Abstract
Highly efficient and effective treatments of hazardous dye-based color effluents are a major problem in the industrial sector. In this research, the cobalt ferrite (CoFe2O4) catalyst was produced and used for the degradation of Congo red (CR) as a model dye from aqueous solution. For a said purpose, cobalt ferrite (CoFe2O4) nanostructures with photocatalytic degradation potential were engineered via co-precipitation method using Fe2(SO4)3, CoO2, and triethylene glycol (as a stabilizing agent). As prepared, CoFe2O4 nanostructures were further surface-functionalized with 3-APTES and tested for CR degradation. The prepared CoFe2O4 nanostructures were characterized by X-ray diffraction, Fourier transform infra-red (FT-IR), scanning electron microscopy (SEM), and Brunauer-Emmitt-Teller (BET) analysis. UV-visible absorption was used to measure the optical band gap of prepared CoFe2O4 nanostructures through Tauc plots. The as-prepared CoFe2O4 nanostructure bandgap was found to be 2.71 EV while using an acidic medium. The degradation rates of CR dye for bs-CoFe2O4, as-CoFe2O4, and fs-CoFe2O4 nanostructures at pH 9 were 84, 87, and 92%, respectively. Furthermore, the influences of various process parameters, i.e., the effect of catalyst dose, contact time, dye dose/concentration, pH effect, and effect of different acids, were checked for the prepared three types of nanostructures, i.e., bs-CoFe2O4, as-CoFe2O4, and fs-CoFe2O4. The kinetics models properly explained that the reaction of degradation following pseudo-first-order kinetics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The removal of organic pollutants from wastewater is a significant measure of environmental protection. Several types of hazardous dyes, extra marketable pigments, and bleaches have developed the attention of environmental remediation efforts (Zhu et al. 2009; Bilal et al. 2019; Rasheed et al. 2019A; Liu et al. 2019). Nowadays, the world is facing the main difficulties of environmental contamination. Organic chemicals are present in effluents from domestic and industrial sources. These pollutants must be destroyed or removed before these are discharged to the near aquatic resources. The discharging of a large amount of wastewater from textile, pharmaceutical, dying, cosmetic, food, printing, and photographic industries produces environmental pollution. These effluents are dangerous in nature because they normally contain considerable amount of different poisonous materials, which are not decomposable (Bilal et al. 2017; Bilal et al. 2018a; Bilal et al. 2018b; Ali et al. 2019).
Dye constituents are one of the main categories of organic compounds, which may find many beneficial uses in everyday life. For example, leather, paper, plastic, and textile industries accumulate up to 80% of the total dyes. Synthetic dye materials with some other dyestuff are comprised of the main group of world water pollutants. In the end, about 1 to 15% of the synthetic fabric dyes used in different industries are disposed to the wastewater channels and in the last reached the main water streams (Bilal and Asgher 2015; Salazar-López et al. 2017). The dying process also produced effluent which contains more than 10–15% dyestuff. The serious problem is to decrease the harmfulness levels and to allowed limits of the effluents before releasing the dye to water channels (Bilal et al. 2017; Rasheed et al. 2019B; Rasheed et al. 2019C). Some traditional or conventional physicochemical methods are used for the handling and treatment of dying polluted wastewater, which comprises adsorption, coagulation, filtration, and advanced oxidation processes such as photochemical oxidation, ozonation, and Fenton’s reagent (Bilal et al., 2018a). Different degradation procedures can be used for wastewater treatment and clarification, like catalysis, adsorption, degradation, electrochemical degradation, biological degradation, sonocatalytic degradation, and photo catalytic degradation (Bilal et al. 2017; Salazar-López et al., 2017; Bilal et al. 2017). Photo catalysis has emerged as a desirable technology for the treatment of wastewater and considered an efficient, eco-friendly, and cost-effective method to resolve problems of environmental pollution (Soylak et al. 2004; Bedoui et al. 2009; Samiey et al. 2014).
The organic pollutants can be decreases using the technique of photo catalysis because it is one of the growing destructive technique and it is oxidation-reduction reaction by using the energy of the photon, and in this process, electron gain energy and get excited and jump to the conduction band from valance shell, which as a result generate photo-generated hole (h+) and enhance catalytic performance (Bedoui et al. 2009; Casbeer et al. 2012; Iqbal et al. 2017). Zhou et al. (Zhou et al. 2008) synthesized CoFe2O4 nanocomposite by combustion reaction and studied the magnetic properties and composition of the CoFe2O4. Hosni et al. (Hosni et al. 2017) prepared CoFe2O4 nanoparticles via co-precipitation method and used different temperature ranges, i.e., (773 to 1223 k) for the annealing process. Rahimi et al. (Rahimi et al. 2013) used solvothermal method for the designed and synthesized Ba-Cd-Sr-Ti-doped Fe3O4 hollow nanosphere and used these nanospheres for the CR dye degradation under UV/visible light. The produced results show that at pH 6, there was about 99.5% degradation. Vijayaraghavan et al. (Vijayaraghavan et al. 2016) conducted research and used AFe2O4 (where A is Ba, Ca, and SR) for the photocatalytic degradation of CR dye under Xe-lamp and sunlight radiation. Under the light of Xe-lamp, BaFe2O4 show the maximum degradation 92% after 75 min. Murcia et al. (2011) utilized XeBr, KrCl, and Cl2 discharged excilamps for the CR dye photodegredation. The experimental results show that about > 90% degradation was achieved in the absence of H2O2 and using KrCl excilamps.
In this research work, we used the co-precipitation method and prepared cobalt ferrite (CoFe2O4) nanostructures. The stabilizing agent was triethylene glycol (TEG), then thermally treated at 600 °C for 6 h, and surfaced functionalized with 3-(APTES). Different analytical techniques were used for the characterization of as-prepared nanostructures. CR dye was used to check the photocatalytic properties of the novel photocatalyst. The effect of various reaction components was also optimized. The kinetic models of the prepared photocatalyst for the degradation of CR dye followed 1st order of kinetic.
2 Materials and Methods
2.1 Chemicals
All chemicals such as iron (III) sulfate hydrate [Fe2(SO4)3. 7H2O], cobalt oxide (CoO2), sodium hydroxide (NaOH), hydrochloric acid (HCl), 3-aminopropyltriethoxysilane (3-APTES), triethylene glycol (TEG), and Congo Red dye were kindly received from Sigma-Aldrich, USA. Figure 1 explains the chemical structure of the targeted CR dye.
2.2 Synthesis of CoFe2O4 Nanostructures
Cobalt ferrite nanostructure was prepared through co-precipitation by the 4:1 reaction of Fe2(SO4)3 and CoO2 (Mahboubeh et al. 2014). The produced aqueous solution contains 0.31 mM of Fe2(SO4)3 and 0.048 mM of CoO2, and about 50 mmol of triethylene glycol in 100 ml were thoroughly mixed for 2 h while constant stirring at room temperature. After vigorous stirring, 10 M of sodium hydroxide solution on 100 ml of volume was added drop wise into 200 ml of salts solution in order to maintain the pH up to 7–8, on these pH reddish-brown color CoFe2O4 precipitate are formed, which are filter off and washed many times using deionized water followed by heating for 24 h at 80 °C. The obtained precipitate was mechanically grinding into a fine powder. The bs-CoFe2O4 nanostructures are subjected to thermal heating in a furnace for 6 h at 600 °C. It was noted that TEG stabilized CoFe2O4 nanostructures at the interface and chemical due to the hydroxyl groups in the glycol, which may oxidize and leads to form a complex with the transition metal (Ruttink et al. 2012; Rishikeshi et al. 2013). The formation of hydrated cobalt ferric acetate hydroxide complex following to reduction and formation of CoFe2O4 nanostructures on heating is also explained in the literature (Skrabalak et al. 2008).
2.3 Surface Modification of CoFe2O4 Nanostructures
The surface of thermally as-CoFe2O4 nanostructures was modified in two phases, first using 1 M HCl by keeping CoFe2O4 nanostructures for 30 min, then filtration and washed twice using deionized water. The hydroxylated CoFe2O4 nanostructures were then treated with a 0.1 M solution of 3-APTES at constant stirring for 2 h at 60 °C temperature, then filtered and dried to obtain fs-CoFe2O4 nanostructure.
2.4 Percent Photocatalytic Degradation CR Dye
The photocatalytic degradation potential of CoFe2O4 nanostructures was tested against CR dye. For a said purpose, a freshly prepared aqueous solution of CR dye with 10 ppm concentration was treated with 10 mg of CoFe2O4 nanostructures. The above reaction mixture was thoroughly mixed and kept at room temperature for 30 min in the dark. To obtain adsorption/desorption equilibrium between CR and CoFe2O4, constant stirring was maintained. UV irradiation was passed from the solutions at different intervals of time and then subjected to centrifugation at 4500 rpm for 15 min. To record the photo catalytic degradation of CR dye, every 30 min of reaction period, up to 5 ml sample was collected and subjected to the UV-Vis spectral analysis. Following Eq. (1) was used to calculate the percent photo catalytic degradation (%D) of CR dye.
where a is initial absorbance and At is observed after time t.
2.5 Contact Time Effect on the Photocatalytic Degradation of CR Dye
The influence of reaction time on the photocatalytic reaction of CR was studied. Briefly, the solutions of a dye were exposed to the radiation of UV light at a different time interval from 30 to 180 min. Then the irradiated solutions were centrifuged for 15 min
at 4500 rpm, and the total degradation was recorded via UV-Vis spectrophotometric analysis.
2.6 Influence of Catalyst Dose on the Photocatalytic Degradation of CR Dye
Variable amount of the photocatalyst dose, i.e., 10, 15, 20, 25, 30, 35, 40, 45, 50, and 60 mg was used in the reaction mixture in order to see its degradation affect. The CR dye sample was treated for 30 min in the dark with each specified dose of the catalyst. After the stipulated reaction period, the reaction mixture was centrifuged at 4500 for 15 min. Finally, the resultant solution was subjected to UV/Vis spectral analysis to measure the percent degradation.
2.7 Influence of Dye Dose on the Photocatalytic Degradation of CR
Different concentrations of dye solutions, i.e., 5, 10, 15, 20, 25, 30, 35, 40, 45, and 50 ppm were used in order to see the dye concentration effect on the percent degradation (Ikram et al. 2018). The reaction solutions, each separately, were kept in the dark for 30 min then exposed to UV light. Finally, the test samples were centrifuged and subjected to UV/Vis spectral analysis to measure the percent degradation.
2.8 Influence of pH on the Photocatalytic Degradation of CR Dye
pH influence on the percent CR dye degradation was tested herein. For a said purpose, CR dye solutions were prepared with different pH ranges from 1 to 14 in order to optimize the pH effect. The reaction was performed using optimized reaction time, catalyst dose, and dye concentration (optimized in earlier sections). Each reaction solution with different pH value was treated for 30 min in the dark, then exposed to UV light. Finally, the test samples were centrifuged and subjected to UV/Vis spectral analysis to measure the percent degradation.
2.9 Influence of Acids on the Photocatalytic Degradation of CR Dye
The effect of different acids on the photocatalytic degradation of CR dye was checked. Freshly prepared 0.01 M dm−1 solution of different acids (HNO3, H2SO4, and HCl) were used in the CR dye solution preparation. This was followed by 30 min reaction in the dark under optimum reaction conditions. Finally, the test samples were centrifuged and subjected to UV/Vis spectral analysis to measure the percent degradation.
3 Results and Discussion
3.1 Structure Morphology and Chemical Compositions
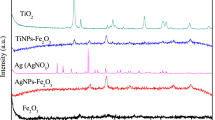
The XRD analysis confirms the crystallinity and phase purity of different types of spinel CoFe2O4 nanostructures. Figure 2 shows the XRD pattern of the bs-CoFe2O4, as-CoFe2O4, and fs-CoFe2O4 nanostructures. The XRD pattern of bs-CoFe2O4 and as-CoFe2O4 show that both are entire of single-phase despite that the bs-CoFe2O4 nanostructure exhibit amorphous nature as shown by the weakening of sharp diffraction peaks. The XRD pattern of as-CoFe2O4 nanostructure show eight peaks that coincide with the cubic spinel CoFe2O4 (Franklinite) phase with the Fd3m space group (Reference Code No. 01079-1744) (Goya and Leite 2003; Deraz and Abd-Elkader 2015). These peaks can be fitted with the following miller indexes corresponding to the crystal plane of spinel CoFe2O4; (003), (012), (104), (113), (006), (024), (205), and (116) at 1.288°, 21.147°, 30.082°, 35.431°, 37.063°, 43.060°, 47.148°, and 48.477° θ position, respectively. The XRD pattern of the bs-CoFe2O4 nanostructure does not exhibit sharp and well-resolved diffraction peaks. However, any additional peaks of the second phase are not observed, which indicates the formation of cubic spinel CoFe2O4 nanocrystals. The XRD spectra of fs-CoFe2O4 nanostructure also exhibit eight peaks with the following miller indexes (111), (220), (311), (222), (400), (31), (422), and (511) at 18.289, 30.085, 35.438, 37.058, 43.059, 47.150, 53.446, and 56.975 θ position, while an additional broad peaks around 23° is observed that indicates the functionalization of fs-CoFe2O4 nanostructure with 3-APTES groups. The crystallites size of different CoFe2O4 nanostructures can be calculated from the diffraction broadening of (003) peak by using the following Scherrer’s formula (Eq. 2).
where D is the diameter of the particles, Ks is Scherrer’s constant, Cuλ is the wavelength of X-ray radiation, β is a full-width half-maximum of (003) peak reduced for instrumental broadening; and θ position. Thus, the crystallite size of the bs-CoFe2O4, as-CoFe2O4, and fs-CoFe2O4is estimated as 5.30, 4.82, and 5.26, respectively.
3.2 Scanning Electron Microscopy Analysis
The morphological analysis of the different types of CoFe2O4 nanostructures is carried out by using SEM techniques. Figure 3 shows the SEM micrographic images and surface morphology of the bs-CoFe2O4, as-CoFe2O4, and fs-CoFe2O4 nanostructures. SEM images show the inhomogeneous distribution of particles with an aggregate of bs-CoFe2O4 in the center (5 μm). The surface morphology of as-CoFe2O4 nanostructures is more uniform, and the average surface roughness is estimated to be lower than as compared with bs-CoFe2O4 nanostructures (3 μm). It is due to the thermal annealing which leads to the removal of water molecules (Rafaqat et al. 2019) and organic species like TEG and its oxidized products and shrinking of the bs-CoFe2O4 crystallites. However, high temperature (i.e., 600 °C) promote the nucleation and growth of CoFe2O4 nanostructure leading to the fusion of smaller crystallites to yield bigger aggregates of structures. These results are in agreement with the previous reports on thermal annealing of ferrite nanostructures (Mahdavi et al. 2013) Moreover, the surface modification procedure involves hydroxylation of as-CoFe2O4 nanostructures, which lead to the separation of loosely bound crystallites in sintered clumps of nanoparticles and hydroxylation of the surface of the nanoparticles. The hydroxylated CoFe2O4 nanostructures are then treated with 3-(APTES). The SEM image of fs-CoFe2O4 nanostructures shows that the bigger aggregates of nanostructures disappear, and the overall distribution of particles in improved after functionalization. The average surface roughness of fs-CoFe2O4 nanostructures is calculated to be (1 μm).
3.3 Fourier Transform Infra-Red Analysis
To investigate different functional groups of CoFe2O4 nanostructures (bs-CoFe2O4, as-CoFe2O4, and fs-CoFe2O4), Fourier transform infra-red (FTIR) spectroscopic characterization was performed. The recorded FTIR spectrum is shown in Fig. 4. FTIR spectrum line of bs-CoFe2O4 photocatalyst show three small peaks at about 3189, 3011, and 1647 cm−1, and these peaks are due to the presence of O-H bond of polyethylene glycol PEG (Priyadharsini et al. 2009). Similarly, peak at about 1400 cm−1 due to vibration of C-O stretching of polyethylene glycol PEG confirms PEG presence, and these bands further explain the interaction of C-O oxygen with Fe or Co on the surface of CoFe2O4, and peak, because of the Co-O stretching, are shown at 605 cm−1 (Zhang et al. 2007; Liu et al. 2007). Similarly, the FTIR spectrum line for as-CoFe2O4 nanostructure shows different peaks in Fig. 4. Absence of peaks at 3013 cm−1 and 3189 is the evidence that there are no more O-H groups, which means there is no water molecule (Priyadharsini et al. 2009). At 1414 cm−1, a peak centered which displays the binding vibration of C-H bond of carboxylate group (Lopez et al. 1998) and C-O stretching vibration of polyethylene glycol PEG was observed at 1027 cm−1 (Wang et al. 2009). Bending vibration of C-H is shown at about 859 cm−1, peak at about 547 cm−1 is because of Co-O binding vibration, and peak at 448 cm−1 is due Fe-O bond (Zhang et al. 2007; Liu et al. 2007). Now finally the third spectrum line in Fig. 4, which shows the FTIR spectrum of fs-CoFe2O4 nanostructures. The comparison of bs-CoFe2O4 and as-CoFe2O4 samples with spectrum of fs-CoFe2O4 has different number of peaks observed at different positions. A peak at 3342 cm−1 is due to O-H vibration present in water and peaks at about 2937, and 2859 cm−1 is for CH2CH2CH2-NH2, which explains that 3-APTES is properly grafted on to the surface of CoFe2O4 (Huang et al. 2003; Zaharieva et al. 2015). Peaks at about 1603 and 1459 cm−1 are due to binding vibration of N-H bond and stretching vibration of the C-H bond of carboxylate group (Huang et al. 2003). C-O stretching vibration of PEG causes peak at about 1278 cm−1, and vibration of N-O of nitrate group show peaks at 1023 and 906 cm−1. Now finally band at 749 cm−1 is due to C-H vibration of carboxylate group, and peak at 543 cm−1 are because of vibration of Co-O and Fe-O (Zhang et al. 2007; Liu et al. 2007).
3.4 Brunauer-Emmett-Taller Analysis of CoFe2O4 Nanostructures
The BET pore structure and surface area of the synthesized CoFe2O4 were determined by the measurement of N2 adsorption-desorption isotherm at 77 K (Fig. 5). Prior to N2 adsorption gas, the sample was degassed at 353 K for about 16 h. The adsorption branch in the three isotherms of mechanochemically treated materials bs-CoFe2O4, as-CoFe2O4, and fs-CoFe2O4 belong to type II of the IUPAC classification. They display hysteresis loops of type H3 that are typical of non-rigid aggregates of plate-like particles giving rise to slit-shape pores (Zaharieva et al. 2015).
The BET surface area of bs-CoFe2O4 is to be found at 111.9 ± 1 m/g, slop is 0.388 ± 1 g/cm and molecular cross-sectional area is 0.1620 nm. BET surface area of as-CoFe2O4 is to be found at 11.9 ± 1 m2/g, slop is 0.361 ± 1 g/cm3 STP and molecular cross-sectional area is 0.1620 nm2. The surface area of bs-CoFe2O4 is higher than as-CoFe2O4, meaning that the annealing treatment (at 600 °C) led to a slight collapse of the structure. BET surface area of fs-CoFe2O4 is to be found at 0.528 ± 1 m2/g, slop is 7.357 ± 1 g/cm3 STP and molecular cross-sectional area is 0.1620 nm2. After the surface modification of the as-CoFe2O4 sample, the surface area is almost equal to zero, indicating that the functionalization molecule is entrapped in the porosity.
3.5 Calculation of Bandgap Energy and Lambda Max
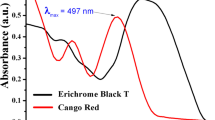
Bandgap energy may have a major role in the semiconductor. Bandgap energy of semiconductors affects different properties such as impurity and size of particles with structure, and it can be calculated experimentally from Touch-plots through the UV-visible spectroscopy data. Here we used the peak area, and the calculated value is divided by 1240 to calculate the binding energy (eV). As obtained binding energy was plotted vs (αhc)1/2 (where α is = (1/log abs), c is the speed light (msec−1) and h is plank constant. The point at which the line breaks and form a tangent on x-axis direction that is the bandgap energy of semiconductor in eV. The calculated band gap energy of CoFe2O4 nanostructures by using the current procedure is 2.71 eV. The calculated band gap energy f CoFe2O4 in acidic medium is illustrated in Fig. 6. Lambda max for CR dye was measured while using UV/vis spectrophotometer at the 200–800 nm of wavelength range, and the maximum absorbance for CR was noted at 497 nm as shown in Fig. 7.
3.6 Mechanism of Photocatalysis
UV light excited the electron from the surface of the exposed photocatalyst and jump from the valence band to the conduction band. The electron leaves a positive hole in the VB, which then, on reaction with water molecules, produces radicals, and these radicals effectively degrade dye (Mahmoodi et al. 2006). The mechanism of photocatalytic degradation is as;
3.7 Factors that Effect on the Photocatalytic Degradation of CR Dye
3.7.1 Effect of Contact Time
The contact time effects ( i.e., 20–180 min) on the photocatalytic degradation of CR dye were studied in the presence of all three types of photocatalysts and indicate that the degradation efficiencies of CR dye increase readily with increasing irradiation time interval. It is because at the start, the dye molecules concentration is more for degradation which later decreased with time. This also may be because initially large number of anionic radicals present in the solution attack on dye molecule with the time the anionic radicals decrease so that way the speed of degradation process may also slow down. The percentage of degradation efficiency of all the three kinds of CoFe2O4 nanostructure for the CR dye degradation was plotted against the contact time explained in Fig. 8. It shows that initially, the degradation rate of CR dye increases very rapidly up to 90 min, and then the degradation rate decreases gradually after the optimum time. The maximum degradation of CR dye in the presence of bs-CoFe2O4, as-CoFe2O4, and fs-CoFe2O4 nanostructures are 83%, 87%, and 89% respectively, after UV/Vis irradiation for 90 min (Shu et al. 2015).
3.7.2 Effect of Adsorbent Dose
To check the absorbance dose effect of CoFe2O4 nanostructures (bs-CoFe2O4, as-CoFe2O4, and fs-CoFe2O4) on the CR dye degradation under UV light, by using a different amount of photocatalyst, i.e., from 10 to 60 mg. The degradation efficiency (%) of all three types of photocatalyst for CR dye wee plotted against adsorbent dose as shown in Fig. 9. It shows that the rate of degradation of CR increases gradually with increase in adsorbent dose, i.e., up to 35 mg, it is due to the fact that a large amount of adsorbent dose provides large surface area for dye degradation, and then to increase further the catalyst dose (35 mg) the organic dye degradation rate almost remained constant (Chen et al. 2012). This is because the high catalyst may increase the rate agglomeration of adsorbent dose which further affects the surface area for degradation is decreased. Also, with increase in catalyst dose from 35 mg, leads to the increase in turbidity, which in turn decreases the penetration power of UV irradiation, this situation may reduce the photo-motivation potential of CoFe2O4 nanostructure (Casbeer et al. 2012; Sakthivel et al. 2003). Maximum dye degradation may occur in the presence of bs-CoFe2O4, as-CoFe2O4, and fs-CoFe2O4 photocatalyst are 84%, 87%, and 92%.
3.7.3 Effect of Dye Concentration
Dye concentration is one of the important parameters affecting the photocatalytic degradation of CR dye. The effect of dye dose on the degradation of CR dye shows that the maximum degradation occurs at 5 ppm of dye solution, and a further increase in dye dose leads to a decrease in the degradation process. It was observed that an increase in the concentration of dye resultant decreases the degradation of dye as shown in Fig. 10. The decreasing rate of photocatalytic degradation of CR dye is because an increase in dye concentration causing more interaction of light with the dye light and molecules unable to reach the surface of the catalyst, which leads to a decrease in the process of photocatalytic degradation. In the start, more dye molecules generate more inorganic ions like nitrate and sulfate in solution which compete with the molecules of the dye. Moreover, Bear-Lambert law says that the initial concentration of solution increases and the path length for the incoming photons to the solution decreases, and as a result, a very small number of photons reach to the surface of the catalyst and consequently a less photocatalytic degradation of dye (Saquib and Muneer 2002; Bhattacharyya and Sharma 2005).
3.7.4 Effect of pH
The effect of pH on the photocatalytic degradation of CR dye in the presence of CoFe2O4 nanostructure was observed in the range from 1.0 to 14.0, as shown in Fig. 11. It is indicated that in the start, the degradation (%) got increases with increase in pH till 9, and then started decrease if we go with further increase in pH above 9, this may be because of the formation of oxygen anions radical by the reaction between photocatalyst electrons (e−) and the oxygen molecules, so that way the speed of the photocatalytic degradation process of CR dye got increased. And further increase in pH decrease in the speed of degradation of CR dye because of the force of repulsion between anionic species and negatively charged surface of the CoFe2O4 nanostructure. It may be due to the fact, the initial pH, linked with the further acidification of the reaction mixture using HCl, a large amount of conjugated base, which is added to the dye solutions. The Cl− ions can react with OH• radicals to produce inorganic radicals (i.e., ClO−•) and this ClO−• anion radicals have lower reactivity as compared with hydroxyl radical, and as a result they do not take part in the dye degradation, there is a high competition between the dye anions radicals and hydroxyl radicals (Murphy 2007; Guo et al. 2017). Therefore, the increase in solution pH results in the increase in dye degradation, but further increase above 9.0 leads to a decrease in the degradation of dye.
3.7.5 Effect of Different Acids
The effect of acid on the mixture and observed the photocatalytic degradation process of CR dye. The obtained results from the experiment are illustrated in Fig. 12. Due to the high amount of hydrogen ions (H•) in the solution, HNO3 produces maximum degradation in percent. As H2SO4 generates sulfate SO42− ions which stop the hydrogen ion in the solution, so their CR dye degradation is much lower than HNO3. The same HCl produces Cl− ions to the solution, which scavenge OH• ions and hence shows very low photocatalytic degradation of CR dye. The photocatalytic degradation of CR dye shows many interesting features. To accomplish this task, we used different acid solutions with different amount, i.e., 1–8 ml of each acid (HNO3, H2SO4, and HCl) to a separate reaction.
3.8 Kinetics Study of the Photocatalytic Degradation of Congo Red Dye
Kinetics of the photocatalytic degradation for CoFe2O4 (bs-CoFe2O4, as-CoFe2O4, and fs-CoFe2O4) nanostructures measured from the graph of 1 + log optical density versus contact time as shown in Fig. 13. The kinetics model of photocatalytic reaction follows pseudo-first order of kinetics. The kinetics data are presented in Table 1. The photocatalytic activity of all three photocatalysts (bs-CoFe2O4, as-CoFe2O4, and fs-CoFe2O4) was calculated by the following Eq. 3.
where k is the rate constant and Ccatalyst is the photocatalyst concentration. The determined efficiencies of photocatalytic were 0.0018 min−1 g−1 for bs-CoFe2O4, 0.001 min−1 mg−1 for as-CoFe2O4, and 0.0020 min−1 mg−1 for fs-CoFe2O4 photocatalyst.
Rate constant obtained from slope were used to calculate the speed of decolorization level for all prepared three types of CoFe2O4 nanostructure. Figure 14 shows the decolorization rate for the decomposition of CR dye in the presence of fs-CoFe2O4 nanostructure, which was found higher as compared with bs-CoFe2O4 and as-CoFe2O4 photocatalyst. The fs-CoFe2O4 particle size is much smaller than as compared with bs-CoFe2O4 and as-CoFe2O4 particles and has significantly higher photocatalytic activity, and this is because of the available active sites on particles surface.
4 Conclusions
Cobalt ferrite nanostructure was successfully synthesized by using the process of co-precipitation, and the as-prepared sample was then heated for about 6 h at 600 °C. A total of 1 M HCl acids were used to treat these thermally annealed nanoparticles, and CoFe2O4 nanoparticles surface was successfully modified with 3-(APTES). XRD was used to confirm the particle size and crystallinity of the prepared CoFe2O4 nanostructures, and the particle size is 5.30, 4.82, and 5.26 nm of bs-CoFe2O4, as-CoFe2O4, and fs-CoFe2O4 nanostructures. The photocatalytic performance CoFe2O4 nanostructures showed that fs-CoFe2O4 nanostructure degrades 91% of Congo red dye at pH 9 in 90 min. In the acidic medium the bandgap energy value is 2.71 eV. The rate of CR dye degradation reaction on the surface of CoFe2O4 nanostructure following pseudo-first-order kinetics model (6.40 × 10−3 s−1). The obtained efficiency of photocatalyst were found 0.0018 min−1 mg−1, 0.0020 min−1 mg−1, and 0.001 min−1 mg−1 for bs-CoFe2O4, as-CoFe2O4, and fs-CoFe2O4 photocatalyst.
References
Ali, N., Zaman, H., Bilal, M., Nazir, M. S., & Iqbal, H. M. (2019). Environmental perspectives of interfacially active and magnetically recoverable composite materials–a review. Science of the Total Environment, 670, 523–538.
Bedoui, A., Ahmadi, M. F., Bensalah, N., & Gadri, A. (2009). Comparative study of Eriochrome black T treatment by BDD-anodic oxidation and Fenton process. Chemical Engineering Journal, 146(1), 98–104.
Bhattacharyya, K. G., & Sharma, A. (2005). Kinetics and thermodynamics of methylene blue adsorption on neem (Azadirachta indica) leaf powder. Dyes and Pigments, 65(1), 51–59.
Bilal, M., & Asgher, M. (2015). Dye decolorization and detoxification potential of Ca-alginate beads immobilized manganese peroxidase. BMC Biotechnology, 15(1), 111.
Bilal, M., & Iqbal, H. M. N. (2019). Lignin peroxidase immobilization on Ca-alginate beads and its dye degradation performance in a packed bed reactor system. Biocatalysis and Agricultural Biotechnology, 20, 101205.
Bilal, M., Asgher, M., Parra-Saldivar, R., Hu, H., Wang, W., Zhang, X., & Iqbal, H. M. (2017). Immobilized ligninolytic enzymes: an innovative and environmental responsive technology to tackle dye-based industrial pollutants–a review. Science of the Total Environment, 576, 646–659.
Bilal, M., Rasheed, T., Iqbal, H. M., Li, C., Wang, H., & Zhang, X. (2018a). Photocatalytic degradation, toxicological assessment and degradation pathway of CI reactive blue 19 dye. Chemical Engineering Research and Design, 129, 384–390.
Bilal, M., Rasheed, T., Iqbal, H. M., Hu, H., Wang, W., & Zhang, X. (2018b). Toxicological assessment and UV/TiO2-based induced degradation profile of reactive black 5 dye. Environmental Management, 61(1), 171–180.
Bilal, M., Rasheed, T., Zhao, Y., & Iqbal, H. M. N. (2019). Agarose-chitosan hydrogel-immobilized horseradish peroxidase with sustainable bio-catalytic and dye degradation properties. International Journal of Biological Macromolecules, 124, 742–749.
Casbeer, E., Sharma, V. K., & Li, X. Z. (2012). Synthesis and photocatalytic activity of ferrites under visible light: a review. Separation and Purification Technology, 87, 1–14.
Chen, C., Zhang, M., Guan, Q., & Li, W. (2012). Kinetics and thermodynamics studies on the adsorption of xylenol orange on to MIL-101 (Cr). Chemical Engineering Journal, 183, 60–67.
Deraz, N. M., & Abd-Elkader, O. H. (2015). Fabrication and characterization of ZnFe2O4/ZnO based anticorrosion pigments. International Journal of Electrochemical Science, 10, 7103–7110.
Goya, G. F., & Leite, E. R. (2003). Ferrimagnetism and spin canting of Zn 57 Fe2O4 nano particles embedded in ZnO matrix. Journal of Physics: Condensed Matter, 15(4), 641.
Guo, X., Liu, J., & Guo, G. (2017). Photocatalytic removal of dye and reaction mechanism analysis over Y2O3 composite nanomaterials. In MATEC Web of Conferences. EDP Sciences, 88, 02003.
Hosni, N., Zehani, K., Bartoli, T., Bessais, L., & Maghraoui-Meherzi, H. (2017). Semi-hard magnetic properties of nanoparticles of cobalt ferrite synthesized by the coprecipitation process. Journal of Alloys and Compounds, 694, 1295–1301.
Huang, H. Y., Yang, R. T., Chinn, D., & Munson, C. L. (2003). Amine-grafted MCM-48 and silica xerogel as superior sorbents for acidic gas removal from natural gas. Industrial & Engineering Chemistry Research, 42(12), 2427–2433.
Khurshid, R., Ali, F., Qureshi, M. T., Afzal, A., & Ali, Z. (2019). Polyol-mediated coprecipitation and aminosilane grafting of superparamagnetic, spinel ZnFe2O4 nanoparticles for room-temperature ethanol sensors. Journal of the Electrochemical Society, 166(4), B258–B265.
Liu, L., Chan, K. C., Sun, M., & Chen, Q. (2007). The effect of the addition of Ta on the structure, crystallization and mechanical properties of Zr–Cu–Ni–Al–Ta bulk metallic glasses. Materials Science and Engineering A, 445, 697–706.
Liu, L., Bilal, M., Duan, X., & Iqbal, H. M. (2019). Mitigation of environmental pollution by genetically engineered bacteria—current challenges and future perspectives. Science of the Total Environment, 667, 444–454.
Lopez, F. A., Lopez-D, A., & de Vidales, J. L. M. (1998). Simple synthesis, structural and optical properties of cobalt ferrite nanoparticles. The European Physical Journal Plus, 134, 296.
Mahboubeh, H., Fatemeh, Z., Zahra, J. R., Ali, A., & Zohreh, A. (2014). Synthesis of cobalt ferrite (CoFe2O4) nanoparticles using combustion, coprecipitation, and precipitation methods: A comparison study of size, structural, and magnetic properties. Journal of Magnetism and Magnetic Materials, 371, 43–48.
Mahdavi, M., Ahmad, M., Haron, M., Namvar, F., Nadi, B., Rahman, M., & Amin, J. (2013). Synthesis, surface modification and characterization of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules, 18(7), 7533–7548.
Mahmoodi, N. M., Arami, M., Limaee, N. Y., & Tabrizi, N. S. (2006). Kinetics of heterogeneous photocatalytic degradation of reactive dyes in an immobilized TiO2 photocatalytic reactor. Journal of Colloid and Interface Science, 295(1), 159–164.
Murcia, M. D., Gómez, M., Gómez, E., Gómez, J. L., & Christofi, N. (2011). Photodegradation of Congo red using XeBr, KrCl and Cl2 barrier discharge excilamps: a kinetics study. Desalination, 281, 364–371.
Murphy, A. B. (2007). Band-gap determination from diffuse reflectance measurements of semiconductor films, and application to photoelectrochemical water-splitting. Solar Energy Materials and Solar Cells, 91(14), 1326–1337.
Priyadharsini, P., Pradeep, A., Rao, P. S., & Chandrasekaran, G. (2009). Structural, spectroscopic and magnetic study of nanocrystalline Ni–Zn ferrites. Materials Chemistry and Physics, 116(1), 207–213.
Rahimi, R., Tadjarodi, A., Rabbani, M., Kerdari, H., & Imani, M. (2013). Preparation, characterization and photocatalytic properties of Ba-Cd-Sr-Ti doped Fe3O4 nanohollow spheres on removal of Congo red under visible-light irradiation. Journal of Superconductivity and Novel Magnetism, 26(1), 219–228.
Rasheed, T., Bilal, M., Iqbal, H. M., Hu, H., & Zhang, X. (2017). Reaction mechanism and degradation pathway of rhodamine 6G by photocatalytic treatment. Water, Air, & Soil Pollution, 228(8), 291.
Rasheed, T., Nabeel, F., Adeel, M., Rizwan, K., Bilal, M., & Iqbal, H. M. (2019a). Carbon nanotubes-based cues: a pathway to future sensing and detection of hazardous pollutants. Journal of Molecular Liquids, 111425.
Rasheed, T., Adeel, M., Nabeel, F., Bilal, M., & Iqbal, H. M. (2019b). TiO2/SiO2 decorated carbon nanostructured materials as a multifunctional platform for emerging pollutants removal. Science of the Total Environment, 688, 299–311.
Rasheed, T., Nabeel, F., Bilal, M., & Iqbal, H. M. N. (2019c). Biogenic synthesis and characterization of cobalt oxide nanoparticles for catalytic reduction of direct yellow-142 and methyl orange dyes. Biocatalysis and Agricultural Biotechnology, 19, 101154.
Rishikeshi, S. N., Joshi, S. S., Temgire, M. K., & Bellare, J. R. (2013). Chain length dependence of polyol synthesis of zinc ferrite nanoparticles: why is diethylene glycol so different? Dalton Transactions, 42(15), 5430–5438.
Ruttink, P. J., Dekker, L. J., Luider, T. M., & Burgers, P. C. (2012). Complexation of divalent metal ions with diols in the presence of anion auxiliary ligands: zinc-induced oxidation of ethylene glycol to glycolaldehyde by consecutive hydride ion and proton shifts. Journal of Mass Spectrometry, 47(7), 869–874.
Sakthivel, S., Neppolian, B., Shankar, M. V., Arabindoo, B., Palanichamy, M., & Murugesan, V. (2003). Solar photocatalytic degradation of azo dye: comparison of photocatalytic efficiency of ZnO and TiO2. Solar Energy Materials and Solar Cells, 77(1), 65–82.
Salazar-López, M., Rostro-Alanis, M. D. J., Castillo-Zacarías, C., Parra-Guardado, A. L., Hernández-Luna, C., Iqbal, H. M. N., & Parra-Saldivar, R. (2017). Induced degradation of anthraquinone-based dye by laccase produced from Pycnoporus sanguineus (CS43). Water, Air, & Soil Pollution, 228(12), –469.
Samiey, B., Cheng, C. H., & Wu, J. (2014). Organic-inorganic hybrid polymers as adsorbents for removal of heavy metal ions from solutions: a review. Materials, 7(2), 673–726.
Saquib, M., & Muneer, M. (2002). Semiconductor mediated photocatalysed degradation of an anthraquinone dye, Remazol Brilliant Blue R under sunlight and artificial light source. Dyes and Pigments, 53(3), 237–249.
Shu, J., Wang, Z., Huang, Y., Huang, N., Ren, C., & Zhang, W. (2015). Adsorption removal of Congo red from aqueous solution by polyhedral Cu2O nanoparticles: kinetics, isotherms, thermodynamics and mechanism analysis. Journal of Alloys and Compounds, 633, 338–346.
Skrabalak, S. E., Wiley, B. J., Kim, M., Formo, E. V., & Xia, Y. (2008). On the polyol synthesis of silver nanostructures: glycolaldehyde as a reducing agent. Nano Letters, 8(7), 2077–2081.
Soylak, M., Narin, I., Divrikli, U., Saracoglu, S., Elci, L., & Dogan, M. (2004). Preconcentration-separation of heavy metal ions in environmental samples by membrane filtration-atomic absorption spectrometry combination. Analytical Letters, 37(4), 767–780.
Ullah, I., Ali, F., Ali, Z., Humayun, M., & wahab, Z. U. (2018). Glycol stabilized magnetic nanoparticles for photocatalytic degradation of xylenol orange. Materials Research Express, 5, 055509–055522.
Vijayaraghavan, T., Suriyaraj, S. P., Selvakumar, R., Venkateswaran, R., & Ashok, A. (2016). Rapid and efficient visible light photocatalytic dye degradation using AFe2O4 (A= Ba, Ca and Sr) complex oxides. Materials Science and Engineering B, 210, 43–50.
Wang, X., Schwartz, V., Clark, J. C., Ma, X., Overbury, S. H., Xu, X., & Song, C. (2009). Infrared study of CO2 sorption over “molecular basket” sorbent consisting of polyethylenimine-modified mesoporous molecular sieve. The Journal of Physical Chemistry C, 113(17), 7260–7268.
Zaharieva, K. L., Milenova, K. I., Rives, V., Trujillano, R., Cherkezova-Zheleva, Z. P., Eliyas, A. E., & Mitov, I. G. (2015). Mixed cobalt-copper ferrite-type materials: synthesis and photocatalytic efficiency in degradation of Reactive Black 5 dye under UV-light irradiation. Bulgarian Chemical Communications, 47, 105–111.
Zhang, G., Li, C., Cheng, F., & Chen, J. (2007). ZnFe2O4 tubes: synthesis and application to gas sensors with high sensitivity and low-energy consumption. Sensors and Actuators B: Chemical, 120(2), 403–410.
Zhou, Z., Zhang, Y., Wang, Z., Wei, W., Tang, W., Shi, J., & Xiong, R. (2008). Electronic structure studies of the spinel CoFe2O4 by X-ray photoelectron spectroscopy. Applied Surface Science, 254(21), 6972–6975.
Zhu, H., Jiang, R., Xiao, L., Chang, Y., Guan, Y., Li, X., & Zeng, G. (2009). Photocatalytic decolorization and degradation of Congo red on innovative crosslinked chitosan/nano-CdS composite catalyst under visible light irradiation. Journal of Hazardous Materials, 169(1–3), 933–940.
Funding
All listed authors are grateful to their representative departments and universities for the financial support and analytical services used in this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors report no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, N., Said, A., Ali, F. et al. Photocatalytic Degradation of Congo Red Dye from Aqueous Environment Using Cobalt Ferrite Nanostructures: Development, Characterization, and Photocatalytic Performance. Water Air Soil Pollut 231, 50 (2020). https://doi.org/10.1007/s11270-020-4410-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-4410-8