Abstract
At present, the concentration of environmental pollutants, such as pesticides and antibiotics exposed in environment, especially in aquatic environment is increasing. Research on environmental pollutants has exploded in the last few years. However, studies on the combined effects of pesticides and antibiotics on fish are rare, especially the toxic damage to gill tissue is vague. In this paper, cypermethrin (CMN) and sulfamethoxazole (SMZ) were analyzed and found that there was a strong correlation between the pathways affected by the first 30 genes regulated by CMN and SMZ, respectively. Therefore, the toxic effects of CMN (0.651 μg L−1) and/or SMZ (0.3 μg L−1) on grass carp gill were studied in this paper. Histopathology, quantitative real-time PCR, and other methods were used to detect the tissue morphology, oxidative stress level, inflammation, and apoptosis-related indicators of the fish gills after exposure of 42 days. It was found that compared with the single exposure (CMN/SMZ) group, the combined exposure (MIX) group had a more pronounced oxidative stress index imbalance. At the same time, nuclear factor-κB (NF-κB) signal pathway was activated and immuno-inflammatory reaction appeared in MIX group. The expression of tumor necrosis factor (TNF-α) in the rising range is 2.94 times that of the C group, while the expression of interleukin 8 (IL-8) is as high as 32.67 times. This study reveals the harm of CMN and SMZ to fish, and provides a reference and basis for the rational use of pesticides and antibiotics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the use of pesticides on a global scale, and antibiotics is increasing (Chen et al. 2015; Klein et al. 2018). In 2018, the use of pesticides in Argentina alone reached 170,000 tons (http://www.fao.org/faostat/en/). Meanwhile, antibiotics are also used a lot. In 2013 alone, the total use of antibiotics in China reached 162,000 tons, and this number is still increasing (Ying et al. 2017). After entering the ecological cycle, these excessive organic matters become organic pollutants, enter rivers and oceans through rain water and the atmosphere, eventually accumulate in aquatic organisms, and threaten human health through the food chain (Chen et al. 2018; Omwenga et al. 2016; LL Zhao et al. 2021). Cypermethrin (CMN) is a broad-spectrum and efficient insecticidal pyrethroid insecticide (Sparks et al. 2021). CMN residues are widespread in fruits, vegetables, air, and water (Bedi et al. 2013; Bedi et al. 2015), and can restrain cholinesterase activity and induce nerve cell decomposition (Raszewski et al. 2015). Sulfamethoxazole (SMZ) is widely found in various water environments and is considered to be one of the main antibiotic pollutants (Chen and Zhou 2014; Harada 2018). Environment-related concentrations of CMN and SMZ were able to inhibit the antioxidant capacity of organisms and threaten developmental health (Guo et al. 2021; Limbu et al. 2018; Martin et al. 2018). Fish exposed to environmental pollutants will cause damage to the tissues and organs of the body (Soltanian and Fereidouni 2017; HJ Zhao et al., 2020a, b). Therefore, we assume that gill, as the first organ in contact with the external environment, long-term exposure to environment-related concentrations of CMN and SMZ can cause gill tissue damage.
Numerous studies have suggested that oxidative stress is a potential way of pesticide and antibiotics induced toxicity (Lushchak et al. 2009; Qiu et al. 2020). Under normal circumstances, the redox in the body is in a dynamic balance, but when the cell is stimulated by the outside, the level of reactive oxygen species (ROS) rises, leading to the occurrence of oxidative stress (Chen and Maltagliati 2018). NF-E2-related factor 2 (Nrf2) signaling pathway is one of the main ways to protect cells from oxidative damage. Nrf2 gains activity by dissociating from inhibitory cytoplasmic protein Kelch-like ECH-associated protein 1 (Keap1) to activate antioxidant-related genes in the nucleus (Lu et al. 2016). Studies have displayed that when the body experiences oxidative stress, Nrf2-Keap1 signaling pathway is activated (Bellezza et al. 2018). CMN can activate the Nrf2 signaling pathway to protect the central nervous system (Zhou et al. 2020). SMZ produces cytotoxicity by stimulating the production of reactive oxygen species and aryl hydroxylamine metabolites. It irritated the sea urchin to generate oxidative stress and eventually destroyed the sea urchin’s defense mechanism (Ragusa et al. 2017). The legally farmed dose of SMZ may enhance the oxidative stress, inflammation, and apoptosis levels of largemouth bass (Xie et al. 2020). However, the effect of CMN and/or SMZ on the Nrf2 pathway of grass carp gills is still unclear.
Nuclear factor-κB (NF-κB) signaling pathway plays a key role in inducing gene expression. NF-κB participates in a variety of biological processes including inflammation and apoptosis (X Wang et al., 2021a). Interleukins (IL-6/8/10, etc.) push forward an immense influence on immune inflammation and can mediate the transmission of inflammatory signals (Li et al. 2021). At present, it is generally believed that apoptosis is caused by the highly regulated cysteine protease caspase cascade, and Caspase-3 is just downstream of the entire cascade and is the executor of apoptotic process (Guerin et al. 2021). Apoptosis has been found as an important mechanism of CMN toxicity (Zhou et al. 2021), which could not only caused male reproductive toxicity (Wang et al., 2021b) but also caused liver damages in grass carp (Ctenopharyngodon idella) through NF-κB signal pathway (H Zhao et al. 2021b). Environmental concentration of SMZ could up-regulate the expression of inflammatory factors and activated apoptosis signals, thus causing cascade damage to the intestines of grass carp (Y Wang et al. 2021a). However, in aquatic organisms, there is still a lack of corresponding research on how the combined use of CMN and SMZ can cause inflammation and apoptosis.
Grass carp is one of the “four domestic fish” in China, and it is an important freshwater fish in aquaculture (Y Wang et al. 2021a). In this study, grass carp was used as the experimental model to study the toxicity and toxicological mechanism of environmental concentrations of CMN and/or SMZ from a comprehensive point of view. The effects of CMN and/or SMZ on gene-gene interaction and phenotype were studied, and the changes of gill tissue structure and physiological indexes were detected by experimental model. Strive to truly reflect the effects of CMN and/or SMZ on aquatic organisms in the water environment, and provide a new basis for standardizing the use of antibiotics, pesticides, and healthy culture of freshwater fish.
Materials and methods
Chemicals and animals
MS-222 and CMN (No. 52315-07-8, purity of 98%), and SMZ (No. 1196157-90-0, purity of 99.8%) were purchased from Sigma Chemical Co. (St Louis, Missouri, USA). CMN and SMZ were dissolved in 99% pure dimethyl sulfoxide (DMSO) and stored in dark at 4 °C.
A total of 120 juvenile grass carps with an average body weight of 105.45 ± 5.68 g (Harbin Aquaculture Farm) were adapted in the laboratory for 2 weeks (Y Wang et al. 2021b). Grass carps (C. idella) were reared with dechlorinated tap water in a 500 L indoor circulation tank. The whole experiment lasted for 42 days with 12 h light/dark period. The water temperature was controlled at 27.0 ± 1.5 °C, the dissolved oxygen content of water was greater than 6.0 mg L−1, the ammonia nitrogen content was lower than 0.05 mg L−1, the nitrite nitrogen content was lower than 0.06 mg L−1, and the pH value was 7.0–8.0.
Experimental design
The study was conducted under the supervision of the Animal Protection and Utilization Management Committee of Northeast Forestry University. One hundred and twenty healthy grass carps were randomly divided into 4 groups, namely control (C) group, CMN group, SMZ group, and CMN + SMZ (Mix) group, with 3 replicates in each group. According to some current environmental-related concentration studies (Chen and Zhou 2014; Zhao et al., 2020a) and environmental surveys (Wang et al. 2018; Zhou et al. 2016), the concentration of aerial CMN is 0.32–9.04 pg m3 − 1, while the common concentration of CMN in water is 0.01–0.68 ng L−1. The concentration of SMZ in the river ranges from 34 to 859 ng L−1, and the common concentration of SMZ is 259.6–385 ng L−1. Referring to the concentrations used in previous studies, the final concentrations of CMN and SMZ were set to 0.651 μg L−1 and 0.30 μg L−1 respectively in this study, and this concentration has been shown to cause chronic poisoning (Y Wang et al. 2021a; H Zhao et al. 2021a).
During the entire experiment, dechlorinated tap water with the same exposure concentration was replaced every 48 h. Water samples were collected at exposure times of 1, 14, 28, and 42 days, and the concentrations of CMN (Enzyme-linked Biotechnology, China) and SMZ (REAGENLLC, USA) were detected with ELISA kit. During the whole experiment, the deviation of the actual average concentration of CMN and SMZ from the nominal concentration was less than 10%, which was relatively stable. After 42 days of exposure, the grass carp was anesthetized with MS-222 (10 mg L−1) and the gill tissue was separated and stored at −80 °C. No death of grass carp was found throughout the experiment.
Histopathological observation
After 42 days exposure, the extracted gill tissue of each group was fixed with 4% paraformaldehyde and made into paraffin sections with a thickness of 4 mm. The tissue sections were stained with hematoxylin-eosin (H&E) and observed under a microscope (Olympus, Japan) for histological analysis.
Oxidative stress index detection
Use Nanjing Jiancheng Biological Company (NJJCBIO, China) testing kits, and follow the instructions to process the tissue for testing, including superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione (GSH) content.
Real-time fluorescence quantitative PCR
Using TriZol reagent (Invitrogen, USA) to extract total RNA from the gill tissue, total RNA was measured (260 nm) spectrophotometer nano concentration, and RNA quality was detected (260/280 nm, 260/230 nm). qPCR was performed with HiScript II Q Select RT Super Mix kit (Vazyme Biotech Co., Ltd.) to obtain cDNA. Use FastStart Universal SYBR Green Master reagent (Roche) and Light Cycler® 480 (Roche, Switzerland) system to complete the experiment. Using 2−ΔΔCT method of data analysis.
Find mRNA sequences on National Center of Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/) and use Integrated Device Technology (IDT, https://sg.idtdna.com/pages) to conduct primer design. Finally, the primer is identified using the BLAST (https://blast.ncbi.nlm.nih.gov/blast.cgi) function on the NCBI website, the primers shown in Table S1.
Immunoblotting analyses
The nucleoprotein and plasma protein extraction kit (Wanleibio, China) was used to extract NF-κB proteins from the gill tissue. The remaining proteins were extracted from the gill tissue using RIPA lysate (add 100 mg tissue to a mixture of 990 μL RIPA lysis buffer and 10 μL PMSF), and the supernatant was collected, quantified with a BCA kit (Beyotime, China), and stored at −80 °C. Western blot and digital imaging equipment (General Electric, USA) were used to detect the signal, and Image-J software was used for quantitative analysis. Primary antibodies include Caspase-3, Caspase-9, Bcl-2-associated X (Bax), B cell lymphoma-2 (Bcl-2), inhibitory protein of nuclear factor of kappa B-alpha (IκB-α), p-iκBα, histone H3 (H3), NF-κB (cytosol) (Wanleibio, China), and β-actin (Abclonal, China).
Selection and enrichment analysis of differential genes
Using DTC database (http://ctdbase.org), the differential genes under the action of CMN and SMZ were selected, and the genes of top 30 (if there are less than 30 genes, select all of them) were selected for follow-up analysis according to the order for P value from large to small. The selected genes were enriched and analyzed by gene ontology (GO), Kyoto gene and genome encyclopedia (KEGG), and Metascape (http://metascape.org/gp/index.html#/main/step1). According to the reference (Sendra et al. 2021), the GO results were divided into three categories according to biological process (BP), cellular component (CC), and molecular functional (MF).
The top 50 genes (if there are less than 50 genes, select all of them) affected by CMN and SMZ were mixed, and the duplicate genes were removed to get a new gene list. After the mixed gene list, the online software Metascape was used for term enrichment analysis, and the interaction network between genes was generated. The online software STRING 11.0 (http://string-db.org/) was used to generate the interaction network of the proteins corresponding to the mixed gene list, and the k-means algorithm was used to mark them as different paths (specific reference: Zhao et al., 2020b).
Statistical analysis
IBM SPSS 22.0 (Version 22.0, Inc., Chicago, IL, USA) software was used to analyze the experimental data. One-way analysis of variance was adopted, and the data results passed the Tukey test. All the data in this article (F < 0.05, P < 0.05) were statistically significant, and all values were expressed as the mean ± SD value. GraphPad Prism 5.0 (version 5.01, Inc., La Jolla, USA) was used to draw statistics.
Result
Results of data analysis
According to the results of GO analysis (Fig. 1), it is found that the gene changes caused by CMN and SMZ have something in common in cell process, cell composition, and molecular function, indicating that the physiological changes caused by CMN and SMZ may be similar.
In order to determine whether there are similarities between them in the mechanism of action, we carried out signal pathway enrichment analysis (Fig. 2) of differential genes. All the results clearly point out that both CMN and SMZ can cause significant changes in oxidative stress. And found that compared with SMZ, CMN can cause obvious apoptosis, while the effect of SMZ on inflammation is more intense. This may be due to the different types of the two and the different functions they perform. CMN is a kind of insecticide, which aims to destroy the target organisms, so it has a stronger toxic effect. SMZ is an antibiotic that may affect target organisms by causing immune inflammatory response and apoptosis.
The results of pathway enrichment analysis. A The results of KEGG pathway enrichment analysis of the top 30 differential genes affected by CMN. B The results of KEGG pathway enrichment analysis of the top 30 differential genes affected by SMZ. C The rich term bar graph of the top 30 differential genes affected by CMN was colored by P value. D The rich term bar graph of the top 30 differential genes affected by SMZ was colored by P value
Interaction analysis
From Fig. 3, it is found that the changes that may be caused by a mixture of CMN and SMZ are not the same as when using CMN or SMZ alone. In the protein-protein interaction analysis (Fig. 3A), the proteins related to oxidative stress, inflammation, and apoptosis are at the center. After enriching the differential genes (Fig. 3C), it was found that the P values of oxidative stress, apoptosis, and inflammation-related pathways were low, indicating that the combined use of CMN and SMZ is very likely to affect these three physiological processes.
Histological observation of gills
In the case of CMN and/or SMZ exposure (Fig. 4, Table 1), the gill tissue has obvious pathological changes, and the gill tissue damage under the combined exposure is clearly more serious than the single exposure. According to the Histology Alteration Index (HAI) index (Rosety-Rodriguez et al. 2002; Salamat et al. 2013) calculation result (Table S2), the histopathological changes of the CMN group and the SMZ group have met moderate changes. The HAI fraction of the MIX group is up to 131, which represents the gill been irreversible damage.
The effect of CMN and/or SMZ on the histological structure of grass carp gills. 41.2×; red arrow: cell swelling; blue arrow: epithelial hyperplasia; black arrow: cell shedding; green arrow: edema; H, hyperemia; GL, curved gill lamella. Black pentagram: marginal canal dilation. Green pentagram: chlorine cell hyperplasia
The control group (Fig. 4A) has normal morphology, clear structure, and neatly arranged gill lamella. Gill epithelial hyperplasia, cell swelling, and epithelial cell shedding occurred in the CMN single exposure group (Fig. 4B), and even local hyperemia and inconspicuous gill lamella structure (gill lamella fusion). The SMZ single exposure group (Fig. 4C) shows cell hyperplasia, edema, hyperemia, and curved gill lamella. The necrosis of the gill epithelium was more serious in the MIX group (Fig. 4D), with more vacuoles in the middle of the gill lamella, and the overall structure gradually deviated from the normal shape.
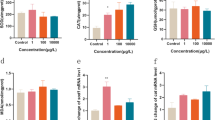
The effect of CMN and/or SMZ on the oxidative stress of gills
As shown in Fig. 5, compared with the control group, the use of CMN and/or SMZ significantly reduced the activity of SOD and the content of GSH, and significantly increased the content of MDA. And compared with CMN or SMZ alone, the MIX group has a stronger ability to induce MDA production, which is 1.9 times that of the control group. It is suggested that the mixed use of CMN and SMZ reduces the antioxidant capacity of grass carp gill tissue and aggravates oxidative damage.
The effect of CMN and/or SMZ on the Nrf2-Keap1 pathway and inflammatory factors
Testing the mRNA expression level of the Nrf2-Keap1 pathway (Fig. 6) to study the mechanism of oxidative stress induced by CMN and SMZ. According to the test results, the MIX group significantly increased the expression level of Keap1 and decreased the expression level of Nrf2. At the same time, the expression levels of HO-1 and NQO1 in the MIX group were also obviously decreased compared with the individual exposure group. It is suggested that the combined use of CMN and SMZ can curb the Nrf2-Keap1 signaling pathway and decrease the antioxidant capacity. The expression level of NQO1 has not been suppressed very seriously.
A–F The effect of CMN and/or SMZ on the transcription of inflammatory factors. Different letters indicate significant differences between groups (F < 0.05, P < 0.05). G The effect of CMN and/or SMZ exposure on the transcription of Nrf2 and its downstream target genes (F < 0.05, *: 0.01 < P < 0.05, **: P < 0.01)
Studies have described those pesticides and/or antibiotics can induce inflammatory effects in the body (F Wang et al. 2020a; Zhao et al., 2020a). In order to verify whether the combined use of the two can cause inflammation in the gill tissue of grass carp, we performed qRT-PCR detection of related inflammatory factors. Compared with group C, the combined use of CMN and SMZ made inducible nitric oxide synthase (iNOS) (5.27 times), tumor necrosis factor (TNF-α) (2.94 times), and IL-1β (9.84 times), IL-6 (3.41 times), and IL-8 (32.67 times) mRNA levels increased, and the anti-inflammatory factor interleukin 10 (IL-10) (0.21 times) expression decreased (Fig. 6), indicating that the gill tissue of the grass carp had an inflammatory response.
The effect of CMN and/or SMZ on NF-κB
Through the changes in protein levels (Fig. 7), compared with the control group, the expression of IκB-α (0.76 times) was significantly reduced in the MIX group, and the expression of p-IκBα (2.26 times) was increased. These results indicate that IκB-α is activated. The level of NF-κB protein increased in the nucleus and decreased in the cytoplasm. These phenomena indicate that NF-κB is activated and transferred to the nucleus to activate the NF-κB signaling pathway. The transcription level of inflammatory factors (Fig. 6A–F) is consistent with the expression of NF-κB, and the trend is identical, suggesting that the combined use of CMN and SMZ may trigger an inflammatory response by activating the NF-κB signaling pathway.
The influence of CMN and/or SMZ on apoptosis
To investigate the relationship between the exposure (CMN and/or SMZ) and apoptosis, we examined the expression of apoptosis-related genes at the protein level, and the results are shown in Fig. 8. Compared with the control group, whether CMN or SMZ exposure is alone or joint, the expression levels of apoptosis-related proteins (Bax, Caspase-3, and Caspase-9) increased significantly. It was also observed that Bcl-2 was significantly inhibited at the level of transcription and translation. According to the experimental results, the change trend of Bax/Bcl-2 protein ratio is consistent with the expression trend of Caspase-3 and Caspase-9 protein. The expression level of the experimental groups is higher than that of the control group, and the MIX group is much higher than the single exposure group. It suggests that the MIX group is more toxic to the tissues and causes more serious damage. The change trend of apoptosis-related proteins is the same as that of nuclear factor NF-κB (Fig. 7C–D), suggesting that apoptosis may be triggered by the activation of NF-κB signaling pathway.
Discussion
The law of natural ecology makes the aquatic system rich in a variety of low concentrations of pollutants (Edwards et al. 1986). Therefore, compared with land animals and birds, aquatic organisms are more likely to be affected by compound pollutants. Gills are the respiratory organs of fish, and they complete gas exchange when blood flows through here. The morphological change of gill is an indicator of early toxicity (Fiedler et al. 2020). From the results of this experiment, the interaction between CMN and SMZ can be observed obviously. In other words, the toxicity of CMN was enhanced by adding SMZ, and many studies by Zhao et al. proved this conclusion (H Zhao et al. 2021a; Zhao et al., 2020a). Wang et al. (2021c) concluded that the mixture of CMN and difenoconazole has stronger toxicity, suggesting the relationship between mixed pollutants and biological health risk. The same results were found in the combined exposure study of SMZ and bisphenol AF (Kwon et al. 2016). Generally, the organic matter in the environment will enhance each other. Up to now, many studies have proved that pesticides and antibiotics may increase the toxicity of other organic pollutants in the environment, but the specific mechanism should be explored for more in-depth study of molecular and metabolic pathways.
Liu et al. found that zebrafish exposed to an environment containing SMZ can consume SMZ through daily activities and cause oxidative stress and inflammation in healthy fish (Liu et al. 2020). GSH is commonly found in various organisms and is the most important antioxidant. GSH scavenges free radicals in the body through the oxidative dehydrogenation of sulfhydryl (-SH) in the molecular structure (Aldini et al. 2018). When the free radicals and peroxidation products in the tissue exceed the regulatory ability of GSH, GSH forms GSSG after the oxidative dehydrogenation of the -SH group. And the content of total GSH in the tissue decreased (Moreno-Sanchez et al. 2018). In this study (Fig. 5), CMN and/or SMZ significantly decreased the content of GSH, indicating that a large number of free radicals and peroxidation products were produced in gill tissue. SOD maintains the oxidation balance in the body by converting superoxide free radicals into hydrogen peroxide, and is a ubiquitous antioxidant enzyme (Sakamoto and Imai 2017). Generally, strength of the antioxidant ability in the organism can be judged by the content of SOD (Qu et al. 2019). MDA is the final product of lipid peroxidation. It can not only affect the function of mitochondria but also aggravated the damage to cell membrane (Tsikas 2017). MDA accumulates in a time-dependent manner under oxidative stress (Y Wang et al. 2020c). According to the results of Fig. 5, the content of MDA increased significantly in the MIX group while the content of SOD was consumed. After observing the results of the oxidative stress indicators detection, it was found that the exposure of CMN and/or SMZ caused oxidative stress in the tissues. Simultaneously, it was observed that the change of the index in the MIX group was more obvious than that in the CMN or SMZ group, indicating that the MIX group had a synergistic effect to some extent and caused more serious damage. Similar results have been found in some literature (Aderemi et al. 2018). Zhao et al. found that the combined use of antibiotics and pesticides aggravated the oxidative stress of the carp spleen and damaged the immune system (Zhao et al., 2020a). In addition to fish, pesticides and antibiotics can also affect the behavior of shrimp, restrain neuro enzyme activity, and induce oxidative stress (Huynh et al. 2010). These studies confirm that our evidence is reliable.
The Nrf2-Keap1 signaling pathway is the main antioxidant pathway in the body, which can resist the damage caused by toxic substances (Casalino et al. 2007), and maintain the body’s oxidative balance (Copple et al. 2008). Normally, when the organism is subjected to oxidative stress, the Nrf2-Keap1 signaling pathway is activated and Nrf2 is released into the nucleus (Qiu et al. 2020). Nrf2 entering the nucleus further promoted the production of antioxidant products such as HO-1 and NQO1 (Chen et al., 2020a; Kaspar and Jaiswal 2010), and induce antioxidant reactions (Kaspar et al. 2009; Zhong et al. 2015). However, in the results of this study, it was found that the Nrf2-Keap1 signaling pathway was inhibited (Fig. 6G). This may be due to the combined toxicity of CMN and SMZ beyond the body’s own regulatory capacity. The Nrf2 pathway has a certain protective ability, but this protective effect is very limited. The protective effect of the Nrf2 pathway depends on the dose of toxic substances and the exposure time to organisms (Zhou et al. 2020). The gill is the first organ of the fish that encounters the external environment. Long-term exposure to poisons puts the gill tissue in a continuous stress state, produces excessive free radicals, and restrains the Nrf2-Keap1 signaling pathway (David et al. 2017). In the study of Wang et al. also found that antibiotics can curb the expression of zebrafish gills antioxidant-related genes (F Wang et al. 2020a). When the antioxidant products in the organism try to restore the oxidative balance but fail, inflammation and apoptosis are triggered (Chi et al. 2021; Y Wang et al. 2020b).
TNF-α is an inflammatory cytokine produced by macrophages or monocytes during acute inflammation. It can induce tissue damage by inducing the production of ROS, and induce cell necrosis and apoptosis (Balkwill 2006). Studies have demonstrated a critical role in NF-κB signaling pathway in induced inflammation (Ji et al. 2019). Many studies have shown that antibiotics or pesticides can cause oxidative stress and inflammation in healthy organisms. Gentamicin can cause severe nephrotoxicity by inducing oxidative stress and inflammation (Ince et al. 2020). Dietary exposure to CMN changes the lipid homeostasis and energy metabolism in the salmon liver (Fuller et al. 2021). Oxidative stress can have a cascade reaction with NF-κB, which in turn triggers inflammation (Wang et al. 2017a). Pesticides can amplify the inflammatory response by increasing the levels of iNOS and cyclooxygenase-2 (COX-2) (Cupic Miladinovic et al. 2021). The iNOS/NF-κB cascade reaction produces peroxynitrate, which in turn increases the toxicity of pesticides (Chi et al. 2018). From our results, it was found that the protein expression level of NF-κB-related molecules increased (Fig. 7). Meanwhile, the protein expression level of IκB-α decreased and its corresponding phosphorylation level increased, which proved that NF-κB played a role (Fang et al. 2021) and produced high expression of TNF-α (Fig. 6D), which proved that the combined exposure to CMN and SMZ can activate the NF-κB signaling pathway and cause inflammation. Combined with the results of Fig. 6E, the content of iNOS in the MIX group was significantly increased, and the combined toxicity enhancement of CMN and SMZ may be achieved through the cascade reaction of iNOS and NF-κB.
Studies have shown that environmental pollutants can activate cell apoptosis through the NF-κB signaling pathway (Arab-Nozari et al. 2020; Chen et al., 2020b). Bcl-2 family of proteins is key regulator of apoptosis, which plays a crucial role in maintaining the homeostasis (Siddiqui et al. 2015). Among them, the pro-apoptotic proteins Bax and Bak can initiate the caspase cascade reaction, which is the programmed cell death (Edlich 2018). The caspase cascade can activate the mitochondrial stress pathway (Guo et al. 2019). When cytochrome c in the mitochondria is released and interacts with Apaf-1, Caspase-9 will be activated and works. Caspase-3 is located downstream of the entire caspase cascade, acting as an effector of the reaction and a target for cell lysis (Fan et al. 2005). Therefore, in apoptosis caused by common environmental pollutants, the up-regulation of caspase family proteins and the down-regulation of Bcl-2 proteins can be seen (Wang et al. 2017b). Combined with the results of Fig. 8, we can find that the apoptotic pathway plays a role under the exposure of CMN and/or SMZ. Studies have shown that environmental toxins can also activate cell apoptosis through oxidative stress (Zhang et al. 2021). It is known that sulfa drugs can induce the production of ROS by accumulating corresponding metabolites, and then activate cell apoptosis and necrosis (Elzagallaai et al. 2020). These indicate that SMZ may induce apoptosis through oxidative stress. CMN can cause apoptosis and affect the cell cycle through the selective toxicity of enantiomers (Ji et al. 2021). From the results of Fig. 8, we observed that the change of apoptosis index was more obvious when CMN was used in combination with SMZ than when it was used alone. Referring to the conclusions of other researchers (Elzagallaai et al. 2020; Ji et al. 2021), CMN and SMZ may induced apoptosis through different ways, resulting in an increase in apoptotic effect.
From the point of view of big data, after analyzing the possible mechanism and results of CMN and/or SMZ, we found that the combined use of CMN and SMZ could cause strong oxidative stress, inflammation, and apoptosis at the same time. By establishing the experimental model of long-term exposure to environmental concentrations of CMN and SMZ in fish gill, we found that CMN and/or SMZ could reduce the activity of antioxidant enzymes, inhibit Nrf2-keap1 signal pathway, and destroy the antioxidant system of gill tissue. Then activated NF-κB signal pathway, to induce inflammatory cytokines and inflammation, finally caused cell apoptosis. At the same time, compared with the control group and single exposure group, the toxicity of MIX group was stronger and the damage to gill tissue was more apparent. These experimental results support the conclusion drawn by big data and prove that the combined use of pesticides and antibiotics at environmental concentrations is harmful to organisms. Furthermore, it is inferred that the organisms living in the polluted water environment are damaged by environmental pollutants.
Conclusion
In general, our research demonstrated that long-term exposure to SMZ (0.651 μg L−1) and/or CMN (0.3 μg L−1) can cause redox imbalance in gill tissue, trigger inflammation and apoptosis, and cause tissue damage. We found that the combined exposure to CMN and SMZ caused more serious damage than single exposure, and this damage may be achieved by activating the NF-κB signaling pathway and inhibiting the Nrf2 signaling pathway. Our research showed for the first time that environment-related concentrations of CMN and SMZ could damage gill tissue and provided a new basis for the combination of pesticides and antibiotics, helping for the study of environmental pollutants.
References
Aderemi AO, Novais SC, Lemos MFL, Alves LM, Hunter C, Pahl O (2018) Oxidative stress responses and cellular energy allocation changes in microalgae following exposure to widely used human antibiotics. Aquat Toxicol 203:130–139. https://doi.org/10.1016/j.aquatox.2018.08.008
Aldini G, Altomare A, Baron G, Vistoli G, Carini M, Borsani L et al (2018) N-acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic Res 52:751–762. https://doi.org/10.1080/10715762.2018.1468564
Arab-Nozari M, Mohammadi E, Shokrzadeh M, Ahangar E, Amiri FT, Shaki F (2020) Co-exposure to non-toxic levels of cadmium and fluoride induces hepatotoxicity in rats via triggering mitochondrial oxidative damage, apoptosis, and NF-kB pathways. Environ Sci Pollut R 27:24048–24058. https://doi.org/10.1007/s11356-020-08791-4
Balkwill F (2006) TNF-alpha in promotion and progression of cancer. Cancer Metast Rev 25:409–416. https://doi.org/10.1007/s10555-006-9005-3
Bedi JS, Gill JPS, Aulakh RS, Kaur P, Sharma A, Pooni PA (2013) Pesticide residues in human breast milk: risk assessment for infants from Punjab, India. Sci Total Environ 463:720–726. https://doi.org/10.1016/j.scitotenv.2013.06.066
Bedi JS, Gill JPS, Aulakh RS, Kaur P (2015) Pesticide residues in bovine milk in Punjab, India: spatial variation and risk assessment to human health. Arch Environ Con Tox 69:230–240. https://doi.org/10.1007/s00244-015-0163-6
Bellezza I, Giambanco I, Minelli A, Donato R (2018) Nrf2-keap1 signaling in oxidative and reductive stress. Bba-Mol Cell Res 1865:721–733. https://doi.org/10.1016/j.bbamcr.2018.02.010
Casalino E, Calzaretti G, Landriscina M, Sblano C, Fabiano A, Landriscina C (2007) The Nrf2 transcription factor contributes to the induction of alpha-class GST isoenzymes in liver of acute cadmium or manganese intoxicated rats: comparison with the toxic effect on NAD(P)H: quinone reductase. Toxicology 237:24–34. https://doi.org/10.1016/j.tox.2007.04.020
Chen D, Ning F, Zhang J, Tang Y, Teng X (2020a) NF-kappaB pathway took part in the development of apoptosis mediated by miR-15a and oxidative stress via mitochondrial pathway in ammonia-treated chicken splenic lymphocytes. Sci Total Environ 729:139017. https://doi.org/10.1016/j.scitotenv.2020.139017
Chen H, Liu S, Xu XR, Zhou GJ, Liu SS, Yue WZ et al (2015) Antibiotics in the coastal environment of the Hailing Bay region, South China Sea: spatial distribution, source analysis and ecological risks. Mar Pollut Bull 95:365–373. https://doi.org/10.1016/j.marpolbul.2015.04.025
Chen H, Liu S, Xu XR, Diao ZH, Sun KF, Hao QW et al (2018) Tissue distribution, bioaccumulation characteristics and health risk of antibiotics in cultured fish from a typical aquaculture area. J Hazard Mater 343:140–148. https://doi.org/10.1016/j.jhazmat.2017.09.017
Chen K, Zhou JL (2014) Occurrence and behavior of antibiotics in water and sediments from the Huangpu River, Shanghai, China. Chemosphere 95:604–612. https://doi.org/10.1016/j.chemosphere.2013.09.119
Chen QM, Maltagliati AJ (2018) Nrf2 at the heart of oxidative stress and cardiac protection. Physiol Genomics 50:77–97. https://doi.org/10.1152/physiolgenomics.00041.2017
Chen X, Han K, Zhang T, Qi GQ, Jiang ZY, Hu CY (2020b) Grass carp (Ctenopharyngodon idella) Nrf2 alleviates the oxidative stress and enhances cell viability through upregulating the expression of HO-1. Fish Physiol Biochem 46:417–428. https://doi.org/10.1007/s10695-019-00729-z
Chi QR, Chi X, Hu XY, Wang S, Zhang HF, Li S (2018) The effects of atmospheric hydrogen sulfide on peripheral blood lymphocytes of chickens: perspectives on inflammation, oxidative stress and energy metabolism. Environ Res 167:1–6. https://doi.org/10.1016/j.envres.2018.06.051
Chi QR, Hu XY, Zhao B, Zhang Q, Zhang KX, Li S (2021) Regulation of H2S-induced necroptosis and inflammation in broiler bursa of Fabricius by the miR-15b-5p/TGFBR3 axis and the involvement of oxidative stress in this process. J Hazard Mater 406. https://doi.org/10.1016/j.jhazmat.2020.124682
Copple IM, Goldring CE, Kitteringham NR, Park BK (2008) The Nrf2-Keap1 defence pathway: role in protection against drug-induced toxicity. Toxicology 246:24–33. https://doi.org/10.1016/j.tox.2007.10.029
Cupic Miladinovic D, Prevendar Crnic A, Pekovic S, Dacic S, Ivanovic S, Santibanez JF et al (2021) Recovery of brain cholinesterases and effect on parameters of oxidative stres and apoptosis in quails (Coturnix japonica) after chlorpyrifos and vitamin B1 administration. Chem Biol Interact 333:109312. https://doi.org/10.1016/j.cbi.2020.109312
David JA, Rifkin WJ, Rabbani PS, Ceradini DJ (2017) The Nrf2/Keap1/are pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. J Diabetes Res. https://doi.org/10.1155/2017/4826724
Edlich F (2018) Bcl-2 proteins and apoptosis: recent insights and unknowns. Biochem Bioph Res Co 500:26–34. https://doi.org/10.1016/j.bbrc.2017.06.190
Edwards R, Millburn P, Hutson DH (1986) Comparative toxicity of cis-cypermethrin in rainbow trout, frog, mouse, and quail. Toxicol Appl Pharmacol 84:512–522. https://doi.org/10.1016/0041-008x(86)90256-5
Elzagallaai AA, Sultan EA, Bend JR, Abuzgaia AM, Loubani E, Rieder MJ (2020) Role of oxidative stress in hypersensitivity reactions to sulfonamides. J Clin Pharmacol 60:409–421. https://doi.org/10.1002/jcph.1535
Fan TJ, Han LH, Cong RS, Liang J (2005) Caspase family proteases and apoptosis. Acta Biochim Biophys Sin Shanghai 37:719–727. https://doi.org/10.1111/j.1745-7270.2005.00108.x
Fang Y, Yang L, He J (2021) Plantanone C attenuates LPS-stimulated inflammation by inhibiting NF-kappaB/iNOS/COX-2/MAPKs/Akt pathways in RAW 264.7 macrophages. Biomed Pharmacother 143:112104. https://doi.org/10.1016/j.biopha.2021.112104
Fiedler S, Wunnemann H, Hofmann I, Theobalt N, Feuchtinger A, Walch A, et al (2020) A practical guide to unbiased quantitative morphological analyses of the gills of rainbow trout (Oncorhynchus mykiss) in ecotoxicological studies. Plos One. https://doi.org/10.1371/journal.pone.0243462
Fuller N, Magnuson JT, Hartz KEH, Fulton CA, Whitledge GW, Acuna S et al (2021) Effects of dietary cypermethrin exposure on swimming performance and expression of lipid homeostatic genes in livers of juvenile Chinook salmon, Oncorhynchus tshawytscha. Ecotoxicology 30:257–267. https://doi.org/10.1007/s10646-021-02352-2
Guerin DJ, Kha CX, Tseng KA (2021) From cell death to regeneration: rebuilding after injury. Front Cell Dev Biol 9:655048. https://doi.org/10.3389/fcell.2021.655048
Guo DM, Liu WP, Yao TS, Ma MM, Wang Q, Qiu J, et al (2021) Combined endocrine disruptive toxicity of malathion and cypermethrin to gene transcription and hormones of the HPG axis of male zebrafish (Danio rerio). Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.128864
Guo JM, Xing HJ, Chen MH, Wang W, Zhang HF, Xu SW (2019) H2S inhalation-induced energy metabolism disturbance is involved in LPS mediated hepatocyte apoptosis through mitochondrial pathway. Sci Total Environ 663:380–386. https://doi.org/10.1016/j.scitotenv.2019.01.360
Harada K (2018) Antibiotic residue in environmental water in Vietnam. Yakugaku Zasshi 138:271–275
Huynh TT, Silvestre F, Nguyen TP, Kestemont P (2010) Effects of pesticides and antibiotics on penaeid shrimp with special emphases on behavioral and biomarker responses. Environ Toxicol Chem 29:929–938. https://doi.org/10.1002/etc.99
Ince S, Kucukkurt I, Demirel HH, Arslan-Acaroz D, Varol N (2020) Boron, a trace mineral, alleviates gentamicin-induced nephrotoxicity in rats. Biol Trace Elem Res 195:515–524. https://doi.org/10.1007/s12011-019-01875-4
Ji CY, Magnuson JT, Zhang W, Zhao MR (2021) New insight into the enantioselective cytotoxicity of cypermethrin: imbalance between cell cycle and apoptosis. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.123893
Ji PY, Li ZY, Wang H, Dong JT, Li XJ, Yi HL (2019) Arsenic and sulfur dioxide co-exposure induce renal injury via activation of the NF-kappaB and caspase signaling pathway. Chemosphere 224:280–288. https://doi.org/10.1016/j.chemosphere.2019.02.111
Kaspar JW, Niture SK, Jaiswal AK (2009) Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radical Bio Med 47:1304–1309. https://doi.org/10.1016/j.freeradbiomed.2009.07.035
Kaspar JW, Jaiswal AK (2010) Antioxidant-induced phosphorylation of tyrosine 486 leads to rapid nuclear export of Bach1 that allows Nrf2 to bind to the antioxidant response element and activate defensive gene expression. J Biol Chem 285:153–162. https://doi.org/10.1074/jbc.M109.040022
Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA et al (2018) Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. P Natl Acad Sci USA 115:E3463–E3470. https://doi.org/10.1073/pnas.1717295115
Kwon B, Kho Y, Kim PG, Ji K (2016) Thyroid endocrine disruption in male zebrafish following exposure to binary mixture of bisphenol AF and sulfamethoxazole. Environ Toxicol Pharmacol 48:168–174. https://doi.org/10.1016/j.etap.2016.10.018
Li SZ, Jiang LQ, Beckmann K, Hojen JF, Pessara U, Powers NE, et al (2021) A novel anti-human IL-1R7 antibody reduces IL-18-mediated inflammatory signaling. J Biol Chem. https://doi.org/10.1016/j.jbc.2021.100630
Limbu SM, Zhou L, Sun SX, Zhang ML, Du ZY (2018) Chronic exposure to low environmental concentrations and legal aquaculture doses of antibiotics cause systemic adverse effects in Nile tilapia and provoke differential human health risk. Environ Int 115:205–219. https://doi.org/10.1016/j.envint.2018.03.034
Liu JY, Wei TZ, Wu X, Zhong HB, Qiu WH, Zheng Y (2020) Early exposure to environmental levels of sulfamethoxazole triggers immune and inflammatory response of healthy zebrafish larvae. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.134724
Lu MC, Ji JA, Jiang ZY, You QD (2016) The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: an update. Med Res Rev 36:924–963. https://doi.org/10.1002/med.21396
Lushchak OV, Kubrak OI, Storey JM, Storey KB, Lushchak VI (2009) Low toxic herbicide roundup induces mild oxidative stress in goldfish tissues. Chemosphere 76:932–937. https://doi.org/10.1016/j.chemosphere.2009.04.045
Martin FL, Martinez EZ, Stopper H, Garcia SB, Uyemura SA, Kannen V (2018) Increased exposure to pesticides and colon cancer: early evidence in Brazil. Chemosphere 209:623–631. https://doi.org/10.1016/j.chemosphere.2018.06.118
Moreno-Sanchez R, Marin-Hernandez A, Gallardo-Perez JC, Vazquez C, Rodriguez-Enriquez S, Saavedra E (2018) Control of the NADPH supply and GSH recycling for oxidative stress management in hepatoma and liver mitochondria. Bba-Bioenergetics 1859:1138–1150. https://doi.org/10.1016/j.bbabio.2018.07.008
Omwenga M, Kanja L, Nguta J, Mbaria J, Irungu P (2016) Assessment of organochlorine pesticide residues in farmed fish in Machakos and Kiambu counties, Kenya. Toxicol Lett 258:S176–S177. https://doi.org/10.1016/j.toxlet.2016.06.1658
Qiu SN, Fu HY, Zhou RY, Yang Z, Bai GD, Shi BM (2020) Toxic effects of glyphosate on intestinal morphology, antioxidant capacity and barrier function in weaned piglets. Ecotox Environ Safe. https://doi.org/10.1016/j.ecoenv.2019.109846
Qu W, Du GL, Feng B, Shao H (2019) Effects of oxidative stress on blood pressure and electrocardiogram findings in workers with occupational exposure to lead. J Int Med Res 47:2461–2470. https://doi.org/10.1177/0300060519842446
Ragusa MA, Costa S, Cuttitta A, Gianguzza F, Nicosia A (2017) Coexposure to sulfamethoxazole and cadmium impairs development and attenuates transcriptional response in sea urchin embryo. Chemosphere 180:275–284. https://doi.org/10.1016/j.chemosphere.2017.04.030
Raszewski G, Lemieszek MK, Lukawski K, Juszczak M, Rzeski W (2015) Chlorpyrifos and cypermethrin induce apoptosis in human neuroblastoma cell line SH-SY5Y. Basic Clin Pharmacol 116:158–167. https://doi.org/10.1111/bcpt.12285
Rosety-Rodriguez M, Ordonez FJ, Rosety M, Rosety JM, Rosety I, Ribelles A et al (2002) Morpho-histochemical changes in the gills of turbot, Scophthalmus maximus L., induced by sodium dodecyl sulfate. Ecotoxicol Environ Saf 51:223–228. https://doi.org/10.1006/eesa.2001.2148
Sakamoto T, Imai H (2017) Hydrogen peroxide produced by superoxide dismutase SOD-2 activates sperm in caenorhabditis elegans. J Biol Chem 292:14804–14813. https://doi.org/10.1074/jbc.M117.788901
Salamat N, Soleimani Z, Safahieh A, Savari A, Ronagh MT (2013) Using histopathological changes as a biomarker to trace contamination loading of Musa creeks (Persian gulf). Toxicol Pathol 41:913–920. https://doi.org/10.1177/0192623312468515
Sendra M, Pereiro P, Figueras A, Novoa B (2021) An integrative toxicogenomic analysis of plastic additives. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.124975
Siddiqui WA, Ahad A, Ahsan H (2015) The mystery of Bcl2 family: Bcl-2 proteins and apoptosis: an update. Arch Toxicol 89:289–317. https://doi.org/10.1007/s00204-014-1448-7
Soltanian S, Fereidouni MS (2017) Immunotoxic responses of chronic exposure to cypermethrin in common carp. Fish Physiol Biochem 43:1645–1655. https://doi.org/10.1007/s10695-017-0399-3
Sparks TC, Storer N, Porter A, Slater R, Nauen R (2021) Insecticide resistance management and industry: the origins and evolution of the insecticide resistance action committee (IRAC) and the mode of action classification scheme. Pest Manag Sci 77:2609–2619. https://doi.org/10.1002/ps.6254
Tsikas D (2017) Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem 524:13–30. https://doi.org/10.1016/j.ab.2016.10.021
Wang Y, Zhao H, Shao Y, Liu J, Li J, Xing M (2017a) Copper or/and arsenic induce oxidative stress-cascaded, nuclear factor kappa B-dependent inflammation and immune imbalance, trigging heat shock response in the kidney of chicken. Oncotarget 8:98103–98116. https://doi.org/10.18632/oncotarget.21463
Wang Y, Zhao H, Shao Y, Liu J, Li J, Xing MJO (2017b) Copper or/and arsenic induce oxidative stress-cascaded, nuclear factor kappa B-dependent inflammation and immune imbalance, trigging heat shock response in the kidney of chicken. Oncotarget 8:98103–98116
Wang SR, Salamova A, Hites RA, Venier M (2018) Spatial and seasonal distributions of current use pesticides (CUPs) in the atmospheric particulate phase in the Great Lakes region. Environ Sci Technol 52:6177–6186. https://doi.org/10.1021/acs.est.8b00123
Wang F, Zheng FF, Liu F (2020a) Effects of triclosan on antioxidant- and apoptosis-related genes expression in the gill and ovary of zebrafish. Exp Anim Tokyo 69:199–206. https://doi.org/10.1538/expanim.19-0115
Wang Y, Zhao H, Liu Y, Nie X, Xing M (2020b) Zinc exerts its renal protection effect on arsenic-exposed common carp: a signaling network comprising Nrf2, NF-kappaB and MAPK pathways. Fish Shellfish Immunol 104:383–390. https://doi.org/10.1016/j.fsi.2020.06.031
Wang Y, Zhao HJ, Guo MH, Fei DX, Zhang LN, Xing MW (2020c) Targeting the miR-122/PKM2 autophagy axis relieves arsenic stress. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2019.121217
Wang HX, Zhang R, Li Z, Wang LS, Yu Y, Wang Q et al (2021a) Cypermethrin induces sertoli cell apoptosis through mitochondrial pathway associated with calcium. Toxicol Res (Camb) 10:742–750. https://doi.org/10.1093/toxres/tfab056
Wang T, Ma M, Chen C, Yang X, Qian Y (2021b) Three widely used pesticides and their mixtures induced cytotoxicity and apoptosis through the ROS-related caspase pathway in HepG2 cells. Food Chem Toxicol 152:112162. https://doi.org/10.1016/j.fct.2021.112162
Wang X, Yuwen T, Yanqin T (2021c) Mangiferin inhibits inflammation and cell proliferation, and activates proapoptotic events via NF-kappaB inhibition in DMBA-induced mammary carcinogenesis in rats. J Environ Pathol Toxicol Oncol 40:1–9. https://doi.org/10.1615/JEnvironPatholToxicolOncol.2021036057
Wang Y, Zhao HJ, Liu YC, Li JY, Nie XP, Huang PY, et al (2021d) Environmentally relevant concentration of sulfamethoxazole-induced oxidative stress-cascaded damages in the intestine of grass carp and the therapeutic application of exogenous lycopene. Environ Pollut. https://doi.org/10.1016/j.envpol.2021.116597
Wang Y, Zhao HJ, Mu MY, Guo MH, Xing MW (2021e) Zinc offers splenic protection through suppressing PERK/IRE1-driven apoptosis pathway in common carp (Cyprinus carpio) under arsenic stress. Ecotox Environ Safe. https://doi.org/10.1016/j.ecoenv.2020.111473
Xie SW, Yin P, Tian LX, Liu YJ, Tan BP, Niu J (2020) Interactions between dietary lipid levels and chronic exposure of legal aquaculture dose of sulfamethoxazole in juvenile largemouth bass Micropterus salmoides. Aquat Toxicol. https://doi.org/10.1016/j.aquatox.2020.105670
Ying GG, He LY, Ying AJ, Zhang QQ, Liu YS, Zhao JL (2017) China must reduce its antibiotic use. Environ Sci Technol 51:1072–1073. https://doi.org/10.1021/acs.est.6b06424
Zhang YM, Hang Y, Bing S, Hua X, Bo H, Liu HG, et al. Antagonistic effect of VDR/CREB1 pathway on cadmium-induced apoptosis in porcine spleen. Ecotox Environ Safe, 2021. 209. ARTN 111819. https://doi.org/10.1016/j.ecoenv.2020.111819
Zhao H, Wang Y, Mu M, Guo M, Yu H, Xing M (2020a) Lycopene alleviates sulfamethoxazole-induced hepatotoxicity in grass carp (Ctenopharyngodon idellus) via suppression of oxidative stress, inflammation and apoptosis. Food Funct 11:8547–8559. https://doi.org/10.1039/d0fo01638a
Zhao H, Wang Y, Guo M, Liu Y, Yu H, Xing M (2021a) Environmentally relevant concentration of cypermethrin or/and sulfamethoxazole induce neurotoxicity of grass carp: involvement of blood-brain barrier, oxidative stress and apoptosis. Sci Total Environ 762:143054. https://doi.org/10.1016/j.scitotenv.2020.143054
Zhao H, Wang Y, Liu Y, Yin K, Wang D, Li B et al (2021b) ROS-induced hepatotoxicity under cypermethrin: involvement of the crosstalk between Nrf2/Keap1 and NF-kappaB/ikappaB-alpha pathways regulated by proteasome. Environ Sci Technol 55:6171–6183. https://doi.org/10.1021/acs.est.1c00515
Zhao HJ, Wang Y, Guo MH, Mu MY, Yu HX, Xing MW (2020b) Grass carps co-exposed to environmentally relevant concentrations of cypermethrin and sulfamethoxazole bear immunodeficiency and are vulnerable to subsequent aeromonas hydrophila infection. Environ Pollut. https://doi.org/10.1016/j.envpol.2020.115156
Zhao LL, Tang G, Xiong C, Han SS, Yang CP, He K, et al (2021) Chronic chlorpyrifos exposure induces oxidative stress, apoptosis and immune dysfunction in largemouth bass (Micropterus salmoides). Environ Pollut. https://doi.org/10.1016/j.envpol.2021.117010
Zhong RZ, Fang Y, Qin GX, Li HY, Zhou DW (2015) Tea catechins protect goat skeletal muscle against H(2)O(2)-induced oxidative stress by modulating expression of phase 2 antioxidant enzymes. J Agric Food Chem 63:7921–7928. https://doi.org/10.1021/acs.jafc.5b00816
Zhou LH, Zhou MQ, Tan HD, Xiao MX (2020) Cypermethrin-induced cortical neurons apoptosis via the Nrf2/are signaling pathway. Pestic Biochem Phys. https://doi.org/10.1016/j.pestbp.2020.02.013
Zhou LH, Chang JR, Zhao WH, Gao YL (2021) Proanthocyanidins regulate the Nrf2/are signaling pathway and protect neurons from cypermethrin-induced oxidative stress and apoptosis. Pestic Biochem Phys. https://doi.org/10.1016/j.pestbp.2021.104898
Zhou LJ, Wu QLL, Zhang BB, Zhao YG, Zhao BY (2016) Occurrence, spatiotemporal distribution, mass balance and ecological risks of antibiotics in subtropical shallow Lake Taihu, China. Environ Sci-Proc Imp 18:500–513. https://doi.org/10.1039/c6em00062b
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This work was supported by the Key Projects of Natural Science Foundation of Heilongjiang Province of China (Grant ZD2020C005).
Author information
Authors and Affiliations
Contributions
Baoying Li: writing — original draft, visualization; Yu Wang: methodology, conceptualization; Hongjing Zhao: data curation, investigation; Kai Yin: project administration; Yachen Liu: resources; Dongxu Wang: visualization; Hui Zong: supervision; Mingwei Xing: funding acquisition.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval and consent to participate of this study was approved by the Animal Protection and Utilization Management Committee of Northeast Forestry University.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 61 kb)
Rights and permissions
About this article
Cite this article
Li, B., Wang, Y., Zhao, H. et al. Oxidative stress is involved in the activation of NF-κB signal pathway and immune inflammatory response in grass carp gill induced by cypermethrin and/or sulfamethoxazole. Environ Sci Pollut Res 29, 19594–19607 (2022). https://doi.org/10.1007/s11356-021-17197-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-17197-9