Abstract
Enrofloxacin (ENR) is a commonly used drug in aquaculture, and it is frequently detected in the aquatic environment. Data on ENR toxicity toward aquatic species are limited. This study was aimed at using different biomarkers to evaluate the possible toxic effects of grass carp (Ctenopharyngodon idella) exposed to 0 (control), 1, 100, and 10,000 μg/L enrofloxacin for 21 days as a sub-chronic exposure trial, oxidative stress biomarkers (including superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH), and malondialdehyde (MDA)), neurotoxicity indicators (including acetylcholinesterase (ACHE) activity, nitric oxide (NO)), and digestive enzyme activities (including lipase (LPS), amylase (AMS) enzymes). In addition, an integrated biomarker response (IBR) index was utilized to evaluate the integrated toxic effects of ENR on grass carp. Our results demonstrated that ENR exposure significantly increased activities of CAT, LPS, and AMS. ENR exposure also significantly upregulated the expression levels of sod1, ACHE, LPL, ATGL, and AMY genes. Furthermore, histopathological changes were observed in the hepatopancreatic tissues of grass carp exposed to ENR. It was observed that higher IBR scores were noticed in the tissues of fish exposed to ENR, suggesting an induced biological response. The comprehensive biomarker index showed that CAT and ACHE activities have a higher response to ENR, and 100 μg/L has a greater impact on grass carp. These results indicate that ENR has a toxic effect on grass carp and impairs their physiological functions. This is the first study to explore the effects of ENR on grass carp, and it provides basic information for assessment of ENR effects in aquaculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Population growth, over-reliance on fossil fuels, and climate change could threaten global food security in the future (Fry et al., 2016; Yogev et al., 2020). Aquatic food has made a significant contribution to alleviating this pressure. In order to safeguard aquaculture production, antibiotics are inevitably used to prevent and treat diseases that occur in aquaculture (Chen et al., 2020; Zhou et al., 2021). Antibiotics have been the focus of many ecotoxicological studies due to their persistence in the environment and high ability to produce physiological effects (Perussolo et al., 2019; Rodrigues et al., 2019). For example, antibiotics can inhibit fish survival, development, and hatching rates by primarily disrupting the intracellular redox balance and inducing oxidative stress (Yang et al., 2020).
ENR, a fluoroquinolone, exerts its antibacterial effect by inhibiting DNA gyrase (a type II topoisomerase) (Sehonova et al., 2019). It is widely used in the prevention and treatment of biological diseases in aquaculture (Li et al., 2017, 2018; Zhang et al., 2019). ENR is released into the water environment because it is not fully metabolized by the body (Ren et al., 2021). Considering that ENR can be rapidly adsorbed by soil particles and 90% dissipation time of ENR in soil and marine sediments is greater than 150 days, it will slowly desorb from soil particles (Dalla Bona et al., 2015; Wei et al., 2012). The continued release of ENR may pose a potential risk to aquatic life. At present, ENR has been found in various concentrations in aquatic environments around the world (Huang et al., 2020a; Andrieu et al., 2015; He et al., 2019; Tang et al., 2015; Teglia et al., 2019; Han et al., 2020). For example, the maximum concentration of ENR detected was 5.68 μg/L in 24 water samples from two rivers in North China (Cheng et al., 2019). The concentration of ENR in seawater around Xiamen Island was 24 ng/L (Chen et al., 2021). Previous studies have detected ENR in fish muscles. For example, the residual levels of ENR in the muscles of six fish species from the Karakaya Dam Reservoir in Turkey ranged from 0.0034 to 0.0073 mg/kg (Varol & Sunbul, 2019).
Adverse reactions of ENR released into the water environment to nontargeted organisms have been reported. For example, in juvenile giant freshwater prawn Macrobrachium rosenbergii, ENR inhibited the growth of shrimp, caused damage to the gill and hepatopancreas tissue, and also showed to induce oxidative stress (Zhang et al., 2019). A previous studies by Du et al. (2022). showed that ENR affects glycolysis/gluconeogenesis and the pentose phosphate pathway, which indirectly affects nutrient absorption and meat quality, in the gut of American shad. Qiu et al. showed that ENR can produce immune suppression on fish and confirmed for the first time that the immune suppression by ENR is closely mediated through alterations of the intestinal microbiome in fish (Qiu et al., 2022). However, there are few studies on toxic effects of ENR on aquatic organisms, and there is a lack of comprehensive analysis of indicators. Integrated biomarker response (IBR) is a comprehensive analysis method, which compares the specific adverse effects of harmful substances by simple calculation, screens sensitive biomarkers, and further accurately and effectively evaluates the ecological risk of the environment (Liao et al., 2021; Samanta et al., 2018). Samanta et al. integrated oxidative stress and histopathological changes in fish gills, liver, and kidneys through IBR to study the adverse effects of domestic, industrial, and hot spring on fish inhabiting polluted streams (Samanta et al., 2018). Superoxide dismutase, catalase, reduced glutathione, and malondialdehyde are sensitive markers to assess the antioxidant response and lipid peroxidation caused by chemicals in the external environment (Huang et al., 2020b). For example, a study has found that greater antioxidant was with the higher IBR index of limpets in polluted environment (Silva et al., 2018). Acetylcholinesterase and nitric oxide are sensitive biomarkers of neurotoxicity when organisms are challenged by toxicants and adverse environmental conditions (Shi et al., 2018; Iheanacho & Odo, 2020; Mukherjee et al., 2019). The activity of analyze lipase and amylase can be used as biomarkers to assess the health status of an organism (Li & Li, 2020). To date, few studies have employed IBR to quantify the effects of ENR on aquatic organisms. In this study, grass carp were exposed to different concentrations of ENR (1, 100, 10,000 μg/L) for 21 days to explore the comprehensive impact of ENR on grass carp. This will enrich our understanding of the ecological risks of ENR in aquaculture.
2 Material and Methods
2.1 Chemicals
Enrofloxacin (ENR) was purchased from Hefei Bomei Biotechnology Co. Ltd (Anhui, China) and the purity was above 98%.
2.2 Animal Experiment
Grass carps (weight 91.975 ± 13.17 g, length: 21.01 ± 0.87 cm) were obtained from Rushan fish breeding base (Shandong, China). After 14 days of adaptation, they were randomly distributed into four treatments (15/group in three plastic tanks (20 L), n = 5 per tank). The concentrations used included 1, 100, and 10,000 μg/L. Exposure doses of ENR were selected based on ambient concentrations and those used in production (Zhang et al., 2019; Zheng et al., 2020; Li et al., 2020b; Liang et al., 2014; Phillips et al., 2016). The water temperature was controlled at 23 °C. The photoperiod was kept at 14:10-h light/dark cycle. The fish were fed with commercial fish food (Xinda, Tianjin, China) twice a day. The water (half of the volume) of each experimental tank was replaced every 48 h to maintain the appropriate concentration and water quality. All solution samples were analyzed by HPLC equipped with a fluorescence detector, which was based on the method (Sun et al., 2014). According to the analyzed results, the measured concentration of ENR (0.92 ± 0.05, 95.14 ± 11.42, and 9012.97 ± 159.32 μg /L, corresponding to the 1, 100, and 10,000 μg /L) was within 20% of the nominal concentration, which meets the OECD guidelines (the OECD guideline for testing of chemicals No. 204, “Fish, Prolonged Toxicity Test”).
All procedures and animal handling comply with the guidelines approved by the Local Animal Ethics Committee. No fish mortality occurred during the toxicity tests.
After 21 days, grass carps were euthanized with MS-222 (0.03%, Sigma-Aldrich Corp.). The weight and length of the fish were measured. Calculate the condition factor (CF, whole fish weight (g)/whole fish length (cm)3 × 100). The brain, hepatopancreas, and intestines were frozen in liquid nitrogen and stored at −80 °C.
2.3 Biochemical Biomarkers
Oxidative stress indexes (SOD, CAT, GSH, and MDA) in the hepatopancreas, neurotoxicity indexes (ACHE and NO) in the brain, and digestive enzymes (LPS and AMS) in the intestine were analyzed using commercial kits (Nanjing Jiancheng Bioengineering Institute, China) according to manufacturer’s protocol. References of all biomarker kits are in the supporting information (Table S1).
2.4 Histopathological Biomarker
Fragments of hepatopancreas were immobilized in 4% paraformaldehyde. They were then dehydrated in alcohol (70%, 80%, 90%, and 100%), transparentized in xylene, and incorporated into Paraplast. They were cut into 5 μm slices and stained with hematoxylin–eosin (HE). For the nuclear area, each sample captured 3 images under a × 1000 microscope. In each image, the area of 12 nuclei was measured and analyzed using ImageJ.
2.5 Quantitative Real-Rime Polymerase Chain Reaction (qPCR)
The RNA was extracted according to the manufacturer protocol from the brain, hepatopancreas, and intestines (n = 3) using Trizol (Accurate Biotechnology Co., Ltd., Hunan, China). Then, use Evo M-MLV RT Kit and gDNA Clean for qPCR II Kit (Accurate Biotechnology Co., Ltd., Hunan, China) for reverse transcription. Use the Roche 96 Light Cycler RT-PCR system (Roche Applied Science, Indianapolis, IN, USA) to perform qRT-PCR on the target gene. The reaction system included 5 μL SYBR® Green Premix Pro Taq HS, 0.2 μL PCR forward primer (10 μmol/L), 0.2 μL PCR reverse primer (10 μmol/L), 2 μL cDNA template, and 2.6-μL DEPC-treated water. The amplification procedure used a two-step method: pre-denaturation at 95 °C for 60 s, followed by 45 cycles of 95 °C for 10 s and 60 °C for 30 s. β-Actin was cited in Wang et al.’s previous work (Wang et al., 2015) (F: GGCTGTGCTGTCCCTGTA, R: GGGCATAACCCTCGTAGAT. GenBank access: M25013). Sod1 was cited in Wang et al.’s previous work (Wang et al., 2019). NCBI primer blast program (https://www.ncbi.nlm.nih.gov/) is used to determine the biochemical biomarker-related pathway primer sequences of other genes. The primers used for qRT-PCR analysis are listed in Table 1. Functional gene primers are synthesized by Tsingke Biotechnology Co., Ltd. Use the 2−ΔΔCt formula to calculate the gene fold change (Livak and Schmittgen, 2001). And the primer amplification efficiency of the selected genes in the study has been shown in Table S2.
2.6 Integrated Multi-level Biomarker Response
This study used SOD, CAT, GSH, MDA, ACHE, NO, LPS, and AMS activity to calculate the biomarker reaction version 2 comprehensive index (IBRv2) (Sanchez et al., 2013; Beliaeff & Burgeot, 2002) results. IBRv2 was calculated following the methods described by Beliaeff and Burgeot (2002) with modifications from Sanchez et al. (2013). Details of IBRv2 can be found in the support information (text S1).
2.7 Statistical Analysis
Mean ± standard error of the mean (SEM) was used to calculate all data. Three biological replicates were set for each treatment. Statistical analyses use SPSS statistics 19 (SPSS Inc., Chicago, Ltd., USA). The data were determined for normality and homogeneity of variances using the Shapiro–Wilk test and Levene’s test, respectively. If the data does not satisfy the normal distribution and variance homogeneity, Log-transformation is performed on the data. If the data is not satisfied after Log-transformation, nonparametric analysis is used. One-way analysis of variance (ANOVA) and Dunnett’s test could be used to analyze statistically significant differences between treatments and the corresponding control. The level of significance was set at P < 0.05 (*) and P < 0.01 (**).
3 Results
3.1 Growth Performance
Exposure to ENR had no effect on CF index of grass carp. CF is shown in the supporting information (Fig. S1).
3.2 Molecular and Biochemical Responses
3.2.1 Oxidative Stress Responses
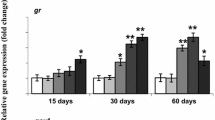
Compared with the control group, the activities of SOD (Fig. 1a) and GSH (Fig. 1c) and the content of MDA (Fig. 1d) showed no significant differences after 21 days of exposure to ENR. Compared with the control group, the activity of CAT (Fig. 1b) was significantly increased in the 1 μg/L (p < 0.05), 100 μg/L (p < 0.01), and 10,000 μg/L (p < 0.01) ENR exposure group.
Enzyme activities of SOD (a), CAT (b), GSH (c), content of MDA (d), and the transcription levels of sod1 (e) and cat (f) in hepatopancreas of grass carp after 21 days of exposure to different concentrations of ENR (0, 1, 100, and 10,000 μg/L). Data are mean ± SEM (n = 3). *p < 0.05; **p < 0.01 relative to the control
Through exposure to ENR, the transcription level of sod1 (Fig. 1e) in hepatopancreas of grass carp in all exposed groups was upregulated compared with the control. When the concentration was 1 μg/L, it was significantly upregulated (p < 0.01). The transcription level of cat (Fig. 1f) in all exposed groups was upregulated compared with the control, but not significantly.
3.2.2 Neurotoxicity Responses
After 21 days of exposure to ENR, the activity of ACHE (Fig. 2a) and the content of NO (Fig. 2b) showed no significant difference compared with the control group. But the activity of ACHE increased with the increase of exposure concentration, and the content of NO was lower than the control group.
3.2.3 Digestive Enzyme Responses
Compared with the control group, LPS (Fig. 3a) activity increased significantly at exposure concentrations of 1 μg/L (p < 0.01) and 100 μg/L (p < 0.05). AMS (Fig. 3b) activity in all exposed groups was higher than that in the control group. And it was significantly increased when exposure concentration was 100 μg/L (p < 0.05).
Enzyme activities of LPS (a), AMS (b), and the transcription levels of LPL (c), ATGL (d), and AMY (e) in the intestines of grass carps after 21 days of exposure to different concentrations of ENR (0, 1, 100, and 10,000 μg/L). Data are mean ± SEM (n = 3). *p < 0.05; **p < 0.01 relative to the control
The expression of LPL (Fig. 3c) was inhibited in the intestinal tract of grass carp exposed to ENR, and the mRNA transcription level of LPL was significantly downregulated (p < 0.05) when the concentration was 1 μg/L. Compared with the control group, the mRNA transcription level of ATGL (Fig. 3d) significantly decreases (p < 0.05) when the concentration is 10,000 μg/L. The mRNA transcription level of AMY (Fig. 3e) was significantly downregulated at 1 μg/L (p < 0.05) and 10000 μg/L (p < 0.05).
3.3 Hepatopancreas Histopathology
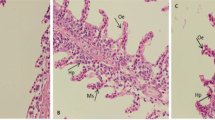
The results of histopathological examination of the hepatopancreas of grass carp are shown in Fig. 4. The structure of normal hepatopancreas tissue is neatly arranged, the cytoplasm is intact, and the nucleus is clear (Fig. 4a, e). Compared with the control group, the exposure group had vascular congestion, especially in the portal vein (Fig. 4b–d, f–h). Exposure to ENR can also cause cell nuclei to become smaller (Fig. 4i). Compared to the control group, the nuclei in the 1 μg/L (p < 0.05) and 100 μg/L (p < 0.05) groups were smaller.
Representative photomicrographs of hepatopancreas tissue sections of grass carp after 21 days of exposure to different concentrations of ENR (0, 1, 100, and 10,000 μg/L). a control × 100; b 1 μg/L × 100; c 100 μg/L × 100; d 10,000 μg/L × 100; e control × 1000; f 1 μg/L × 1000; g 100 μg/L × 1000; h 10,000 μg/L × 1000; i hepatopancreas cell nuclear area. Data are mean ± SEM (n = 12). V, blood vessel; arrow, hyperemia
3.4 Integrated Biomarker Response
In this study, eight biomarkers were selected to explore whether grass carp exposed to ENR would be toxic. The star plot of the IBR index of grass carp exposed to different concentrations of ENR is shown in Fig. 5a, and the obtained IBR value is shown in Fig. 5b. In all exposed groups, the content of NO was inhibited, while the activities of CAT, ACHE, LPS, AMS, and the contents of MDA were induced. Among all the biomarkers measured, the changes in CAT and ACHE were obvious. IBR values show that the stress caused by 100 μg/L is the highest.
a Related star maps and comprehensive biomarker response index (IBRv2) values of grass carp after 21 days of exposure to different concentrations of ENR (0, 1, 100, and 10,000 μg/L). Abbr.: SOD superoxide dismutase activity, CAT catalase activity, MDA malondialdehyde, GSH glutathione, ACHE acetylcholinesterase activity, NO nitric oxide, LPS lipase, AMS amylase. The area above 0 reflects induction of the biomarker and below 0 indicates reduction of the biomarker. b Histogram of IBRv2
4 Discussion
Oxidative stress is closely related to fish growth and health during fish culture. Fish under oxidative stress are often weak in immunity and disease resistance, so their health status and growth performance are negatively affected. Studies have shown that organisms exposed to pollutants increase the production of reactive oxygen species (ROS) due to increased metabolic and biotransformation activities, which further increase the antioxidant enzyme activity and/or transcription level in the body (Regoli & Giuliani, 2014). SOD and CAT play a huge role in removing active oxygen and the body’s protective defense response (Zhang et al., 2017). SOD can effectively remove the superoxide anion free radicals generated during the oxidation process in organisms and convert them into H2O2 and O2. GSH can be used as a cofactor to combine with exogenous compounds to degrade toxic substances out of the body (Ajima et al., 2021; Sehonova et al., 2019; Su et al., 2019). MDA is a toxic product of lipid peroxidation, which can indirectly reflect the degree of oxidative damage in the body (Zhang et al., 2017). When the antioxidant system is unable to eliminate free radicals produced by xenobiotics, the content of MDA will increase (Lin et al., 2016). For example, Sehonova et al. exposed zebrafish to 5, 10, and 500 μg/L enrofloxacin for 14 days, and the results showed that enrofloxacin can cause oxidative stress in exposed fish and lipid peroxidation was observed at the highest concentration (Sehonova et al., 2019). The enhanced expression of antioxidant genes and enzyme activity observed in this study indicated increased ROS production. However, MDA content did not change, which may be due to the antioxidant response to avoid the oxidation of macromolecules due to oxidative stress (Grott et al., 2021). In this study, SOD activity did not change significantly, while CAT activity increased significantly compared with the control, possibly because CAT involved in the removal of H2O2 was more active compared with SOD activity (Magara et al., 2018). It is not surprising that the gene transcription level of SOD increases without changing at the functional level. Because there are multiple regulatory steps involved between transcription and translation in eukaryotic cells, there is a delay (Defo et al., 2015).
ACHE activity has been widely recognized as a sensitive biomarker of neurotoxicity of organisms (Shi et al., 2018; Iheanacho & Odo, 2020). It not only inactivates acetylcholine, ensuring the normal function of the neuromuscular system, but also participates in neurodevelopment (Yang et al., 2018). Alterations in ACHE activity may lead to changes in cholinergic neurotransmission (Yuan et al., 2018). The increased levels of ACHE hydrolyzed the acetylcholine produced in the body, which eventually led to the reduction of neurotoxicity (Li et al., 2020a). In this study, the activity of ACHE increased. The results of this study may be due to overcompensation (Badiou et al., 2008; Pan et al., 2012). NO is a neurotransmitter that is enzymatically synthesized by nitric oxide synthase (NOS) in various cell types (Carreno Gutierrez et al., 2020; Serafini et al., 2020). NO plays an important role in cell signal transmission and neurotransmission (Jay et al., 2014). In this study, the level of NO decreased compared to the control group, which may be due to the generation of ROS that reduced the level of NO in the brain. NO reacts quickly with oxygen free radicals (O2∙-) to produce peroxynitrite anion (ONOO-), which can further form peroxynitrous acid (ONOOH), thereby reducing the level of NO (Liu et al., 2013).
In general, most pollutants entering an organism enter the intestines, where they alter the activity of intestinal enzymes that play an important role in digestion (Adeyemi et al., 2020; Xie et al., 2019). Therefore, the activity of digestive enzymes can be used as a biological indicator of fish growth and health (Xie et al., 2019). Changes in the activity of biological digestive enzymes may change the processing of dietary inputs, which in turn may affect energy metabolism (Kong et al., 2019). Wang et al. showed that under severe ammonia stress, fish consume more energy by increasing digestive enzymes in the intestine to maintain the balance of ammonia accumulation and metabolism in the body (Wang et al., 2021). Previous studies have fed zebrafish with oxytetracycline (OTC) at a therapeutic concentration (80 mg/kg body weight) for 6 weeks. The results showed increased amylase and lipase activities, indicating that fish need more energy to resist the pressure caused by antibiotics (Limbu et al., 2020; Zhou et al., 2018). In this study, the activity of amylase and lipase increased significantly, which may be due to the increase of energy supply by fish to enhance the body’s adaptability to ENR. Previous studies have shown that there was a low correlation between gene expression and enzyme activity of AMS and trypsin. For example, the study by Xie et al. showed that the activities of lipase and amylase under Cd2+ exposure were significantly inhibited, while significantly downregulating the activity of the respective encoded enzymes (Xie et al., 2019). In Houde et al.’s previous study, AMS gene expression was upregulated and AMS enzyme activity was inhibited under hexachlorocyclopentadiene exposure (Houde et al., 2013). Our research results show that the activities of lipase and amylase are significantly increased, while the mRNA expression level of related enzymes is significantly downregulated. These results suggest that gene expression and enzyme activity may show different responses under different conditions (Schwarzenberger & Fink, 2018). The difference between mRNA expression level and related biochemical reactions needs further study (Kim & Jung, 2016; Defo et al., 2015).
Histopathology can reflect the health status of organisms exposed to a variety of environmental pollutants (Correia et al., 2020; Lam et al., 2013). It can be employed as biomarkers of contaminant exposure and consequences (Iftikhar et al., 2022). Because of its sensitivity to aquatic pollution, the hepatopancreas is often used as a histopathological index to assess the health of fish (Yancheva et al., 2016). Our histopathological results indicate that grass carp hepatopancreas may be affected by ENR. The hepatopancreas of fish exposed to ENR will show congestion and a decrease in the area of the nucleus. Congestion may be caused by increased blood pressure after exposure to poison (Bernet et al., 1999). Compared with the control group, the area of hepatocyte nucleus is smaller. Similar to the results of this study, Zhang et al. exposed Macrobrachium rosenbergii to 5 mg/L ENR for 14 days and found that its hepatopancreas nucleus had coagulated and became smaller (Liu et al., 2015; Zhang et al., 2019). A small nucleus is a sign of apoptosis. Previous studies have shown that ENR can induce hepatocyte apoptosis (Liu et al., 2015; Zhang et al., 2019).
The IBR index is considered to be a general description of the “health” of the animal in the environment (Adeyemi et al., 2020). It is used to prove the environmental stress caused by animals exposed to xenobiotics (Adeyemi et al., 2020). It can help integrate all the parameters to show changes that could not be so easily noticed (Mukherjee et al., 2022). In this study, the method was used to integrate biochemical indicators to understand the impact of ENR on grass carp more comprehensively. These results showed that the response of CAT and ACHE activity might imply its high sensitivity to ENR. This is consistent with the analysis results of a single indicator. The results of these two indicators were more representative, which helped us better understand the toxicity of ENR to grass carp. Interestingly, the stress caused by ENR exposure is stronger at 100 μg/L. This may be because high concentrations of ENR trigger defense mechanisms more rapidly, and after a short-time response, the biomarker gradually adjusts and returns to a baseline condition. IBR indicators can be a quantitative and effective tool for monitoring ENR on fish toxicology.
5 Conclusions
This study was a contribution to the assessment of adverse effects of ENR on grass carp. Multi-biomarker methods indicate that exposure to ENR can cause grass carp oxidative damage and neurotoxicity and affect digestive enzyme activity. In addition, our results indicate that CAT and ACHE activities have a higher response to ENR. CAT and ACHE activities are more sensitive to ENR. Further, it was observed that a concentration of 100 μg/L ENR was the most toxic to grass carp after 21 days of exposure. Histopathological results indicate that ENR may induce cell apoptosis and cause hepatopancreas cell damage in grass carp. Considering the large-scale use of ENR in aquaculture, it may pose a major risk to aquatic organisms. The concentration of ENR in the aquatic environment needs to be controlled to avoid further harm to humans.
Data Availability
The data and materials that support the findings of this study are available from the corresponding authors upon reasonable request.
Code Availability
Not applicable.
References
Adeyemi, J. A., Olise, C. C., Bamidele, O. S., & Akinola, B. K. (2020). Effects of ultraviolet photooxidation of cypermethrin on the activities of phosphatases and digestive enzymes, and intestinal histopathology in African catfish, Clarias gariepinus (Burchell, 1822). Journal of Experimental Zoology Part A: Ecological and Integrative Physiology, 333, 543–549. https://doi.org/10.1002/jez.2392
Ajima, M. N. O., K. Kumar, N. Poojary, and P. K. Pandey. (2021). Oxidative stress biomarkers, biochemical responses and Na+ -K+ -ATPase activities in Nile tilapia, Oreochromis niloticus exposed to diclofenac. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology, 240, 108934. https://doi.org/10.1016/j.cbpc.2020.108934.
Andrieu, M., Rico, A., Phu, T. M., Huong, D. T. T., Phuong, N. T., & Van den Brink, P. J. (2015). Ecological risk assessment of the antibiotic enrofloxacin applied to Pangasius catfish farms in the Mekong Delta, Vietnam. Chemosphere, 119, 407–414. https://doi.org/10.1016/j.chemosphere.2014.06.062
Badiou, A., Meled, M., & Belzunces, L. P. (2008). Honeybee Apis mellifera acetylcholinesterase–A biomarker to detect deltamethrin exposure. Ecotoxicology and Environmental Safety, 69, 246–253. https://doi.org/10.1016/j.ecoenv.2006.11.020
Beliaeff, B., & Burgeot, T. (2002). Integrated biomarker response: A useful tool for ecological risk assessment. Environmental Toxicology and Chemistry, 21, 1316–1322. https://doi.org/10.1002/etc.5620210629
Bernet, D., Schmidt, H., Meier, W., Burkhardt-Holm, P., & Wahli, T. (1999). Histopathology in fish: Proposal for a protocol to assess aquatic pollution. Journal of Fish Diseases, 22, 25–34. https://doi.org/10.1046/j.1365-2761.1999.00134.x
Carreno Gutierrez, H., O’Leary, A., Freudenberg, F., Fedele, G., Wilkinson, R., Markham, E., van Eeden, F., Reif, A., & Norton, W. H. J. (2020). Nitric oxide interacts with monoamine oxidase to modulate aggression and anxiety-like behaviour. European Neuropsychopharmacology, 30, 30–43. https://doi.org/10.1016/j.euroneuro.2017.09.004
Chen, J., Sun, R., Pan, C., Sun, Y., Mai, B., & Li, Q. X. (2020). Antibiotics and food safety in aquaculture. Journal of Agriculture and Food Chemistry, 68, 11908–11919. https://doi.org/10.1021/acs.jafc.0c03996
Chen, H., Chen, W., Guo, H., Lin, H., & Zhang, Y. (2021). Pharmaceuticals and personal care products in the seawater around a typical subtropical tourist city of China and associated ecological risk. Environmental Science and Pollution Research International, 28, 22716–22728. https://doi.org/10.1007/s11356-020-12335-1
Cheng, J., Jiang, L., Sun, T., Tang, Y., Du, Z., Lee, L., & Zhao, Q. (2019). Occurrence, seasonal variation and risk assessment of antibiotics in the surface water of North China. Archives of Environmental Contamination and Toxicology, 77, 88–97. https://doi.org/10.1007/s00244-019-00605-0
Correia, A. T., Rodrigues, S., Ferreira-Martins, D., Nunes, A. C., Ribeiro, M. I., & Antunes, S. C. (2020). Multi-biomarker approach to assess the acute effects of cerium dioxide nanoparticles in gills, liver and kidney of Oncorhynchus mykiss. Comparative Biochemistry and Physiology, Part C: Toxicology & Pharmacology, 238, 108842. https://doi.org/10.1016/j.cbpc.2020.108842
Dalla Bona, M., Zounkova, R., Merlanti, R., Blaha, L., & De Liguoro, M. (2015). Effects of enrofloxacin, ciprofloxacin, and trimethoprim on two generations of Daphnia magna. Ecotoxicology and Environmental Safety, 113, 152–158. https://doi.org/10.1016/j.ecoenv.2014.11.018
Defo, M. A., Bernatchez, L., Campbell, P. G. C., & Couture, P. (2015). Transcriptional and biochemical markers in transplanted Perca flavescens to characterize cadmium- and copper-induced oxidative stress in the field. Aquatic Toxicology, 162, 39–53. https://doi.org/10.1016/j.aquatox.2015.02.014
Du, J., Liu, Q., & Fu, L. (2022). Metabolic and transcriptional disruption of American shad (Alosa sapidissima) by enrofloxacin in commercial aquaculture. Environmental Science and Pollution Research International, 29, 2052–2062. https://doi.org/10.1007/s11356-021-15330-2
Fry, J. P., Love, D. C., MacDonald, G. K., West, P. C., Engstrom, P. M., Nachman, K. E., & Lawrence, R. S. (2016). Environmental health impacts of feeding crops to farmed fish. Environment International, 91, 201–214. https://doi.org/10.1016/j.envint.2016.02.022
Grott, S. C., D. Bitschinski, N. G. Israel, G. Abel, S. P. da Silva, T. C. Alves, D. Lima, A. C. D. Bainy, J. J. Mattos, E. B. da Silva, C. A. C. de Albuquerque, and E. A. de Almeida. (2021). Influence of temperature on biomarker responses and histology of the liver of American bullfrog tadpoles (Lithobates catesbeianus, Shaw, 1802) exposed to the herbicide Tebuthiuron. Science of the Total Environment, 771, 144971. https://doi.org/10.1016/j.scitotenv.2021.144971.
Han, Q. F., Zhao, S., Zhang, X. R., Wang, X. L., Song, C., & Wang, S. G. (2020). Distribution, combined pollution and risk assessment of antibiotics in typical marine aquaculture farms surrounding the Yellow Sea, North China. Environment International, 138, 105551. https://doi.org/10.1016/j.envint.2020.105551
He, K., Hain, E., Timm, A., Tarnowski, M., & Blaney, L. (2019). Occurrence of antibiotics, estrogenic hormones, and UV-filters in water, sediment, and oyster tissue from the Chesapeake Bay. Science of the Total Environment, 650, 3101–3109. https://doi.org/10.1016/j.scitotenv.2018.10.021
Houde, M., Carter, B., & Douville, M. (2013). Sublethal effects of the flame retardant intermediate hexachlorocyclopentadiene (HCCPD) on the gene transcription and protein activity of Daphnia magna. Aquatic Toxicology, 140–141, 213–219. https://doi.org/10.1016/j.aquatox.2013.06.008
Huang, F., An, Z., Moran, M. J., & Liu, F. (2020a). Recognition of typical antibiotic residues in environmental media related to groundwater in China (2009–2019). Journal of Hazardous Materials, 399, 122813. https://doi.org/10.1016/j.jhazmat.2020.122813
Huang, J. N., Wen, B., Meng, L. J., Li, X. X., Wang, M. H., Gao, J. Z., & Chen, Z. Z. (2020b). Integrated response of growth, antioxidant defense and isotopic composition to microplastics in juvenile guppy (Poecilia reticulata). Journal of Hazardous Materials, 399, 123044. https://doi.org/10.1016/j.jhazmat.2020.123044
Iftikhar, N., Zafar, R., & Hashmi, I. (2022). Multi-biomarkers approach to determine the toxicological impacts of sulfamethoxazole antibiotic on freshwater fish Cyprinus carpio. Ecotoxicology and Environmental Safety, 233, 113331. https://doi.org/10.1016/j.ecoenv.2022.113331
Iheanacho, S. C., & Odo, G. E. (2020). Neurotoxicity, oxidative stress biomarkers and haematological responses in African catfish (Clarias gariepinus) exposed to polyvinyl chloride microparticles. Comparative Biochemistry and Physiology, Part C: Toxicology & Pharmacology, 232, 108741. https://doi.org/10.1016/j.cbpc.2020.108741
Jay, M., Bradley, S., & McDearmid, J. R. (2014). Effects of nitric oxide on neuromuscular properties of developing zebrafish embryos. PLoS One, 9, e86930. https://doi.org/10.1371/journal.pone.0086930
Kim, W. K., & Jung, J. (2016). In situ impact assessment of wastewater effluents by integrating multi-level biomarker responses in the pale chub (Zacco platypus). Ecotoxicology and Environmental Safety, 128, 246–251. https://doi.org/10.1016/j.ecoenv.2016.02.028
Kong, H., Wu, F., Jiang, X., Wang, T., Hu, M., Chen, J., Huang, W., Bao, Y., & Wang, Y. (2019). Nano-TiO2 impairs digestive enzyme activities of marine mussels under ocean acidification. Chemosphere, 237, 124561. https://doi.org/10.1016/j.chemosphere.2019.124561
Lam, S. H., Ung, C. Y., Hlaing, M. M., Hu, J., Li, Z. H., Mathavan, S., & Gong, Z. Y. (2013). Molecular insights into 4-nitrophenol-induced hepatotoxicity in zebrafish: Transcriptomic, histological and targeted gene expression analyses. Biochimica Et Biophysica Acta-General Subjects, 1830, 4778–4789. https://doi.org/10.1016/j.bbagen.2013.06.008
Li, P., & Li, Z. H. (2020). Tributyltin induces the tissue-specific stresses in zebrafish, a study in various tissues of muscle, gill and intestine. Bulletin of Environment Contamination and Toxicology, 105, 847–852. https://doi.org/10.1007/s00128-020-03048-9
Li, K., Liu, L., Zhan, J., Scippo, M. L., Hvidtfeldt, K., Liu, Y., & Dalsgaard, A. (2017). Sources and fate of antimicrobials in integrated fish-pig and non-integrated tilapia farms. Science of the Total Environment, 595, 393–399. https://doi.org/10.1016/j.scitotenv.2017.01.124
Li, Y., Mou, Y., Thunders, M., Wu, Y., Ai, X., Zhou, X., & Qiu, J. (2018). Effects of enrofloxacin on antioxidant system, microsomal enzymatic activity, and proteomics in porcine liver. Journal of Veterinary Pharmacology and Therapeutics, 41, 562–571. https://doi.org/10.1111/jvp.12493
Li, W., Wang, S., Li, J., Wang, X., Cui, L., Chen, J., & Liu, Z. (2020a). Antioxidative enzyme activities in the Rhodeinae sinensis Gunther and Macrobrachium nipponense and multi-endpoint assessment under tonalide exposure. Ecotoxicology and Environmental Safety, 199, 110751. https://doi.org/10.1016/j.ecoenv.2020.110751
Li, Z., Li, M., Zhang, Z., Li, P., Zang, Y., & Liu, X. (2020b). Antibiotics in aquatic environments of China: A review and meta-analysis. Ecotoxicology and Environmental Safety, 199, 110668. https://doi.org/10.1016/j.ecoenv.2020.110668
Liang, J. P., Li, J., Li, J. T., Liu, P., Chang, Z. Q., & Nie, G. X. (2014). Accumulation and elimination of enrofloxacin and its metabolite ciprofloxacin in the ridgetail white prawn Exopalaemon carinicauda following medicated feed and bath administration. Journal of Veterinary Pharmacology and Therapeutics, 37, 508–514. https://doi.org/10.1111/jvp.12115
Liao, T., Li, B., Zhang, Z., Feng, W., Chen, Y., Ding, Y., Chen, H., Zhao, T., Mao, G., Wu, X., & Yang, L. (2021). Short-term exposure of decabromodiphenyl ether in female adult Balb/c mice: Immune toxicity and self-recovery. Toxicology Letters, 342, 26–37. https://doi.org/10.1016/j.toxlet.2021.02.002
Limbu, S. M., Chen, L.-Q., Zhang, M.-L., & Zhen-Yu, Du. (2020). A global analysis on the systemic effects of antibiotics in cultured fish and their potential human health risk: A review. Reviews in Aquaculture, 13, 1015–1059. https://doi.org/10.1111/raq.12511
Lin, T., Zhou, D., Dong, J., Jiang, F., & Chen, W. (2016). Acute toxicity of dichloroacetonitrile (DCAN), a typical nitrogenous disinfection by-product (N-DBP), on zebrafish (Danio rerio). Ecotoxicology and Environmental Safety, 133, 97–104. https://doi.org/10.1016/j.ecoenv.2016.06.047
Liu, C. M., Zheng, G. H., Ming, Q. L., Sun, J. M., & Cheng, C. (2013). Protective effect of puerarin on lead-induced mouse cognitive impairment via altering activities of acetyl cholinesterase, monoamine oxidase and nitric oxide synthase. Environmental Toxicology and Pharmacology, 35, 502–510. https://doi.org/10.1016/j.etap.2013.02.009
Liu, B., Cui, Y., Brown, P. B., Ge, X., Xie, J., & Xu, P. (2015). Cytotoxic effects and apoptosis induction of enrofloxacin in hepatic cell line of grass carp (Ctenopharyngodon idellus). Fish & Shellfish Immunology, 47, 639–644. https://doi.org/10.1016/j.fsi.2015.10.007
Livak, K. J., & Schmittgen, T. J. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCt method. Methods, 25(402), 08.
Magara, G., Elia, A. C., Syberg, K., & Khan, F. R. (2018). Single contaminant and combined exposures of polyethylene microplastics and fluoranthene: Accumulation and oxidative stress response in the blue mussel, Mytilus edulis. Journal of Toxicology and Environmental Health. Part A, 81, 761–773. https://doi.org/10.1080/15287394.2018.1488639
Mukherjee, A., Bhowmick, A. R., Mukherjee, J., & Moniruzzaman, M. (2019). Physiological response of fish under variable acidic conditions: A molecular approach through the assessment of an eco-physiological marker in the brain. Environmental Science and Pollution Research International, 26, 23442–23452. https://doi.org/10.1007/s11356-019-05602-3
Mukherjee, D., Ferreira, N. G. C., & Saha, N. C. (2022). Effects of 2,4,6-Trichlorophenol on Clarias batrachus: A biomarkers approach. Environmental Science and Pollution Research International, 29, 47011–47024. https://doi.org/10.1007/s11356-022-19213-y
Pan, J. F., Buffet, P. E., Poirier, L., Amiard-Triquet, C., Gilliland, D., Joubert, Y., Pilet, P., Guibbolini, M., Risso de Faverney, C., Romeo, M., Valsami-Jones, E., & Mouneyrac, C. (2012). Size dependent bioaccumulation and ecotoxicity of gold nanoparticles in an endobenthic invertebrate: The Tellinid clam Scrobicularia plana. Environmental Pollution, 168, 37–43. https://doi.org/10.1016/j.envpol.2012.03.051
Perussolo, M. C., Guiloski, I. C., Lirola, J. R., Fockink, D. H., Corso, C. R., Bozza, D. C., Prodocimo, V., Mela, M., Ramos, L. P., Cestari, M. M., Acco, A., & Silva de Assis, H. C. (2019). Integrated biomarker response index to assess toxic effects of environmentally relevant concentrations of paracetamol in a neotropical catfish (Rhamdia quelen). Ecotoxicology and Environmental Safety, 182, 109438. https://doi.org/10.1016/j.ecoenv.2019.109438
Phillips, B. E., Harms, C. A., Lewbart, G. A., Lahner, L. L., Haulena, M., Rosenberg, J. F., & Papich, M. G. (2016). Population pharmacokinetics of enrofloxacin and its metabolite ciprofloxacin in the green sea urchin (Strongylocentrotus droebachiensis) following intracoelomic and immersion administration. Journal of Zoo and Wildlife Medicine, 47, 175–186. https://doi.org/10.1638/2015-0110.1
Qiu, W., Liu, T., Liu, X., Chen, H., Luo, S., Chen, Q., Magnuson, J. T., Zheng, C., Xu, E. G., & Schlenk, D. (2022). Enrofloxacin Induces intestinal microbiota-mediated immunosuppression in zebrafish. Environmental Science and Technology, 56, 8428–8437. https://doi.org/10.1021/acs.est.1c08712
Regoli, F., & Giuliani, M. E. (2014). Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Marine Environmental Research, 93, 106–117. https://doi.org/10.1016/j.marenvres.2013.07.006
Ren, Z., Xu, H., Wang, Y., Li, Y., Han, S., & Ren, J. (2021). Combined toxicity characteristics and regulation of residual quinolone antibiotics in water environment. Chemosphere, 263, 128301. https://doi.org/10.1016/j.chemosphere.2020.128301
Rodrigues, S., Antunes, S. C., Correia, A. T., Golovko, O., Zlabek, V., & Nunes, B. (2019). Assessment of toxic effects of the antibiotic erythromycin on the marine fish gilthead seabream (Sparus aurata L.) by a multi-biomarker approach. Chemosphere, 216, 234–247. https://doi.org/10.1016/j.chemosphere.2018.10.124
Samanta, P., Im, H., Yoo, J., Lee, H., Kim, N. Y., Kim, W., Hwang, S. J., Kim, W. K., & Jung, J. (2018). Comparative assessment of the adverse outcome of wastewater effluents by integrating oxidative stress and histopathological alterations in endemic fish. Journal of Hazardous Materials, 344, 81–89. https://doi.org/10.1016/j.jhazmat.2017.10.016
Sanchez, W., Burgeot, T., & Porcher, J. M. (2013). A novel “Integrated Biomarker Response” calculation based on reference deviation concept. Environmental Science and Pollution Research International, 20, 2721–2725. https://doi.org/10.1007/s11356-012-1359-1
Schwarzenberger, A., & Fink, P. (2018). Gene expression and activity of digestive enzymes of Daphnia pulex in response to food quality differences. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 218, 23–29. https://doi.org/10.1016/j.cbpb.2018.01.009
Sehonova, P., Tokanova, N., Hodkovicova, N., Kocour Kroupova, H., Tumova, J., Blahova, J., Marsalek, P., Plhalova, L., Doubkova, V., Dobsikova, R., Chloupek, P., Dolezalova, P., Faldyna, M., Svobodova, Z., & Faggio, C. (2019). Oxidative stress induced by fluoroquinolone enrofloxacin in zebrafish (Danio rerio) can be ameliorated after a prolonged exposure. Environmental Toxicology and Pharmacology, 67, 87–93. https://doi.org/10.1016/j.etap.2019.02.002
Serafini, S., de Freitas Souza, C., Baldissera, M. D., Baldisserotto, B., & Da Silva, A. S. (2020). Nitric oxide levels in brain, liver, and gills of silver catfish (Rhamdia quelen) exposed to the antiparasitic eprinomectin. Fish Physiology and Biochemistry, 46, 1867–1872. https://doi.org/10.1007/s10695-020-00836-2
Shi, Q., Wang, M., Shi, F., Yang, L., Guo, Y., Feng, C., Liu, J., & Zhou, B. (2018). Developmental neurotoxicity of triphenyl phosphate in zebrafish larvae. Aquatic Toxicology, 203, 80–87. https://doi.org/10.1016/j.aquatox.2018.08.001
Silva, C. S. E., Novais, S. C., Simoes, T., Caramalho, M., Gravato, C., Rodrigues, M. J., Maranhao, P., & Lemos, M. F. L. (2018). Using biomarkers to address the impacts of pollution on limpets (Patella depressa) and their mechanisms to cope with stress. Ecological Indicators, 95, 1077–1086. https://doi.org/10.1016/j.ecolind.2017.09.046
Su, H., Sun, J., Fang, S., Wei, Y., Zheng, R., Jiang, Y., & Hu, K. (2019). Effects of lactic acid on drug-metabolizing enzymes in Chinese mitten crab (Eriocheir sisnensis) after oral enrofloxacin. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 223, 9–14. https://doi.org/10.1016/j.cbpc.2019.04.017
Sun, X. L., Wang, J. C., Li, Y., Yang, J. J., Jin, J., Shah, S. M., et al. (2014). Novel dummy molecularly imprinted polymers for matrix solid-phase dispersion extraction of eight fluoroquinolones from fish samples. Journal of Chromatography A, 1359, 1–7. https://doi.org/10.1016/j.chroma.2014.07.007
Tang, J., Shi, T., Wu, X., Cao, H., Li, X., Hua, R., Tang, F., & Yue, Y. (2015). The occurrence and distribution of antibiotics in Lake Chaohu, China: Seasonal variation, potential source and risk assessment. Chemosphere, 122, 154–161. https://doi.org/10.1016/j.chemosphere.2014.11.032
Teglia, C. M., Perez, F. A., Michlig, N., Repetti, M. R., Goicoechea, H. C., & Culzoni, M. J. (2019). Occurrence, distribution, and ecological risk of fluoroquinolones in rivers and wastewaters. Environmental Toxicology and Chemistry, 38, 2305–2313. https://doi.org/10.1002/etc.4532
Varol, M., & Sunbul, M. R. (2019). Environmental contaminants in fish species from a large dam reservoir and their potential risks to human health. Ecotoxicology and Environmental Safety, 169, 507–515. https://doi.org/10.1016/j.ecoenv.2018.11.060
Wang, B., Liu, Y., Feng, L., Jiang, W. D., Kuang, S. Y., Jiang, J., Li, S. H., Tang, L., & Zhou, X. Q. (2015). Effects of dietary arginine supplementation on growth performance, flesh quality, muscle antioxidant capacity and antioxidant-related signalling molecule expression in young grass carp (Ctenopharyngodon idella). Food Chemistry, 167, 91–99. https://doi.org/10.1016/j.foodchem.2014.06.091
Wang, S., Li, X., Zhang, M., Jiang, H., Wang, R., Qian, Y., & Li, M. (2021). Ammonia stress disrupts intestinal microbial community and amino acid metabolism of juvenile yellow catfish (Pelteobagrus fulvidraco). Ecotoxicology and Environmental Safety, 227, 112932. https://doi.org/10.1016/j.ecoenv.2021.112932
Wang, Y. L., X. Q. Zhou, W. D. Jiang, P. Wu, Y. Liu, J. Jiang, S. W. Wang, S. Y. Kuang, L. Tang, and L. Feng. (2019). Effects of dietary zearalenone on oxidative stress, cell apoptosis, and tight junction in the intestine of juvenile grass carp (Ctenopharyngodon idella). Toxins (Basel), 11. https://doi.org/10.3390/toxins11060333.
Wei, R., Ge, F., Chen, M., & Wang, R. (2012). Occurrence of ciprofloxacin, enrofloxacin, and florfenicol in animal wastewater and water resources. Journal of Environmental Quality, 41, 1481–1486. https://doi.org/10.2134/jeq2012.0014
Xie, D. M., Li, Y. W., Liu, Z. H., & Chen, Q. L. (2019). Inhibitory effect of cadmium exposure on digestive activity, antioxidant capacity and immune defense in the intestine of yellow catfish (Pelteobagrus fulvidraco). Comparative Biochemistry and Physiology C-Toxicology & Pharmacology, 222, 65–73. https://doi.org/10.1016/j.cbpc.2019.04.012
Yancheva, V., Velcheva, I., Stoyanova, S., & Georgieva, E. (2016). Histological biomarkers in fish as a tool in ecological risk assessment and monitoring programs: A review. Applied Ecology and Environmental Research, 14, 47–75.
Yang, Y., Xiao, Y., Chang, Y., Cui, Y., Klobucar, G., & Li, M. (2018). Intestinal damage, neurotoxicity and biochemical responses caused by tris (2-chloroethyl) phosphate and tricresyl phosphate on earthworm. Ecotoxicology and Environmental Safety, 158, 78–86. https://doi.org/10.1016/j.ecoenv.2018.04.012
Yang, C., Song, G., & Lim, W. (2020). A review of the toxicity in fish exposed to antibiotics. Comparative Biochemistry and Physiology, Part C: Toxicology & Pharmacology, 237, 108840. https://doi.org/10.1016/j.cbpc.2020.108840
Yogev, U., Barnes, A., Giladi, I., & Gross, A. (2020). Potential environmental impact resulting from biased fish sampling in intensive aquaculture operations. Science of the Total Environment, 707, 135630. https://doi.org/10.1016/j.scitotenv.2019.135630
Yuan, Z., Shao, X., Miao, Z., Zhao, B., Zheng, Z., & Zhang, J. (2018). Perfluorooctane sulfonate induced neurotoxicity responses associated with neural genes expression, neurotransmitter levels and acetylcholinesterase activity in planarians Dugesia japonica. Chemosphere, 206, 150–156. https://doi.org/10.1016/j.chemosphere.2018.05.011
Zhang, C., Wang, J., Zhang, S., Zhu, L., Du, Z., & Wang, J. (2017). Acute and subchronic toxicity of pyraclostrobin in zebrafish (Danio rerio). Chemosphere, 188, 510–516. https://doi.org/10.1016/j.chemosphere.2017.09.025
Zhang, Y., Wang, L., Zhuang, H., Li, X., Gao, X., An, Z., Liu, X., Yang, H., Wei, W., & Zhang, X. (2019). Excessive use of enrofloxacin leads to growth inhibition of juvenile giant freshwater prawn Macrobrachium rosenbergii. Ecotoxicology and Environmental Safety, 169, 344–352. https://doi.org/10.1016/j.ecoenv.2018.11.042
Zheng, X., Zhou, S., Hu, J., Yang, R., Gu, Z., Qin, J. G., Ma, Z., & Yu, G. (2020). Could the gut microbiota community in the coral trout Plectropomus leopardus (Lacepede, 1802) be affected by antibiotic bath administration? Veterinary Medicine and Science, 6, 649–657. https://doi.org/10.1002/vms3.267
Zhou, L., Limbu, S. M., Qiao, F., Du, Z. Y., & Zhang, M. (2018). Influence of long-term feeding antibiotics on the gut health of zebrafish. Zebrafish, 15, 340–348. https://doi.org/10.1089/zeb.2017.1526
Zhou, M., Yu, S., Hong, B., Li, J., Han, H., & Qie, G. (2021). Antibiotics control in aquaculture requires more than antibiotic-free feeds: A tilapia farming case. Environmental Pollution, 268, 115854. https://doi.org/10.1016/j.envpol.2020.115854
Funding
This work was financially supported by National Key R&D Program of China (2018YFD0900905, 2018YFD0900905).
Author information
Authors and Affiliations
Contributions
Xu-Qian Cao: writing original draft preparation; Xu Wang: methodology and software; Bin Liu: index measurement; Shu-Wen He: index measurement; Zhi-Han Cao: fish culture and sampling; Shao-Ying Xing: fish culture and sampling; Ping Li: writing, reviewing, and editing; Zhi-Hua Li: conceptualization and overall guidance.
Corresponding authors
Ethics declarations
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Approval of Animal Ethics Committee of Shandong University was taken.
Consent to Participate
It is not applicable.
Consent for Publication
All the authors are in agreement with the publishment.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, XQ., Wang, X., Liu, B. et al. Evaluation of Physiological Stress of Grass Carp Chronically Exposed to Enrofloxacin Based on IBR Index. Water Air Soil Pollut 234, 548 (2023). https://doi.org/10.1007/s11270-023-06574-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06574-4