Abstract
The increased use of pyrethroid insecticides raises concern for exposure to non-target aquatic species, such as Chinook salmon (Oncorhynchus tshawytscha). Cypermethrin, a type II pyrethroid, is frequently detected in surface waters and sediments at concentrations that exceed levels that induce toxicity to several invertebrate and salmonid species. To better understand the effects of cypermethrin to salmonids following dietary exposure, juvenile Chinook salmon were dietarily exposed to a 0, 200, or 2000 ng/g cypermethrin diet for a duration of 7, 14, or 21 days and assessed for body burden residues, swimming performance, lipid content, and lipid homeostatic gene expression. The average cypermethrin concentrations in fish dietarily exposed to cypermethrin for 21 days were 155.4 and 952.1 ng cypermethrin/g lipid for the 200 and 2000 ng/g pellet treatments, respectively. Increased trends of fatty acid synthase (fasn, r2 = 0.10, p < 0.05) and ATP citrate lyase (acly, r2 = 0.21, p < 0.001) mRNA expression were found in the fish livers relative to increasing cypermethrin body burden residues, though no significant changes in the mRNA expression of farnesoid X receptor or liver X receptor were observed. Furthermore, Chinook salmon dietarily exposed to cypermethrin did not have a significantly altered burst swimming performance (Umax). These results support studies that have suggested Umax may not be a sensitive endpoint when assessing the effects of certain pesticide classes, such as pyrethroids, but that dysregulation of fasn and acly expression may alter lipid homeostasis and energy metabolism in the liver of fish dietarily exposed to cypermethrin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of synthetic pyrethroid insecticides in both urban and agricultural applications has increased substantially over the last two decades (Li et al. 2017). The increased usage of pyrethroids is largely attributed to their high efficiency, broad spectrum, and low toxicity towards mammals (Bradbury and Coats 1989). However, pyrethroids are highly toxic to aquatic species with acute lethality thresholds for some fish species several orders of magnitude lower than corresponding values for mammalian species (Coats et al. 1989; Weston et al. 2015). Though pyrethroids are typically thought to degrade more rapidly than organochlorine-based insecticides, pyrethroids may persist for months to years in sediments owing to greater chemical stability (Gan et al. 2005; Meyer et al. 2013). Consequently, pyrethroid residues are found ubiquitously in sediments globally, with bifenthrin, cyfluthrin, lambda-cyhalothrin, cypermethrin, esfenvalerate, and permethrin among the most frequently detected (Hunt et al. 2016; Li et al. 2017; Lu et al. 2019).
Due to their high hydrophobicity, pyrethroids are generally not thought to be bioavailable in the freely dissolved water phase at concentrations sufficient to cause acute toxicity in fish (Budd et al. 2007). However, the increased agricultural and urban usage of pyrethroids has led to an elevated risk of fish exposure. The high hydrophobicity of pyrethroids coupled with their known uptake by invertebrate prey items inhabiting pyrethroid-contaminated field sediments has emphasized the importance of the dietary route of exposure for fish (Werner et al. 2002; Zhang et al. 2018). Furthermore, studies of wild-caught fish have demonstrated the potential for pyrethroid exposure. In a monitoring study of four Iberian rivers, Corcellas et al. (2015) documented the presence of bifenthrin, cyhalothrin, and cypermethrin in tissues of all fish sampled, with total pyrethroid concentrations ranging from 12 to 4938 ng/g lipid weight.
Pyrethroids are divided into classes based on chemical structure, with type II compounds generally thought to be more potent due to a greater degree of modification of sodium currents (Motomura and Narahashi 2000; Nasuti et al. 2003). Type I and type II pyrethroids are differentiated by the presence of a cyano moiety at the alpha position relative to the ester, with type I pyrethroids lacking the cyano moiety. Cypermethrin is a type II pyrethroid which is commonly used as treatment for insect pests of cotton, fruit, and vegetable crops (US EPA 2008). Due to its high toxicity to crustaceans, cypermethrin is commonly used in salmon aquaculture in Norway, Scotland, and Ireland to treat sea lice (Burridge et al. 2010). Residential runoff also represents an important source of cypermethrin to the aquatic environment (Weston et al. 2009). Maximum cypermethrin concentrations of 940 ng/g dry weight were detected in suspended sediments discharged from urban stormwater drains in Northern California (Weston et al. 2009). Furthermore, usage of cypermethrin and other pyrethroids have been related to declines in the abundance of six pelagic fish species in the San Francisco Estuary (Fong et al. 2016). Additionally, high concentrations of cypermethrin have been detected in run-off from agricultural areas following pesticide applications, reaching 194 µg/l in watersheds adjacent to soybean production areas in Argentina (Hunt et al. 2017; Marino and Ronco 2005). In a global review of pyrethroid contamination of various environmental media (soil, water and sediment) and organisms, cypermethrin was the most frequently detected (Tang et al. 2018), further emphasizing the importance of elucidating the effects of cypermethrin on biota.
The primary site of toxic action for pyrethroids in target organisms are voltage-gated sodium channels, leading to repetitive neuronal firing and subsequent neurotoxic effects (Vijverberg and vanden Bercken 1990). In fish, acute waterborne exposure to pyrethroids may lead to loss of balance, respiratory problems, and immobilization (Yilmaz et al. 2004). Given the known neurotoxicity of pyrethroids, swimming performance represents an important organismal-level measure of neuromuscular function which has implications for a wide range of processes including foraging, migration, and predator avoidance (Goulding et al. 2013).
The application of molecular tools, such as transcriptomics and metabolomics, has led to a further advancement in understanding the modes of action associated with pyrethroid exposure in fish species (Magnuson et al. 2020a, b; Jeffries et al. 2015). Studies linking effects at the whole-organism and molecular levels are of particular importance in interpreting the potential ecological effects of a contaminant. Previous studies have reported altered regulation of mRNAs that encode proteins that control lipid function and utilization as well as impairments to energy metabolism following pyrethroid treatment (Jin et al. 2015; Beggel et al. 2011). Furthermore, lipid metabolism and fatty acid oxidation are fundamental in providing energy for processes such as swimming and reproduction, with studies demonstrating a link between activities of mitochondrial enzymes involved in lipid metabolism (e.g. β–hydroxyacyl coenzymeA dehydrogenase) and swimming performance (Ucrit, Farrell et al. 1991). Though a number of studies have assessed the relationships between energy homeostasis and swimming performance of fish (e.g. Gibb and Dickson 2002; Goertzen et al. 2011), the effects of pyrethroids on lipid metabolism and synthesis pathways in fish have not been well characterized.
Uptake and accumulation of cypermethrin in benthic invertebrate prey items inhabiting contaminated sediments has been observed (Maund et al. 2002; Zhang et al. 2018), confirming the importance of diet as a pathway of exposure in the natural environment. As such, dietary exposure was chosen to simulate the potential effects of consuming pyrethroid-contaminated prey items by Chinook salmon. Salmon were fed cypermethrin spiked pellets for 7, 14, or 21 days and analyzed for cypermethrin body residues, swimming performance, and liver lipid homeostatic genes. Consequently, the present study aimed to determine the effects of dietary cypermethrin exposure on swimming performance (measured here as Umax) and expression of genes involved in lipid homeostasis and energetics in juvenile Chinook salmon. It is anticipated that exposure to dietary cypermethrin would lead to alterations in expression of liver homeostatic genes, such as fatty acid synthase and ATP citrate lyase, with a concomitant reduction in swimming performance.
Materials and methods
Chemicals
Cypermethrin (99% purity) for spiking pellets and dibromooctafluorobiphenyl (DBOFB, 99% purity) and decachlorobiphenyl (DCBP, 99% purity, 200 µg/ml in acetone) for measuring recovery were obtained from Sigma-Aldrich (St. Louis, MO, USA). 2,3′,4,4′,5′,6-hexachlorobiphenyl (PCB-168, 99% purity, 35 µg/ml in isooctane) for measuring recovery was obtained from AccuStandard (New Haven, CT, USA). Cypermethrin for analysis was obtained from AccuStandard as a custom standard (99% purity, 10 µg/ml in hexane). All solvents were Optima grade (Fisher Scientific, Waltham, MA, USA). Silica (60–200 mesh, grade 62), sea sand, sodium sulfate (ACS grade), acetic acid (ACS grade), and copper powder were obtained from Fisher Scientific. Internal standards 2,2′,3,4,4′,5,6,6′-octachlorobiphenyl (PCB-204, 99% purity), 13C12-DCBP (99% purity, 40 µg/ml in isooctane), and d6-cypermethrin (99% purity) were obtained from AccuStandard, Cambridge Isotope Laboratories (Tewksbury MA, USA) and Kalexsyn (Kalamazoo, MI, USA), respectively.
Juvenile Chinook salmon rearing
Juvenile Chinook salmon were obtained from the Illinois Department of Natural Resources’ Jake Wolf Hatchery (Topeka, IL, USA). Fish were maintained in recirculating-water mesocosms supplied with biofiltered, dechlorinated municipal water (pH 6.8) with the temperature held at 12 ± 1 °C. In addition, they were acclimated for nine months and fed a commercial pellet diet (BioVita Fry, Bio-Oregon, WA, USA) ad libitum daily under a 12:12 h light:dark photoperiod. All experiments were performed in accordance with the Institutional Animal Care and Use Committee (IACUC; Protocol number: 17-027) approved by Southern Illinois University.
Experimental treatment and water quality
Juvenile Chinook salmon (average fork length = 17.91 ± 1.64 cm; average weight = 52.83 ± 18.54 g) were dietarily exposed to cypermethrin-dosed (nominal concentrations; 200 or 2000 ng/g) or solvent control (acetone) pellets for a duration of 7, 14, or 21 days (n = 6 per time point per treatment). Juvenile Chinook salmon were selected for exposure to elucidate the potential for contaminants to affect the health of out-migrating salmonids. These dietary concentrations and timepoints were chosen to simulate a worst-case scenario for benthic invertebrates rearing in highly contaminated sediments within the Sacramento-San Joaquin Delta (Nowell 2003). Fish were fed 1% of their body weight daily. A preliminary study (data not shown) demonstrated the palatability of cypermethrin-spiked pellets over a seven-day period. Two fish were present per 38 l glass aquarium, with a total of three replicate aquaria for each treatment (n = 6 per treatment, per timepoint). Each aquarium contained dechlorinated municipal water and was equipped with carbon filtration. Water changes were conducted twice per week throughout the experiment, and water quality parameters assessed three times a week. The following water quality parameters were assessed; dissolved oxygen (mg/l), temperature (°C), salinity (ppt), pH and total ammonia (mg/l). Briefly, a 500 ml water sample was taken from a randomly selected control tank and temperature and salinity assessed using an YSI-30 meter. Dissolved oxygen, pH and total ammonia were assessed using an YSI Pro20 meter, Oakton pH 150 meter, and an API total ammonia kit, respectively. The following water quality parameters were maintained throughout the duration of the study: temperature (11.6 ± 0.3 °C), pH (6.6 ± 0.3), dissolved oxygen (9.9 ± 1.5 mg/l), conductivity (474.5 ± 55 µS/cm), and total ammonia (0.25 ± 0.0 mg/l). All values are presented as average ± standard deviation.
Pellet dosing and extraction of cypermethrin from pellet feed
Commercial pellet feed (BioVita Fry 2.5 mm, Bio-Oregon, WA, USA) was spiked with a solvent control (acetone) or 200 and 2000 ng cypermethrin/g pellet (nominal) following Meador et al. (2006). Briefly, pellets were added to amber glass bottles and covered with acetone. A pre-calculated amount (1.08 mg/ml) of cypermethrin stock in acetone was added to the 2000 ng pellet group and mixed thoroughly. For the 200 ng treatment and solvent control pellets, an equivalent amount of dilute (0.108 mg/ml) cypermethrin stock and clean acetone, respectively, was added. All pellets were mixed on an orbital shaker (Thermo Scientific MaxQ2000, Thermo Fisher Scientific) at 100 rotations per minute for 24 h and dried under a gentle nitrogen flow until a constant mass was achieved.
To assess actual concentrations of cypermethrin in the pellets, four replicate sub-samples of control (0.3 ± 0.01 g) and treated (0.1 ± 0.01 g) pellets were ground to a homogenous powder using a mortar and pestle and 5 ml of acetone was added. A matrix spike, matrix spike duplicate, two solvent blanks and a matrix blank were included to demonstrate the accuracy of the extraction procedure. Matrix blank and spiked samples were clean commercial pellet feed (BioVita Fry 2.5 mm). All samples were spiked with DCBP and DBOFB surrogates and a target analyte mixture added to the matrix spike samples only to determine the efficiency of the extraction techniques (Supplementary Information). To determine the potential for other legacy and current-use insecticide contaminants in the fish food pellets, a target analyte mixture containing a suite of 32 compounds was tested (Supplementary Information, Table S1). Pellets were ultrasonicated at 30% amplitude (Tekmar, Newton, CT, USA) for 10 s in triplicate and 5 ml of hexane was added. Next, samples were bath sonicated (Branson Ultrasonic 5800, Branson, Danbury, CT, USA) for an additional 10 min and extracts transferred to clean vials followed by a total of three hexane washes. Extracts were evaporated to ~1 ml and passed through a sodium sulfate column to remove residual water. Samples were transferred to GC vials and evaporated to a final volume of 0.5 ml under a gentle nitrogen flow. Nine calibration standards were prepared at levels of 0.5, 1, 2, 5, 10, 25, 50, 100 and 200 µg/l of target analytes and surrogates (DCBP and DBOFB) in hexane and internal standards were added (d6-cypermethrin, PCB-204, and 13C12-DCBP, 20 ng each). Cypermethrin was quantified as the sum of four structural isomers. Samples were acidified with acetic acid (You and Lydy 2007) and quantified on an Agilent 7890A GC equipped with an Agilent 5975A inert XL MS (Santa Clara, CA, USA) using negative chemical ionization (NCI). An Agilent HP-5ms (30 m × 0.25 mm; 0.25 µm) column was used. Pellets spiked to 2000 ng/g were diluted by a factor of 10 for analysis.
Solid phase extraction
To determine the potential for leaching of cypermethrin from unconsumed feed, solid phase extraction (SPE) was conducted on water samples at 7, 14 and 21 days using a method adapted from Wang et al. (2009). At each time point, 167 ml water samples were taken from each of the three replicate tanks from each treatment along with three randomly selected aquaria (from other time points) and composited to form 500 ml samples. Cypermethrin concentrations were quantified on an Agilent 7890A GC equipped with an Agilent 5975A inert XL MS (Santa Clara, CA, USA). Detailed information regarding extractions, quantification, and QA/QC parameters are provided in Supplementary Information.
Swimming performance (Umax)
Burst swimming speed (Umax) was chosen as a measure of swimming performance in juvenile Chinook salmon and conducted as previously described in Goulding et al. (2013). At each timepoint, individual fish were placed in a 30 l impeller-driven swim tunnel (Loligo, Denmark) and allowed to acclimate to the chamber for 20 min at a flow rate of approximately 1.5 body lengths per second (BL/s). Body lengths per second was based on average measurements conducted on a random subset of fish (n = 20). Water flow was increased every 2 min by 0.2 BL/s until fish were fatigued. Fatigue was determined when the fish rested against the rear of the test chamber and did not respond to gentle mechanical stimulation. At this point, the fish was removed from the swim chamber and immediately euthanized with buffered (sodium bicarbonate) tricaine mesylate (MS-222, 120 mg/l). Swimming performance assays were conducted on a total of 52 fish during the course of the experiment. Temperature and dissolved oxygen were monitored between assays and water changed as needed. Umax was calculated according to Farrell (2008).
Morphometric measures and tissue extractions
Weight, total and fork length, and tissue extractions were taken following euthanasia. Approximately 30 mg of liver tissue was extracted, preserved in RNAlater (Ambion), and stored at −20 °C until subsequent molecular analysis. The remaining fish carcass was weighed and stored at −20 °C and used for body burden analysis.
Cypermethrin body burden analysis
Dissected fish carcasses were freeze dried for 48 h using a Freezone 1 freeze drier (Labconco, Kansas City, MO, USA) and homogenized using a Waring 7010S commercial lab blender equipped with a stainless-steel cup (Stamford, CT, USA). Cypermethrin was extracted from homogenized tissue using Accelerated Solvent Extraction (ASE). ASE was performed by first loading homogenized and freeze-dried juvenile Chinook tissue (2 g) into a stainless steel cell (33 ml) equipped with a cellulose filter, copper powder (0.5 g), and silica (5 g, activated at 130 °C for 4 h) for inline extract clean up and lab sand as filler. The tissue was extracted using an ASE 200 (Thermo Fisher Scientific, Waltham, MA, USA) using two heat-static cycles of acetone:dichloromethane (1:1, volume:volume) at 100 °C and at 1.03 × 107 Pa. The extract was cleaned first using freezing lipid precipitation (FLP) followed by SPE (Hong et al. 2004). Further details of these procedures are available in the Supplementary Information.
A set of five QA/QC samples were analyzed with each batch of 18 samples: a matrix-free lab blank and a matrix blank sample were used to assess for contamination in the preparation methods, a sample matrix spiked with cypermethrin (40 ng) was used to assess the accuracy of the analysis, and a matrix spike duplicate sample and a batch duplicate was used to assess the reproducibility of the analysis. Sample recovery was assessed using DBOFB and PCB-168 surrogates, 40 ng each, added to the ASE cell prior to extraction and measured relative to a spike check. Acceptability criteria for QA/QC samples is described in the Supplementary Information (section S1). The method detection limit (MDL) was estimated by analyzing seven replicate matrix samples spiked with 2.5 ng cypermethrin and calculated as the product of the standard deviation of the concentration and the one-sided Student’s t value at 99% confidence level (Glaser et al. 1981). The MDL for cypermethrin in Chinook salmon was 0.89 ng/g dry weight (dw), and all concentrations below the MDL were considered to be not detected. Detections of cypermethrin greater than the MDL, but below the method reporting limit (set as 3 × MDL, i.e. <2.67 ng/g dw) were reported as below the reporting limit. Further details on QA/QC for the cypermethrin body burden analysis is available in the Supplementary Information.
Lipid content analysis
Lipid content of juvenile Chinook salmon was assessed to normalize measured cypermethrin body residues using a vanillin-phosphoric acid colorimetric method as described in Van Handel (1985). Further methodological details for lipid analysis are available in Supplementary Information.
Gene expression analyses
Several genes that regulate lipid metabolism and energetics in livers of fish were selected for mRNA evaluation (see Table S2) including fatty acid synthase (fasn), ATP citrate lyase (acly), farnesoid X receptor (fxr) and liver X receptor (lxrɑ) in an attempt to link molecular responses to swimming metrics. Total RNA was isolated from individual, homogenized liver tissue (N = 52) using a RNeasy Mini Kit (Qiagen). RNA concentration and purity were analyzed on a NanoDrop-1000 (Thermo Fisher Scientific, Waltham, MA, USA). RNA was diluted to 1 µg and reverse transcribed to cDNA using an iScript Reverse Transcription Supermix RT-qPCR kit (Bio-Rad, Hercules, CA, USA), per manufacturer’s instructions. qPCR was performed using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) on a Bio-Rad CFX Connect Real-Time PCR Detection System. Each reaction had a concentration of 100 ng cDNA with specific primer pairs (10 µM) for the genes of interest (Supplementary Table S2) with the following thermal cycling conditions used for qPCR analysis: A denaturation at 95 °C for 5 min, with 40 cycles of a 10 s denaturation step at 95 °C and an annealing and extension step for 60 s at 60 °C. A melt curve was carried out from 54–95 °C in increments of 0.5 °C. All genes were normalized to the housekeeping gene, 18S. All samples were run in triplicate and relative fold change calculated using the 2−ΔΔCt method (Livak and Schmittgen 2001).
Statistical analysis
RStudio (version 1.3.3) and SPSS (version 24) were used to perform statistical analyses. Linear regressions were used to compare cypermethrin body residues to gene expression and swimming performance. The effects of cypermethrin and duration of exposure on total lipid content were assessed using ANOVA. An α < 0.05 was used to determine statistical differences among treatments.
Results
Pellet and water cypermethrin concentrations
Measured cypermethrin concentrations in spiked feed are displayed in Table 1. Average cypermethrin values were 7.6 ± 0.3, 263.8 ± 16.7 and 2252.3 ± 87.3 for the solvent control, 200 and 2000 ng/g (nominal) pellet concentrations, respectively. Cypermethrin was detected in matrix blank (n = 1), but not lab blank (n = 2) samples, indicating that control pellets contained low levels of cypermethrin. Pellets did not contain detectable levels of other insecticides (Supplementary Information). Cypermethrin was not detected in water samples taken from the solvent control or 200 ng pellet tanks at 7, 14, or 21 days. For the 2000 ng treatment, cypermethrin was not detected in water samples after 7 days of feeding, though 1.8 and 5.1 ng/l cypermethrin was detected in the water after 14 and 21 days, respectively. Surrogate recoveries and further QA/QC details for both matrices are provided in the Supplementary Information.
Cypermethrin body residues
Average lipid-normalized body residues following a 7-day treatment were 97.0 ± 55.8 and 563.7 ± 618.9 ng cypermethrin/g lipid for the 200 and 2000 ng/g groups, respectively (n = 6). For the solvent control treatment across all timepoints, cypermethrin was not detected (<0.89 ng/g dry weight) in 53% of samples, and below the reporting limit (<2.67 ng/g dry weight) in 47% of samples. The average lipid normalized body residues in juvenile Chinook salmon across 7, 14 and 21 days are shown in Fig. 1. The larger than expected variance within treatments at each timepoint was attributed to one of the two fish in each tank consuming the majority of the supplied feed, leading to body residues below detection levels in some fish fed contaminated pellets, a trend which continued throughout the study. At the 14 days timepoint, average body residues were 123.9 ± 62.1 and 1523.6 ± 1523.3 ng/g lipid. After a 21-day treatment, average body residues were 155.4 ± 73.3 and 952.1 ± 665.6 ng/g lipid at the 200 and 2000 ng pellet treatments, respectively.
Swimming performance (Umax)
Swimming performance trials lasted between 29 and 77 min, with an average trial duration of 56 ± 8.4 min. The average Umax values across all timepoints were 4.28 ± 1.06, 3.69 ± 1.10 and 3.99 ± 0.96 BL/s for the solvent control, 200 ng and 2000 ng pellet treatments, respectively (average ± standard deviation). Swimming performance was analyzed against measured cypermethrin body residues irrespective of timepoints assessed, as there was variability in tissue burden concentrations within treatment groups (Fig. 2). There was no significant relationship between swimming performance and measured cypermethrin body residues observed (linear regression, r2 = 0.0125, p = 0.454; Fig. 2).
Total lipid analysis
Total lipid content in juvenile Chinook salmon ranged from 4–19% across all timepoints and treatments. For the control, 200 and 2000 ng/g pellet groups, average lipid content across all timepoints was 9.0 ± 4.9%, 9.5 ± 3.4% and 8.9 ± 3.6%, respectively (average ± standard deviation). No significant relationship was found between total lipid content and cypermethrin residues or duration of exposure (ANOVA, p > 0.05).
Gene expression analysis
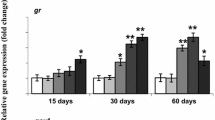
There were increased trends in fasn (r2 = 0.10, p < 0.05) and acly (r2 = 0.21, p < 0.001) mRNA expression in the livers of juvenile Chinook that were statistically significant when compared to cypermethrin body residues, though no significant relationship between fxr or lxrα expression, regardless of cypermethrin concentration (Fig. 3).
Discussion
The present study represents the first known evaluation of the impact of dietary exposure of a pyrethroid insecticide to swimming performance, total lipids, and subsequent alterations to genes involved in lipid utilization, transport, and energy metabolism in the liver of a salmonid species. Swimming performance and gene expression analyses were related to concentrations of cypermethrin that were accumulated as body residues following 7, 14, or 21 days of exposure. Molecular level endpoints were more sensitive to cypermethrin exposures than burst swimming performance (Umax), with trends of increased mRNA expression of fasn, involved in the synthesis of long-chain fatty acids, and acly, involved in energy metabolism pathways.
Cypermethrin represents one of the most ubiquitously recorded pyrethroids in sediments globally (Tang et al. 2018), with sediment concentrations as high as 1.92 µg/g organic carbon recorded in sediments collected from Guangzhou, China (Sun et al. 2015). The bioaccumulation of cypermethrin by invertebrates inhabiting spiked field sediments has been demonstrated for Chironomus tentans, an important dietary item for juvenile Chinook salmon (Goertler et al. 2018), with a biota-sediment accumulation factor (BSAF) of 0.63 recorded for a 1% organic carbon sediment (Maund et al. 2002). Assuming a BSAF value of 0.63 and a worst-case sediment concentration of 1.92 µg/g, resultant concentrations for C. tentans would be 1.21 µg/g. Though analysis of cypermethrin in field-collected benthic invertebrates is limited, this emphasizes the potential for dietary exposure of juvenile Chinook salmon to cypermethrin in the natural environment in concentrations similar in magnitude to those used in the present study.
Juvenile Chinook salmon exposed to cypermethrin spiked pellets for 21 days accumulated an average of 155.4 ± 73.3 and 952.1 ± 665.6 ng/g lipid (average ± standard deviation) in the 200 and 2000 ng/g pellet treatment groups, respectively. These concentrations are similar in magnitude to those recorded for brown trout (Salmo trutta) collected from the Llobregat River, Spain, where cypermethrin concentrations were measured up to 1520 ng/g lipid weight, though effects on fish health were not considered (Corcellas et al. 2015). The higher concentrations observed in wild caught fish are likely due to the influence of both waterborne and dietary exposures, whereas the present study focused solely on the latter. Laboratory studies quantifying cypermethrin accumulation in fish are limited (Bonansea et al. 2017; Muir et al. 1994), and no known previous study has assessed the dietary route of exposure and the downstream effects following treatment. A species of killifish, Jenynsia multidentata, exposed to 40 ng/l of waterborne cypermethrin for 96 h, had an estimated mean accumulation of 6 ng/g cypermethrin in whole fish, based on amounts measured in individual tissues (Bonansea et al. 2017), and subsequently reduced swimming behavior and altered biochemical responses in treated fish that may dysregulate metabolic processes (Bonansea et al. 2016).
In the present study, juvenile Chinook salmon expression of fasn mRNA in the liver was statistically correlated with cypermethrin body burden residues, following dietary exposure (Fig. 3). Fasn plays a key role in the synthesis of fatty acids, and has previously been shown to be altered by exposure to xenobiotics in dietary exposures (Olsvik et al. 2019). Adult male rats dietarily supplemented with another type II pyrethroid, lambda-cyhalothrin, at a dose of 0.15 mg/ kg body weight for two months exhibited significantly increased expression of fasn in liver tissue and subsequently supported immunohistochemical increases in the livers of exposed rats (Moustafa and Hussein 2016). HepG2 cells exposed to 1 × 10−7 M of a type I pyrethroid, cis-bifenthrin, for 24 h had significantly upregulated expression of fasn, along with a similar dysregulation of genes involved in lipid metabolism (Xiang et al. 2018). Fasn mRNA expression has also been reported to be altered in other in vivo-based studies following exposure to non-pyrethroid pesticides. Atlantic cod (Gadus morhua) dietarily exposed to 23.22 µg/g chlorpyrifos-methyl for 30 days had significant increases in liver fasn expression, which was used to predict altered synthesis and metabolism of hepatic cholesterol through informatic pathway analyses of diseases and functions (Olsvik et al. 2019).
In addition to fasn, the expression of acly also was significantly correlated with cypermethrin body residues in juvenile Chinook salmon (Fig. 3). ATP citrate lyase (acly) is highly expressed in the liver where it plays an important role in energy metabolism through the synthesis of acetyl-CoA, which serves as a precursor for downstream cholesterol and fatty acid synthesis (Chypre et al. 2012). A similar upregulation of acly mRNA expression was reported in mice administered a 10 mg/kg dose of cypermethrin for four weeks (Jin et al. 2015). Additionally, following a trend previously mentioned regarding altered fasn expression, Atlantic cod dietarily exposed to 23.2 mg/kg (equivalent to 23,200 ng/g) chlorpyrifos-methyl for 30 days also had significant increases in liver acly expression (Olsvik et al. 2019). Induction of each of these genes suggests that fasn and acly share similar lipogenic pathways in the livers of exposed murine models and fish and could potentially be sensitive biomarkers of impaired lipid synthesis following pesticide treatment. The mechanism of induction is unclear, however, as expression of upstream regulating receptors (eg. lxrα or fxr) was unaltered by treatment. Since previous studies have indicated pyrethroids may act as agonists and/or antagonists on these receptors (Yang et al. 2019), additional studies evaluating receptor activation may be needed to determine how lipogenic genes are upregulated.
Taken together, these findings suggest that dietary cypermethrin exposure may lead to alterations to lipid metabolism to promote lipidogenesis and accumulation, and that fasn and acly expression may serve as potential biomarkers of exposure to pesticide-treated fish. Studies have suggested that increased lipid levels in pesticide exposed fish may be an adaptive response to sequester residues in greater amounts and thereby increase tolerance (Thangavel et al. 2005); however, this was not supported by analysis of total lipid content in the present study. The lack of a downstream response in total lipid content suggests that the induction of fasn and acly was not of a magnitude necessary to elicit physiological changes in lipid utilization, or that lipid changes were tissue-specific and not detected using whole-body lipid determinations.
Furthermore, studies have demonstrated changes in hepatic expression of genes relating to fatty acid synthase in fish exposed to phytoestrogens and endocrine disrupting compounds (Cleveland and Manor 2015), with the degree of upregulation related to compound estrogenicity. Cypermethrin has been demonstrated to have endocrine disrupting effects on fish (Eni et al. 2019; Ullah et al. 2018), thus the observed upregulation of fasn and acly in the present study may be related to synthesis of long-chain fatty acids for vitellogenin or oocyte development, indicative of endocrine disruption. Additional dose-response studies linking expression of lipogenic enzymes to tissue-specific lipid content and markers of endocrine disruption including vitellogenin and serum hormone levels is needed to fully explore these mechanisms as an adverse outcome of cypermethrin exposure.
Proper lipid metabolism and fatty acid oxidation are paramount for providing a sustained energy source for fish (Watanabe 1982), particularly during energetically taxing functions such as reproduction and swimming. In rainbow trout (Oncorhynchus mykiss) elevated activities of mitochondrial enzymes involved in lipid metabolism (e.g. β–hydroxyacyl coenzymeA dehydrogenase) were observed in fish with a higher critical swimming speed (Ucrit), indicating that lipid metabolism is positively correlated with swimming performance (Farrell et al. 1991). However, this relationship is not ubiquitous, with some studies finding no relationship between metabolic enzyme activity and swimming performance (Gibb and Dickson 2002; Goertzen et al. 2011). The two most common assessments of swimming performance in fish are critical swimming speed (Ucrit) and burst swimming speed (Umax) (Brett 1964; Farrell 2008; Goulding et al. 2013). Juvenile Chinook salmon dietarily exposed to cypermethrin did not exhibit a significantly different Umax, regardless of body burden residue (Fig. 1). Similarly, juvenile rainbow trout exposed up to 300 ng/l deltamethrin or up to 3 µg/l permethrin for 4 days did not have significant changes in Umax nor subsequent changes in Ucrit in trout exposed to permethrin (Goulding et al. 2013). Though a reduced swim performance was noted in permethrin-exposed rainbow trout previously (Kumaraguru and Beamish 1983), contradicting findings were thought to be due to differences in developmental stage and fish size (Goulding et al. 2013). Furthermore, sheepshead minnow (Cyprinodon variegatus) chronically exposed to an organophosphate, guthion, at concentrations up to 0.50 µg/l for 216 days did not exhibit significant changes in Ucrit when compared to control treatments (Cripe et al. 1984). However, guthion significantly inhibited acetylcholinesterase (78%) and caused reproductive effects (Cripe et al. 1984). Additionally, juvenile sockeye salmon (Oncorhynchus nerka) exposed to a suite of neonicotinoids, including imidacloprid, clothianidin, thiamethoxam, and their mixture, at concentrations up to 300 µg/l for 96 h did not exhibit significant changes in Umax (Engelking 2018). However, Coho salmon (Oncorhynchus kisutch) exposed to 20 µg/l chlorpyrifos had reduced Umax compared to controls and the 5 and 10 µg/l treatment groups (Tierney et al. 2007). This suggests that Umax may not be a sensitive endpoint when assessing all classes of pesticides, such as pyrethroids, and fish species, size, exposure duration, and route of exposure (waterborne versus dietary) may influence swimming performance following treatment. Additionally, molecular-level changes, biochemical alterations, or other swimming-based assessments, such as olfactory-driving responses, may be more sensitive endpoints to pesticides, relative to Umax or Ucrit.
Data availability
Data will be available on request.
References
Beggel S, Connon R, Werner I, Geist J (2011) Changes in gene transcription and whole organism responses in larval fathead minnow (Pimephales promelas) following short-term exposure to the synthetic pyrethroid bifenthrin. Aquat Toxicol 105(1–2):180–188. https://doi.org/10.1016/j.aquatox.2011.06.004
Bonansea RI, Wunderlin DA, Amé MV (2016) Behavioral swimming effects and acetylcholinesterase activity changes in Jenynsia multidentata exposed to chlorpyrifos and cypermethrin individually and in mixtures. Ecotoxicol Environ Saf 129:311–319. https://doi.org/10.1016/j.ecoenv.2016.03.043
Bonansea RI, Marino DJ, Bertrand L, Wunderlin DA, Amé MV (2017) Tissue‐specific bioconcentration and biotransformation of cypermethrin and chlorpyrifos in a native fish (Jenynsia multidentata) exposed to these insecticides singly and in mixtures. Environ Toxicol Chem 36(7):1764–1774. https://doi.org/10.1002/etc.3613
Bradbury SP, Coats JR (1989) Comparative toxicology of the pyrethroid insecticides. In: Ware GW (ed.) Reviews of environmental contamination and toxicology. Springer, New York, NY, p 133–177. https://doi.org/10.1007/978-1-4613-8850-0_4
Brett JR (1964) The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Res Board Canada 21:1183–1226. https://doi.org/10.1139/f64-103
Budd R, Bondarenk S, Haver D, Kabashima J, Gan J (2007) Occurrence and bioavailability of pyrethroids in a mixed land use watershed. J Environ Qual 36:1006–1012. https://doi.org/10.2134/jeq2006.0249
Burridge L, Weis JS, Cabell F, Pizarro J, Bostick K (2010) Chemical use in salmon aquaculture: a review of current practices and possible environmental effects. Aquaculture 306:7–23. https://doi.org/10.1016/j.aquaculture.2010.05.020
Chypre M, Zaidi N, Smans K (2012) ATP-citrate lyase: a mini-review. Biochem Biophys Re Commun 422:1–4. https://doi.org/10.1016/j.bbrc.2012.04.144
Cleveland BM, Manor ML (2015) Effects of phytoestrogens on growth-related and lipogenic genes in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol C Toxicol Pharmacol 170:28–37. https://doi.org/10.1016/j.cbpc.2015.02.001
Coats JR, Symonik DM, Bradbury SP, Dyer SD, Timson LK, Atchison GJ (1989) Toxicology of synthetic pyrethroids in aquatic organisms: an overview. Environ Toxicol Chem 8:671–679. https://doi.org/10.1002/etc.5620080805
Corcellas C, Eljarrat E, Barceló D (2015) First report of pyrethroid bioaccumulation in wild river fish: a case study in Iberian river basins (Spain). Environ Int 75:110–116. https://doi.org/10.1016/j.envint.2014.11.007
Cripe GM, Goodman LR, Hansen DJ (1984) Effect of chronic exposure to EPN and to guthion on the critical swimming speed and brain acetylcholinesterase activity of Cyprinodon variegatus. Aquat Toxicol 5:255–266. https://doi.org/10.1016/0166-445X(84)90024-9
Engelking S (2018) Viability, growth, development, and performance of juvenile sockeye salmon (Oncorhynchus nerka) exposed to neonicotinoid pesticides. Dissertation, Simon Fraser University
Eni G, Ibor OR, Andem AB, Oku EE, Chukwuka AV, Adeogun AO, Arukwe A (2019) Biochemical and endocrine-disrupting effects in Clarias gariepinus exposed to the synthetic pyrethroids, cypermethrin and deltamethrin. Comp Biochem Physiol C Toxicol Pharmacol 225:108584. https://doi.org/10.1016/j.cbpc.2019.108584
Farrell AP, Johansen JA, Suarez RK (1991) Effects of exercise-training on cardiac performance and muscle enzymes in rainbow trout, Oncorhynchus mykiss. Fish Physiol Biochem 9(4):303–312. https://doi.org/10.1007/BF02265151
Farrell AP (2008) Comparisons of swimming performance in rainbow trout using constant acceleration and critical swimming speed tests. J Fish Biol 72:693–710. https://doi.org/10.1111/j.1095-8649.2007.01759.x
Fong S, Louie S, Werner I, Davis J, Connon RE (2016) Contaminant effects on California Bay–Delta species and human health. San Franc Estuary Watershed Sci 14:4. https://doi.org/10.15447/sfews.2016v14iss4art5
Gan J, Lee SJ, Liu WP, Haver DL, Kabashima JN (2005) Distribution and persistence of pyrethroids in runoff sediments. J Environ Qual 34:836–841. https://doi.org/10.2134/jeq2004.0240
Gibb AC, Dickson KA (2002) Functional morphology and biochemical indices of performance: is there a correlation between metabolic enzyme activity and swimming performance? Integr Comp Biol 42(2):199–207. https://doi.org/10.1093/icb/42.2.199
Glaser JA, Foerst DL, McKee GD, Quave SA, Budde WL (1981) Trace analyses for wastewaters. Environ Sci Tech 15(12):1426–1435. https://doi.org/10.1021/es00094a002
Goertler P, Jones K, Cordell J, Schreier B, Sommer T (2018) Effects of extreme hydrologic regimes on juvenile Chinook salmon prey resources and diet composition in a large river floodplain. Trans Am Fish Soc 147(2):287–299. https://doi.org/10.1002/tafs.10028
Goertzen MM, Driessnack MK, Janz DM, Weber LP (2011) Swimming performance and energy homeostasis in juvenile laboratory raised fathead minnow (Pimephales promelas) exposed to uranium mill effluent. Comp Biochem Physiol C Toxicol Pharmacol 4:420–426. https://doi.org/10.1016/j.cbpc.2011.07.012
Goulding AT, Shelley LK, Ross PS, Kennedy CJ (2013) Reduction in swimming performance in juvenile rainbow trout (Oncorhynchus mykiss) following sublethal exposure to pyrethroid insecticides. Comp Biochem Physiol C Toxicol Pharmacol 157:280–286. https://doi.org/10.1016/j.cbpc.2013.01.001
Hong J, Kim HY, Kim DG, Seo J, Kim KJ (2004) Rapid determination of chlorinated pesticides in fish by freezing-lipid filtration, solid-phase extraction and gas chromatography–mass spectrometry. J Chromatogr A 1038(1–2):27–35. https://doi.org/10.1016/j.chroma.2004.03.003
Hunt L, Bonetto C, Marrochi N, Scalise A, Fanelli S, Liess M, Lydy MJ, Chiu MC, Resh VH (2017) Species at Risk (SPEAR) index indicates effects of insecticides on stream invertebrate communities in soy production regions of the Argentine Pampas. Sci Total Environ 580:699–709. https://doi.org/10.1016/j.scitotenv.2016.12.016
Hunt L, Bonetto C, Resh VH, Buss DF, Fanelli S, Marrochi N, Lydy MJ (2016) Insecticide concentrations in stream sediments of soy production regions of South America. Sci Total Environ 547:114–124. https://doi.org/10.1016/j.scitotenv.2015.12.140
Jeffries KM, Komoroske LM, Truong J, Werner I, Hasenbein M, Hasenbein S et al. (2015) The transcriptome-wide effects of exposure to a pyrethroid pesticide on the Critically Endangered delta smelt Hypomesus transpacificus. Endanger Species Res 28(1):43–60. https://doi.org/10.3354/esr00679
Jin Y, Lin X, Miao W, Wang L, Wu Y, Fu Z (2015) Oral exposure of pubertal male mice to endocrine-disrupting chemicals alters fat metabolism in adult livers. Far East Entomol 30:1434–1444. https://doi.org/10.1002/tox.22013
Kumaraguru AK, Beamish FWH (1983) Bioenergetics of acclimation to permethrin (NRDC-143) by rainbow trout. Comp Biochem Physiol 75C:247–252
Li H, Cheng F, Wei Y, Lydy MJ, You J (2017) Global occurrence of pyrethroid insecticides in sediment and the associated toxicological effects on benthic invertebrates: an overview. J Hazard Mater 324:258–271. https://doi.org/10.1016/j.jhazmat.2016.10.056
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Lu Z, Gan J, Cui X, Delgado-Moreno L, Lin K (2019) Understanding the bioavailability of pyrethroids in the aquatic environment using chemical approaches. Environ Int 129:194–207. https://doi.org/10.1016/j.envint.2019.05.035
Magnuson JT, Giroux M, Cryder Z, Gan J, Schlenk D (2020a) The use of non-targeted metabolomics to assess the toxicity of bifenthrin to juvenile Chinook salmon (Oncorhynchus tshawytscha). Aquat Toxicol 224:105518. https://doi.org/10.1016/j.aquatox.2020.105518
Magnuson JT, Cryder Z, Andrzejczyk NE, Harraka G, Wolf DC, Gan J, Schlenk D (2020b) Metabolomic profiles in brains of juvenile steelhead (Oncorhychus mykiss) following bifenthrin treatment. Environ Sci Tech 54(19):12245–12253. https://doi.org/10.1021/acs.est.0c04847
Marino D, Ronco A (2005) Cypermethrin and chlorpyrifos concentration levels in surface water bodies of the Pampa Ondulada, Argentina. Bull Environ Contam Toxicol 75:820–826. https://doi.org/10.1007/s00128-005-0824-7
Maund SJ, Hamer MJ, Lane MCG, Farrelly E, Rapley JH, Goggin UM, Gentle WE (2002) Partitioning, bioavailability, and toxicity of the pyrethroid insecticide cypermethrin in sediments. Environ Toxicol Chem 21:9–15. https://doi.org/10.1002/etc.5620210102
Meador JP, Sommers FC, Ylitalo GM, Sloan CA (2006) Altered growth and related physiological responses in juvenile Chinook salmon (Oncorhynchus tshawytscha) from dietary exposure to polycyclic aromatic hydrocarbons (PAHs). Can J Fish Aquat Sci 63:2364–2376. https://doi.org/10.1139/F06-127
Meyer BN, Lam C, Moore S, Jones RL (2013) Laboratory degradation rates of 11 pyrethroids under aerobic and anaerobic conditions. J Agric Food Chem 61:4702–4708. https://doi.org/10.1021/jf400382u
Motomura H, Narahashi T (2000) Temperature dependence of pyrethroid modification of single sodium channels in rat hippocampal neurons. J Membr Biol 177(1):23–39. https://doi.org/10.1007/s002320001097
Moustafa GG, Hussein MMA (2016) Lambda cyhalothrin toxicity induces alterations in lipogenic genes and inflammatory factors in rat liver. Jpn J Vet Res 64:25–38. https://doi.org/10.14943/jjvr.64.1.25
Muir DC, Hobden BR, Servos MR (1994) Bioconcentration of pyrethroid insecticides and DDT by rainbow trout: uptake, depuration, and effect of dissolved organic carbon. Aquat Toxicol 29(3-4):223–240. https://doi.org/10.1016/0166-445X(94)90070-1
Nasuti C, Cantalamessa F, Falcioni G, Gabbianelli R (2003) Different effects of type I and type II pyrethroids on erythrocyte plasma membrane properties and enzymatic activity in rats. Toxicology 191:233–244. https://doi.org/10.1016/S0300-483X(03)00207-5
Nowell L (2003) Organochlorine pesticides and PCBs in bed sediment and whole fish from United States rivers and streams: Summary statistics: preliminary results from cycle I of the National Water Quality Assessment Program (NAWQA 1992-2001). http://ca.water.usgs.gov/pnsp/oc_doc.html. Accessed 12 Dec 2020
Olsvik PA, Larsen AK, Berntssen MHG, Goksøyr A, Karlsen OA, Yadetie F, Sanden M, Kristensen T (2019) Effects of agricultural pesticides in aquafeeds on wild fish feeding on leftover pellets near fish farms. Front Genet 10 https://doi.org/10.3389/fgene.2019.00794
Sun BQ, Wang F, Li HZ, You J (2015) Occurrence and toxicity of sediment-associated contaminants in Guangzhou College City and its adjacent areas: the relationship to urbanization. Arch Environ Contam Toxicol 68(1):124–131. https://doi.org/10.1007/s00244-014-0097-4
Tang W, Wang D, Wang J, Wu Z, Li L, Huang M, Yan D et al. (2018) Pyrethroid pesticide residues in the global environment: an overview. Chemosphere 191:990–1007. https://doi.org/10.1016/j.chemosphere.2017.10.115
Thangavel P, Sumathiral K, Karthikeyan S, Ramaswamy M (2005) Endocrine response of the freshwater teleost, Sarotherodon mossambicus (Peters) to dimecron exposure. Chemosphere 61(8):1083–1092. https://doi.org/10.1016/j.chemosphere.2005.03.045
Tierney K, Casselman M, Takeda S, Farrell T, Kennedy C (2007) The relationship between cholinesterase inhibition and two types of swimming performance in chlorpyrifos-exposed coho salmon (Oncorhynchus kisutch). Environ Toxicol Chem 26:998–1004. https://doi.org/10.1897/06-459R.1
Ullah S, Zuberi A, Alagawany M, Farag MR, Dadar M, Karthik K et al. (2018) Cypermethrin induced toxicities in fish and adverse health outcomes: Its prevention and control measure adaptation. J Environ Manage 206:863–871. https://doi.org/10.1016/j.jenvman.2017.11.076
US Environmental Protection Agency (2008) Reregistration eligibility decision for cypermethrin (revised 01/14/08). EPA, Washington, DC
Van Handel E (1985) Rapid determination of total lipids in mosquitoes. J Am Mosq Control Assoc 1(3):302–304
Vijverberg HPM, vanden Bercken J (1990) Neurotoxicological effects and the mode of action of pyrethroid insecticides. Crit Rev Toxicol 21:105–126. https://doi.org/10.3109/10408449009089875
Wang D, Weston DP, Lydy MJ (2009) Method development for the analysis of organophosphate and pyrethroid insecticides at low parts per trillion levels in water. Talanta 78(4–5):1345–1351. https://doi.org/10.1016/j.talanta.2009.02.012
Watanabe T (1982) Lipid nutrition in fish. Comp Biochem Physiol 73B:3–15
Werner I, Geist J, Okihiro M, Rosenkranz P, Hinton DE (2002) Effects of dietary exposure to the pyrethroid pesticide esfenvalerate on medaka (Oryzias latipes). Mar Environ Res 54(3–5):609–614. https://doi.org/10.1016/S0141-1136(02)00151-4
Weston DP, Holmes RW, Lydy MJ (2009) Residential runoff as a source of pyrethroid pesticides to urban creeks. Environ Pollut 157:287–294. https://doi.org/10.1016/j.envpol.2008.06.037
Weston DP, Schlenk D, Riar N, Lydy MJ, Brooks ML (2015) Effects of pyrethroid insecticides in urban runoff on Chinook salmon, steelhead trout, and their invertebrate prey. Environ Toxicol Chem 34:649–657. https://doi.org/10.1002/etc.2850
Xiang D, Chu T, Li M, Wang Q, Zhu G (2018) Effects of pyrethroid pesticide cis-bifenthrin on lipogenesis in hepatic cell line. Chemosphere 201:840–849. https://doi.org/10.1016/j.chemosphere.2018.03.009
Yang JS, Qi W, Farias-Pereira R, Choi S, Clark JM, Kim D, Park Y (2019) Permethrin and ivermectin modulate lipid metabolism in steatosis-induced HepG2 hepatocyte. Food Chem Toxicol 125:595–604. https://doi.org/10.1016/j.fct.2019.02.005
Yilmaz M, Gül A, Erbaşli K (2004) Acute toxicity of alpha-cypermethrin to guppy (Poecilia reticulata, Pallas, 1859). Chemosphere 56:381–385. https://doi.org/10.1016/j.chemosphere.2004.02.034
You J, Lydy MJ (2007) A solution for isomerization of pyrethroid insecticides in gas chromatography. J Chromatogr A 1166:181–190. https://doi.org/10.1016/j.chroma.2007.08.014
Zhang J, You J, Li H, Tyler Mehler W, Zeng EY (2018) Particle-scale understanding of cypermethrin in sediment: desorption, bioavailability, and bioaccumulation in benthic invertebrate Lumbriculus variegatus. Sci Total Environ 642:638–645. https://doi.org/10.1016/j.scitotenv.2018.06.098
Acknowledgements
We thank the anonymous reviewers for their thoughtful comments which improved the manuscript.
Funding
This research was funded through the California Department of Fish and Wildlife Proposition 1 Restoration Grant Program (#P1896015).
Author information
Authors and Affiliations
Contributions
All authors contributed to the production of the manuscript and research project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments were performed in accordance with the Institutional Animal Care and Use Committee (Protocol number: 17-027) approved by Southern Illinois University IACUC. No studies with human participants are included in this research project.
Informed consent
All authors are aware of the informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Fuller, N., Magnuson, J.T., Huff Hartz, K.E. et al. Effects of dietary cypermethrin exposure on swimming performance and expression of lipid homeostatic genes in livers of juvenile Chinook salmon, Oncorhynchus tshawytscha. Ecotoxicology 30, 257–267 (2021). https://doi.org/10.1007/s10646-021-02352-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-021-02352-2