incubation, soil samples were cultured at 35 °C in an oscillating incubator (150 rpm) for 7 days. A portion (5%) of the cultures were then transferred into 45 mL of fresh MSM medium, and the crude oil content was gradually increased to 5% (1% increase per transfer). This operation was repeated five times. Three strains of efficient petroleum hydrocarbon–degrading bacteria, numbered GNJ1, GNJ2, and GNJ3, were isolated using MSM agar plates containing crude oil and transported to Shanghai Biotechnology Co. for 16S rDNA sequencing.
2.3 Bioremediation Experimental Design
Soil samples were air-dried, crushed, and sieved by a 20-mesh metal sieve. Microcosm devices were prepared in a cylindrical opaque plastic container (120 mm diameter, 180 mm height). As shown in Fig. 1, the bottom of each device was filled with 30 mm of quartz sand and 100 mm of contaminated soil. The devices were filled with deep soil, then with medium soil, and finally with topsoil (25, 25, and 50 mm for surface, medium, and deep soil samples, respectively).
Three treatments were carried out: (1) natural attenuation (NA) to study the biological depletion of petroleum hydrocarbons in contaminated soil by indigenous microbial communities by adding only sterile water; (2) bioaugmentation (BA) to study the effect of adding native dominant degrading bacteria on petroleum hydrocarbon degradation by supplementing the soil with 2% (w/v) dominant petroleum hydrocarbon–degrading strains, resulting in a soil density of 108 CFU g−1; (3) biostimulation (BS) to study the effect of native microorganisms on the degradation of petroleum hydrocarbons when nutrients were added. Soils were biostimulated by adding NH4NO3 and KH2PO3 nutrients to maintain a C:N:P ratio of 100:10:1. A mixture of NH4NO3 and KH2PO3 was added after dissolving in water which assisted in nitrogen and phosphorous dissolution in the soil. All treatments were conducted in triplicate. The experimental device was incubated at a greenhouse for 300 days, and the water content (20%) in microcosms was maintained by regularly adding sterile water. On 120, 210, and 300 days of the experiment, 10 g of soil samples (in triplicate) was collected from each microcosm device for hydrocarbon extraction in layers.
2.4 Extraction and Analysis of Petroleum Hydrocarbons
Petroleum hydrocarbon degradation was assessed using gas chromatography. Soil samples were dried in a freeze-dryer at − 20 °C for 48 h. Soil petroleum hydrocarbons were extracted with dichloromethane in a Soxhlet Leacher (ST310, FOSS Analytical Instruments Ltd.). The extracts were mixed and water was removed by anhydrous sodium sulfate. The solution was dried and re-dissolved with hexane. The TPH degradation rate (%) was calculated using the following equation.
Petroleum hydrocarbons were analyzed using gas chromatography (Agilent 6890N) with a hydrogen flame ionization detector and an Agilent DB-5MS capillary column. The column temperature ramp-up procedure was as follows: initial temperature was 60 °C, held for 1 min, ramped up to 280 °C at a rate of 8 °C/min, held for 1 min, then ramped up to 310 °C at a rate of 15 °C/min and held for 18 min, for a total run time of 49.5 min.
2.5 Phytotoxicity Test
Mung bean (Vigna radiata) seeds were selected as materials and germination percentages were used to assess soil toxicity after bioremediation (Sun et al., 2019; Varjani et al., 2021). Vigna radiata were disinfected with sodium hypochlorite (0.5% v/v) for 10 min; then, the seeds were washed three times with distilled water (Bhattacharya et al., 2019). Test soils were collected at the end of bioremediation and placed in 40 × 40 mm (D × H) nursery pots. Eight seeds were sown in each nursery pot. All pots were incubated in the greenhouse at 37 °C and supplied with regular additions of sterilized distilled water to maintain soil moisture. After 7 days, the number of seeds that germinated was recorded in each pot and germination percentages were calculated as (number of seeds germinated seeds/total number of seeds) × 100.
2.6 Illumina Sequencing
The control soil without remediation (GN1, GN2, and GN3) and soil samples after 300 days of remediation were collected for MiSeq sequencing. DNA was extracted according to the Power Soil DNA extraction kit (MoBio Laboratories Inc.). DNA quality was determined on 1% agarose gel. Polymerase chain reaction was performed using bacterial universal primers 341 F and 805 R to amplify the V3‒V4 region of the bacterial 16S rDNA gene.
2.7 Statistical Analysis
All experiments were conducted in triplicate and results were expressed as mean ± standard deviation. A one-way analysis of variance (Statistical Projections for Social Sciences [SPSS], version 23.0, China) was used for statistical analysis using experimental results (p < 0.05), and Origin 2022 was used for graphing.
3 Results
3.1 Basic Physicochemical Properties of Original Soil
As shown in Table 1, the soil pH of each layer was higher than 7.66, which was weakly alkaline. The C:N:P ratio in the initially contaminated soil was 946:10:45 (Table 1). In all samples, the moisture content, organic carbon, and total phosphorus were highest in the surface soil layer, which were 35.92%, 66,531.32 mg kg−1, and 1202.80 mg kg−1, respectively. In the deep soil layer, the highest total nitrogen was 501.99 mg kg−1. In the surface soil, the TPH concentration was 888,615.75 mg kg−1, accounting for 98.13%, and was 15,342.13 mg kg−1 and 1630.06 mg kg−1 in the middle and deep soils, respectively. As shown in Fig. 2, the main petroleum hydrocarbon fractions in the surface, middle, and deep soils were C14‒C36+, C17‒C36+, and C17‒C21, respectively. The C14‒C16 and C17‒C21 fractions were detected only in surface and deep soils, respectively. The short-chain alkanes were degraded in the surface layer, whereas the long-chain alkanes were inherently difficult to biodegrade or migrate to the deep layer (Liu et al., 2019).
3.2 Highly Efficient Petroleum Hydrocarbon–Degrading Strains
Table 2 shows the strains with the highest sequence homology to strains in this study. Upon searching the National Center for Biotechnical Information (NCBI) 16S database and performing the 16S rDNA sequence homology study, GNJ1 and GNJ2 belong to Firmicutes, and GNJ3 belongs to Proteobacteria.
3.3 Bioremediation of Petroleum Hydrocarbon–Contaminated Soil
3.3.1 Degradation of TPHs in Each Soil Layer
Figure 3 shows the TPH degradation rates in each soil layer after microtreatment. The TPH degradation rate in the surface soil was > 95% after 120 days of treatment, and most TPHs had been removed. TPH degradation rates in the middle and deep soil layers increased with time. After 300 days of treatment, TPH degradation rates in the middle soil exceeded 95.37% for BS and BA treatments; in the deep soil, the TPH degradation rate was 99.84% for the BS treatment group and only 71.63% for the BA group. Compared to NA, the TPH removal effect was significantly improved by feeding bacteria and adding nutrient salt, and addition of nutrient salts was shown to have superior effects compared to that of addition of bacteria (Wei et al., 2021). These results indicate that the TPH degradation rate decreased with the increase in soil depth. This result agrees with that of Suja et al. (2014), who found a higher percentage of TPH degradation in the topsoil than that in the bottom soil in soil columns microcosmic experiments and field trials.
3.3.2 Variation of Petroleum Hydrocarbon Components in Each Soil Layer
The degradation rate of the TPH fraction in the surface soil (0‒25 mm) exceeded 99% after 300 days of bioremediation (Fig. 4A and B). The C14‒C16 fraction was completely removed within 120 days of bioremediation, as short-chain alkanes are considered highly biodegradable (Li et al., 2022). Throughout the bioremediation process, the C22‒C26 fraction concentrations reached a minimum within 210 days and its residual concentration increased at the end. Possibly, the bacteria first degraded the long-chain alkanes to shorter-chain alkanes before further degradation, which is consistent with the results of Li et al. (2022). Overall, BS showed better degradation of long-chain alkanes (C27‒C36 +) than that of short-chain alkanes.
Residual concentrations of petroleum hydrocarbon fractions in the surface and middle soils after bioremediation. Error bars represent standard deviation. Different letters on the same day represent a significant difference at p < 0.05 (A and B the surface soil, C and D the middle soil, A and C biostimulation, B and D bioaugmentation)
As shown in Fig. 4C and D, the residual concentrations of most of the petroleum hydrocarbon fractions in the middle soil were very low, with maximum concentrations of 717.1 mg kg−1 (C17‒C21 fractions in the BS group) and 633.7 mg kg−1 (C27‒C31 fractions in the BA group) at the end of the experiment. The degradation rates of C22‒C26 and C32‒C36+ fractions were better than those of C17‒C21 and C27‒C31 fractions, respectively. On day 210, the C17‒C21 fraction was completely degraded. However, at the end of treatment, the residual concentrations of this component were 717.1 mg kg−1 (BS) and 550.75 mg kg−1 (BA). The residual concentration of the C22‒C26 fractions first decreased and then increased, showing the same pattern as the surface soil. Presumably, the long-chain alkanes were degraded to short-chain alkanes before being further degraded or that the alkanes in the surface layer penetrated the middle layer soil particles (Li et al., 2012, 2022). Among all the fractions, the C32‒C36+ fraction showed the most significant change, and its degradation rate increased by 65% in the BS group.
In the deep soils, only C17‒C21 fractions were detected (Fig. 2). During 120 days of microtreatment, C17‒C21 fractions were completely removed and small amounts of C27, C34, and C36 fractions were detected (data not shown). Some petroleum hydrocarbon fraction, which was not detected in the original soil sample, appeared in the lower soil layer of the experiment. The petroleum fraction flow from the upper layer to the lower layer implies the downward migration of petroleum pollutants (Li et al., 2012).
3.3.3 Seed Germination Test
In 7 days, the highest germination rate observed in the NA group was 12.5% (Fig. 5). The germination rates in BS and BA groups were > 72.5%. Compared with the NA group, the germination rates of bioremediation were significantly higher (Varjani et al., 2020). The germination rate increased with increasing soil depth, and the highest germination rate of 82.5% was observed in deeper soils.
3.4 Soil Microbial Community Composition and Relative Abundance
The initial sequence numbers of the original contaminated soils GN1, GN2, and GN3 were 22,034,187, 22,469,052, and 24,555,251, respectively; after removing the low-quality reads from the original sequences, the valid sequences were 21,324,043, 21,601,005, and 23,635,793, respectively. The initial and valid sequences of each soil layer after 300 days of bioremediation are shown in Table 3. Sequencing optimization for all samples was > 96%. The details of the microbial taxonomy (phylum, class, order, family, genus, and species) in different soil samples are shown in Table 3.
Figure 6 shows the 10 most abundant phyla and the top 20 predominant genera and their relative abundance in each layer of soil samples. Compared with the original soil, the bacterial communities in the treated soil layers were more diverse. In all soil samples, Proteobacteria was the dominant phylum, with relative abundance ranging from 38.26 to 85.70%. Bacteroidetes and Actinobacteria were the minor phyla. After bioremediation, the relative abundance of Proteobacteria phylum in surface soil showed a decreasing trend, whereas it increased in the middle and deep soils. In the BA group, the relative abundances of the Firmicutes phylum increased to 8.19%, 9.67%, and 10.09% in surface, middle, and deep soil layers, respectively.
At the genus level, Marinobacter and Halomonas were the dominant genera in original surface soil, with relative abundances of 36.11% and 15.81%, respectively. Somee et al., (2018) found that Marinobacter and Halomonas predominate in oil-based drill cuttings. After 300 days, Marinobacter declined sharply across treatments. Halomonas showed a decreasing trend in surface and deep soils, whereas there was an opposite change in the middle layer. In the BA group, the relative abundance of Pseudomonas increased sharply, accounting for 41.53% (GN1BA), 12.91% (GN2BA), and 38.59% (GN3BA) of the dominant genus. In the GN3BS group, Lysobacter was the dominant genus with a relative abundance of 42.20%. There was a positive correlation between Lysobacter and the TPH removal rate (Lee et al., 2022). The NA group had a more diverse microbial community composition and a more stable structure than the original soil.
4 Discussion
In this study, the TPH concentrations in contaminated soil samples collected from different depths varied greatly (Fig. 2). The TPH contents in surface soil and middle soil accounted for 98.13% and 1.69% of the total content, respectively. This result indicated that TPHs accumulated mainly in the 0‒20 cm soil layer. Petroleum hydrocarbons are adsorbed when they enter the soil, and the TPH concentration decreases with increasing soil depth (Geng et al., 2022; Teng et al., 2013). In this study, short-chain alkanes (C14‒C16) were not detected in medium and deep soils, and the C17‒C21 fraction posed the greatest threat to deep soils (Fig. 2). Short-chain alkanes had higher bioavailability, whereas long-chain alkanes are more readily adsorbed by soil minerals, and the heavier the TPH fraction, the stronger its adsorption (Kwon et al., 2016; Liu et al., 2019). In deeper soils, the C14‒C16 fraction may have been bioavailable, or completely volatilized in the surface soil, and the long-chain fraction (C22‒C36 +) may have been blocked in the middle soil layer due to its excessive molecular weight.
The TPH removal rate exceeded 99% after 300 days of treatment with bacteria or nutrient salt addition (Fig. 3). The indigenous microorganisms in the NA group could degrade the soil petroleum hydrocarbons, and the addition of dominant degrading bacteria improved the degradation efficiency. Abena et al. (2019) found that TPH degradation by treatment with injected strains was superior to NA treatment. However, this approach is limited by nutrient inhibition (Liu et al., 2010). Inappropriate nutrient levels can severely limit the remediation capacity of indigenous microorganisms, especially the lack of nitrogen and phosphorus that are required for microbial growth and metabolism (Sarkar et al., 2016; Smith et al., 2015). The results of this study demonstrated that BS should be the treatment of choice for the remediation of petroleum hydrocarbon–contaminated soils. This study obtained results that were similar to those of previous studies, where BS was more effective than BA (Polyak et al., 2017; Wu et al., 2016).
The degradation rate of the TPH fraction in the surface soil ranged from 93.34 to 100% after 120 days of bioremediation. The degradation of the C14‒C16 fraction was the highest, and the C32‒C36 + fraction had only 93.34% (Fig. 4A and B). Alkane degradation decreases with an increase in the carbon number (Liu et al., 2019). However, in the middle soil, the residual concentrations of C17‒C21 and C27‒C31 fractions were higher than those of the C22‒C26 and C32‒C36 + fractions, respectively. Studies indicate that high-molecular-weight hydrocarbons are preferentially degraded in low TPH concentrations (Chang et al., 2010). During bioremediation, soil particles containing petroleum hydrocarbon fractions migrated with moisture to the lower soil layers, allowing C27, C34, and C36 to be detected in deeper soils. Li et al. (2012) studied the vertical variation in the TPH concentration in five soil profiles and found a downward migration trend for TPHs.
In this study, the hydrocarbon-degrading flora obtained from the contaminated soil were from the Firmicutes phylum and Pseudomonas genus. These two strains have been identified as hydrocarbon-degrading bacteria (Devi et al., 2021; Liu et al., 2020; Wu et al., 2019). This indicated that the petroleum hydrocarbon–degrading flora isolated from different petroleum pollutants had a certain degree of homology.
Proteobacteria, Bacteroidetes, Actinobacteria, and Firmicutes were the dominant phyla in all soil samples (Fig. 6). This result is consistent with the results of the study by Feng et al. (2019). These phyla are widely distributed in petroleum-contaminated soils (Shahi et al., 2016b; Wang et al., 2021; Wei et al., 2021). The relative abundance of Pseudomonas increased in the BA group. In particular, Pseudomonas replaced Helicobacter as the dominant genus in the surface soil. The above results implied that the growth of native microorganisms was inhibited by the addition of degrading bacteria (Wu et al., 2019). The increased relative abundances of Salegentibacter, Halomonas, and Lysobacter genera suggested that these strains were associated with biostimulatory treatment of TPHs. The bacterial community composition in the NA group was relatively stable, which was consistent with previously reported results (Silva-Castro et al., 2013).
Mung beans are commonly used in phytotoxicity tests for testing the acute toxicity of various contaminants (Gogoi et al., 2022). We found that the germination rate in bioremediated soil samples was much higher than that in the NA group. This was consistent with the results obtained by other researchers, indicating that bioremediation can efficiently remediate contaminated soils (Varjani et al., 2020, 2021).
5 Conclusion
This study showed that short-chain alkanes were only present in the surface soils, and that the C17‒C21 fractions posed the greatest threat to the deeper soils. Compared to NA, BA using native petroleum-degrading flora and BS using nitrogen and phosphorus nutrients could effectively promote TPH degradation in contaminated soils. In surface and middle soils, the two remediation strategies were very similar in terms of TPH degradation, whereas nitrogen and phosphorus BS were more effective remediation methods for TPH degradation in deep soils. The addition of dominant petroleum-degrading bacteria could rapidly multiply into dominant genera, and the diversity of microbial communities in the BS group increased, whereas the structure of bacterial communities in the NA group was relatively stable. At 300 days, the germination rate of mung beans in the soil exposed to biological treatments was much higher than that in soil under the NA group. This result indicated that bioremediation can reduce the toxicity of contaminated soil and restore seed germination capacity in a relatively short period, thus aiding soil remediation.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Andrea Silva-Castro, G., Rodriguez-Calvo, A., Laguna, J., Gonzalez-Lopez, J., & Calvo, C. (2016). Autochthonous microbial responses and hydrocarbons degradation in polluted soil during biostimulating treatments under different soil moisture. Assay in pilot plant. International Biodeterioration & Biodegradation, 108, 91–98.
Bhattacharya, S., Das, A., Srividya, S., Prakruti, P. A., Priyanka, N., & Sushmitha, B. (2019). Prospects of Stenotrophomonas pavanii DB1 in diesel utilization and reduction of its phytotoxicity on Vigna radiata. International Journal of Environmental Science and Technology, 17, 445–454.
Bidja Abena, M. T., Li, T., Shah, M. N., & Zhong, W. (2019). Biodegradation of total petroleum hydrocarbons (TPH) in highly contaminated soils by natural attenuation and bioaugmentation. Chemosphere, 234, 864–874.
Chang, W. J., Dyen, M., Spagnuolo, L., Simon, P., Whyte, L., & Ghoshal, S. (2010). Biodegradation of semi- and non-volatile petroleum hydrocarbons in aged, contaminated soils from a sub-Arctic site: Laboratory pilot-scale experiments at site temperatures. Chemosphere, 80, 319–326.
Deng, Z., Jiang, Y., Chen, K., Gao, F., & Liu, X. (2020). Petroleum depletion property and microbial community shift after bioremediation using Bacillus halotolerans T-04 and Bacillus cereus 1–1. Frontiers Microbiology, 11, 353.
Devi, S. S. S. S. P., Jani, K., Sharma, A., & Jha, D. K. (2021). Bacterial communities and their bioremediation capabilities in oil-contaminated agricultural soils. Environmental Monitoring and Assessment, 194, 9.
Feng, X., Liu, Z., Jia, X., & Lu, W. (2019). Distribution of bacterial communities in petroleum-contaminated soils from the Dagang Oilfield, China. Transactions of Tianjin University, 26, 22–32.
Gautam, P., Bajagain, R., & Jeong, S. W. (2020). Combined effects of soil particle size with washing time and soil-to-water ratio on removal of total petroleum hydrocarbon from fuel contaminated soil. Chemosphere, 250, 126206.
Geng, P., Ma, A., Wei, X., Chen, X., Yin, J., Hu, F., Zhuang, X., Song, M., & Zhuang, G. (2022). Interaction and spatio-taxonomic patterns of the soil microbiome around oil production wells impacted by petroleum hydrocarbons. Environmental Pollution, 307, 119531.
Gogoi, B., Das, I., Gogoi, M., Charingia, D., Bandyopadhyay, T., & Borah, D. (2022). Bioremediation of motor oil-contaminated soil and water by a novel indigenous Pseudomonas otitidis strain DU13 and characterization of its biosurfactant. 3 Biotech, 12, 68.
Hoang, S. A., Lamb, D., Seshadri, B., Sarkar, B., Choppala, G., Kirkham, M. B., & Bolan, N. S. (2021). Rhizoremediation as a green technology for the remediation of petroleum hydrocarbon-contaminated soils. Journal of Hazardous Materialls, 401, 123282.
Kwon, M. J., Lee, G., Hwang, Y. H., Lee, M., & Yang, J.-S. (2016). Effects of soil micro-particles and micropores on petroleum hydrocarbons released from contaminated soils during solvent extraction with ultrasound. Water, Air, & Soil Pollution, 227, 357.
Lee, Y. Y., Lee, S. Y., Lee, S. D., & Cho, K. S. (2022). Seasonal dynamics of bacterial community structure in diesel oil-contaminated soil cultivated with tall fescue (Festuca arundinacea). Interinational Journal Environmental Research and Public Health, 19, 4629.
Li, J., Zhang, J., Lu, Y., Chen, Y., Dong, S., & Shim, H. (2012). Determination of total petroleum hydrocarbons (TPH) in agricultural soils near a petrochemical complex in Guangzhou, China. Environmental Monitoring Assessment, 184, 281–287.
Li, J., Liu, Q., Sun, S., Zhang, X., Zhao, X., Yu, J., Cui, W., & Du, Y. (2022). Degradation characteristics of crude oil by a consortium of bacteria in the existence of chlorophenol. Biodegradation, 33, 461–476.
Liu, H., Gao, H., Wu, M., Ma, C., Wu, J., & Ye, X. (2020). Distribution characteristics of bacterial communities and hydrocarbon degradation dynamics during the remediation of petroleum-contaminated soil by enhancing moisture content. Microbial Ecology, 80, 202–211.
Liu, Q., Tang, J., Liu, X., Song, B., Zhen, M., & Ashbolt, N. J. (2019). Vertical response of microbial community and degrading genes to petroleum hydrocarbon contamination in saline alkaline soil. Journal of Environmental Sciences (china), 81, 80–92.
Liu, W., Luo, Y., Teng, Y., Li, Z., & Ma, L. Q. (2010). Bioremediation of oily sludge-contaminated soil by stimulating indigenous microbes. Environmental Geochemistry and Health, 32, 23–29.
Ma, W., & You, X. (2016). Numerical simulation of plant–microbial remediation for petroleum-polluted soil. Soil and Sediment Contamination: An International Journal, 25, 727–738.
Polyak, Y. M., Bakina, L. G., Chugunova, M. V., & Mayachkina, N. V. (2017). Effect of remediation strategies on biological activity of oil-contaminated soil - A field study. International Biodeterioration & Biodegradation, 126, 57–68.
RezaeiSomee, M., Shavandi, M., Dastgheib, S. M. M., & Amoozegar, M. A. (2018). Bioremediation of oil-based drill cuttings by a halophilic consortium isolated from oil-contaminated saline soil. 3 Biotech, 8, 229.
Ruffini Castiglione, M., Giorgetti, L., Becarelli, S., Siracusa, G., Lorenzi, R., & Di Gregorio, S. (2016). Polycyclic aromatic hydrocarbon-contaminated soils: Bioaugmentation of autochthonous bacteria and toxicological assessment of the bioremediation process by means of Vicia faba L. Environmental Science and Pollution Research, 23, 7930–7941.
Sarkar, J., Kazy, S. K., Gupta, A., Dutta, A., Mohapatra, B., Roy, A., Bera, P., Mitra, A., & Sar, P. (2016). Biostimulation of indigenous microbial community for bioremediation of petroleum refinery sludge. Frontiers in Microbiology, 7, 1407.
Shahi, A., Aydin, S., Ince, B., & Ince, O. (2016a). Evaluation of microbial population and functional genes during the bioremediation of petroleum-contaminated soil as an effective monitoring approach. Ecotoxicology and Environmental Safety, 125, 153–160.
Shahi, A., Aydin, S., Ince, B., & Ince, O. (2016b). Reconstruction of bacterial community structure and variation for enhanced petroleum hydrocarbons degradation through biostimulation of oil contaminated soil. Chemical Engineering Journal, 306, 60–66.
Shaoping, K., Zhiwei, D., Bingchen, W., Huihui, W., Jialiang, L., & Hongbo, S. (2021). Changes of sensitive microbial community in oil polluted soil in the coastal area in Shandong, China for ecorestoration. Ecotoxicology and Environmental Safety, 207, 111551.
Silva-Castro, G. A., Rodelas, B., Perucha, C., Laguna, J., Gonzalez-Lopez, J., & Calvo, C. (2013). Bioremediation of diesel-polluted soil using biostimulation as post-treatment after oxidation with Fenton-like reagents: Assays in a pilot plant. Science of the Total Environment, 445–446, 347–355.
Singha, L. P., & Pandey, P. (2021). Rhizosphere assisted bioengineering approaches for the mitigation of petroleum hydrocarbons contamination in soil. Critical Reviews in Biotechnology, 41, 749–766.
Suja, F., Rahim, F., Taha, M. R., Hambali, N., Rizal Razali, M., Khalid, A., & Hamzah, A. (2014). Effects of local microbial bioaugmentation and biostimulation on the bioremediation of total petroleum hydrocarbons (TPH) in crude oil contaminated soil based on laboratory and field observations. International Biodeterioration & Biodegradation, 90, 115–122.
Smith, E., Thavamani, P., Ramadass, K., Naidu, R., Srivastava, P., & Megharaj, M. (2015). Remediation trials for hydrocarbon-contaminated soils in arid environments: Evaluation of bioslurry and biopiling techniques. International Biodeterioration & Biodegradation, 101, 56–65.
Sun, Y., Jing, R., Zheng, F., Zhang, S., Jiao, W., & Wang, F. (2019). Evaluating phytotoxicity of bare and starch-stabilized zero-valent iron nanoparticles in mung bean. Chemosphere, 236, 124336.
Teng, Y., Feng, D., Song, L., Wang, J., & Li, J. (2013). Total petroleum hydrocarbon distribution in soils and groundwater in Songyuan oilfield, Northeast China. Environmental Monitoring Assessment, 185, 9559–9569.
Varjani, S., Pandey, A., & Upasani, V. N. (2021). Petroleum sludge polluted soil remediation: Integrated approach involving novel bacterial consortium and nutrient application. Science of the Total Environent, 763, 142934.
Varjani, S., Upasani, V. N., & Pandey, A. (2020). Bioremediation of oily sludge polluted soil employing a novel strain of Pseudomonas aeruginosa and phytotoxicity of petroleum hydrocarbons for seed germination. Science of the Total Environment, 737, 139766.
Varjani, S. J. (2017). Microbial degradation of petroleum hydrocarbons. Bioresource Technology, 223, 277–286.
Varjani, S. J., & Upasani, V. N. (2017). A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants. International Biodeterioration & Biodegradation, 120, 71–83.
Wang, Y. G., Wang, J. Q., Leng, F. F., & Chen, J. X. (2021). Effects of oil pollution on indigenous bacterial diversity and community structure of soil in Fushun, Liaoning Province, China. Geomicrobiology Journal, 38, 115–126.
Wei, Y., Chen, J., Wang, Y., Meng, T., & Li, M. (2021). Bioremediation of the petroleum contaminated desert steppe soil with Rhodococcus erythropolis KB1 and its effect on the bacterial communities of the soils. Geomicrobiology Journal, 38, 842–849.
Wolińska, A., Kuźniar, A., Szafranek-Nakonieczna, A., Jastrzębska, N., Roguska, E., & Stępniewska, Z. (2016). Biological activity of autochthonic bacterial community in oil-contaminated soil. Water, Air, & Soil Pollution, 227, 130.
Wu, B., Guo, S., & Wang, J. (2021). Spatial ecological risk assessment for contaminated soil in oiled fields. Journal of Hazardous Materials, 403, 123984.
Wu, M., Dick, W. A., Li, W., Wang, X., Yang, Q., Wang, T., Xu, L., Zhang, M., & Chen, L. (2016). Bioaugmentation and biostimulation of hydrocarbon degradation and the microbial community in a petroleum-contaminated soil. International Biodeterioration & Biodegradation, 107, 158–164.
Wu, M., Li, W., Dick, W. A., Ye, X., Chen, K., Kost, D., & Chen, L. (2017). Bioremediation of hydrocarbon degradation in a petroleum-contaminated soil and microbial population and activity determination. Chemosphere, 169, 124–130.
Wu, M., Wu, J., Zhang, X., & Ye, X. (2019). Effect of bioaugmentation and biostimulation on hydrocarbon degradation and microbial community composition in petroleum-contaminated loessal soil. Chemosphere, 237, 124456.
Xu, X., Liu, W., Tian, S., Wang, W., Qi, Q., Jiang, P., Gao, X., Li, F., Li, H., & Yu, H. (2018). Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: A perspective analysis. Frontiers in Microbiology, 9, 2885.
Yu, W., Zheng, J., Song, Q., Zhao, C., Chen, C., Chen, H., Gao, C., & Song, J. (2019). Bio-char influenced indigenous microbial stimulation for oil contaminated soil remediation in Northwest China. Petroleum Science and Technology, 37, 1338–1345.
Funding
This work was supported by the National Natural Science Foundation of China (No. 51978189).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Jieliang Nong participates in collection of sampling; experimental work; compilation of resulted data; writing—original draft; and writing—review and editing. Peng Peng: investigation; visualization; software; writing—original draft; and writing—review and editing. Jiayuan Pan: methodology; investigation; writing—original draft; and writing—review and editing. Taiming Shen: software and writing—review and editing. Qinglin Xie: supervision, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The C17–C21 fraction poses the greatest threat to deep soils.
• Biostimulation was more effective than bioaugmentation in TPH biodegradation.
• Lack of nutrients is a major factor affecting petroleum hydrocarbon degradation.
• More structural diversity of microorganisms in the biostimulation group.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nong, J., Peng, P., Pan, J. et al. Effect of Bioaugmentation and Biostimulation on Hydrocarbon Degradation and Bacterial Community Composition in Different Petroleum-Contaminated Soil Layers. Water Air Soil Pollut 234, 189 (2023). https://doi.org/10.1007/s11270-023-06161-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06161-7