Abstract
To ensure the safety of drinking water, 51 groundwater samples were collected from a semi-arid area of China and various physicochemical parameters were analyzed. Groundwater quality for drinking purposes along with the associated health risks was assessed using a water quality index (WQI) which was improved using the Criteria Importance Through Inter-criteria Correlation weighting method. The results show that the groundwater was slightly alkaline and the total dissolved solids ranged from 497.26 to 2198.82 mg/L. The ionic dominance pattern was in the order of K+ + Na+ > Ca2+ > Mg2+ > NH4+ for cations, and HCO3− > SO42+ > Cl− > NO2− > NO3− > CO32− > F− for anions, respectively. In the study region, HCO3–Na and HCO3–Ca·Mg were the dominant water types, followed by the SO4·Cl–Na type, which are mainly controlled by rock weathering, leaching, and evaporation. 94.12% of the total samples are suitable for drinking; the poor and extremely poor water for human consumption are mainly located in the center and northeast of the study area. The non-carcinogenic health risk for males ranged from 0.0002 to 38.7575, for females 0.0002 to 49.2935, and for children 0.0003 to 84.3167, respectively. The health risk for children was approximately 2.18 times and 1.71 times higher than that for males and females, indicating that children are more susceptible to water contamination. The major pollutants in the study region are nitrite, nitrate, and fluoride. Therefore, the necessary steps to be taken to clean up this highly nitrite-, nitrate-, and fluorine-contaminated groundwater and health risks in this study region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater accounts for about one-third of freshwater consumption globally, which is important for domestic, industrial, and agricultural use, especially in arid and semi-arid areas where water source is scarce and unevenly distributed (Wu et al. 2017; Chen et al. 2018; Li et al. 2018a, b; Zhang et al. 2018). It is reported that more than 1.5 billion people worldwide rely on groundwater for primary needs (He et al. 2015; Adimalla and Wu 2019). However, with the swift population growth, rapid industrial development, and extensive agricultural activities, groundwater pollution has become a serious problem in many countries and regions (Adimalla et al. 2018, 2019; Li et al. 2017a, 2019a). Groundwater pollutants mainly include inorganic salts, toxic metals, cations (potassium (K+), sodium (Na+), calcium (Ca2+), and magnesium (Mg2+)), and anions (chloride (Cl−), bicarbonate (HCO3−), carbonate (CO32−), and sulfate (SO42−)) (Khanoranga and Khalid 2019). Therefore, groundwater quality issues have become a major concern in the last several decades, and groundwater quality assessment along with health risk evaluation has widely been studied across the globe, including in China, India, and the USA (Qiu 2010; Yu et al. 2011; Li et al. 2018c). Adimalla and Wu (2019) conducted a study on groundwater quality and the related health risk assessment in a semi-arid region of south India and found that the nitrate and fluoride were the principal contaminants affecting drinking water safety in the Siddipeta-Vagu (SDV) region. Xu et al. (2019a) investigated the hydrogeochemical characterization of shallow groundwater in the Central-Western Guanzhong Basin, China, and indicated that HCO3–Ca Mg and HCO3–Na are the main hydrochemical facies, controlled by rock weathering, cation exchange, and evaporation. Karakus (2019) evaluated the groundwater quality in Sivas province, Turkey, showing that TDS, NO3−, SO42−, Cr, and As negatively affect groundwater quality. Li et al. (2019a) studied fluoride in groundwater of a loess aquifer in Tongchuan, China, finding that high-fluoride groundwater is mainly prevalent in the southeast part of the study area. Similar studies in other regions have been carried out by researchers (Kihumba et al. 2016; Rasool et al. 2016; Adimalla and Qian 2019a; Chen et al. 2019; Ganyaglo et al. 2019; He and Wu 2019; Iticescu et al. 2019; Jia et al. 2019; Rezaei et al. 2019; Zhang et al. 2019).
Groundwater researchers have used many methods to assess groundwater quality. Some of these methods include a fuzzy comprehensive assessment method (Wu et al. 2019), Technique for Order of Preference by Similarity to Ideal Solution (TOPSIS) (Li et al. 2013a, b; Gorgij et al. 2019), set pair analysis (Tian and Wu 2019; Su et al. 2019; Lu et al. 2019), Hierarchical analysis (Deng et al. 2017), and water quality index (WQI) (Chen et al. 2019; Li et al. 2014a, 2018d, 2019b). The water quality index (WQI) is an efficient tool to assess water quality using various water quality parameters (Abbasi and Abbasi 2012; Chen et al. 2019). The parameters are often weighted according to their importance to water quality. However, a small change in weighting will affect the overall interpretation of water quality (Mukate et al. 2019). To overcome this problem, the Criteria Importance Though Inter-criteria Correlation (CRITIC) method was used to generate relative weights of parameters in this study. The idea is based on the two concepts of standard deviation and the conflict among the different parameters. The CRITIC weighting method also overcomes the shortcomings of conventional information entropy which considers only the effects of the factor variation and ignores the effects of conflicts between factors (Yu et al. 2019). Therefore, combining the CRITIC weighting theory and WQI analysis is reasonable and can take the advantage of the two methods.
The study area is part of the Guanzhong Basin, located at the starting point of the “Silk Road Economic Belt,” and occupies an important position in China’s regional economic pattern (Li et al. 2015; Xu et al. 2019a). The source of drinking water in the study region is mainly groundwater (Luo et al. 2014). In Guanzhong Basin, the groundwater quality is poor in some areas duo to the pollution such as high salinity and the presence of other toxic elements (Luo et al. 2014; Li et al. 2014b, 2016a, b; Xu et al. 2019b). Thus, proper assessment and reporting of groundwater quality are important issues in the study region. The main objectives of this research are to (1) analyze the hydrogeochemical characteristics and hydrochemical facies of the groundwater and their formation mechanisms; (2) appraise the overall groundwater quality for drinking purposes using WQI based on CRITIC weighting, and (3) assess the non-carcinogenic risks via drinking intake and dermal contact pathways for males, females, and children. The study provides essential information for local groundwater quality protection and management, which is helpful for supporting the sustainable development of drinking water in the study region and establishing a long-term harmonious relationship among humans, society, and the environment.
Materials and Methods
Study Area
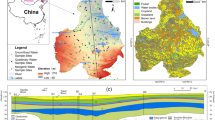
The study area (34° 15′–34° 45′ N, 109° 23′–109° 45′ E) is a part of the Guanzhong Basin (Fig. 1), where groundwater is one of the main water supply sources. Lei and Ju (2008) and Zhang (2009) reported that the Weinan City drinking water was unsafe and the urban area groundwater was overexploited (Zhang 2017). The southern part of study area is the Qinling Mountains and the loess platform, with an altitude of 600–2400 m, while the central and northern parts are the Weihe Plain, with an altitude of 330–600 m. The study region is geographically located in warm temperate semi-humid and semi-arid monsoon climate region (Xu et al. 2019b) with an average annual temperature of 13.6 °C, an average of 2200–2500 h annual daylight, and a frost-free period of 199–255 days. The annual average rainfall in this area is about 600 mm and the annual average evaporation rate ranges between 1000 and 1200 mm (Xu et al. 2019b).
Geologically, the study region was mainly occupied by the Quaternary alluvial rock group of the alluvial fan and the Quaternary aeolian rock group of the loess plateau. The groundwater in the study area is mainly loose rock pore water. The groundwater level is shallow and varies from 14 to 37 m below the ground level with good recharge conditions. The recharge of groundwater mainly includes rainfall infiltration replenishment, irrigation infiltration recharge, and recharge of Wei River. In addition, the discharge of groundwater mainly includes evaporation and exploiting and the runoff direction of groundwater generally flows from west to east.
Sampling and Analysis
A total of fifty-one phreatic samples were collected in study area from existing hand pumps and bore wells using thoroughly prewashed polyethylene bottles. The samples were stored at 4 °C until analysis. Figure 1 shows the groundwater sampling points in the study region. Groundwater quality parameters, including pH, total dissolved solids (TDS), major ions (sodium (Na+), potassium(K+), calcium(Ca2+), magnesium(Mg2+), bicarbonate (HCO3−), carbonate (CO32−), chloride (Cl−), sulfate (SO42−)), heavy metals [manganese (Mn) and Hexavalent chromium (Cr6+)], and other ions [ammonia nitrogen (NH4+), nitrate (NO3−), nitrite (NO2−), and fluoride (F−)], were analyzed for all groundwater samples. pH was measured immediately in the field using portable devices on site. TDS was determined by drying the samples at 105 °C and weighing them with an analytical balance. Na+, K+, Ca2+, Mg2+, Cl−, SO42−, NH4+, NO3−, NO2− , and F− were tested using ion chromatograph (ICS-600). HCO3− and CO32− were determined by alkalinity titration. Mn and Cr6+ were measured using plasma emission spectrometry (ICAP6300).
The analytical accuracy was cross-checked by calculating ionic balance error (IBE) as follows:
where all cations and anions were expressed in meq/L. The computed IBE was within the acceptable limit of ± 5%. In this study, the calculated results showed that the IBE ranged from − 4.13 to 4.47, which confirms the reliability of the ion analysis results.
Methods
Improved Water Quality Index (WQI)
Water quality index (WQI) is frequently used to determine the suitability of the groundwater for drinking purposes throughout the world and is an effective tool for appraising the groundwater quality (Li et al. 2010; Adimalla et al. 2018; Adimalla and Qian 2019b; Iticescu et al. 2019). When calculating WQI, the first step is to calculate the weights of the parameters. Criteria Importance Through Inter-criteria Correlation (CRITIC), proposed by Diakoulaki, is an objective weighting method, which is mainly composed of two parts (Wang et al. 2018). These two parts are represented by the following equations:
where \(C_{j}\) represents the information amount of the jth parameter. \(\delta_{j}\) indicates the standard deviation of the jth parameter. m is the number of different parameters. \(r_{ij}\) is the correlation coefficient. \(W_{j}\) is the weight of the jth parameter.
In order to eliminate the unit influence between different parameters, the data need to be normalized before calculating the weight. Let \(y_{ij}\) represent the normalized value, the correlation coefficient is calculated using the formula (4).
where \(x_{ij}\) is the jth evaluation index of the ith groundwater sample. \(\overline{x}_{ij}\) and \(\overline{y}_{ij}\) express the average value of \(x_{ij}\) and \(y_{ij}\), respectively.
The second step is to assign a quality rating scale (\(Q_{j}\)) for each parameter. \({\text{Q}}_{j}\) is calculated by the following formula:
where \(C_{j}\) is the concentration of each chemical parameter in water sample in mg/L. \(C_{{{\text{jp}}}}\) is the ideal value of the parameter in pure water (consider \(C_{{{\text{jp}}}}\) = 0 for all, except pH where \(C_{{{\text{jp}}}}\) = 7). \(S_{j}\) is the standard value for each chemical parameter in mg/L according to Chinese Quality Standard for Groundwater.
Lastly, the WQI can be calculated by the formula below:
Computed WQI values were classified into five categories, excellent, good, moderate, poor, and very poor (Li et al. 2010; Adimalla et al. 2018; Zotou et al. 2019). The WQI range and type of water are shown in Table 1.
Human Health Risk Assessment (HHRA) Model
The human health risk assessment (HHRA) model established by the United States Environmental Protection Agency (USEPA) is a widely used method to evaluate the potentially harmful effects of groundwater contaminants on the health of children and adults (Li et al. 2016c, 2019b; Adimalla et al. 2019; Adimalla and Qian 2019a; Adimalla and Wu 2019). Based on the Ministry of Environmental Protection (MEP) of the P.R. China, there are two main channels through which are human body absorbs harmful substances from groundwater, they are orally drinking water and dermal contact (Li et al. 2016c; Wu and Sun 2016; Adimalla and Qian 2019a). The HHRA includes non-carcinogenic risks and carcinogenic risks. The non-carcinogenic risks were assessed using NH4+, NO3−, NO2−, Mn2+, F−, and Cr6+ as the risk assessment parameters. The models for non-carcinogenic risks via ingestion and dermal contact are as follows (Li et al. 2016c; Wu and Sun 2016; Adimalla et al. 2019).
The non-carcinogenic risk through dermal contact is expressed as (Li et al. 2017b):
Therefore, the total non-carcinogenic risks are calculated as follows:
where CDI: chronic daily dose via ingestion (mg/kg day), C: concentration of pollutant for groundwater (mg/L), HQoral and HQdermal: hazard quotient through oral and dermal exposure pathways, CDD: chronic daily dose via dermal contact (mg/kg day), DA: exposure dosage (mg/cm2), SA: skin surface area (cm2), RfDoral and RfDdermal: reference dosage via oral and dermal contact (mg/kg day), and ABSgi: gastrointestinal absorption factor. The meanings and index values of other parameters are shown in Tables 2 and 3.
In addition to the non-carcinogenic risk, Cr6+ can also create carcinogenic risks for humans (Li et al. 2016c). The carcinogenic risks of Cr6+ through drinking water intake and dermal contact are calculated as follows:
where CR denotes the carcinogenic risk. SF is the slope factor for the carcinogenic contaminants (mg/kg day)−1. The SForal value for Cr6+ was set at 0.42 (mg/kg day)−1 according to the Chinese technical guidelines for risk assessments of contaminated sites (Ministry of Health of the P.R. China, S. A. o. t. P. R. C. 2006). The EF × ED of CDI for carcinogenic risk assessments is set at 25,550 days for both adults and children. The acceptable limit for CR is 1 × 10–6.
Results and Discussion
Groundwater Chemistry
Physiochemical Parameters
The statistical results of water quality for the 51 groundwater samples are illustrated in Table 4. Table 4 also shows the details of drinking water quality limits. The pH values of the groundwater are in the range of 7.1–8.4 (mean = 7.67), which does not exceed the limits of pH (6.5–8.5) and reveals that the groundwater in this area is alkaline. TDS values varied in a wide range of 497.26–2198.82 mg/L, with a mean value of 734.64 mg/L. According to the standard limits of TDS (< 1000 mg/L), 9.8% groundwater samples show unhealthy and unpalatable for human health.
The ionic dominance pattern was in the order of K+ + Na+ > Ca2+ > Mg2+ > NH4+ for cations and HCO3− > SO42− > Cl− > NO2− > NO3− > CO32− > F− for anions. The average concentrations of K+ + Na+, Ca2+, Mg2+, Cl−, SO42−, HCO3−, CO32−, NH4+, NO3−, NO2−, and F− in groundwater were 91.35, 55.78, 37.62, 35.38, 79.32, 418.17, 4.28, 0.048, 16.3, 16.98, and 1.89 mg/L (Table 4). Concentrations of sodium and bicarbonate were the highest among the cations and anions, respectively. A certain amount of sodium is very essential to maintain a human health, whereas excess sodium intake will cause adverse health risks such as hypertension and osteoporosis (Adimalla and Qian 2019a; Li et al. 2019a). According to Drinking Water Quality Standard of P.R. China (Ministry of Health of the P.R. China 2006), the percentage of K+ + Na+ below the permissible limits is 92.2. Ca2+ and Mg2+ are also essential to human health. When the human body lacks Ca2+ , it leads to several diseases such as stroke, osteoporosis, and colorectal cancer. High Mg2+ concentration acts as a laxative agent (WHO 2011; Adimalla and Qian 2019a). In the present study, 98% of the groundwater sampling locations were within the maximum allowable limit for Ca2+ and Mg2+ (Table 4). In addition, the concentrations of HCO3− and CO32− ranged from 300.81 to 1020.22 mg/L and 0 to 12 mg/L, respectively. The SO42− concentration of groundwater in study area varied from 0 to 903.02 mg/L. 94.1% of the groundwater samples were within the desirable limit of 250 mg/L for SO42− in the study region. Chloride concentration ranged from 5.32 to 242.9 mg/L and all groundwater samples were within the upper limit (≤ 250 mg/L) for drinking water.
The study area is an agricultural region with wide fertilizer and pesticide use (Quan 2018; https://www.sxzx.gov.cn/zxhy/ydxszth/15351.html). Therefore, the study assessed the presence of nitrogen pollution. The NH4+ concentration of all groundwater samples was within the desirable limit for drinking (Table 4). However, the concentration of NO3− and NO2− for all groundwater samples in study region varied from 0 to 36 mg/L and 0 to 180 mg/L, respectively. 94.1% and 62.7% of groundwater samples were within the upper limit of 20 and 0.02 mg/L for NO3− and NO2− in the study region. Despite high levels of nitrite ions at two sampling points (44 and 47) in the study area, no reports of blue infant disease have been heard. Moreover, fluoride (F−) is a necessary element for human health at low concentration, but has non-carcinogenic risks at a high level, causing endemic fluorosis (dental and skeletal) and damage to the soft tissues (liver, kidney, lung, testis, etc.) (Duan et al. 2018; Ganyaglo et al. 2019). In this study, the F− concentration ranged from 0 to 13.2 mg/L. Six groundwater samples were not suitable for drinking, based on this parameter with 11.8% of all samples exceeding the upper limit (≤ 1.0 mg/L). High fluoride content in the study area leads to high incidence of dental and skeletal fluorosis (Li et al. 2009; Liu 2009). Therefore, the pollution from nitrogen (NO2− and NO3−) and fluoride was the most serious in the study region. In addition, the Mn2+ concentrations for all samples ranged from 0 to 0.36 mg/L, with a mean of 0.05 mg/L (Table 4). In the study region, 96.1% of groundwater samples were within the upper limit of 0.1 mg/L for Mn2+. The Cr6+ concentration of all groundwater samples was within the desirable limit for drinking.
The Dominant Water Types
Hydrochemical types are governed by major ions and are usually classified by a piper diagram (Piper 1944; He and Li 2019; Li et al. 2016c; Xu et al. 2019a). As shown in Fig. 2, cations of groundwater samples in the study region were mainly plotted in zones B and D, indicating that groundwater in the study area is mainly of the “no dominant’’ type and the “sodium’’ type. Anions were mainly plotted in zone E, followed by B, indicating that the groundwater is mainly of the “bicarbonate” type and “no dominant” type. Almost all groundwater samples were plotted in zones III and IV, followed by zone II, illustrating that HCO3–Na and HCO3–Ca·Mg were the dominant water types, followed by the SO4·Cl–Na type. The hydrochemical types are mainly related to the carbonate-rich material dissolution within the aquifers (Xu et al. 2019a).
Groundwater Chemistry Formation
The Gibbs diagrams are helpful to analyze the relationship between water chemistry and aquifer lithology (Gibbs 1970; He and Li 2019; Li et al. 2016c; Adimalla and Qian 2019b; Chen et al. 2019; Xu et al. 2019a). There are three main natural mechanisms forming the water chemistry in these diagrams: evaporation dominance, rock dominance, and precipitation dominance (Fig. 3). As shown in Fig. 3, the groundwater samples of “bicarbonate type” were mainly in the zone of rock dominance, which suggested that rock weathering and leaching are the major mechanisms controlling groundwater chemistry in these areas. However, the distributions of “no dominant type” and “chloride type” showed a slightly increasing trend toward the evaporation-dominant zone. This is related to the local semi-arid climate with little rainfall and large evaporation. Therefore, the main mechanisms governing groundwater chemistry of “chloride type” in this area are rock weathering and evaporation.
Groundwater Quality for Drinking
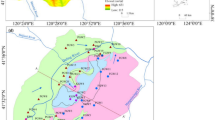
Groundwater samples (n = 51) and its WQI values and ranks are presented in Table 5. The results of WQI ranged from 21.02 to 966.98. Out of 51 groundwater samples, water quality of 1 and 2 samples was categorized as poor and extremely poor, and 48 samples were suitable for drinking purposes (rank = 1, 2, 3) (Table 5). The assessment results indicated that the samples suitable for drinking water account for 94.12% of the total samples, while the samples unsuitable for drinking account for 5.88%.
Spatial distribution of WQI is also shown in Fig. 4. It can be seen from Fig. 4 that the poor and extremely poor water samples are mainly located in the Weihe Plain in the central and northern parts of study area. It implies that human activities, including extensive use of fertilizers, septic tank leakage, and effluent of organic matter, are considered to have a greater impact on the groundwater quality of the study area. In addition, the lower the elevation, the worse is the groundwater quality in the study region (Figs. 1 and 4). This phenomenon indicated that groundwater will be affected by the geological environment and human factors during the flow process, and the flow of groundwater is basically the same as that of surface water in the study region, flowing from a high elevation to a low elevation.
Human Health Risk Assessment
The health risk assessment (HRA) model is the most effective tool for calculating the non-carcinogenic health risk in the different age groups (Li et al. 2016c, 2019b; Adimalla and Qian 2019a, b). The calculated results of non-carcinogenic health risks for adults and children in the study region through oral intake and dermal contact are explicitly presented in Table 5. As shown in Table 5, the HQoral values ranged from 0.0002 to 38.5714, with a mean of 2.6540 for males in the study region. The HQoral values for females ranged from 0.0002 to 49.0909, with a mean of 3.3778. And the HQoral values for children varied from 0.0003 to 84.0000, with a mean of 5.7797. The results of the HQdermal values were smaller than HQoral, ranging from 7.38 × 10–7 to 1.86 × 10–1 for males, 8.04 × 10–7 to 2.03 × 10–1 for females, and 1.26 × 10–6 to 3.17 × 10–1 for children, with means of 1.27 × 10–2, 1.38 × 10–2, and 2.16 × 10–2, respectively. This suggests that for non-carcinogenic risk, dermal contact pathway is quite low as compared with the ingestion pathway. The results of the HQtotal values ranged from 0.0002 to 38.7575 for males and from 0.0002 to 49.2935 for females, with means of 2.6666 and 3.3916, respectively. For children, the HQtotal values varied from 0.0003 to 84.3167, with a mean of 5.8013 (Table 6). 23.53%, 25.49%, and 37.25% of the samples have HQtotal exceeding 1, suggesting that most samples may induce non-carcinogenic risk to males, females, and children, respectively. These results showed the health risk of children and females is much higher than males. The reason is that children are more vulnerable is that they have smaller body weights than that of adult females and males (Li et al. 2016c).
As discussed earlier, non-carcinogenic risks are high in study region for adults and children. Table 7 shows the interval values for non-carcinogenic risks of different ions in drinking water. As shown in Table 7, the non-carcinogenic risk for adults and children was generally observed in the order of NO2− > F− > NO3− > Cr6+ > Mn2+ > NH4+. The pattern also indicated that the pollution from nitrogen (NO2− and NO3−) and fluoride was the most serious in the study region. The extensive use of fertilizers in agricultural applications is typically the cause for the high nitrogen concentration in groundwater of the study region. Also, nitrogen contamination can potentially originate from septic tank leakage and effluent organic matter (Kihumba et al. 2016; Adimalla et al. 2019). This is a serious issues because high nitrogen concentration could cause debilitating health disorders, such as gastric cancer, goiters, methemoglobinemia, birth defects, and hypertension (Zhang et al. 2018; Adimalla and Wu 2019; He et al. 2019). In addition to nitrogen, fluorine is another concern and widely distributed in the Earth’s crust and exists in a number of fluoride rich minerals, such as fluorite (CaF2), fluorapatite (Ca5(PO4)3F), villiaumite (NaF), and topaz (Al2(SiO4)F2), and so on (Adimalla et al. 2019; Adimalla and Wu 2019). Alkaline conditions favor the dissolution of fluoride minerals (Jia et al. 2019). Also, industrial sources, such as coal combustion, brick kilns, aluminum smelting, glass, and coal-based power stations, also can release fluoride into the environment, depositing and entering the water. Therefore, there is also a high fluorine concentration in the study region. The higher the concentration of hazardous substances in drinking water is, the greater the risk of disease on human health is.
The HQtotal limits for non-carcinogenic risk for human health should not exceed 1, so the existence of Cr6+ would merely cause non-carcinogenic risk to children, but no non-carcinogenic risk to adult males and females in the study region. As an aside, the presence of Mn2+ in the study area does not cause non-carcinogenic risks for adults and children.
Additionally, the results of carcinogenic health risk for Cr6+ are shown in the Fig. 5. It can be seen from Fig. 5 that the carcinogenic health risks for adult male, adult female, and children mainly occur in the south of the study region, especially in the mountains in the southeast. Since there is no large industrial distribution in this area, the reason for the high Cr6+ content in the southeast in the study region may be related to the geological environment of the area. Gu et al. (2015) conducted the similar research. Furthermore, the health risk of children is also much higher than males and females, and the distribution area of carcinogenic health risk for children is wider than females and males. It can be seen that although the content of Cr6+ does not exceed the limit (0.05 mg/L) of drinking water, there is also a great carcinogenesis risk for different people groups.
Sustainable Groundwater Quality Management and Possible Options
Groundwater is critical for the life of humans, animals, and plants, especially in areas where surface water is scarce. However, groundwater pollution is getting worse in areas where water treatment procedures are absent (Jia et al. 2019). The results of this study have indicated that the groundwater in the study region that is available for consumption is not totally healthy for humans. Therefore, some possible strategies are recommended to enhance the sustainable groundwater quality management in the study area.
-
In consideration of saving financial, material, and time costs, it is advisable to avoid exploiting high-fluorine and high-nitrogen water sources as much as possible. However, in the long run, the treatment measures, such as distillation and fluoride removal techniques, are necessary.
-
In order to raise residents’ awareness of protecting the water sources, education on water conservation should be carried out. Governments and non-governmental organizations should also take measures to optimize the monitoring network and enhancing cooperation and data sharing to improve the groundwater quality.
-
Experts and scholars who study water resources should also increase their research on water quantity and quality to provide certain help for government decision-making and achieve sustainable development of the earth's water resources.
Conclusions
In this study, 51 groundwater samples were collected and analyzed for various physicochemical parameters to assess the quality using WQI and its health risk using HHRA model. The major conclusions of the study are as follows:
-
(1)
The groundwater is slightly alkaline, and the TDS varied in a wide range of 497.26–2198.82 mg/L. The ionic dominance pattern was in the order of K+ + Na+ > Ca2+ > Mg2+ > NH4+ for cations and HCO3− > SO42+ > Cl− > NO2− > NO3− > CO32− > F− for anions, respectively. HCO3–Na and HCO3–Ca·Mg were the dominant water types, followed by the SO4·Cl–Na. Rock weathering and leaching are major mechanisms that contribute to the “bicarbonate type” groundwater, while rock weathering and evaporation are main mechanisms that govern the “chloride type” water.
-
(2)
According to the water quality index (WQI), groundwater samples suitable for drinking account for 94.12% of the total, while the samples unsuitable for drinking account for 5.88%. The poor and extremely poor water for human consumption are mainly located in the center and northeast of study area.
-
(3)
The assessment of non-carcinogenic risk showed that the risk ranged from 0.0002 to 38.7575 for males and between 0.0002 and 49.2935 for females, with means of 2.6666 and 3.3916, respectively. Also, the risk for children was even greater, ranging from 0.0003 to 84.3167, with a mean of 5.8013. The health risk for children was approximately 2.18 times and 1.71 times higher than that for adult males and females, indicating that children are more susceptible to water contamination. The pollution from nitrogen (NO2− and NO3−) and fluoride was the most serious for human health risks in the study region.
-
(4)
Extensive use of fertilizers, septic tank leakage, and the effluent of organic matter cause the high nitrogen concentration in groundwater for the study region. Also, the dissolution of a large amount of fluoride in the earth's crust in an alkaline environment causes high fluorine concentration of groundwater. This implies that anthropogenic activity and water rock interaction play dominant roles for the high nitrogen and fluorine concentrations. Therefore, there should be steps taken to abolish activities that contribute to this highly nitrogen- and fluorine-contaminated groundwater so the health risks can be lowered in this study region.
References
Abbasi T, Abbasi SA (2012) Water quality indices. Elsevier, New York
Adimalla N, Li P, Venkatayogi S (2018) Hydrogeochemical evaluation of groundwater quality for drinking and irrigation purposes and integrated interpretation with water quality index studies. Environ Process 5(2):363–383
Adimalla N, Qian H (2019a) Hydrogeochemistry and fluoride contamination in the hard rock terrain of central Telangana, India: analyses of its spatial distribution and health risk. SN Appl Sci 1(3):202
Adimalla N, Qian H (2019b) Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, South India. Ecotoxicol Environ Saf 176:153–161
Adimalla N, Wu J (2019) Groundwater quality and associated health risks in a semi-arid region of South India: implication to sustainable groundwater management. Hum Ecol Risk Assess 25(1–2):191–216
Adimalla N, Li P, Qian H (2019) Evaluation of groundwater contamination for fluoride and nitrate in semi-arid region of Nirmal Province, South India: a special emphasis on human health risk assessment (HHRA). Hum Ecol Risk Assess 25(5):1107–1124
Chen J, Wu H, Qian H, Li X (2018) Challenges and prospects of sustainable groundwater management in an agricultural plain along the Silk Road Economic Belt, north-west China. Int J Water Resour Dev 34(3):354–368
Chen J, Huang Q, Lin Y, Fang Y, Qian H, Liu R, Ma H (2019) Hydrogeochemical characteristics and quality assessment of groundwater in an irrigated region, Northwest China. Water 11(1):18
Deng H, Dai D, Li S (2017) Comprehensive operation risk evaluation of overhead transmission line based on hierarchical analysis-entropy weight method. Power System Prot Control 45(1):28–34
Duan Q, Jiao J, Chen X, Wang X (2018) Association between water fluoride and the level of children's intelligence: a dose–response meta-analysis. Public Health 154:87–97
Ganyaglo SY, Gibrilla A, Teye EM, Owusu-Ansah EDGJ, Tettey S, Diabene PY, Asimah S (2019) Groundwater fluoride contamination and probabilistic health risk assessment in fluoride endemic areas of the Upper East Region, Ghana. Chemosphere 233:862–872
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088
Gorgij AD, Wu JH, Moghadam AA (2019) Groundwater quality ranking using the improved entropy TOPSIS method: a case study in Azarshahr plain aquifer, east Azerbaijan, Iran. Hum Ecol Risk Assess 25(1–2):176–190. https://doi.org/10.1080/10807039.2018.1564235
Gu X, Dang X, Yang B, Chang L, Li X, You X, Wang H, Wang Q (2015) Discussion on distribution and source of Cr6 + in groundwater in Wuqi County, Yan'an. Northwestern Geol 48(4):190–203
He JH, Ma JZ, Zhao W, Sun S (2015) Groundwater evolution and recharge determination of the Quaternary aquifer in the Shule River basin, Northwest China. Hydrogeol J 23(8):1745–1759
He S, Li P (2019) A MATLAB based graphical user interface (GUI) for quickly producing widely used hydrogeochemical diagrams. Geochemistry. https://doi.org/10.1016/j.chemer.2019.125550
He S, Wu J (2019) Hydrogeochemical characteristics, groundwater quality, and health risks from hexavalent chromium and nitrate in groundwater of Huanhe formation in Wuqi County, Northwest China. Expo Health 11(2):125–137. https://doi.org/10.1007/s12403-018-0289-7
He X, Wu J, He S (2019) Hydrochemical characteristics and quality evaluation of groundwater in terms of health risks in Luohe aquifer in Wuqi County of the Chinese Loess Plateau, northwest China. Hum Ecol Risk Assess 25(1–2):32–51. https://doi.org/10.1080/10807039.2018.1531693
Iticescu C, Georgescu LP, Murariu G, Topa C, Timofti M, Pintilie V, Arseni M (2019) Lower danube water quality quantified through WQI and multivariate analysis. Water 11(6):20
Jia H, Qian H, Qu W, Zheng L, Feng W, Ren W (2019) Fluoride occurrence and human health risk in drinking water wells from Southern Edge of Chinese Loess Plateau. Int J Environ Res Public Health 16(10):1683. https://doi.org/10.3390/ijerph16101683
Karakus CB (2019) Evaluation of groundwater quality in Sivas province (Turkey) using water quality index and GIS-based analytic hierarchy process. Int J Environ Health Res 29(5):500–519
Khanoranga KS (2019) An assessment of groundwater quality for irrigation and drinking purposes around brick kilns in three districts of Balochistan province, Pakistan, through water quality index and multivariate statistical approaches. J Geochem Explor 197:14–26
Kihumba AM, Longo JN, Vanclooster M (2016) Modelling nitrate pollution pressure using a multivariate statistical approach: the case of Kinshasa groundwater body, Democratic Republic of Congo. Hydrogeol J 24(2):425–437
Lei L, Ju H (2008) Investigation and thoughts on rural drinking water safety in Linwei District. Shaanxi Water Conserv S2:120–121
Li G, Qian H, Zhang X, Guo S, Zhang M (2009) Investigation and analysis of drinking fluorosis in Weinan City. Chin J Endemiol 28:68–69
Li P, Qian H, Wu J (2010) Groundwater quality assessment based on improved water quality index in Pengyang County, Ningxia, Northwest China. E-J Chem 7(S1):S209–S216. https://doi.org/10.1155/2010/451304
Li P, Qian H, Wu J, Chen J (2013a) Sensitivity analysis of TOPSIS method in water quality assessment: I. Sensitivity to the parameter weights. Environ Monit Assess 185:2453–2461. https://doi.org/10.1007/s10661-012-2723-9
Li P, Wu J, Qian H, Chen J (2013b) Sensitivity analysis of TOPSIS method in water quality assessment II: sensitivity to the index input data. Environ Monit Assess 185:2463–2474. https://doi.org/10.1007/s10661-012-2724-8
Li P, Wu J, Qian H, Lyu X, Liu H (2014a) Origin and assessment of groundwater pollution and associated health risk: a case study in an industrial park, northwest China. Environ Geochem Health 36(4):693–712. https://doi.org/10.1007/s10653-013-9590-3
Li P, Qian H, Wu J, Chen J, Zhang Y, Zhang H (2014b) Occurrence and hydrogeochemistry of fluoride in shallow alluvial aquifer of Weihe River, China. Environ Earth Sci 71(7):3133–3145. https://doi.org/10.1007/s12665-013-2691-6
Li P, Qian H, Howard KWF, Wu J (2015) Building a new and sustainable "Silk Road economic belt". Environ Earth Sci 74(10):7267–7270. https://doi.org/10.1007/s12665-015-4739-2
Li P, Wu J, Qian H (2016a) Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: a case study in and around Hua County, China. Arab J Geosci 9(1):15. https://doi.org/10.1007/s12517-015-2059-1
Li P, Wu J, Qian H (2016b) Preliminary assessment of hydraulic connectivity between river water and shallow groundwater and estimation of their transfer rate during dry season in the Shidi River, China. Environ Earth Sci 75(2):99. https://doi.org/10.1007/s12665-015-4949-7
Li P, Li X, Meng X, Li M, Zhang Y (2016c) Appraising groundwater quality and health risks from contamination in a Semiarid Region of Northwest China. Expos Health 8(3):361–379. https://doi.org/10.1007/s12403-016-0205-y
Li P, Tian R, Xue C, Wu J (2017a) Progress, opportunities and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environ Sci Pollut Res 24(15):13224–13234. https://doi.org/10.1007/s11356-017-8753-7
Li P, Feng W, Xue C, Tian R, Wang S (2017b) Spatiotemporal variability of contaminants in lake water and their risks to human health: a case study of the Shahu Lake tourist area, northwest China. Expo Health 9(3):213–225. https://doi.org/10.1007/s12403-016-0237-3
Li P, He S, Yang N, Xiang G (2018a) Groundwater quality assessment for domestic and agricultural purposes in Yan’an City, northwest China: implications to sustainable groundwater quality management on the Loess Plateau. Environ Earth Sci 77(23):775. https://doi.org/10.1007/s12665-018-7968-3
Li P, Qian H, Wu J (2018b) Conjunctive use of groundwater and surface water to reduce soil salinization in the Yinchuan Plain, North-West China. Int J Water Resour Dev 34(3):337–353. https://doi.org/10.1080/07900627.2018.1443059
Li P, He S, He X, Tian R (2018c) Seasonal hydrochemical characterization and groundwater quality delineation based on matter element extension analysis in a Paper Wastewater Irrigation Area, Northwest China. Expos Health 10(4):241–258. https://doi.org/10.1007/s12403-17-0258-6
Li P, Wu J, Tian R, He S, He X, Xue C, Zhang K (2018d) Geochemistry, hydraulic connectivity and quality appraisal of multilayered groundwater in the Hongdunzi Coal Mine, Northwest China. Mine Water Environ 37(2):222–237. https://doi.org/10.1007/s10230-017-0507-8
Li P, He X, Li Y, Xiang G (2019a) Occurrence and health implication of fluoride in groundwater of loess aquifer in the Chinese Loess Plateau: a case study of Tongchuan, Northwest China. Expos Health 11(2):95–107. https://doi.org/10.1007/s12403-018-0278-x
Li P, He X, Guo W (2019b) Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: a case study in Yan’an City on the Loess Plateau of northwest China. Hum Ecol Risk Assess 25(1–2):11–31. https://doi.org/10.1080/10807039.2018.1553612
Liu R (2009) Study on transference and transform simulation of fluoride in groundwater and relation between fluoride and human body health in Dali Region, Guanzhong Basin. Chang’an University, Xi’an (in Chinese)
Lu S, Shang Y, Li W (2019) Assessment of the Tarim River basin water resources sustainable utilization based on entropy weight set pair theory. Water Sci Technol Water Supply 19(3):908–917
Luo K, Zhang S, Tian Y, Gao X (2014) Arsenic distribution pattern in different sources of drinking water and their geological background in Guanzhong Basin, Shaanxi, China. Acta Geol Sin Engl Ed 88(3):984–994
Ministry of Health of the P.R. China, S. A. o. t. P. R. C (2006) Standards for drinking water quality (GB 5749–2006). China Standard Press, Beijing (in Chinese)
Mukate S, Wagh V, Panaskar D, Jacobs JA, Sawant A (2019) Development of new integrated water quality index (IWQI) model to evaluate the drinking suitability of water. Ecol Ind 101:348–354
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Eos Trans Am Geophys Union 25(6):914–928
Qiu J (2010) China faces up to groundwater crisis. Nature 466(7304):308–308
Quan J (2018) Status of fertilizer application in Weinan City and countermeasures to achieve zero growth. Agric Sci Technol Newslett 3:39–40 (in Chinese)
Rasool A, Farooqi A, Masood S, Hussain K (2016) Arsenic in groundwater and its health risk assessment in drinking water of Mailsi, Punjab, Pakistan. Hum Ecol Risk Assess 22(1):187–202
Rezaei H, Jafari A, Kamarehie B, Fakhri Y, Ghaderpoury A, Karami MA, Ghaderpoori M, Shams M, Bidarpoor F, Salimi M (2019) Health-risk assessment related to the fluoride, nitrate, and nitrite in the drinking water in the Sanandaj, Kurdistan County, Iran. Hum Ecol Risk Assess 25(5):1242–1250
Su F, Wu J, He S (2019) Set pair analysis-Markov chain model for groundwater quality assessment and prediction: a case study of Xi’an City, China. Hum Ecol Risk Assess 25(1–2):158–175. https://doi.org/10.1080/10807039.2019.1568860
Tian R, Wu J (2019) Groundwater quality appraisal by improved set pair analysis with game theory weightage and health risk estimation of contaminants for Xuecha drinking water source in a loess area in Northwest China. Hum Ecol Risk Assess 25(1–2):132–157. https://doi.org/10.1080/10807039.2019.1573035
Wang S, Huang T, Chen H, Liu M, Xue H (2018) Application of fuzzy comprehensive evaluation model based CRITIC weighting in water quality evaluation. Hydropower Energy Sci 36(06):48–51
WHO (2008). World Health Organisation Guidelines for Drinking Water Quality, third ed. 20 Avenue Appia, 1211 Geneva 1227, Switzerland
WHO (2011). World Health Organisation Guidelines for Drinking Water Quality, 4rd ed. Incorporating the First and Second Addenda, vol 1. Recommendation, Geneva
Wu J, Sun Z (2016) Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Expos Health 8(3):311–329. https://doi.org/10.1007/s12403-015-0170-x
Wu J, Wang L, Wang S, Tian R, Xue C, Feng W, Li Y (2017) Spatiotemporal variation of groundwater quality in an arid area experiencing long-term paper wastewater irrigation, northwest China. Environ Earth Sci 76(13):460. https://doi.org/10.1007/s12665-017-6787-2
Wu J, Zhou H, He S, Zhang Y (2019) Comprehensive understanding of groundwater quality for domestic and agricultural purposes in terms of health risks in a coal mine area of the Ordos basin, north of the Chinese Loess Plateau. Environ Earth Sci 78(15):446. https://doi.org/10.1007/s12665-019-8471-1
Xu P, Feng W, Qian H, Zhang Q (2019a) Hydrogeochemical characterization and irrigation quality assessment of shallow groundwater in the Central-Western Guanzhong Basin, China. Int J Environ Res Public Health 16(9):18
Xu P, Zhang Q, Qian H, Li M, Hou K (2019b) Characterization of geothermal water in the piedmont region of Qinling Mountains and Lantian-Bahe Group in Guanzhong Basin, China. Environ Earth Sci 78(15):17
Yu C, Gong P, Yin Y (2011) China's water crisis needs more than words. Nature 470(7334):307–307
Yu S, Liu H, Bai L, Han F (2019) Study on the suitability of passive energy in public institutions in China. Energies 12(12):2446
Zhang P (2009) Rural drinking water safety engineering technology and analysis in Linwei District. Shaanxi Water Conserv 4:119–120
Zhang R (2017) Groundwater status and evaluation of over-exploitation area in Shaanxi Province. Groundwater 39(04):73–76
Zhang Q, Xu P, Qian H (2019) Assessment of groundwater quality and human health risk (HHR) evaluation of nitrate in the Central-Western Guanzhong Basin, China. Int J Environ Res Public Health 16(21):4246
Zhang Y, Wu J, Xu B (2018) Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest China. Environ Earth Sci 77(7):273. https://doi.org/10.1007/s12665-018-7456-9
Zotou I, Tsihrintzis VA, Gikas GD (2019) Performance of seven water quality indices (WQIs) in a Mediterranean River. Environ Monit Assess 191(8):505
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Grant Nos. 41572236 and 41931285). And the completion of this article was inseparable from the contributions of all authors. Their support is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Q., Xu, P. & Qian, H. Groundwater Quality Assessment Using Improved Water Quality Index (WQI) and Human Health Risk (HHR) Evaluation in a Semi-arid Region of Northwest China. Expo Health 12, 487–500 (2020). https://doi.org/10.1007/s12403-020-00345-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-020-00345-w