Abstract
Coloured wastewater is a major issue of today for human health and ecology. Among all available processes such as physical, chemical, biological and electrochemical methods, photocatalysis can be a promising solution because of its ability to degrade colour-causing compounds completely by converting them into simpler molecules (H2O, CO2) depending on dye structure. In this work, NiFe2O4 was synthesized by the co-precipitation method. Furthermore, the composites of NiFe2O4 with TiO2 were synthesized by varying amounts of TiO2. The spinel and composites were characterized by XRD, ZETA analysis and UV–DRS. Their photocatalytic activities were investigated using the photocatalytic degradation of reactive turquoise blue 21 (RB 21) dye as model pollutants under sunlight. The increased absorption of the visible light and the enhanced separation of the electron–hole pairs due to the relative energy band positions in NiFe2O4 and TiO2 are considered as the main advantages. Our results showed that NiFe2O4-based nanocomposites could be used as an effective and magnetic retrievable photocatalyst.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wastewater is one of the biggest challenges to the scientific community. Because of increasing industrialization has questioned the quality of water. A very small part of water available on earth is useful for human uses (nearly 0.03%). Chemical and typical dyes and dying industries consume maximum water and produce a large amount of wastewater, which adversely affects aquatic life because of its colour-causing compounds, which hindered the sunlight penetration through the surface of water bodies (Ahmed et al. 2012; Osman 2014; Rajaram and Das 2008; Robinson et al. 2000; Seow et al. 2016; Singh 2015). The main constituents of these types of effluents are dyes. Dyes have mainly four parts namely skeleton, chromophore, auxochrome and soluble part. Dyes could be classified into two basic types: based on structure and based on application (Benkhaya et al. 2020; Forgacs et al. 2004; Jonstrup et al. 2011; Khehra et al. 2006; Kiernan 2001; Klaus 2008; Popli and Patel 2015; Sandhya et al. 2005).
Dyes from wastewater could be removed via different methods such as physical (Hethnawi et al. 2017; Katheresan et al. 2018; Yagub et al. 2014), biological (Chacko and Subramaniam 2011; Manavi et al. 2017; Mojsov et al. 2016; Srinivasan and Viraraghavan 2010), chemical (Forgacs et al. 2004; Gusain et al. 2019; Joshi et al. 2004; Nidheesh et al. 2018). Physical methods do not destroy the dyes but carry out the mass transfer from the liquid phase into the solid phase commonly, hence there are the chances of colour regain after some time, while in biological processes only limited dyes can be degraded and have a limited range of pH and temperature. Even though biological processes are used to a large extent for effluent treatment in ETP plants, too long operation time makes it unfavourable for dye degradation. And chemical processes are faster than biological processes, hence these processes can be a promising option for dyes removal. Typically, with AOPs complete mineralization and partial oxidation to inert and less concerning products such as H2O, CO2 and other simpler products (Anwer et al. 2019; Babuponnusami and Muthukumar 2014; Hodges et al. 2018). Among all AOPs, photocatalysis serves as a promising method as it makes use of sunlight and relatively cheaper process than other methods as separation of catalyst is easy (Anwer et al. 2019; Babuponnusami and Muthukumar 2014; Joseph et al. 2009; Paździor et al. 2018; Krishnan et al. 2017; Verma and Samanta 2018).
Semiconductors are widely used photocatalysts, such as TiO2, ZnO, ZnS, Fe3O4, CdS, C3N4 and many others (Chen et al. 2020; Cui et al. 2018; Davar et al. 2015; Hu et al. 2020; Mahmoodi 2014; Saravanan et al. 2016; S. Wang et al. 2014; Ye et al. 2012). But among widely explored are TiO2 and ZnO. But problems associated with semiconductors are wide bandgap, nonmagnetic nature. Because of these properties, semiconductors can be only used with UV radiation and are difficult to remove after completion of degradation, while this problem can be resolved by using spinel catalyst which has a narrow bandgap, and if ferrite spinels are used it shows better magnetic properties than semiconductors (Eiichi et al. 2003; Kirankumar and Sumathi 2020). While even spinels are not as effective as semiconductors even under UV radiation, combination can be a promising solution to the hurdles of both types of catalysts (Mamba and Mishra 2016). Composites of semiconductors and spinel have advantages of easy separation, utilize almost whole UV and visible spectrum of sunlight which is nearly 50% (combine) of sunlight, higher surface area and a large number of active sites (Gawande et al. 2015; Govan and Gun’ko 2014; Johnson 2017; Parsons et al. 2009; Sun et al. 2019; Wang and Astruc 2017; Zhang et al. 2010).
Jing et al.(Jing et al. 2016) have investigated photocatalytic performance of Ag/Ag3VO4 and 5% CoFe2O4/Ag/Ag3VO4 composite. The result has shown that 49.75% and 61.48% tetracycline was photo-degraded, respectively. This shows that incorporation of CoFe2O4 improves the activity, and even composite shows magnetic separability. They have confirmed the better performance for several runs. Photocatalytic activity of core–shell structured Fe3O4/SiO2/TiO2 nanocomposites synthesized using the sol–gel process was compared with SiO2 TiO2 by Ye et al. (Ye et al. 2010). This composite shows additional advantages of high chemical stability, fast magnetic separation and maintenance of the photocatalytic activity for at least eighteen cycles. Similarly, Wang et al. ( Wang et al. 2012) synthesized Fe3O4/SiO2/TiO2 NCs using the sol–gel method and investigated its photocatalytic activity on MB that was present in an aqueous solution at room temperature and pH = 10. Under UV irradiation, the NCs showed higher photodegradation of MB (78%) within 5 min. Here, in Table 1 some of the other composites are briefed. Other works on CoCr2O4 / TiO2 for methyl orange degradation under UV light by Shojaei et al. (Shojaei et al. 2013), while Ni0.65Zn0.35Fe2O4 / r-GO has been investigated as photocatalyst by Javed et al. (Javed et al. 2019).
In this work, dye degradation performance of photocatalyst nickel ferrite and nickel ferrite/ titanium dioxide synthesized via co-precipitation method has been investigated. Materials from two different generations are combined to obtain photocatalyst with desired properties to sequester ultraviolet and visible ranges from whole solar spectrum reaching to the earth, better dye decolouration and mineralization along with effective separation at the end of the process. Some of the characterizations have been done to investigate the properties of prepared catalysts.
Materials and methods
Material
All chemicals used were analytical or laboratory-grade reagents which were supplied by Ranbaxy, Spectrochem, HPLC, NICK, CDH, MERCK. The detailed purity and phase of precursors and other reagents are as follows: citric acid (Ranbaxy, 99.9%), ethylene glycol (Spectrochem, 99.9%), sulfuric acid (HPLC, 98%), sodium hydroxide (NICK, 99.9%), potassium dichromate (HPLC), Ag2SO4 (NICK, 99.9%), ferroin indicator (HPLC), ferrous ammonium sulphate (HPLC, 99.9%), HgSO4 (HPLC, 99.9%), Fe2O3 (MERCK, 99.5%), distilled water (MERCK), iron nitrate—Fe(NO3)3∙9H2O (HPLC, 99.0%), nickel nitrate—Ni(NO3)2.6H2O (HPLC, 99.0%), TiO2 (HPLC, 80% anatase and 20% rutile), Na2CO3 (NICK, 99.9%).
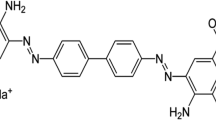
Reactive turquoise blue 21 (RB21), a copper phthalocyanine reactive group with (molecular weight = 1282.97 g/mol), was generously supplied by Avni Dye Chem Industries, Ahmedabad (Fig. 1).
Preparation method
Preparation of NiFe2O4
The co-precipitation method was adopted for the synthesis of NiFe2O4 and NiFe2O4/ TiO2. A stoichiometric amount of nickel nitrate (hexahydrate) and ferric nitrate (nonahydrate) are mixed. This mixture is stirred until it became a homogenous solution. Na2CO3 (sodium hydroxide, 1 M) was used as a precipitating agent and added dropwise until pH ≥ 10. Once the desired pH is obtained solution is kept for ageing for 1 h at 80 ℃. After ageing, filtration, several times washing of precipitates has been carried out and precipitates dried at 110 ℃ overnight and calcined at 700 ℃ for 5 h. Refer to Fig. 2.
Preparation of NiFe2O4 / TiO2
A typical procedure for preparing nanocomposite NiFe2O4/TiO2catalysts was prepared by adding nickel ferrite into suspension of TiO2, TiO2 was added in varying amounts for preparation of NF: TiO2 = 90:10, 80:20, 50:50, 10:90. The suspension was stirred further for proper mixing. Heating was given to the solution then it was calcined in a muffle furnace under 700 °C (ramp = 10 °C/min) for 5 h to form NiFe2O4/TiO2. Experimental set-up for the NiFe2O4/TiO2 nanocomposite is reported in Fig. 3. Samples are named as follows: NFT90, NFT80, NFT50, NFT10, NF, T for NF:TiO2 = 90:10, 80:20, 50:50, 10:90, and last two for bare nickel ferrite and TiO2. Refer to Fig. 3.
Characterization
Some of the basic characterizations were carried out such as FTIR, zeta sizer analysis and UV–DRS analysis, respectively, for phase check, practical size and for bandgap measurements Fourier transform infrared spectroscopy was carried out to check bond stretching and whether the prepared spinel is inverse type or normal. Zeta potential analysis was carried out to check the particle size of the prepared catalyst. It must be within the nanometric size range to give higher surface area and better catalytic activity. To find out the bandgap of catalyst, which is essential to be known for predicting whether the catalyst activation is possible under sunlight or not. As discussed above, it is observed that if the catalyst bandgap is within 1.98 to 3.2 eV range it can be activated under sunlight.
Photocatalytic activity
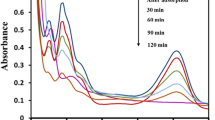
Photocatalytic activity of spinel and nanocomposite were measured by degradation ofRB21dye under natural sunlight irradiation at neutral, acidic and basic pH values. A 150 ml of 40 mg/l RB21 dye aqueous solution and its corresponding dose of photocatalyst were added in a quartz glass container and stirred for 30 min in dark. After every 30 min up to 150 min irradiation time, 25 ml of the suspension sample was withdrawn and then solution and particles are separated by an extra magnate. The photocatalytic degradation process can be confirmed by UV–Vis spectrophotometer for colour removal analysis by checking absorbance at 645 nm and by COD degradation by APHA procedure—close reflux. Figure 4 shows the experimental set-up for photocatalytic dye degradation.
Results and discussion
Characterization
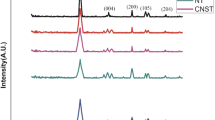
Zeta sizer results of catalyst show good agreement with JCPDS card no. 742081 for NF and JCPDS card #84–1286 as TiO2 was used as prepared and NF was used as synthesized. As per analysis, particle sizes are in the range of 103 nm, refer to Fig. 4, while FTIR spectroscopy shows bond stretching of metal and oxygen for each site atoms (A and B both sites) for these results, refer to Figs. 5 and 6. The bandgap of the as-prepared composite is assumed to be in between bandgap of spinel and metal oxide and from the literature it is 2.69 eV for composites which may provide better efficiency under sunlight and it is lower than that of TiO2 (3.2 eV) (Baig et al. 2020). As-prepared NiFe2O4 (NF) shows bandgap of 2.07 eV which is in agreement with what the other researchers have obtained, while composite shows bandgap of 2.927 eV, which is very close to bandgap of TiO2 (Fig. 8). But not only bandgap is factor that affects the catalyst performance but other parameters such as separation ability and surface area are also important, here in the main objective to obtain better catalyst for dye gradation. Ease of separation of NF is also one of add-on benefit. And partially it was observed that even though bandgap of composite is quite higher than the NF but it shows better dye degradation compared to NF only that might be because of that TiO2 can absorb in ultraviolet region, and spinel can absorb wavelengths from visible range of solar spectrum which to gather shares nearly half of the solar spectrum reaching to the surface. And even good magnetic properties of NF facilitate ease of separation of catalyst too. That is also true that some active sites are not available due to composite formation hence shows bit lesser dye degradation than theoretically assumed. FTIR analysis shows that pure phases are formed as no other vibrational spectra other than that related to Ni–O, Fe–O, O–O, Ni–Fe and Ti–O bonds could be observed. Major peaks were observed around 590 cm−1 and 400 cm−1 which shows stretching of metal oxygen bond those are present in spinel structure of NF sample, which is in agreement with other reported work as well (Li et al. 2014; Ojemaye et al. 2017) (Figs. 6 and 7).
Photocatalytic activity
Photocatalytic dye degradation was carried out under solar light. Refer to Table 1 for COD removal efficiency of composites and parent catalysts, i.e. NFTs and NiFe2O4 and TiO2. As depicted in the table, it is clear that NiFe2O4 shows better COD removal but at the cost of colour removal efficiency. Referring to Fig. 9, it is clear that for colour removal TiO2 performs far better than other catalysts but is accompanied by less COD removal. Hence, for optimum performance with the removal of both COD and colour composite NFT90 best fits the requirements.
Effect of various parameters is also investigated such as pH, catalyst dosage, initial dye concentration and irradiation time. Refer to Figs. 10, 11 and 12 for the effect of pH, catalyst dosage and effect of initial dye concentration, respectively, for selected catalyst NFT90. Results show that at neutral pH (= 7) better performance was observed than other pH values (i.e. 3, 5, 9, 12). The main reason is the pKa value of dye and catalyst, pKa value for dye is 5.5 and the same for the catalyst is 8.5, which restrict the range of pH for better performance between 5.5 and 8.5. At lower pH better COD removal (nearly 30%) can be observed while at higher pH better colour removal (nearly complete removal of colour) is observed, because of repulsion of anionic dye molecules with negatively charged catalyst surface at higher pH and vice versa. Another parameter is catalyst dosage, generally, an increase in catalyst dosage improves the dye degradation performance. But observations suggest that as catalyst dosage increases above 0.5 g, solar light penetration is reduced because of a large amount of suspended solids particles. Higher dye degradation is observed for the lowest concentration of 10 ppm of dye. But all other parameters are checked for a dye concentration of 40 ppm. Further, as the time of irradiation increases, the dye degradation also increases but at a different rate for each catalyst.
Conclusion
Our observation shows that composites show better results for COD removal and dye degradation compared to spinel and TiO2 alone, as this combination of photocatalysts from two different generations facilitates the use of a large part of the solar spectrum, better separation due to magnetic properties of NiFe2O4. From the observations, we can hypothesize that there can be the insertion of a new energy level in between the conduction band and valance band of TiO2 and NiFe2O4 because of the difference in the bandgap of both the materials, which may also promote the separation of photoinduced electrons and photons. Over this lower dye concentration (~ 40 ppm) and neutral pH and 0.5 g catalyst dosage are favourable conditions for dye degradation under daytime sunlight, while other characterizations show agreement with other literature.
Data availability
All relevant data and material are presented in the main paper.
References
Ahmed TF, Sushil M, Krishna M (2012) Impact of dye industrial effluent on physicochemical characteristics of Kshipra river, Ujjain City, India 1(June), 41–45. http://www.isca.in/IJENS/Archive/v1/i2/8.ISCA-IRJEvsS-2012-041.php
Anwer H, Mahmood A, Lee J, Kim K, Park J, Yip ACK (2019) Photocatalysts for degradation of dyes in industrial effluents : Opportunities and challenges 12(1):955–972. https://doi.org/10.1007/s12274-019-2287-0
Babuponnusami A, Muthukumar K (2014) A review on Fenton and improvements to the Fenton process for wastewater treatment. J Environ Chem Eng 2(1):557–572. https://doi.org/10.1016/j.jece.2013.10.011
Baig MM, Pervaiz E, Afzal MJ (2020) Catalytic activity and kinetic studies of core@shell nanostructure NiFe2O4@TiO2FOR photocatalytic degradation of methyl orange dye. J Chem Soc Pakistan 42(4):531–541. https://doi.org/10.52568/000669
Benkhaya S, Souad M, El A (2020) Classifications, properties, recent synthesis and applications of azo dyes 6(October 2019). https://doi.org/10.1016/j.heliyon.2020.e03271
Chacko JT, Subramaniam K (2011) Enzymatic Degradation of Azo Dyes – A Review. Int J Environ Sci 1(6):1250–1260. https://doi.org/10.6088/ijes.00106020018
Chen C, Zhou J, Geng J, Bao R, Wang Z, Xia J, Li H (2020) Perovskite LaNiO3/TiO2 step-scheme heterojunction with enhanced photocatalytic activity. Appl Surf Sci 503. https://doi.org/10.1016/j.apsusc.2019.144287
Cui H, Li B, Li Z, Li X, Xu S (2018) Z-scheme based CdS/CdWO 4 heterojunction visible light photocatalyst for dye degradation and hydrogen evolution. Appl Surf Sci 455(May):831–840. https://doi.org/10.1016/j.apsusc.2018.06.054
Davar F, Majedi A, Mirzaei A (2015) Green Synthesis of ZnO Nanoparticles and Its Application in the Degradation of Some Dyes. 1746:1739–1746. https://doi.org/10.1111/jace.13467
Eiichi H, Yutaka K, Takashima H, Sato T, Tomita A (2003) Optical properties of transition-metal doped spinels. 12(4):467–473. https://doi.org/10.1142/S0218863503001584
Forgacs E, Cserháti T, Oros G (2004) Removal of synthetic dyes from wastewaters: A review. Environ Int 30(7):953–971. https://doi.org/10.1016/j.envint.2004.02.001
Gawande MB, Monga Y, Zboril R, Sharma RK (2015) Silica-decorated magnetic nanocomposites for catalytic applications. Coord Chem Rev 288:118–143. https://doi.org/10.1016/j.ccr.2015.01.001
Govan J, Gun’ko Y (2014) Recent Advances in the Application of Magnetic Nanoparticles as a Support for Homogeneous Catalysts. Nanomaterials 4(2):222–241. https://doi.org/10.3390/nano4020222
Gusain R, Gupta K, Joshi P, Khatri OP (2019) Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites : A comprehensive review Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites : A co. Adv Coll Interface Sci 272(August):102009. https://doi.org/10.1016/j.cis.2019.102009
Hethnawi A, Nassar NN, Manasrah AD, Vitale G (2017) Polyethylenimine-functionalized pyroxene nanoparticles embedded on Diatomite for adsorptive removal of dye from textile wastewater in a fixed-bed column. In Chemical Engineering Journal Vol. 320 Elsevier B.V. https://doi.org/10.1016/j.cej.2017.03.057
Hodges BC, Cates EL, Kim JH (2018) Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat Nanotechnol 13(8):642–650. https://doi.org/10.1038/s41565-018-0216-x
Hu H, Lin Y, Hu YH (2020) Core-shell structured TiO2 as highly efficient visible light photocatalyst for dye degradation. Catal Today 341(October 2018):90–95. https://doi.org/10.1016/j.cattod.2019.01.077
Javed H, Rehman A, Mussadiq S, Shahid M, Khan MA, Shakir I, Agboola PO, Aboud MFA, Warsi MF (2019) Reduced graphene oxide-spinel ferrite nano-hybrids as magnetically separable and recyclable visible light driven photocatalyst. Synth Met 254(February):1–9. https://doi.org/10.1016/j.synthmet.2019.05.013
Jing L, Xu Y, Huang S, Xie M, He M, Xu H, Li H, Zhang Q (2016) Novel magnetic CoFe2O4/Ag/Ag3VO4 composites: Highly efficient visible light photocatalytic and antibacterial activity. Appl Catal B 199:11–22. https://doi.org/10.1016/j.apcatb.2016.05.049
Johnson BFG (2017) Model nanoparticles in catalysis. Metal Nanopart Clusters: Advances in Synthesis, Properties and Applications 24(October):165–199. https://doi.org/10.1007/978-3-319-68053-8_5
Jonstrup M, Kumar N, Murto M, Mattiasson B (2011) Sequential anaerobic-aerobic treatment of azo dyes: Decolourisation and amine degradability. Desalination 280(1–3):339–346. https://doi.org/10.1016/j.desal.2011.07.022
Joseph CG, Li Puma G, Bono A, Krishnaiah D (2009) Sonophotocatalysis in advanced oxidation process: A short review. Ultrason Sonochem 16(5):583–589. https://doi.org/10.1016/j.ultsonch.2009.02.002
Joshi M, Bansal R, Purwar R (2004) Colour removal from textile effluents. Indian J Fibre Text Res 29(June):239–259
Katheresan V, Kansedo J, Lau SY (2018) Efficiency of various recent wastewater dye removal methods : A review. 6(June), 4676–4697. https://doi.org/10.1016/j.jece.2018.06.060
Khehra MS, Saini HS, Sharma DK, Chadha BS, Chimni SS (2006) Biodegradation of azo dye C.I. Acid Red 88 by an anoxic - Aerobic sequential bioreactor. Dyes Pigments 70(1):1–7. https://doi.org/10.1016/j.dyepig.2004.12.021
Kiernan JA (2001) Classification and naming of dyes, stains and fluorochromes. 2111:261–278. https://doi.org/10.1080/bih.76.5-6.261.278
Kirankumar VS, Sumathi S (2020) A review on photodegradation of organic pollutants using spinel oxide. Mater Today Chem 18:100355. https://doi.org/10.1016/j.mtchem.2020.100355
Klaus H (Ed.) (2008) Industrial Dyes: Chemistry, Properties, Applications, Wiley. https://doi.org/10.1002/3527602011
Li X, Tan G, Peng Y, Mellors N (2014) Nanostructural and magnetic studies of virtually monodispersed NiFe2O4 nanocrystals synthesized by a liquid – solid-solution assisted hydrothermal route Nanostructural and magnetic studies of virtually monodispersed NiFe 2 O 4 nanocrystals synthesized by a liquid – solid-solution assisted hydrothermal route. March 2012, 0–9. https://doi.org/10.1007/s11051-012-0751-6
Mahmoodi NM (2014) Binary catalyst system dye degradation using photocatalysis. 15(2):273–280. https://doi.org/10.1007/s12221-014-0273-1
Mamba G, Mishra A (2016) Advances in magnetically separable photocatalysts: Smart, Recyclable materials for water pollution mitigation. Catalysts 6(6):1–34. https://doi.org/10.3390/catal6060079
Manavi N, Kazemi AS, Bonakdarpour B (2017) The development of aerobic granules from conventional activated sludge under anaerobic-aerobic cycles and their adaptation for treatment of dyeing wastewater. Chem Eng J 312:375–384. https://doi.org/10.1016/j.cej.2016.11.155
Mojsov KD, Andronikov D, Janevski A, Kuzelov A, Gaber S (2016) The Application of Enzymes for the Removal of Dyes From. Adv Technol 5(1):81–86
Nidheesh PV, Zhou M, Oturan MA (2018) An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 197:210–227. https://doi.org/10.1016/j.chemosphere.2017.12.195
Ojemaye MO, Okoh OO, Okoh AI (2017) Performance of NiFe2O4-SiO2-TiO2 Magnetic Photocatalyst for the Effective Photocatalytic Reduction of Cr(VI) in Aqueous Solutions. J Nanomater 2017(Vi). https://doi.org/10.1155/2017/5264910
Osman M (2014) Waste Water Treatment in Chemical Industries: The Concept and Current Technologies. J Waste Water Treat Anal 05(01):1–12. https://doi.org/10.4172/2157-7587.1000164
Parsons JG, Lopez ML, Peralta-Videa JR, Gardea-Torresdey JL (2009) Determination of arsenic(III) and arsenic(V) binding to microwave assisted hydrothermal synthetically prepared Fe3O4, Mn3O4, and MnFe2O4 nanoadsorbents. Microchem J 91(1):100–106. https://doi.org/10.1016/j.microc.2008.08.012
Paździor K, Bilińska L, Ledakowicz S (2018) A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem Eng J. https://doi.org/10.1016/j.cej.2018.12.057
Popli S, Patel UD (2015) Destruction of azo dyes by anaerobic–aerobic sequential biological treatment: a review. Int J Environ Sci Technol 12(1):405–420. https://doi.org/10.1007/s13762-014-0499-x
Rajaram TÃ, Das A (2008) Water pollution by industrial effluents in India : Discharge scenarios and case for participatory ecosystem specific local regulation 40:56–69. https://doi.org/10.1016/j.futures.2007.06.002
Robinson T, McMullan G, Marchant R, Nigam P (2000) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Biores Technol 21(3):211–222. https://doi.org/10.1504/IJEP.2004.004190
Krishnan S, Rawindran H, Sinnathambi CM, Lim JW (2017) Comparison of various advanced oxidation processes used in remediation of industrial wastewater laden with recalcitrant pollutants Comparison of various advanced oxidation processes used in remediation of industrial wastewater laden with recalcitrant poll. https://doi.org/10.1088/1757-899X/206/1/012089
Sandhya S, Padmavathy S, Swaminathan K, Subrahmanyam Y, Kaul S (2005) Microaerophilic–aerobic sequential batch reactor for treatment of azo dyes containing simulated wastewater. Proc Biochem
Saravanan R, Sacari E, Gracia F, Mansoob M, Mosquera E, Kumar V (2016) Conducting PANI stimulated ZnO system for visible light photocatalytic degradation of coloured dyes. J Mol Liq 221:1029–1033. https://doi.org/10.1016/j.molliq.2016.06.074
Seow T, Lim C, Nor M, Mubarak M, Lam C, Yahya A, Ibrahim Z (2016) Review on Wastewater Treatment Technologies. Int J Appl Environ Sci 11(1):111–126
Shojaei AF, Tabari AR, Loghmani MH (2013) Normal spinel CoCr2O4 and CoCr2O 4/TiO2 nanocomposite as novel photocatalysts, for degradation of dyes. Micro and Nano Lett 8(8):426–431. https://doi.org/10.1049/mnl.2013.0114
Singh S (2015) Study of Waste Water Effluent Characteristics Generated from Paper Industries. 2(17):1505–1509. https://www.krishisanskriti.org/vol_image/25Jan201610015432%20%20Shivnarayan%20Singh%201505-1509.pdf
Srinivasan A, Viraraghavan T (2010) Decolorization of dye wastewaters by biosorbents: A review. J Environ Manage 91(10):1915–1929. https://doi.org/10.1016/j.jenvman.2010.05.003
Sun M, Han X, Chen S (2019) Synthesis and photocatalytic activity of nano-cobalt ferrite catalyst for the photo-degradation various dyes under simulated sunlight irradiation. Mater Sci Semicond Proc 91(May 2018):367–376. https://doi.org/10.1016/j.mssp.2018.12.005
Verma P, Samanta SK (2018) Microwave-enhanced advanced oxidation processes for the degradation of dyes in water. In Environmental Chemistry Letters, Springer Sci Rev 16(3):969–1007. https://doi.org/10.1007/s10311-018-0739-2
Wang D, Astruc D (2017) The recent development of efficient Earth-abundant transition-metal nanocatalysts. Chem Soc Rev 46(3):816–854. https://doi.org/10.1039/c6cs00629a
Wang R, Wang X, Xi X, Hu R, Jiang G (2012) Preparation and photocatalytic activity of magnetic Fe3O 4/SiO2/TiO2 composites. Adv Mater Sci Engi. https://doi.org/10.1155/2012/409379
Wang S, Li D, Sun C, Yang S, Guan Y, He H (2014) Applied Catalysis B : Environmental Synthesis and characterization of g-C 3 N 4 / Ag 3 VO 4 composites with significantly enhanced visible-light photocatalytic activity for triphenylmethane dye degradation. Appl Catal B, Environ 144:885–892. https://doi.org/10.1016/j.apcatb.2013.08.008
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: A review. Adv Coll Interface Sci 209:172–184. https://doi.org/10.1016/j.cis.2014.04.002
Ye A, Fan W, Zhang Q, Deng W, Wang Y (2012) CdS – graphene and CdS – CNT nanocomposites as visible-light photocatalysts for hydrogen evolution and organic dye degradation. Catal Sci Technol paper 969–978. https://doi.org/10.1039/c2cy20027a
Ye M, Zhang Q, Hu Y, Ge J, Lu Z, He L, Chen Z, Yin Y (2010) Magnetically Recoverable Core-Shell Nanocomposites with Enhanced Photocatalytic Activity. Chem Eur J 16(21):6243–6250. https://doi.org/10.1002/chem.200903516
Zhang S, Niu H, Cai Y, Zhao X, Shi Y (2010) Arsenite and arsenate adsorption on coprecipitated bimetal oxide magnetic nanomaterials: MnFe2O4 and CoFe2O4. Chem Eng J 158(3):599–607. https://doi.org/10.1016/j.cej.2010.02.013
Acknowledgements
The authors are grateful to Department of Chemical Engineering, Vishwakarma Government Engineering College and Department of Chemical Engineering, School of Technology, Pandit Deendayal Petroleum University, for the permission to publish this research.
Funding
Not Applicable.
Author information
Authors and Affiliations
Contributions
All the authors make a substantial contribution to this manuscript. PS, KJ, MS, AU and FP participated in drafting the manuscript. PS, KJ, MS, AU and FP wrote the main manuscript. All the authors discussed the results and implications on the manuscript at all stages.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shah, P., Joshi, K., Shah, M. et al. Photocatalytic dye degradation using nickel ferrite spinel and its nanocomposite. Environ Sci Pollut Res 29, 78255–78264 (2022). https://doi.org/10.1007/s11356-022-21248-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21248-0