Abstract
Rising sea levels, groundwater exploitation, and urbanization were the primary causes of seawater intrusion, exerting pressure on coastal aquifers. In Sri Lanka’s urban coastal region, a comprehensive physicochemical description of groundwater has yet to be identified. Therefore, the objectives of this research were to (a) use a Geographic Information System (GIS) to designate spatial distribution of various water physicochemical characteristics, (b) detect “suitable” groundwater zones for drinking, and (c) estimate groundwater quality by developing a groundwater quality index (GWQI) in Sri Lanka’s urban coastal region. The physiochemical parameters of 18 groundwater samples [pH, electrical conductivity (EC), turbidity, total dissolved solids (TDS), Na+, K+, Ca2+, Mg2+, Cl−, and HCO3−] were studied in terms of their spatial and temporal variation. According to the World Health Organization (WHO) and Sri Lankan Standard Institution (SLS), EC levels in 11% of samples were above the acceptable range, while turbidity levels in 22% of samples were above the acceptable range. Water was consumable in 77.78% of the locations and unsatisfactory in 22.22%. The main hydrochemical facies detected in groundwater samples were Na+– Cl− and the mixed Ca2+– Mg2+– Cl− face, which indicated carbonate dissolution and weathering of silicate minerals and the main mechanism controlling the water chemistry in the study area is water–rock interaction. Based on daily water consumption, it was discovered that the HQ is greater than one, in 61% of males, 78% of females, and 89% of children, indicating a health hazard. Furthermore, groundwater quality in the study region is deteriorating due to significant coastal erosion, making it critical to maintain a comprehensive groundwater management strategy to promote sustainable water consumption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater pollution has a significant impact on the environment and human existence today, as it is the primary source of water (Farzaneh et al. 2021; Khanoranga 2019; Yang et al. 2016). Agriculture, domestic consumption, industrial activities, and other activities all depend on groundwater (Mukherjee and Singh 2018; Tiwari et al. 2017). Groundwater, on the other hand, meets the needs of majority (2/3) of the world’s inhabitants through their water-dependent activities (Adimalla and Li 2019). The major groundwater contaminants include inorganic salts, cations (Na+, Ca2+, Mg2+, etc.), anions (CO32−, HCO3−, Cl−, etc.), and heavy metals (Wijesinghe et al. 2016). Excessive concentrations of certain pollutants in water can lead to health problems, but excessive amounts of other parameters have no effect but do impair appetite (Gunarathna et al. 2016; Wanasinghe et al. 2018). To address the issue of water scarcity, pollution, and management, the United Nations (UN) has prioritized the achievement of clean drinking water for everyone in their Millennium Development Goals and in the Sustainable Development Goals by 2030 (WHO 2017; Weerasooriya et al. 2021). As a result, more consideration should be given to combating groundwater pollution, as many countries are currently experiencing a scarcity of fresh water resources (Pant 2011; UNEP 2018). Furthermore, the United States Environmental Protection Agency (USEPA) has proposed a method for determining the health risk (hazard quotient—HQ) for humans when exposed to harmful levels of chemicals in water (Narsimha and Rajitha 2018; USEPA 1993). This was commonly used by different researchers around the world to assess potential health risks caused by ingestion, oral intake, or dermal intake of harmful elements (Chen et al. 2017).
Groundwater contamination is widespread in several areas of Sri Lanka, particularly in the Maha Oya river basin. In the western urban coastal region, 74% of residents use their land for residential and industrial purposes (tourism, hotels, and restaurants), while 24% use their land for agricultural production. Groundwater contamination in the Western urban coastal region is induced by nutrient and toxic inputs from agriculture, sea water intrusion, as well as urban and industrial development. Furthermore, in the Maha Oya basin, untreated domestic water is usually released into rivers (Hayzoun et al. 2015). In light of these many issues, policymakers and decision-makers depend heavily on WQI to estimate the efficiency of a water source while also determining the usefulness of initiatives and activities aimed at improving groundwater quality.
The “water quality index, WQI” was an essential indicator for determining the degree of water pollution (Singh et al. 2019; Tian et al. 2019; Wang and Zhang 2018); it may be used to assess the quality of groundwater (Jain et al. 2010; Nihalani and Meeruty 2020). Horton (1965) developed the concept of WQI, and there are now numerous methodologies to determine WQI that have been refined by different authors in different regions of the world (Aouiti et al. 2020; Lopes et al. 2020; Şener et al., 2021). By aggregating and evaluating various water quality sub-indices using a single mathematical classification scheme, this method can be used to evaluate water quality and facilitate in decision-making (Gitau et al. 2016). According to Vaiphei et al. (2020), the WQI is calculated by combining physicochemical and biological properties of water.
In recent years, a great percentage of groundwater contamination and intrusion studies have primarily been carried out in various parts of Sri Lanka (Herath and Ratnayake 2007; IGES 2007; International Water Management Project 2005; Perera et al. 2018) and have used a variety of approaches to realize groundwater contamination and identify the cause (Villholth and Rajasooriyar 2010). According to Herath et al. (2017) seawater intrusion and contamination of groundwater are quite common along the Jaffna peninsula of Sri Lanka. Furthermore, the area’s groundwater quality measurements were above the SLS permissible range. Mikunthan and De Silva (2008) assessed groundwater quality in the Thirunelvely and Kondavil areas using geostatistical techniques. They concluded that positive management strategies, such as soil conservation and fertilizer reduction (N—top dressing), had a significant impact on high groundwater chemistry. Cooray et al. (2019) discovered that, with the exception of 6.4% of samples, all water sources in Sri Lanka’s dry zone required additional treatment before consumption.
However, in the western coastline region of Sri Lanka, a precise physicochemical description of groundwater content has yet to be determined. As a result, the study’s main objectives were to (a) use a Geographic Information System (GIS) to create maps to indicate spatial distribution different physicochemical parameters; (b) identify “suitable” and “unsuitable” groundwater zones for drinking; and (c) evaluate groundwater quality by developing a GWQI. The findings of this study may be useful to decision-makers and the scientific community in determining the best course of action for groundwater quality conservation.
Materials and methodology
The research was consisted with physicochemical analysis of groundwater chemistry, calculation, spatialization of GWQI with sub-indices, and human health risk assessment. The methodology employed is summarized in the flowchart shown in Fig. 1.
Study area
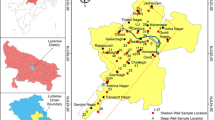
The targeted case study region (Waikkala, 7.2838° N, 79.8578° E, Fig. 2) is located in the Puttlam District of Sri Lanka’s North Western Province where groundwater is a major source of water consumption. It covers an area of 3072 km2, to the north, the Kala Oya and Modaragam Aru; to the east, the Anuradhapura District and Kurunegala District; to the south, the Ma Oya; and to the west, the Indian Ocean (Phok et al. 2021).
The study region encompasses 24 km2 and extends from the left bank of the Maha Oya River, which serves as the southern boundary of Puttlam District, to the north. The case study area includes the mouth of the Gin Ganga River as well (Mahagamage and Jayakody 2020). This region includes 28 Grama Niladhari divisions and is part of the Wennappuwa Divisional Secretariat Division. This region has a population density distribution of 1746km2 (Statista 2021). Groundwater is retained by alluvial deposits and ferruginous gravels, as well as unconsolidated sands and spits in the coastal region. Diverse, confined aquifers have been identified within the sedimentary limestone and sandstone formations of the northwestern and northern coastal plains (Herath 2006). The most common abstraction technologies for groundwater are “dug wells, dug-cum bore wells, and bore wells,” and their yields are largely determined by the recharge levels in the region. The district’s yearly average precipitation is 1174 mm, with November being the wettest month of the year. Puttalam, which is located on the seashore, is mostly flat, with the land rising to about 60 m inland. In land, there are reddish brown earth and low humic gley soils, and the soils are mostly red-yellow latosols (Abeykoon et al. 2021; Wickramasinghe 2013).
Physicochemical analysis of groundwater
Sample collection
Between January 2019 and January 2020, groundwater samples were collected in triplicate at 18 different locations once a month, from a previously drilled well or a deep bore hole. Groundwater samples were collected in accordance with APHA guidelines and the most recent research (APHA 2012; Mao et al. 2021; Senthilkumar et al. 2021). Pre-cleaned, 1-L high-density polythene sample vials were used to collect water samples. The samples were labeled and delivered to a chemical laboratory for physicochemical analysis at 4 °C (Jehan et al. 2020).
Using Arc GIS 10.2 software, the case study region was partitioned into a 9×5 grid. A GPS position at the mid-point of selected spots for water quality monitoring method has been navigated in each cell of the grid in the study region (Naik et al. 2021).
Sample analysis
A handheld optical pH/EC/TDS meter (Hanna HI 9811–5) was used to determine water physical parameters such as EC, pH, and TDS in the field immediately after collection (Maspalma et al. 2018; Narsimha and Sudarshan 2017; Şener et al. 2017). EUTECH waterproof Cyber scan pH650 handheld meter kit was used to determine turbidity (APHA 2012). Calibration was done by using standard solutions. The basic protocols of the American Public Health Association were used to examine other chemical parameters. The AgNO3 back titration (Argentometric method) (Shukla et al. 2018) was used to determine the Cl− concentration in the laboratory. The Mg2+ and Ca2+ were determined using EDTA titration method which used for hardness calculation (Chakraborty 2021; Yadav et al. 2021). The following equations (Eqs. 1 and 2) were used to calculate final Mg2+ and Ca2+ concentrations (Adimalla and Taloor 2020). Furthermore, K+ and Na+ concentrations of the water samples were analyzed by using flame photometer (Model 130 Systronics) (Nath et al. 2021; Singh et al. 2021) and HCO3− were measured by titration with 0.02 N H2SO4 using methyl orange indicator (Şener et al. 2017) (Adimalla and Taloor 2020).
All the reagents and chemicals used for water sample analysis were of analytical standard and they were prepared in Type I (18.2 MΩcm) Milli-Q water. [KCl (99%), Silver nitrate (AgNO3, 98%), potassium chromate (K2CrO4, 99%), sodium hydroxide (NaOH, 97%), sodium chloride (NaCl, 98%), sulfuric acid (H2SO4, 98%), ethylenediamine tetra acetic acid (EDTA) (99%), Eriochrome Black T (EBT), and murexide].
Development of groundwater quality index (GWQI)
The groundwater quality index (GWQI) was developed using the collected data to assess the water’s acceptability for drinking (Adimalla et al. 2018; Chathuranga et al. 2018; Ramakrishnaiah et al. 2009; Rabeiy 2018). The first step in the GWQI classification process was to assign weights (wi) to each parameter such as chloride, TDS, Na+, pH, Ca2+, K+, Mg2+, and HCO3− and then calculate relative weight (Wi) (Eq. 3). Based on expert opinions gathered from various previous studies (Barbosa Filho and de Oliveira 2021; Harun et al. 2021; Kayemah et al. 2021), a minimum weight of one (1) has been assigned to parameters such as Na+ and K+ because they are less important in groundwater quality evaluation (Rajmohan 2021), and the maximum weight of five (5) was allocated to the parameters which are more pertinent in groundwater quality evaluation such as TDS (Table 1) (Adimalla and Taloor 2020).
According to WHO guidelines, each parameter’s consistency rating scale (Qi) is calculated by multiplying its concentration in each water sample by its corresponding standard and then multiplying by 100 (Eq. 4) (Adimalla and Taloor 2020).
where ci is the concentration of each parameter, Qi is the quality rating, and Si is the chemical parameter’s recommended guideline value. Using Eqs. 5 and 6, the sub-index (SIi) and GWQI were determined (Adimalla and Taloor 2020).
Development of spatial distribution maps
The Geographic Information System (GIS) has risen to prominence as the most popular tool for gathering, classifying, and displaying groundwater spatial data, as well as for using the data to make decisions, in many domains, including geographical and geo-environmental disciplines (Eltarabily and Moghazy 2021; Gunaalan et al. 2018; Rajasooriyar et al. 2013). Hence, the exact sampling locations were marked using a portable Global Positioning System (Garman eTrex 30), and the marked coordinates were imported into GIS program. The spatiotemporal behavior analysis and geographic distribution map of groundwater quality were accomplished using the spatial observer model of ArcGIS version 10.1 (Şener et al. 2021).
Geochemical characterization of groundwater
Hydrochemical type and facies of groundwater samples were determined by piper plots. Piper plots are an effective method in graphical illustration of water types and facilitate to decide the sources of the dissolved constituents in groundwater (Aouiti et al. 2020; Elsayed et al. 2021; Moreno et al. 2021). Piper diagram categorized four dominants for cations [(A) Ca2+ type, (B) Mg2+ type, (C) Na+, and K+ type], four dominants for anions [(E) SO42− type, (G) HCO3− type, (F) Cl− type], a (D) dominant type which is common for both anions and cations, and six facies [(1) Ca2+–Mg2+–HCO3−, (2) Na+–Cl−, (3) mixed Ca2+–Na+–HCO3−, (4) mixed Ca2+–Mg2+–Cl−, (5) Ca2+–Cl−, and (6) Na+–HCO3−] for groundwater. Groundwater chemistry controlling factors were determined by Gibbs plots and it was created by TDS versus anions (Cl−/Cl− + HCO3−) and cations (Na+/Na+ + Ca2+). The following calculations (Eq. 7 and 8) have been used to determine Gibbs ratios and controlling factors and then categorized into three groups such as evaporation dominance, precipitation dominance, and rock dominance. All the ionic concentrations were expressed in mill equivalents per liter (meq/L) (Khan et al. 2021).
Human health risk assessment
Examination of health risks due to non-carcinogenic elements in different gender and age groups was done by considering the oral or ingestion pathway of drinking water. According to Chen et al. (2017), element inhalation and dermal contact were always very low, so the impact on human health is negligible. Thus, the non-carcinogenic risk through ingestion of the groundwater was calculated using hazard quotient (HQ). The following Eqs. 9 and 10 were used to calculate HQ and the chronic daily intake dose (CDI) (Adimalla et al., 2019; Bodrud-Doza et al., 2020; Zhang et al., 2020) (Adimalla et al., 2019).
where CDI is the chronic daily intake, C is the element concentration in the groundwater (mg/L), IR is the ingestion rate (L/day), ED is the exposure duration (years), HQ is hazard quotient, RfD is the reference dose for chronic oral exposure of particular element in mg/kg/day, EF is the exposure frequency (days/years), ABW is the average body weight (kg), and AET is the averaging time for non-carcinogens (days) (Adimalla et al., 2019; Zhang et al., 2020).
Furthermore, Table 3 shows the recommended values of selected non-carcinogenic health risk parameters for assessing chronic daily intake via oral ingestion for males, females, and children. According to the USEPA’s recommendation, the limit of HQ is 1, and groundwater samples with HQ values greater than 1 (HQ > 1) posed a substantial health risk due to a specific element (Achary et al., 2016; Adimalla et al., 2019; Amarasinghe, 2020; Bodrud-Doza et al., 2020; Zhang et al., 2020).
Results and discussion
Physicochemical parameters of the groundwater
The significance of groundwater resource quality is critical since it is a fundamental factor in determining its suitability for consumption in the studied area. The data were also compared to the WHO (2017) and SLS guidelines to see if they were suitable for drinking.
pH and EC
The groundwater acidity and alkalinity can be measures using the hydrogen ion concentration or the pH. Despite the fact that pH has no direct impact on human health, it is one of the most important water quality parameters. The groundwater samples in the study area were acidic to alkaline in nature, with a pH ranging from 6.21 to 7.68, with an average of 6.93. Figure 3 a depicts the pH geographic distribution in the research area. According to the WHO guidelines, a suitable pH range of 6.5 to 8.5 is recommended by the WHO (2017) and the pH range for groundwater in SLS was 6.50–9.00, with a maximum permissible level of 9.00. However, no location was found to be exceeding the permissible level in any of the groundwater standards. According to Sampath et al. (2011), the pH range of groundwater in Sri Lanka’s Puttlam district was 6.30–8.20, and Young et al. (2011) discovered a pH range of 5.76 to 8.70 in northwestern province. The pH variation in groundwater in Sri Lanka’s western province was below the SLS permitted level (4.0 to 8.2) and was not hazardous for drinking (Premalal and Jayewardene 2015).
According to Kanga et al. (2020), the ionic concentration of groundwater was commonly measured by calculating the EC, which fluctuates depending on the concentration, ion types available in the water, and temperature. The EC in the research area ranged from 430.10 to 99,144.72 μS/cm, with an average of 10,709.76 μS/cm. When referring to the WHO drinking water recommendations, the maximum permitted EC in water is 1500 μS/cm (WHO 2017) and the maximum allowed level in Sri Lanka is 750 μS/cm. According to the results obtained from the present study, only 11% of groundwater samples were founded to be above the maximum allowable level. The geographic distribution of EC is depicted in Fig. 3b. Furthermore, in 76% of areas in the Puttlam district of Sri Lanka, EC levels exceeded the acceptable values of the WHO drinking water quality guidelines, according to a study conducted by Sampath et al. (2011). Several other studies in Sri Lanka revealed that the Puttlam district had exceeded the maximum permissible level of EC in groundwater (Arasaretnam et al. 2018; Edirisinghe et al. 2016). Furthermore, Young et al. (2011) discovered that EC ranged from 143 to 3549 S/cm in the northwestern province. Gopalakrishnan et al. (2020) discovered high salinity groundwater in Sri Lanka’s Jaffna peninsula (EC = 20,000 S/cm). As a result, salinization could have an effect on groundwater quality in the research area in the coming years.
Total dissolved solids (TDS)
TDS is a term that refers to the various minerals that are present in dissolved form in water (Narsimha and Sudarshan 2017). Large carbonates, bicarbonates, sulfates, chlorides, silica, phosphates, sodium, potassium, calcium, and magnesium are the most common dissolved solids in water (Adimalla et al. 2018; Gnanachandrasamy et al. 2015). As a result, it is an important factor to consider when assessing the consistency of drinking water and other types of water. The TDS content in the current study ranges from 168 to 749 mg/L, with a mean value of 299 mg/L. However, all the samples had TDS levels below the WHO limit (1000 mg/L) and SLS levels (2000 mg/L). According to previous studies, TDS concentrations in the Puttlam district were below the ideal threshold level in 80% of the locations and 20% of the sites exceeded the ideal level. Furthermore, TDS in several other areas in western urban coastal region were of sufficient quality for drinking (Sampath et al. 2011; Wickramasinghe et al. 2021). In the study conducted by Adimalla and Taloor (2020), almost 95% of the samples were below the ideal threshold levels for drinking (TDS: 1000 mg/L), while the remaining samples were suitable for irrigation (TDS: 1000–3000 mg/L). The geographic distribution map of TDS is depicted in Fig. 3c.
Cations (Mg 2+ , Ca 2+ , Na + , and K + )
According to the WHO (2017) standards, the permissible Mg2+ concentration is 150 mg/L. Groundwater Mg2+ values in the study region varied from 1.6 to 23 mg/L, with an average of 10.5 mg/L. The concentration of Mg2+ in the sample region’s groundwater was determined to be below the WHO- (2017) or SLS-recommended maximum permissible level. However, closer sampling points to the coastal area have showed higher Mg2+ content than inside sampling points (Fig. 4a).
According to the findings, the Ca2+ concentration in groundwater in the research area varied from 5 to 43 mg/L, with an average value of 20 mg/L (Fig. 4b). Although closer sampling points to the coastal area showed a higher Ca2+ content than inside sampling points, all of the samples were well within the WHO standard level (WHO 2017). Mg2+ and Ca2+ concentrations in Sri Lanka’s northwestern province ranged from 4.98 to 112 mg/L and 1.29 to 98 mg/L, respectively, according to Young et al. (2011). The dissolution of carbonate minerals resulted in relatively high amounts of Mg2+ and Ca2+ in various sections of the northwestern province. Total hardness (Ca2+ + Mg2+) levels in Puttlam district water have been found to be higher than the allowable levels, with only 16% of places having sufficient quality for potable water (Sampath et al. 2011).
Figure 4 c and d show the geographical distributions of Na+ and K+. Higher Na+ concentrations have been found in the study region’s central and northern regions, while higher K+ concentrations were also found in the northwestern and southeastern regions closer to the shoreline. The Na+ content of groundwater, on the other hand, ranged from 22 to 173 mg/L, with a mean value of 60 mg/L. The results showed that none of the groundwater samples tested fulfilled the WHO and SLS guidelines (WHO, 2017). According to Young et al. (2011), the average Na+ and K+ concentrations in the northwestern province were 79.77 mg/L and 6.12 mg/L, respectively. K+ is the most important nutrient for humans, and too much of it can cause constipation (Adimalla and Venkatayogi 2018). K+ concentrations ranged from 2 to 25 mg/L in the sampling location, with mean value of 12.52 mg/L. Nevertheless, the WHO recommendations for K+ have yet to be established because it occurs naturally in drinking water at levels far below those deemed harmful to human health (Adimalla and Venkatayogi 2018; WHO 2017).
Anions (HCO3 − and Cl−) and turbidity
The concentration of HCO3− in the sample ranged from 26 to 54 mg/L, with a mean value of 37.11 mg/L. The geographic distribution of HCO3− is shown in Fig. 5a, with higher concentrations found in the south shoreline and lower concentrations found in the north shoreline. HCO3− concentrations in the northwestern province ranged from 10 to 240 mg/L, according to Young et al. (2011), while Jayasena et al. (2008) found it to be between 3.5 and 966 mg/L.
Excessive Cl− concentrations in groundwater are regarded as a sign of contamination from a variety of sources, and they impart a salty flavor to the water (Marghade et al. 2012). The Cl− concentration in the sample area varied from 110 to 443 mg/L. However, the average Cl− concentration in the groundwater samples studied (191 mg/L) is lower than the overall permissible limit of 600 mg/L (WHO 2017). The geographic distribution map of Cl− is depicted in Fig. 5b and higher concentrations found in the north shoreline. Previous studies in the coastal region of Sri Lanka have revealed desirable Cl− values (32–1100 mg/L) in accordance with the SLS (Mikunthan and Silva 2010). Despite the fact that no health risks have been identified, residents of coastal areas are reluctant to drink water due to texture and taste concerns.
Turbidity refers to the relative clarity of the water, which prevents light transmission. Groundwater turbidity in the study area ranged from 0.62 to 41.67 NTU (Fig. 5c). According to the SLS and WHO drinking water quality guidelines, the maximum turbidity level was exceeded in 22% of locations. As a result, it is unlikely to be suitable for consumption. Several previous investigations discovered that the turbidity in the Puttlam district’s groundwater was much higher than the WHO and SLS permitted values. Turbidity ranged from 1.6 to 164 NTU, according to Galhenage et al. (2021), while Gunarathna et al. (2021) discovered turbidity ranged from 44 to 723 NTU. Furthermore, Gunarathna et al. (2021) discovered that the mean turbidity of groundwater was significantly higher than that of surface water.
Groundwater quality index (GWQI)
Ramakrishnaiah et al. (2009) presented several categories for GWQI such as excellent water (WQI = 50), good water (WQI = 50 to 100), poor water (WQI = 100 to 200), very poor water (WQI = 200 to 300) and unsuitable water for drinking (WQI = > 300) are some of the categories for GWQI. According to this classification, 16.67% of samples in the study area were excellent (NW4, NW11, NW13), 61.11% are good (NW1, NW2, NW3, NW7, NW9, NW10, NW12, NW15, NW16, NW17, NW18), and 11.11% are poor (NW6, NW8) for drinking purposes (Fig. 7). NW5 and NW14 were unsafe to drink (11.11%). Furthermore, values were ranged from 45.12 to 2700.55, with an average of 337.78 (Figs. 6 and 7).
Geochemical depiction of groundwater
Piper diagrams were drawn using dissolved ion (anions and cations) concentrations. It represented the hydrochemical progression of groundwater parameters as well as the overall effects of major ion reactions. According to Fig. 8, the cations in the water samples belonged to the Na+ and K+ types, with no dominant type, whereas the anions belonged to the Cl− type. Overall, the majority of the water samples were assigned to zones 2 and 4, which represent the Na+–Cl− face and the mixed Ca2+– Mg2+– Cl− face, respectively.
The interaction between aquifer materials and groundwater was the most important factor in determining groundwater genesis and quality. As a result, the Gibbs diagrams were used to examine the evolution mechanisms. Most previous studies used Gibbs plots to interprit the three dominant factors involved in groundwater or surface water chemistry (evaporation, precipitation, and rock-water interaction) (Liu et al. 2021; Meng et al. 2021; Wanda et al. 2021). Gibbs ratios were ranged from 0.71 to 0.94 (ratio I) and 0.47 to 0.91 (ratio II), with a mean of 0.78 and 0.82, respectively. All of the sampling sites, as shown in Fig. 9, were in the middle of both cation- and anion-based plots, representing rock dominance or water–rock interaction category. According to the findings, rock weathering was a major factor in controlling the ionic composition and chemistry of groundwater.
However, the Na+– Cl− and the mixed Ca2+– Mg2+– Cl− water types specify the silicate mineral weathering in the study area as well as carbonate dissolution. Moreover, agricultural activities and saline water intrusion can be a major reason for Na+– Cl− water type in the study area. Limestone and sandstone weathering also can be affected for Ca2+ and Mg2+ elements in the water samples (Adimalla and Taloor 2020; Khan et al. 2021).
Health risk assessment of groundwater
The groundwater was the major source of drinking water in the urban western coastal region in Sri Lanka (Amarasinghe, 2020). According to the physicochemical analysis and hydrochemistry of the study region, Na+– Cl− and Ca2+– Mg2+– Cl− were the distinct facies of the groundwater samples. Therefore, the potential health risk for human through these elements (Na+, Ca2+, Mg2+, Cl−) might be high, and its assessment is critical (Nta et al., 2020). The total HQ limit for non-carcinogenic health risk, which always safe for human, has been set for less than 1 (HQ < 1) (Achary et al., 2016; Adimalla et al., 2019; Bodrud-Doza et al., 2020; Zhang et al., 2020).
The results of the non-carcinogenic risk assessment revealed that the total HQ values of drinking water containing Na+, Ca2+, Mg2+, and Cl− to adults and children in Sri Lanka’s western coastal region ranged from 0.15 to 2.85 for males, 0.19 to 3.58 for females, and 0.86 to 6.86 for children, with a mean of 1.19, 1.50, and 2.01, respectively. Furthermore, the locations that were above the recommended total HQ limit (HQ = 1) were 61% for males, 78% for females, and 89% for children, respectively. However, NW2, NW3, NW5, NW6, NW7, and NW15 were not used for human consumption, whereas other wells were used for a variety of domestic purposes, including drinking.
As a result, the non-carcinogenic health risk associated with ingesting excessive Na+, Ca2+, Mg2+, and Cl− via groundwater/drinking water had a significant health impact on adults and children in the study region (Fig. 10). Children were more vulnerable than adults when exposed to these elements.
The majority of previous studies have revealed a number of non-carcinogenic health risks associated with groundwater contamination in various parts of the world, including Sri Lanka (Achary et al., 2016; Amarasinghe, 2020; Bodrud-Doza et al., 2020; Zhang et al., 2020).
Conclusions
The groundwater condition in Sri Lanka’s western urban coastal region has begun to deteriorate as a result of seawater intrusion. Hence, investigating water quality in groundwater was critical, as it is the primary source for drinking and domestic use. This study used the GIS to indicate spatial distribution of different water physicochemical parameters and locate “suitable” and “unsuitable” groundwater zones for drinking among 18 locations, and evaluated water quality by developing a groundwater quality index (GWQI) using water quality sub-indices.
According to the study, the groundwater in the research area is neutral to slightly alkaline in composition. Na+ and Cl− were the major abundant cations and anions, respectively. This could be due to coastal erosion and seawater intrusion in the region. Ca2+ (5 – 43 mg/L), Na+ (22 – 173 mg/L), and K+ (2 – 24.67 mg/L) ion concentrations in the research region’s groundwater are within the maximum allowable limits when compared to the WHO drinking water quality guidelines (Mg2+ 300 mg/L, Na+ 200 mg/L, K+, not recommended) and Sri Lankan water quality standards (Ca2+ 100 mg/L, Na+ 200 mg/L, K+, not recommended). Excessive turbidity (22%) and EC (11%) have also been discovered at a few groundwater sample sites in the research area. The groundwater quality in the study region ranges from excellent to poor for drinking, according to the GWQI. The most significant influences on the groundwater quality index were turbidity and EC. Groundwater samples that are suitable for drinking account for 77.78%, while samples that are not suitable for drinking account for 22.22%. The study of groundwater chemistry, Gibbs and Piper diagram in urban coastal region, Sri Lanka, revealed that the rock-water interaction were the major cause for groundwater quality. In the urban coastal region, water types such as Na+ and K+, Cl−, Na+– Cl−, and Ca2+– Mg2+– Cl− were discovered. According to the non-carcinogenic risk assessment, total HQ values of drinking water containing Na+, Ca2+, Mg2+, and Cl− to adults and children in Sri Lanka’s western coastal region ranged from 0.15 to 2.85 for males, 0.19 to 3.58 for females, and 0.86 to 6.86 for children, with a mean of 1.19, 1.50, and 2.01, respectively. Furthermore, the locations that were above the recommended total HQ limit (HQ = 1) were 61% for males, 78% for females, and 89% for children, respectively.
According to the spatial distribution maps, the research area’s poor and unfit water for human consumption is mostly concentrated in the central and western coastal areas. The spatialization of the WQI using GIS can be a useful tool for decision-makers to evaluate the effectiveness of water management projects and to take actions to reduce the impacts on groundwater pollution. As a result, consistent monitoring of seawater intrusion in Sri Lanka’s western urban coastal areas is critical to ensuring community well-being and safety.
References
Achary MS, Panigrahi S, Satpathy KK, Prabhu RK, Panigrahy RC (2016) Health risk assessment and seasonal distribution of dissolved trace metals in surface waters of Kalpakkam, southwest coast of Bay of Bengal. Reg Stud Mar Sci 6:96–108. https://doi.org/10.1016/J.RSMA.2016.03.017
Abeykoon LCK, Thilakarathne EPDN, Abeygunawardana AP, Warnasuriya TWS, Egodauyana KPUT (2021) Are coastal protective hard structures still applicable with respect to shoreline changes in Sri Lanka? https://think-asia.org/handle/11540/13450. Accessed 16 June 2021
Adimalla N, Li P (2019) Occurrence, health risks, and geochemical mechanisms of fluoride and nitrate in groundwater of the rock-dominant semi-arid region, Telangana State, India. Hum Ecol Risk Assess Int J 25(1–2):81–103. https://doi.org/10.1080/10807039.2018.148035
Adimalla N, Li P, Venkatayogi S (2018) Hydrogeochemical evaluation of groundwater quality for drinking and irrigation purposes and integrated interpretation with water quality index studies. Environ Process 5(2):363–383. https://doi.org/10.1007/s40710-018-0297
Adimalla N, Taloor AK (2020) Hydrogeochemical investigation of groundwater quality in the hard rock terrain of South India using Geographic Information System (GIS) and groundwater quality index (GWQI) techniques. Groundw Sustain Dev 10(126):100288. https://doi.org/10.1016/j.gsd.2019.100288
Adimalla N, Marsetty SK, Xu P (2019). Assessing groundwater quality and health risks of fluoride pollution in the Shasler Vagu (SV) watershed of Nalgonda, India, 26(6), 1569–1588. https://doi.org/10.1080/10807039.2019.1594154
Amarasinghe SR (2020). Health risk assessment of heavy metals in drinking water: a case study in Lenabatuwa Division of Southern Sri Lanka. Rajarata University Journal 5(2). http://www.rjt.ac.lk/journals/vol6/15RUJ
Aouiti S, Hamzaoui AF, El MF, Hamdi M, Celico F, Zammouri M (2020). Groundwater quality assessment for different uses using various water quality indices in semi-arid region of central Tunisia. Environ Sci Pollut Res 28(34), 46669–46691. https://doi.org/10.1007/S11356-020-11149-5
APHA (2012). Standard methods for the examination of water and wastewater, twenty-second ed. American Public Health Association, Washington, DC.
Arasaretnam S, Prasadini HRP, Venujah K (2018). Assessment of open well water quality around Puttalam District, Sri Lanka. Int J Adv Sci Res Eng, 4(12), 225–232. https://doi.org/10.31695/ijasre.2018.33024
Barbosa FJ, de Oliveira IB (2021). Development of a groundwater quality index: GWQI, for the aquifers of the state of Bahia, Brazil using multivariable analyses. Scientific Reports 11(1), 1–22. https://doi.org/10.1038/s41598-021-95912-9.
Bodrud-Doza M, Islam SMDU, Rume T, Quraishi SB, Rahman MS, Bhuiyan MAH (2020) Groundwater quality and human health risk assessment for safe and sustainable water supply of Dhaka City dwellers in Bangladesh. Groundw Sustain Dev 10:100374. https://doi.org/10.1016/J.GSD.2020.100374
Chakraborty SK (2021) Methodologies for the assessment of river ecosystem in Southern West Bengal, India. Riverine Ecology 2:747–855. https://doi.org/10.1007/978-3-030-53941-2_9
Chathuranga AGL, Perera TANT, De Silva PMCS, Jayasinghe GY (2018). Assessment of physico-chemical parameters of drinking water quality in selected DS divisions - Hambanthota District. 11th International Research Conference of KDU at: General Sir John Kotelawala Defence University, 47.
Chen J, Wu H, Qian H, Gao Y (2017) Assessing nitrate and fluoride contaminants in drinking water and their health risk of rural residents living in a semiarid region of Northwest China. Expo Health 9:183. https://doi.org/10.1007/s12403-016-0231-9
Cooray T, Wei Y, Zhong H, Zheng L, Weragoda SK, Weerasooriya R (2019). Assessment of groundwater quality in CKDu affected areas of Sri Lanka: implications for drinking water treatment. International Journal of Environmental Research and Public Health, 16(10). https://doi.org/10.3390/ijerph16101698
Edirisinghe EANV, Karunarathne GRR, Samarakoon ASMNB, Pitawala HMTGA, Dharmagunawardhane HA, Tilakarathna IANDP (2016) Assessing causes of quality deterioration of groundwater in Puttalam, Sri Lanka, using isotope and hydrochemical tools. Isot Environ Health Stud 52(4–5):513–528. https://doi.org/10.1080/10256016.2015.1127918
Elsayed GM, Soussa H, Fattouh E (2021) Groundwater quality evaluation for drinking and irrigation uses in Dayrout city Upper Egypt. Ain Shams Eng J 12(1):327–340. https://doi.org/10.1016/J.ASEJ.2020.05.010
Eltarabily MG, Moghazy HEM (2021) GIS-based evaluation and statistical determination of groundwater geochemistry for potential irrigation use in El Moghra, Egypt. Environ Monit Assess 193(5):306. https://doi.org/10.1007/s10661-021-09058-2
Farzaneh G, Khorasani N, Ghodousi J, Panahi M (2021) Assessment of surface and groundwater resources quality close to municipal solid waste landfill using multiple indicators and multivariate statistical methods. Int J Environ Res 15(2):383–394. https://doi.org/10.1007/s41742-020-00307-9
Galhenage AC, Kumari EGSS, Kumara AMLU, Thalgaspitiya TWLR, Edirisinghe V, Vithanage M, Athapattu BCL (2021) Assessment of groundwater quality for drinking water from deep confined aquifer in Wanathawilluwa. Lect Notes Civ Eng 94:503–516. https://doi.org/10.1007/978-981-15-7222-7_41
Gitau MW, Chen J, Ma Z (2016) Water quality indices as tools for decision making and management. Water Resour Manage 30(8):2591–2610
Gnanachandrasamy G, Ramkumar T, Venkatramanan S (2015) Accessing groundwater quality in lower part of Nagapattinam district, Southern India: using hydrogeochemistry and GIS interpolation techniques. Appl Water Sci 5(1):39–55. https://doi.org/10.1007/s13201-014-0172-z
Gopalakrishnan T, Kumar L, Mikunthan T (2020). Assessment of spatial and temporal trend of groundwater salinity in Jaffna Peninsula and its link to paddy land abandonment. Sustainability (Switzerland), 12(9). https://doi.org/10.3390/su12093681
Gunaalan K, Ranagalage M, Gunarathna M, Kumari M, Vithanage M, Srivaratharasan T, Saravanan S, Warnasuriya TWS (2018) Application of geospatial techniques for groundwater quality and availability assessment: a case study in Jaffna Peninsula, Sri Lanka. ISPRS Int J Geo Inf 7(1):20. https://doi.org/10.3390/ijgi7010020
Gunarathna MHJP, Kumari MKN, Nirmanee KGS, Jayasinghe GY (2016) Spatial and seasonal water quality variation of Yan Oya in tropical Sri Lanka. Int J Appl Nat Sci 5(4):45–56
Gunarathna N, Amarasinghe A, Wijesundara S, Iddawela D, Wickramasinghe S (2021). Isolation, molecular characterization and phylogeny of Naegleria species in water bodies of North-Western Province, Sri Lanka. PLoS ONE, 16(3 March), e0248510. https://doi.org/10.1371/journal.pone.0248510.
Harun HH, Kasim MRM, Nurhidayu S, Ash’aari ZH, Kusin FM, Karim MKA (2021). Association of physicochemical characteristics, aggregate indices, major ions, and trace elements in developing groundwater quality index (GWQI) in agricultural area. International Journal of Environmental Research and Public Health 18(9), 4562. https://doi.org/10.3390/IJERPH18094562
Hayzoun H, Garnier C, Durrieu G, Lenoble V, Le Poupon C, Angeletti B (2015) Organic carbon, and major and trace element dynamic and fate in a large river subjected to poorly regulated urban and industrial pressures (Sebou River, Morocco). Sci Total Environ 502(2015):296–308. https://doi.org/10.1016/j.scitotenv.2014.09.014
Herath G (2006). International review for environmental strategies special feature on groundwater management and policy overview of groundwater management, the agro well program, and the impact of the 2004 Tsunami in Sri Lanka. 6(2), 373–386.
Herath G, Ratnayake U (2007). Urban groundwater management issues in Sri Lanka. Engineer: Journal of the Institution of Engineers, Sri Lanka, 40(4), 123. https://doi.org/10.4038/engineer.v40i4.7163
Herath HAS, Kubota K, Kawakami T, Nagasawa S, Motoyama A, Weragoda SK, Chaminda GGT, Yatigammana SK (2017) Potential risk of drinking water to human health in Sri Lanka. Environ Forensics 18(3):241–250. https://doi.org/10.1080/15275922.2017.1340364
Horton RK (1965). An index number system for rating water quality. J Water Pollut Control Fed, 37, 300–306. http://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/reference/ReferencesPapers.aspx?ReferenceID=1020268.
IGES Freshwater Management Project (2007). The Study of the management of groundwater resources in Sri Lanka. Sustainable Groundwater Management in Asian Cities 2, 110–136. http://enviroscope.iges.or.jp/modules/envirolib/upload/981/attach/08_chapter3-5srilanka.pdf.
International Water Management Project (2005). Planning groundwater use for sustainable rural development Why must groundwater be managed better ? Water Policy, 14.
Jain CK, Bandyopadhyay A, Bhadra A (2010) Assessment of ground water quality for drinking purpose District Nainital, Uttarakhand, India. Environ Monit Assess 166(1–4):663–676. https://doi.org/10.1007/s10661-009-1031-5
Jayasena HAH, Chandrajith R, Dissanayake CB (2008) Hydrogeochemistry of the groundwater flow system in a crystalline terrain: a study from the Kurunegala district, Sri Lanka. Environ Geol 55(4):723–730. https://doi.org/10.1007/s00254-007-1024-z
Jehan S, Ullah I, Khan S, Muhammad S, Khattak SA, Khan T (2020) Evaluation of the Swat River, Northern Pakistan, water quality using multivariate statistical techniques and water quality index (WQI) model. Environ Sci Pollut Res 27(31):38545–38558. https://doi.org/10.1007/s11356-020-09688-y
Kanga IS, Naimi M, Chikhaoui M (2020) Groundwater quality assessment using water quality index and geographic information system based in Sebou River Basin in the North-West region of Morocco. International Journal of Energy and Water Resources 4(4):347–355. https://doi.org/10.1007/s42108-020-00089-y
Kayemah N, Al-Ruzouq R, Shanableh A, Gokhan YA (2021). Evaluation of groundwater quality using groundwater quality index (GWQI) in Sharjah, UAE. E3SWC, 241, 01005. https://doi.org/10.1051/E3SCONF/202124101005.
Khan F, Krishnaraj S, Raja P, Selvaraj G, Cheelil R (2021) Impact of hydrogeochemical processes and its evolution in controlling groundwater chemistry along the east coast of Tamil Nadu and Pondicherry India. Environ Sci Pollut Res 28(15):18567–18588. https://doi.org/10.1007/S11356-020-10912-Y
Khanoranga KS (2019) An assessment of groundwater quality for irrigation and drinking purposes around brick kilns in three districts of Baluchistan province, Pakistan, through water quality index and multivariate statistical approaches. J Geochem Explor 197:14–26
Liu X, Zhou Z, Ding Y (2021) Vegetation coverage change and erosion type’s impacts on the water chemistry in western China. Sci Total Environ 772:145543. https://doi.org/10.1016/J.SCITOTENV.2021.145543
Lopes OF, de Jesus RM, de Sousa LF, Rocha FA, da Silva DML, Amorim AF, da Silva VHC, Navoni JA (2020). Comparison between water quality indices in watersheds of the Southern Bahia (Brazil) with different land use. Environmental Science and Pollution Research 28(10), 12944–12959. https://doi.org/10.1007/S11356-020-10941-7
Mahagamage MGYL, Jayakody S (2020) Influence of current culture practices on disease outbreaks in shrimp farms located in North Western Province of Sri Lanka. 24(7):453–469
Mao M, Guo P, Zhu X, Yang J, Pan D (2021). Hydrochemical characterization and quality assessment of groundwater in the hilly area of the Taihang Mountains in Henan Province, China. Environmental Science and Pollution Research 28(32), 43853–43871. https://doi.org/10.1007/S11356-021-13579-1
Marghade D, Malpe DB, Zade AB (2012) Geochemical characterization of groundwater from northeastern part of Nagpur urban. Central India Environ Earth Sci 62(7):1419–1430. https://doi.org/10.1007/s12665-010-0627-y
Maspalma G, Musa N, Mohammad B (2018) Article no. ADSUJSR 06020195). Temp (29.30–30.10) 6(2):2251–0702. http://www.adsujsr.com. Accessed 15 June 2021
Meng Y, Jia L, Dai J (2021) Using groundwater chemistry to identify soil cave development in karst terrain: a case study in Guangzhou China. Geochem Int 59(2):199–205. https://doi.org/10.1134/S0016702921020063
Mikunthan T, De Silva CS (2008) Vulnerability assessment for shallow aquifers using chemical quality of groundwater: a case study from Thirunelvely and Kondavil in Jaffna District. Trop Agric Res 20:303–312
Mikunthan T, De Silva CS (2010). Sources of extent of groundwater pollution in shallow aquifer: a case study from Jaffna District in Sri Lanka. Third International Conference on Hydrology and Watershed Management in India, 41–48.
Moreno ML, Aguilera H, González-Jiménez M, Díaz-Losada E (2021) D-Piper, a modified piper diagram to represent big sets of hydrochemical analyses. Environ Model Softw 138:104979. https://doi.org/10.1016/J.ENVSOFT.2021.104979
Mukherjee I, Singh UK (2018). Groundwater fluoride contamination, probable release, and containment mechanisms: a review on Indian context. Environmental Geochemistry and Health.
Naik MR, Barik M, Prasad KV, Kumar A, Verma AK, Sahoo SK, Jha V, Sahoo NK (2021) Hydro-geochemical analysis based on entropy and geostatistics model for delineation of anthropogenic ground water pollution for health risks assessment of Dhenkanal district, India. Ecotoxicology 2021:1–16. https://doi.org/10.1007/S10646-021-02442-1
Narsimha A, Sudarshan V (2017) Contamination of fluoride in groundwater and its effect on human health: a case study in hard rock aquifers of Siddipet, Telangana State. India Appl Water Sci 7(5):2501–2512. https://doi.org/10.1007/s13201-016-0441-0
Narsimha A, Rajitha S (2018) Spatial distribution and seasonal variation in fluoride enrichment in groundwater and its associated human health risk assessment in Telangana State, South India. Hum Ecol Risk Assess Int J 24(8):2119–2132. https://doi.org/10.1080/10807039.2018.1438176
Nath AV, Selvam S, Reghunath R, Jesuraja K (2021) Groundwater quality assessment based on groundwater pollution index using Geographic Information System at Thettiyar watershed Thiruvananthapuram district, Kerala, India. Arab J Geosci 14(7):1–26. https://doi.org/10.1007/S12517-021-06820-1
Nihalani S, Meeruty A (2020). Water quality index evaluation for major rivers in Gujarat. Environ Sci Pollut Res, 1–9. https://doi.org/10.1007/s11356-020-10509-5.
Nta SA, Ayotamuno MJ, Igoni AH, Okparanma RN, Udom IJ (2020). Application of hazard quotient (HQ) for the assessment of potential health risk of groundwater users around Uyo main dumpsite. Asian Journal of Advanced Research and Reports, 17–23. https://doi.org/10.9734/AJARR/2020/V14I130321
Pant BR (2011) Ground water quality in the Kathmandu valley of Nepal. Environ Monit Assess 178(1–4):477–485. https://doi.org/10.1007/s10661-010-1706-y
Perera MDND, Ranasinghe TKGP, Piyadasa RUK, Jayasinghe GY (2018) Risk of seawater intrusion on coastal community of Bentota river basin Sri Lanka. Procedia Eng 212:699–706. https://doi.org/10.1016/J.PROENG.2018.01.090
Phok R, Kosgallana DWN, Sumana BW, Herath MTGAP, Hingure AD (2021) Using intrinsic vulnerability and anthropogenic impacts to evaluate and compare groundwater risk potential at northwestern and western coastal aquifers of Sri Lanka through coupling DRASTIC and GIS approach. Appl Water Sci 11(7):1–18. https://doi.org/10.1007/S13201-021-01452-Y
Premalal WAV, Jayewardene DT (2015) Effect of low pH of groundwater in Rathupaswala area, Sri Lanka: A case study, pp 8–10. https://www.researchgate.net/publication/272175683. Accessed 11 June 2021
Rabeiy RES (2018) Assessment and modeling of groundwater quality using WQI and GIS in Upper Egypt area. Environ Sci Pollut Res 25(31):30808–30817. https://doi.org/10.1007/s11356-017-8617-1
Rajasooriyar LD, Boelee E, Prado MCCM, Hiscock KM (2013) Mapping the potential human health implications of groundwater pollution in southern Sri Lanka. Water Resour Rural Devs 1–2:27–42. https://doi.org/10.1016/j.wrr.2013.10.002
Rajmohan N (2021) Application of water quality index and chemometric methods on contamination assessment in the shallow aquifer Ganges River basin India. Environ Sci Pollut Res 28(18):23243–23257. https://doi.org/10.1007/s11356-020-12270-1
Ramakrishnaiah CR, Sadashivaiah C, Ranganna G (2009) Assessment of water quality index for the groundwater in Tumkur taluk, Karnataka state. India E J Chem 6(2):523–530
Sampath GRA, Hewawasam ALT, Dias P (2011). Assessment of groundwater quality status in Puttalam and Chillaw area to demonstrate its suitability as a source of potable water.
Şener Ş, Varol S, Şener E (2021). Evaluation of sustainable groundwater utilization using index methods (WQI and IWQI), multivariate analysis, and GIS: the case of Akşehir District (Konya/Turkey). Environmental Science and Pollution Research, 1–20. https://doi.org/10.1007/s11356-021-14106-y.
Şener Ş, Şener E, Davraz A (2017) Evaluation of water quality using water quality index (WQI) method and GIS in Aksu River (SW-Turkey). Sci Total Environ 584–585:131–144. https://doi.org/10.1016/j.scitotenv.2017.01.102
Senthilkumar M, Mohapatra B, Gnanasundar D, Gupta S (2021). Identifying fluoride endemic areas and exposure pathways for assessment of non-carcinogenic human health risk associated with groundwater fluoride for Gujarat state, India. Environmental Science and Pollution Research 2021, 1–16. https://doi.org/10.1007/S11356-021-14156-2
Shukla M, Arya S, Scholar R, Professor A (2018). Determination of chloride ion (Cl-) concentration in Ganga River Water by Mohr method at Kanpur, India. Green Chemistry & Technology Letters, 4(1), 6–08. https://doi.org/10.18510/gctl.2018.412
Singh S, Ghosh NC, Krishan G, Kumar S, Gurjar S, Sharma MK (2019) Development of indices for surface and ground water quality assessment and characterization for Indian conditions. Environ Monit Assess 191(3):1–20. https://doi.org/10.1007/s10661-019-7276-8
Singh A, Gogoi A, Saikia P, Karunanidhi D, Kumar M (2021) Integrated use of inverse and biotic ligand modelling for lake water quality resilience estimation: a case of Ramsar wetland, (Deepor Beel), Assam. India Environmental Research 200:111397. https://doi.org/10.1016/J.ENVRES.2021.111397
Singh CK, Shashtri S, Mukherjee S, Kumari R, Avatar R, Singh A, Singh RP (2011) Application of GWQI to assess effect of land use change on groundwater quality in Lower Shiwaliks of Punjab: remote sensing and GIS based approach. Water Resour Manag 25(7):1881–1898. https://doi.org/10.1007/S11269-011-9779-0
Statista (2021) Sri Lanka: urban population 2011–2020 | Statista. Available at: https://www.statista.com/statistics/603515/sri-lanka-urban-population/ (Accessed: 16 June 2021).
Tian Y, Jiang Y, Liu Q, Dong M, Xu D, Liu Y (2019) Using a water quality index to assess the water quality of the upper and middle streams of the Luanhe River, northern China. Sci Total Environ 667(219):142–151. https://doi.org/10.1016/j.scitotenv.2019.02.356
Tiwari AK, De Maio M, Amanzio G (2017). Evaluation of metal contamination in the groundwater of the Aosta Valley Region, Italy. Int J Environ Res, 11(3), 291–300. https://doi.org/10.1007/s41742-017-0027-1.
United Nations Environment Programme (2018) Progress on ambient water quality: piloting the monitoring methodology and initial findings for SDG 6 indicator 6.3.2. https://www.unwater.org/publications/progress-on-ambient-water-quality-632/. Accessed 5 May 2021
USEPA (1993) Reference dose (RfD): description and use in health risk assessments. http://www.epa.gov/iris/rfd.htm. Accessed 31 Aug 2021
USEPA (2016) Risk-based concentration table | Mid-Atlantic Risk Assessment | US EPA. https://archive.epa.gov/region9/superfund/web/html/index-23.htmlAccessed 31 Aug 2021
Vaiphei SP, Kurakalva RM, Sahadevan DK (2020) Water quality index and GIS-based technique for assessment of groundwater quality in Wanaparthy watershed Telangana, India. Environ Sci Pollut Res 27(36):45041–45062. https://doi.org/10.1007/s11356-020-10345-7
Villholth KG, Rajasooriyar LD (2010) Groundwater resources and management challenges in Sri Lanka-an overview. Water Resour Manage 24(8):1489–1513. https://doi.org/10.1007/s11269-009-9510-6
Wanasinghe WCS, Gunarathna MHJP, Herath HMPIK, Jayasinghe GY (2018). Drinking Water Quality on Chronic Kidney Disease of Unknown Aetiology (CKDu) in Ulagalla Cascade, Sri Lanka. Sabaragamuwa University Journal, 16(1), 2386–2041. http://repo.lib.sab.ac.lk:8080/xmlui/handle/123456789/632.
Wanda EMM, Chavula G, Tembo FM (2021) Hydrogeochemical characterization of water quality evolution within Livingstonia coalfield mining areas in Rumphi district, northern Malawi. Physics and Chemistry of the Earth, Parts a/b/c 123:103045. https://doi.org/10.1016/J.PCE.2021.103045
Wang X, Zhang F (2018) Multi-scale analysis of the relationship between landscape patterns and a water quality index (WQI) based on a stepwise linear regression (SLR) and geographically weighted regression (GWR) in the Ebinur Lake oasis. Environ Sci Pollut Res 25(7):7033–7048. https://doi.org/10.1007/s11356-017-1041-8
Weerasooriya RR, Liyanage LPK, Rathnappriya RHK, Bandara WBMAC, Perera TANT, Gunarathna MHJP, Jayasinghe GY (2021) Industrial water conservation by water footprint and sustainable development goals: a review. Environ Dev Sustain 23(9):12661–12709. https://doi.org/10.1007/S10668-020-01184-0
WHO (2017). Guidelines for drinking-water quality. In: Incorporating 1st Addendum, fourth ed. World Health Organization, Geneva.
Wickramasinghe BNB, Jayasena HAH, Perera KVGS, Rajapakse RRGR (2021) Assessment of hydrogeological scenario in a cross-section from Anamaduwa to Kalpitiya in Northwest Sri Lanka. Ceylon J Sci 50(1):83. https://doi.org/10.4038/cjs.v50i1.7850
Wickramasinghe W (2013). Change in Other Field Crop Cultivation in the North Western Province Hector Kobbekaduwa Agrarian Research and Training Institute 114, Wijerama Mawatha Colombo 7 Sri Lanka.
Wijesinghe DTN, Dassanayake KB, Sommer SG, Jayasinghe GY, Scales PJ, Chen D (2016). Ammonium removal from high-strength aqueous solutions by Australian zeolite. 51(8), 614–625. https://doi.org/10.1080/10934529.2016.1159861
Yadav K, Raphi M, Jagadevan S (2021) Geochemical appraisal of fluoride contaminated groundwater in the vicinity of a coal mining region: spatial variability and health risk assessment. Geochemistry 81(1):125684. https://doi.org/10.1016/J.CHEMER.2020.125684
Yang Y, Yin X, Yang Z (2016) Environmental flow management strategies based on the integration of water quantity and quality, a case study of the Baiyangdian Wetland China. Ecol Eng 96(2016):150–161. https://doi.org/10.1016/j.ecoleng.2015.12.018
Young SM, Pitawala A, Ishiga H (2011) Factors controlling fluoride contents of groundwater in north-central and northwestern Sri Lanka. Environ Earth Sci 63(6):1333–1342. https://doi.org/10.1007/s12665-010-0804-z
Zhang Q, Xu P, Qian H (2020) Groundwater quality assessment using improved water quality index (WQI) and human health risk (HHR) evaluation in a semi-arid region of Northwest China. Expos Health 12(3):487–500. https://doi.org/10.1007/S12403-020-00345-W
Author information
Authors and Affiliations
Contributions
T.A.N.T. Perera—conceptualization, data curation, formal analysis, methodology, validation, visualization, writing (original draft), writing (review and editing).
HMMSD Herath—conceptualization, resources, software, validation, visualization.
Ranjana UK Piyadasa—conceptualization, funding acquisition, investigation, project administration, resources, supervision.
Liu Jianhui—conceptualization, funding acquisition, investigation, methodology, resources.
Li Bing—conceptualization, funding acquisition, investigation, methodology, resources.
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Xianliang Yi.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Perera, T.A.N.T., Herath, H.M.M.S.D., Piyadasa, R.U.K. et al. Spatial and physicochemical assessment of groundwater quality in the urban coastal region of Sri Lanka. Environ Sci Pollut Res 29, 16250–16264 (2022). https://doi.org/10.1007/s11356-021-16911-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16911-x