Abstract
Bacterial communities play critical roles in biogeochemical cycles and serve as sensitive indicators of environmental fluctuation. However, the influence of mineral resource exploitation on shaping the bacterial communities in the urban river is still ambiguous. In this study, high-throughput sequencing was used to determine the spatial distribution of the sediment bacterial communities along an urban river in the famous mining city Panzhihua of China. The results showed that mineral resource exploitation had a significant impact on the urban river bacterial community structure but not on the bacterial ecological functions. Distinct families of bacteria often associated with nutrients (i.e., Comamonadaceae and Sphingomonadaceae) and metal contaminants (i.e., Rhodobacteraceae) were more predominant in the residential and mining area, respectively. Relative to dispersal dynamics, environmentally induced species sorting may primarily influence bacterial community structure. Heavy metals and sediment physicochemical properties had both similar and significant influence on shaping bacterial community structure. Among heavy metals, essential metal elements explained more rates of bacterial variation than toxic metals at moderate contaminant levels. Moreover, the bacteria with multiple metal resistances identified in culture-dependent experiments were probably not suitable for indicating heavy metal contamination in field research. Thus, several sensitive bacterial genera such as Rhodobacter, Hylemonella, and Dechloromonas were identified as potential bioindicators to monitor metals (iron and titanium) and nutrients (phosphorus and organic carbon) in the river ecosystem of the Panzhihua region. Together, these results profiled the coupling effect of urbanization and mineral resource utilization on shaping sediment bacterial communities in urban rivers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rivers and mineral resources are closely associated with the development of civilization (Jing et al. 1997; Rickard 1934). Many famous cities such as Pittsburgh in the USA, Dortmund in Germany, and Panzhihua in China are perched on riversides and have abundant mineral resources. The mineral resources are the basis of economic development whereas the rivers flowing through these cities are important sources of drinking water for citizens, but the rapid urbanization in mining cities may cause environmental pollution and geological hazards, which in turn, make the urban river a sink for municipal sewage and mining wastewater. Bacterial communities are vital components of biogeochemical processes and food webs in the sediments of river ecosystems (Fernandez-Gonzalez et al. 2016). As the sink and source for chemicals and bacteria, sediments always contain most of contaminant and bacterial biomass in the urban river (Fischer and Pusch 1999). Previous studies indicated that sediment bacterial communities in urban rivers are subjected to anthropogenic stress during rapid urbanization (Gourvendu et al. 2015) because urban effluents like stormwater and sewage are continually discharged into the river (Paul and Meyer 2008). Urban effluents contaminate river water with nutrients, organic pollutants, suspended solids, and metals (Lewis et al. 2007; Wenger et al. 2009), and consequently alter sediment bacterial communities (Roberto et al. 2018). River water deterioration may be especially severe in mining city because the rivers receive not only urban discharges but also a large amount of metal inputs (Cao et al. 2017). However, there is no consensus as to how sediment bacterial communities respond and resilient to the multiple anthropogenic stressors in the urban rivers of mining cities (Sun et al. 2013).
Bacterial communities are extremely sensitive to environmental disturbances and spatial variations in freshwater sediments (Gibbons et al. 2014; Ibekwe et al. 2016). Geographic distance (Isabwe et al. 2018) and environmental factors such as hydrology (Roberto et al. 2018), nutrient levels (Wang et al. 2019), and heavy metals (Jroundi et al. 2020) are the major factors influencing bacterial communities. However, how bacterial communities are assembled under multiple disturbances in urban rivers still remains unclear (Roberto et al. 2018). Among the mentioned factors, heavy metals are always considered as toxic to microorganisms and can damage ecosystems, but some metals are essential for several cellular functions (Seiler and Berendonk 2012). In many cases, high concentrations of heavy metals may adversely affect bacterial communities (Li et al. 2021; Zhang et al. 2015). In contrast, some metals may promote microbial growth and increase bacterial diversity (Jingxin et al. 2013). Those contrasting conclusions shadowed the knowledge on effect of heavy metals on bacterial communities in river sediment. Therefore, an attempt to determine the how heavy metals influence sediment bacterial community has also been emphasized.
The variations of metal resistance bacterial are useful for monitoring heavy metal pollution in freshwater sediments because bacterial community function is a better indicator of environmental perturbation than bacterial community structure (Burke et al. 2011; Steffen et al. 2012). Since culture-dependent methods can more accurately represent physiological state of bacteria than culture-independent methods (Ellis et al. 2003), metal-resistant bacteria are usually identified by culture-dependent tests. However, it is unlikely that the metal-resistant bacteria found in laboratory studies could survive under field conditions. For this reason, metal resistance bacteria reported in the literature must be verified under field conditions. If these metal resistance bacteria fail to monitor heavy metal pollutants, some other sensitive candidate bacteria should be identified as potential indicators to monitor heavy metal contaminants.
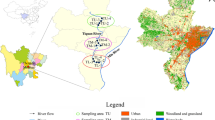
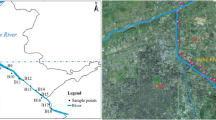
Panzhihua is a famous mining city located along the Jinsha River in Sichuan Province, China. It has abundant mineral resources and accounts for 20% of the iron, 64% of the vanadium, and 53% of the titanium supply in China (Cao et al. 2017). However, its topography interlaces residential area with the mining industry (Teng et al. 2011). This configuration may increase potential human health risks by contaminating the drinking water with heavy metals discharged from the mining areas. Furthermore, the Jinsha River is the upper reach of the Yangtze River, a major source of fresh water in China (Wang et al. 2015). Heavy metal accumulation in the Jinsha River will probably deteriorate the water quality and alter the bacterial community structure downstream of the Yangtze River, which might pose potential risk for both public health and ecosystems in China. Therefore, it is necessary to understand how the sediment bacterial community shifts along the urban river in Panzhihua and to identify sensitive bacteria which can monitor heavy metal contamination. It is hypothesized that high input of nutrient elements and heavy metals from various pollutant sources associated with mining city are important impetus for shifting sediment bacterial community. In this study, we investigated the following: (1) the alterations in the Jinsha River sediment bacterial community caused by inputs from Panzhihua city; (2) the major factors influencing dynamic changes in the sediment bacterial community, and (3) the feasibility of certain sensitive bacterial genera as bioindicators of heavy metals and nutrient contamination.
Materials and methods
Study area and sampling procedure
The Panzhihua region is located in Sichuan Province, China, along the middle stream of the Jinsha River. In this study, sediment samples were collected from eight sites in the Panzhihua region along the Jinsha River (Fig. 1). To minimize variance, three sediment samples were collected within meters of each other at each sampling site along the river. PZH-1 and PZH-3 are pristine sites. PZH-2, PZH-4, and PZH-5 represent residential areas. Within residential areas, PZH-2 is downstream from a village in the rural area. PZH-4 and PZH-5 are located in the urban area. PZH-6 is located in an area affected by both residential effluents and mining industry discharges (hereafter, the comprehensive area). PZH-7 and PZH-8 are influenced by V-Ti and Cu mining industry, respectively.
Each sediment sample was collected at 0 ~ 6 cm depth with an Ekman-Brige grab sampler (Hydro-Bios, Altenholz, Germany) then homogenized. For the sediment physicochemical characteristics and heavy metal analyses, 500 g of homogenized sediment was stored at − 20 °C before returned to the laboratory. Another 50 g of homogenized sediment was stored at − 20 °C in the field to acquire bacterial DNA. Samples were maintained at − 80 °C in the laboratory before DNA extraction.
Physicochemical characteristics and heavy metal analysis
Sampling location data were obtained with a GPS device (GPSMAP 62s; Garmin Ltd., Olathe, KS, USA) during filed sampling. In the laboratory, all sediment samples were freeze-dried using a lyophilizer (Labconco, Kansas City, MO, USA) for environmental factor determinations. Electrical conductivity (EC) and pH were measured for 1:5 sediment: water suspensions with a multiparameter water quality analyzer (HQ40d; Hach, Loveland, CO, USA). Total nitrogen (TN) and total phosphorus (TP) were determined by the Kjeldahl and sulfuric acid-perchloric acid oxidation methods, respectively (Ebina et al. 1983). Ammonium nitrogen (NH4) was extracted with 2 M KCl and then measured with a flow injection autoanalyzer (Lachat QC8500; Lachat Instruments, Loveland, CO, USA). Available phosphorus (AP) was extracted with NaHCO3 and measured by the molybdenum blue method in a spectrophotometer (Persee, Beijing, China). Total organic carbon (TOC) was measured by an Elementar Liqui TOCII analyzer (Frankfurt, Germany).
Heavy metals (Hg, Cu, Cd, Pb, V, Fe, and Ti) in the sediment were digested with concentrated hydrofluoric acid and determined by inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7500 series, USA) as described in the previous study (Gillan et al. 2015). Heavy metal pollution in the river sediment was defined using geoaccumulation and potential ecological risk indices in order to profile the pollution condition of heavy metals properly (Fu et al. 2009; Zhang et al. 2016). Heavy metal pollution in each area was represented by the average geoaccumulation and potential ecological risk indices of their sampling sites. The methods of these pollution assessments were presented in Text. S1 in the Supplementary material.
DNA extraction, high-throughput sequencing, and metal-resistant bacteria identification
DNA was extracted from 0.5 g samples using a PowerSoil DNA extraction kit (Mo Bio, Carlsbad, CA, USA) following the manufacturer’s instructions. Agarose gel electrophoresis (1%) and spectrophotometry (NanoDrop ND 2000, Thermo Scientific, DE, USA) were applied to verify the quality and quantity of extracted DNA.
The V4 region of 16S rRNA was amplified as follows: 94 °C for 5 min, 31 cycles at 94 °C for 30 s, 52 °C for 30 s, 72 °C for 45 s, and a final extension at 72 °C for 10 min. Universal primers were 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Triplicate PCRs were conducted for all samples to minimize potential bias and were combined equally for each sample. Amplicons were measured by the PicoGreen dsDNA assay kit (Invitrogen, Carlsbad, CA, USA).
The PCR product was sequenced on the Illumina Hiseq 2000 platform (Illumina, Inc., San Diego, CA, USA). Data processing was conducted according to a previous study (Li et al. 2017). Briefly, the raw sequences were trimmed to remove low-quality reads (Trimmomatic, version 0.33). Forward- and reverse-end sequences were attributed to their corresponding samples by MOTHUR (version 1.35.1) and were matched using FLASH (version 1.2.11). The matched reads containing an “N” or shorter than 200 bp were removed. Chimeras were discarded via the UCHIME algorithm in USEARCH. The filtered sequences were clustered into operational taxonomic units (OTUs) at 97% identification with the UPARSE algorithm in USEARCH (version 8.0.1517). The OTUs were then assigned taxonomically by aligning representative sequences to the Greengenes database (version 13.5) with the Ribosomal Database Project (RDP) Classifier (Cole et al. 2009).
Metal-resistant bacteria were identified from the literature based on the results of high-throughput sequencing (Mejias Carpio et al. 2018), which including 6 common genera of metal-resistant bacteria Bacillus, Cupriavidus, Ochrobactrum, Paenibacillus, Pseudomonas, and Ralstonia revealed by culture-independent methods in previous studies.
Bioinformatics analysis
The bioinformatics analysis was conducted based on the sequence data of bacterial community. Alpha-diversity of the bacterial community was evaluated by the Chao1 richness and Shannon diversity indices, which were calculated by QIIME (version 1.8.0) (Caporaso et al. 2010). The hierarchical clustering method UPGMA was used to present phylogenetic structure of the bacterial communities based on bacterial abundance-unweighted UniFrac distances. Analysis of similarities (ANOSIM) was performed to identify whether bacterial community structures significantly differed among areas. PICRUSt (Langille et al. 2013) was applied to predict bacterial community functions in the river sediment. It used the associations between the 16S rRNA markers found in the Greengenes database and the functional genes found in the Kyoto Encyclopedia of Genes and Genomes (KEGG) database to reconstruct the potential functional genes in the samples (Roberto et al. 2018). Canonical correspondence analysis (CCA), variance partitioning analysis (VPA), and multivariate regression tree (MRT) were applied to reveal significant drivers influencing bacterial community. CCA was applied to calculate the influence of variation on the shift in bacterial community structure and tested by Monte Carlo permutation. VPA was conducted to compare the effect of geographic distance vs. local environmental condition as well as physicochemical characteristics vs. heavy metal based on CCA analysis. MRT was conducted to cluster bacterial community data by environmental condition and identify the crucial environmental factors that best impacted the bacterial community. A network was constructed with Cytoscape v. 3 (Saito et al. 2012) to visualize the correlations between bacterial taxa and environmental factors. The random matrix theory-based method was used to automatically define the threshold and Pearson correlation network were constructed based on bacterial genera level. (Ding et al. 2015; Xie et al. 2016). In addition, environmental parameters were tested for their associations and significances of the observed variations by Pearson correlation analysis and one-way ANOVA, respectively. All statistical analyses were conducted in the R packages vegan and mvpart (version 3.1.2, R Foundation for Statistical Computing, Vienna, Austria).
Results
Sediment physicochemical characteristics and heavy metal pollution
Sediment physicochemical properties (pH, EC, TOC, TN, NH4, TP, and AP) are listed in Table S1 in the Supplemental Files. Both pH and EC were higher in the pristine site than in the other areas. TN was significantly lower in the mining area than in the other areas (P < 0.05). Minimum NH4 was measured for the pristine site and was relatively higher for the comprehensive area and the mining area. In contrast, TP was the highest for the mining area and significantly lower for the pristine site (P < 0.05). The pristine site had the highest AP of all. TOC was comparatively higher in the pristine site and ranged from 2.61–29.22 mg kg-1.
Hg, Cu, Cd, Pb, V, Fe, and Ti were profiled by the geoaccumulation index (Igeo) shown in Fig. S1A. The geoaccumulation indices of all metals were < 1 in the pristine and residential areas. The highest accumulation level was measured for Ti in the Panzhihua region especially in the comprehensive area and the mining area. According to the geoaccumulation indices, all areas ranged from uncontaminated to moderately contaminated with Hg, Cu, Cd, Pb, and Fe. Moreover, the levels of Ti, Fe, and V were significantly correlated along the river (r > 0.7; P < 0.001). The ecological risk indices of heavy metal along the Jinsha River around the Panzhihua region were shown in Fig. S1B. The toxicities of Hg and Cd were the highest of all the metals analyzed because they are inherently highly toxic. Their potential ecological risk index was the highest in the comprehensive area, and even in this area, the ecological risks of Hg and Cd were moderate.
Bacterial community diversity, structure, and ecological function affected by the mining city
The high-quality sequences sampled from all sediments ranged from 55,286–76,925 (Table S2 in the Supplemental Files). The OTUs ranged from 1342–5287 in all samples and the average was 3120. PZH-5 had the maximum OTUs and was located in the residential quarters. The α-diversity was calculated based on the 55,286 reads randomly selected from each sample to minimize the effect of uneven sampling on the analysis. According to the ANOVA, both the Chao1 richness and Shannon diversity indices were significantly lower in the pristine site than in the other area (P < 0.05).
The ANOSIM indicated that the bacterial community structures in the pristine and residential areas were significantly different with other areas (Table S3). The bacterial community structure in the comprehensive area more nearly resembled that of the mining area than the other areas. UPGMA was applied to determine the phylogenetic structures of the bacterial communities (Fig. 2a). It clustered the sampling sites into three groups: PZH-1 and PZH-3 were clustered into the first group representing the pristine site; PZH-2, PZH-4, and PZH-5 were clustered into the second group and comprised the residential area; and PZH-6, PZH-7, and PZH-8 were clustered into the third group and constituted the comprehensive and mining areas.
The taxonomic distributions of all samples are illustrated in Fig. 2a. Proteobacteria was the dominant phylum in the bacterial community, which accounted for 28.51–57.55% of the total bacteria (average: 43.36%). The second dominant phylum was Firmicutes (20.15%). Bacteroidetes (11.59%), Actinobacteria (8.67%), and Verrucomicrobia (8.48%) were the other major phyla in the community at relative abundances > 5%. The bacterial communities in pristine sites consisted mainly of Proteobacteria and Firmicutes (> 90%). In the mining area, the proportion of Bacteroidetes was higher than other areas. The relative abundance of Actinobacteria was the highest for the residential area, especially at PZH-2 (downstream from the village in the rural area). The relative abundance of Verrucomicrobia was the highest for PZH-6, which was affected both by residential effluents and mining industry discharges.
The most abundant bacterial family in all areas was the Exiguobacteraceae (Firmicutes) and followed by the Moraxellaceae (Proteobacteria). The Exiguobacteraceae and the Moraxellaceae comprised 18.16% and 10.66% of the total bacterial community, respectively (Fig. 2c). Other bacterial families with relative abundances > 1% were the Xanthomonadaceae, Pseudomonadaceae, Enterobacteriaceae, Oxalobacteraceae, Comamonadaceae, Sphingomonadaceae, Erythrobacteraceae, Rhodobacteraceae, Caulobacteraceae (Proteobacteria), [Weeksellaceae], Cytophagaceae, Chitinophagaceae (Bacteroidetes), Pirellulaceae (Planctomycetes), Nocardioidaceae, Micrococcaceae (Actinobacteria), Deinococcaceae ([Thermi]), and Verrucomicrobiaceae (Verrucomicrobia). The proportion of Proteobacteria was high in the residential area and pristine site but it consisted of a greater number of distinct families and the Xanthomonadaceae, Oxalobacteraceae, Comamonadaceae, Sphingomonadaceae, Erythrobacteraceae, Rhodobacteraceae, and Caulobacteraceae were higher in residential area whereas Moraxellaceae, Pseudomonadaceae, and Enterobacteriaceae were peaked in pristine site. The Pirellulaceae (Planctomycetes), Nocardioidaceae, and Micrococcaceae (Actinobacteria) also had the highest relative abundances in the residential areas. The Verrucomicrobiaceae (of the Verrucomicrobia) were the most abundant in the comprehensive area. The Deinococcaceae ([Thermi]), Cytophagaceae, and Chitinophagaceae (Bacteroidetes) had the highest relative abundances in the mining area.
The major functional groups included related cellular processes, environmental information processing, genetic information processing, human diseases, metabolism, and organismal systems, and were investigated with a PICRUSt analysis (Fig. 2b). In all samples, the genes associated with metabolism were more abundant than those for all other genes. There were no significant differences among the areas in terms of functional groups.
Effect of environmental parameters on bacterial community
Bacterial community diversity was more highly correlated with physicochemical characteristics than with heavy metals. The Pearson correlation analysis indicated that both the Chao1 richness and Shannon diversity indices were significantly negatively correlated with TOC (r = − 0.672, P < 0.001; r = − 0.733, P < 0.001) and positively correlated with TP (r = 0.682, P < 0.001; r = 0.694, P < 0.001). No significant correlation was observed between bacterial diversity and heavy metals.
The influences of environmental parameters (including heavy metals and physicochemical characteristics) and spatial distance on the bacterial community structure were determined by CCA. The environmental parameters (89.21%) more strongly influenced the bacterial community than the geographic distance (19.63%) along the urban river (Fig 4a). Among the environmental parameters, heavy metals and physicochemical characteristics had similar and strong influences on bacterial community composition and explained 69.31% and 70.69% of the variation, respectively (Fig 4b). Fe and TOC significantly influenced changes in the bacterial community structure and accounted for 22.95% (P = 0.001) and 21.00% (P = 0.001) of the variation, respectively (Table 1). The MRT analysis was conducted to evaluate the impact of the environmental parameters on the bacterial community structure. As illustrated in Fig. 3b, the MRT was formed by three splits and four leaves according to the sediment environmental factors. The 24 samples were clustered by TOC into two groups. Then, the group with TOC < 15.51 g kg-1 was further split by Fe concentration. Samples with Fe < 9.505 gkg-1 were further separated by Ti concentration (2.055 g kg-1). Therefore, Fe and TOC were the key factors influencing the bacterial community structure.
Relative influences of determined factors on bacterial community structure according to VPA (a and b) and MRT (c). VPA is conducted to compare the effect of geographic distance vs. local environmental parameters (a) as well as physicochemical characteristics vs. heavy metals (b) based on CCA analysis. HM stands for heavy metals while PC stands for physicochemical characteristics. Each split in MRT (c) is represented graphically as a branch in the tree. Bar plots show the multivariate means of different OTUs at each branch. CV Error stands for cross-validated relative error while SE stands for standard error
Moreover, the heavy metals with relatively greater potential ecological risk (Hg and Cd) explained a comparatively lower percentage of the variation in the bacterial communities than other metals. The relationship between the percentage explanation of bacterial community variation and heavy metal toxicity was also evaluated by a Pearson correlation analysis. The explanation percentage of bacterial community variation was significantly negatively correlated with potential ecological risk index (r = − 0.723, P < 0.05).
Correlation network of sensitive bacteria to environmental variation
Seven metal-resistant bacterial genera reported in the literature were screened by Next Generation Sequencing. These included Bacillus, Cupriavidus, Klebsiella, Ochrobactrum, Paenibacillus, Pseudomonas, and Ralstonia. According to the results, 6 genera metal resistance bacteria were identified in the sediment of the Panzhihua region along the Jinsha River, and Bacillus, Pseudomonas, and Ralstonia occurred in every sample (Table S4 in the Supplemental Files). Metal-resistant bacteria constituted only 1% of the total bacterial community in all sediments, and the mining area harbored the lowest amount of these metal-resistant bacteria in the Panzhihua region. Bacillus was the dominant metal-resistant bacterial genus, accounting for 56.93% of the total population of metal-resistant bacteria. The relative abundance of Bacillus was higher in the pristine and comprehensive areas than the other areas. Pseudomonas and Ralstonia also comprised an important fraction of these heavy metal-resistant bacteria in the Panzhihua region. The relative abundance of Pseudomonas and Ralstonia was the highest in the pristine and residential areas, respectively. Moreover, no significant correlation was observed between the potential ecological risk index and metal-resistant bacterial population in the sediment along the Jinsha River.
Network analyses were conducted to determine the correlations between individual bacteria and environmental variables. It is suggested that metals (Fe and Ti) and nutrients (NH4, TOC, and TP) were the main factors influencing bacterial community. Fe, Ti, NH4, and TP were positively correlated with most of the bacteria in the sediment of the Panzhihua region, while TOC was negatively correlated with them (Fig. 4a). The abundances of Rhodobacter, Hylemonella, and Dechloromonas were directly correlated with these environmental variables. Rhodobacter was negatively correlated with TOC and positively correlated with Fe, Ti, and TP. Hylemonella was negatively correlated with TOC and positively correlated with Fe and TP. Dechloromonas was strongly negatively correlated with TOC and strongly positively correlated with Fe and Ti. Since that Fe had the strongest impacts on the bacterial community, it was solely used to run the network analysis with the bacterial community. Figure 4b shows that Fe was a hub node directly connected by 15 bacterial nodes. Fe and most of these bacterial nodes presented with the highest connectivity in the system. Therefore, Fe and its directly connected nodes played important roles in maintaining the network structure.
Network interaction analysis between the bacterial genera community and environmental factors (a) and Fe alone (b). In figure (a), yellow nodes stood for environmental factors, green nodes stood for the bacterial genera which have directly correction with more than on environmental factor, and blue nodes stood for bacterial genera indirectly connected with environmental variables. Red line represented negative correlation and green line represented positive correlation. The diameter of nodes indicated connectivity between bacterial genera community and environmental factors. In figure (b), red nodes stood for Fe, green nodes stood for the bacterial genera which have directly correction with more than on environmental factor, and purple nodes stood for bacterial genera indirectly connected with environmental variables. The diameter of nodes directly corrected to Fe indicated connectivity between bacterial genera community and Fe while the diameter of nodes indirectly corrected to Fe was meaningless
Discussion
Composition of the sediment bacterial community along the urban river in the mining area
The sediment bacterial community was detected to have significant differences in terms of diversity and taxonomic composition along the river (Ibekwe et al. 2012; Xia et al. 2013). However, it was largely unknown how bacterial communities shift along urban rivers in mining cities. In the present study, relatively higher bacterial diversity was detected in the anthropogenically disturbed areas than the pristine areas. This might due to the oligotrophic bacteria are dominant in river sediment of pristine areas and these oligotrophic bacteria could not prevail in anthropogenically disturbed and eutrophic areas, while other bacterial taxa capable of using these resources, which leads to the prevalence of more diverse bacteria. This result is not in consistent with the previous studies reported that the lowest bacterial diversity was associated with the sediment in urban runoff (Wang et al. 2018). In our opinion, this observation may be explained by the fact that the region they investigated was located in a megalopolis with extremely high pollutant levels and only the bacteria with high tolerance of contamination can survive in these conditions (Chen et al. 2018). In contrast, the contaminant concentrations in Panzhihua City were comparatively moderate (Teng et al. 2011).
Moreover, the bacterial community structures in the various areas of the Panzhihua region were significantly different from each other based ANOSIM and UPGMA analysis, except that no significant different was observed between comprehensive and mining areas. This result indicated that the sediment bacterial community structure in the urban river was significantly altered by the mining city. The sediment bacterial community in Panzhihua City is predominated by Proteobacteria, which agrees with previous studies conducted in several urban rivers (Gibbons et al. 2014; Xie et al. 2016). Both the pristine and residential areas harbored relatively high abundances of Proteobacteria but the compositions of their proteobacterial families were different. The proteobacterial families mostly known for dominating in oligotrophic environments (Moraxellaceae and Enterobacteriaceae) were found in high proportions in the pristine sites. On the other hand, the eutrophic proteobacterial families, including Xanthomonadaceae, Oxalobacteraceae, Comamonadaceae, Sphingomonadaceae, Erythrobacteraceae, Rhodobacteraceae, and Caulobacteraceae (Tang et al. 2017; Vetterli et al. 2015), were comparatively more abundant in the residential area. Therefore, these bacterial families may serve as urbanization bioindicators. With the increase of eutrophication, Firmicutes (mostly Exiguobacteraceae) could not thrive in other areas than pristine areas (Liu et al. 2018b). Consequently, other phyla could flourish in these regions along the river (Li et al. 2011). Families within the Acidobacteria prevail in a wide range of contaminated regions (Debarati et al. 2010; Sanchez-Peinado et al. 2010) whereas most Bacteroidete families are resistant to organic pollutants and heavy metals (Chen et al. 2018; Obernosterer et al. 2011). Therefore, the dominance of Acidobacteria and Bacteroidetes in the residential and mining areas reflects the diversity of pollutant sources in residential areas and heavy metal contamination in mining areas, respectively. The higher abundance of Verrucomicrobiaceae (Verrucomicrobia) in the comprehensive areas may indicate that the Verrucomicrobiaceae remain competitive under multiple anthropogenic contaminants including nutrients and heavy metals. The fact that different bacterial families prevailed in each area may reflect distinct contaminant sources in each case. The residential area concentrated industrial contaminants and other chemicals from WWTP effluent and storm drain runoff from residential areas (Roberto et al. 2018). In contrast, the mining areas were impacted primarily by mining industry discharges. The comprehensive area was affected by all the mentioned pollution sources. Mining industry discharges have a relatively stronger impact on bacterial community structure in the comprehensive area because it clustered into the same group with the mining areas rather than the group including the residential areas.
Unlike bacterial diversity and community structure, bacterial community function was not significantly affected by the mining city. Some bacterial groups may have served as seed banks in the environment and helped stabilized the ecosystem by facilitating functional redundancy (Loreau et al. 2001). As environmental conditions change, bacteria with relatively lower tolerance for the new condition revert to dormancy (Cardinale et al. 2012). Conversely, the functionally complimentary groups proliferate and have minimal impact on ecological function (Jung et al. 2016). Therefore, the adaptation of local bacterial communities to alterations in environmental conditions may be independent of bacterial functional change (Bier et al. 2014; Fernandez-Gonzalez et al. 2016).
Vital factors influencing the sediment bacterial community along the urban river
There is no consensus on the vital factors influence sediment bacterial community composition. For this reason, the effects of multiple factors (physicochemical properties, heavy metals, and geographic location) were investigated on sediment bacterial communities. In the present study, the bacterial communities located at adjacent geographical positions were not clustered into the same group according to the UPGMA. For instance, the bacterial community impacted by resident in the rural area (PZH-2) had a structure more alike the distant sites in the residential quarter of the urban area (PZH-4 and PZH-5), rather than the adjacent pristine sites in the rural area (PZH-1 and PZH-3). On the other hand, environmental stressors accounted for a larger proportion of the bacterial community structure variation (89.21%) than the river length or the geographical position (21.06%). Therefore, the shift of the bacterial community structure along the river may follow environmental stressors rather than spatial distance in the local scale, which corroborates several previous studies (Logue and Lindström 2010; Staley et al. 2015; Winter et al. 2013).
Sediment bacterial communities may be directly or indirectly affected by environmental stressors (Chen et al. 2018; Li et al. 2017). In this study, the effects of metals and physicochemical characteristics on bacterial community structure were evaluated simultaneously. In general, both heavy metals and physicochemical characteristics explained high and nearly equal levels of the variation in bacterial community structure (69.31% and 70.69%, respectively). Metals with relatively low toxicity (Fe and Ti) and nutrients (TOC and TP) most strongly influenced bacterial community structure. Nutrients are essential environmental factors that significantly affect bacterial community structure because they provide necessary materials for the growth of river microorganisms (Davidson et al. 2007). In this study, TOC was the nutrient with the most powerful influence on bacterial community structure. Large amounts of carbon are discharged from terrestrial to aquatic ecosystems (Jones et al. 2010). In the sediment, carbon is the main energy source for heterotrophic bacteria (Kritzberg et al. 2004). Increases in TOC are often correlated with rises in cellulolytic and chitinolytic activities and may further alter bacterial metabolism (Larson et al. 2002; Phillips et al. 2002). High inputs of TOC in the sediment may favor the proliferation of microorganisms which degrade carbon polymers (He et al. 2012). Under these conditions, the heterotrophic bacteria may effectively compete with autotrophs like Chloroflexus and cyanobacteria (Celine et al. 2010). The present study also confirmed that TP could also impact bacterial community structure. Phosphorus plays a major role in the riverine ecosystem (Miettinen et al. 1997), and serves as an essential nutrient for bacterial growth. The lack of phosphorus may be a principal factor limiting microbial productivity (Hu et al. 2014). Therefore, TOC and TP are essential nutrients influencing bacterial community structure.
Heavy metals are always consider to have negative effect on the bacterial community since they are toxic and inactivate the sulfhydryl groups in proteins (Valls and De 2003). However, many metals are necessary for the bacterial community especially at the proper concentrations. In the present study, the percentage explanation of the variation in bacterial community structure was significantly negatively correlated with heavy metal toxicity. The metal contamination level is moderate in the Panzhihua region. At low concentrations, metals may function as essential nutrients rather than hazardous materials, which may have a more positive effect on bacterial communities (Jingxin et al. 2013; Liu et al. 2018a). It was determined that Fe was the most essential metal for the bacterial communities. Fe is widely distributed in mining cities because the mining industry discharges harbor very high Fe2+ and Fe3+ concentrations (Yang et al. 2007). Fe2+ is one of the key energy source for bacteria and many bacterial species are capable of making use of ferrous iron (Yang et al. 2007). On the other hand, Fe3+ addition increases bacterial diversity and alters the bacterial community by changing the electric field. This effect enriches bacteria with Fe3+-reducing capabilities, including the Comamonadaceae and the Rhodocateraceae (Jingxin et al. 2013). Low concentrations of Fe are also necessary for bacteria to catalyze enzymatic reactions and maintain protein structures, which may enhance the production of extracellular polymeric substances (EPS) (Stacy et al. 2016). Cytoactivity may also increase with Fe level (Jingxin et al. 2013). Therefore, the present study confirmed that Fe is a vital factor influencing bacterial community structure.
Networking analysis demonstrated that there are rules to bacterial community assembly which reflect ecological processes like cooperation and competition among different bacterial genera under environmental stress (Fuhrman 2009). Since Fe explained the highest percentage of bacterial community variation, it was selected as the main environmental stressor for the network analysis of bacterial species. Network analysis described a strong interaction between the bacteria and Fe stress. This association may be explained by certain deterministic processes like habitat filtering, reduction in trait dispersal, and common ecological tolerances (Cornwell et al. 2006). The high interaction indicated that the microbial system is stable and resistant to environmental alterations, particularly at the functional level (Ding et al. 2015).
Response patterns of individual bacteria to contaminants
Metal-resistant bacteria in the sediment can indicate environmental perturbations (Xie et al. 2016). Bacteria with multiple metal resistances like Bacillus, Cupriavidus, Paenibacillus, and Pseudomonas were investigated in previous laboratory studies based on culture-dependent methods (Mejias Carpio et al. 2018). Nevertheless, their relative abundances were low in the present field study. Bacteria with multiple metal resistances only gain a competitive advantage for resource acquisition under extreme environmental conditions (Zhang et al. 2015). These metal-resistant bacteria in the river sediment may be considered alien species discharged from the mining industry, and the competition for vital resources and direct antagonism may lead to the suppression of these metal-resistant bacteria in the river sediment (Moynihan et al. 2015). Moreover, heavy metal contamination levels and the abundances of metal-resistant bacteria were not significantly correlated. Therefore, this type of bacteria derived from culture-dependent research may not be feasible as indicators of heavy metal contamination in the river. For this reason, effective river sediment contaminant indicators are still required further investigation.
Several bacterial genera were positively correlated with the contaminant levels according to the network analysis. Therefore, these bacteria could tolerate contaminants or actually utilize them as energy sources (Xie et al. 2016). These bacterial genera could be applied as contamination indicators. For instance, Rhodobacter has been reported to resist certain metals (Mishra and Malik 2013). In our study, Rhodobacter abundance was negatively correlated with Fe, Ti, and TP and positively correlated with TOC. For this reason, Rhodobacter may be a suitable candidate to monitor these substances. Dechloromonas are active denitrifiers in the river sediments (Price et al. 2017; Wang et al. 2017) and could also be contaminant indicators because their abundance was negatively correlated with Fe and Ti and positively correlated with TOC. Hylemonella can indicate the levels of Fe, Ti, and TOC, and its abundance was negatively correlated with TOC and positively correlated with Fe and TP.
There is a potential limitation of this study that needs further discussion. The quantitative relationships between sensitive bacteria and contaminant types and levels described in this study may also be influenced by other environmental factors unique to the Panzhihua region. Therefore, the indicator potential of these bacteria should also be investigated under other environmental conditions (Xie et al. 2016). Nevertheless, this study reveals the potential contaminant stressors affecting the river sediment bacterial community and identifies possible bacterial indicators of environmental contamination, and our results show that ecogenomics could be effectively applied towards the improvement of river management (Gibson et al. 2015).
Conclusions
The present study showed that anthropogenic activities in both urban and mining areas had significant impacts on bacterial community structure and diversity but not on bacterial ecological function. Distinct bacterial families associated with municipal pollution, such as the Comamonadaceae and Sphingomonadaceae, and metal contamination, such as the Rhodobacteraceae, were more abundant in the urban and mining areas, respectively, than the other areas. Relative to dispersal dynamics, environmentally induced species sorting may be substantially more important in shaping bacterial community structure. Heavy metal concentrations and sedimentary physicochemical properties both significant influenced bacterial community structure. In contrast, since the metal contamination levels were only moderate in the mining city, metals were more likely to be used by the bacteria as essential nutrients and less likely to be toxic to the bacterial community. Moreover, bacteria with multiple metal resistances identified by culture-dependent research may be unable to indicate heavy metals contamination in field studies. Therefore, several sensitive bacterial genera like Rhodobacter, Hylemonella, and Dechloromonas were identified as potential bioindicators for iron, titanium, phosphorus, and organic carbon in the riverine ecosystem. Overall, these results reveal that urbanization and mineral resource utilization jointly affect urban river sediment bacterial communities and provide a useful tool to monitor sediment contaminant levels.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Bier RL, Voss KA, Bernhardt ES (2014) Bacterial community responses to a gradient of alkaline mountaintop mine drainage in Central Appalachian streams. ISME J 9:1378
Burke C, Steinberg P, Rusch D, Kjelleberg S, Thomas T (2011) Bacterial community assembly based on functional genes rather than species. Proc Natl Acad Sci U S A 108:14288–14293
Cao X, Diao M, Zhang B, Liu H, Wang S, Yang M (2017) Spatial distribution of vanadium and microbial community responses in surface soil of Panzhihua mining and smelting area, China. Chemosphere 183:9–17
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, Narwani A, Mace GM, Tilman D, Wardle DA (2012) Biodiversity loss and its impact on humanity. Nature 489:59–67
Celine L, Dimitris P, Sean MC, Bernard O, Steven S, Safiyh T, Donald Z, Daniel VDL (2010) Elevated atmospheric CO2 affects soil microbial diversity associated with trembling aspen. Environ Microbiol 10:926–941
Chen Y, Jiang Y, Huang H, Mou L, Ru J, Zhao J, Xiao S (2018) Long-term and high-concentration heavy-metal contamination strongly influences the microbiome and functional genes in Yellow River sediments. Sci Total Environ 637–638:1400–1412
Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM (2009) The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic acids research 37:D141–D145
Cornwell WK, Schwilk DW, Ackerly DD (2006) A trait-based test for habitat filtering: convex hull volume. Ecology 87:1465–1471
Davidson K, Gilpin LC, Hart MC, Fouilland E, Mitchell E, Álvarez Calleja I, Laurent C, Miller AEJ, Leakey RJG (2007) The influence of the balance of inorganic and organic nitrogen on the trophic dynamics of microbial food webs. Limnol Oceanogr 52:2147–2163
Debarati P, Gunjan P, Christoph M, Jan Roelof VDM, Jain RK (2010) Bacterial community structure of a pesticide-contaminated site and assessment of changes induced in community structure during bioremediation. FEMS Microbiol Ecol 57:116–127
Ding J, Zhang Y, Deng Y, Cong J, Lu H, Sun X, Yang C, Yuan T, Van Nostrand JD, Li D (2015) Integrated metagenomics and network analysis of soil microbial community of the forest timberline. Sci Rep 5:7994
Ebina J, Tsutsui T, Shirai T (1983) Simultaneous determination of total nitrogen and total phosphorus in water using peroxodisulfate oxidation. Water Res 17:1721–1726
Ellis RJ, Morgan P, Weightman AJ, Fry JC (2003) Cultivation-dependent and -independent approaches for determining bacterial diversity in heavy-metal-contaminated soil. Appl Environ Microbiol 69:3223–3230
Fernandez-Gonzalez N, Huber JA, Vallino JJ (2016): Microbial communities are well adapted to disturbances in energy input. mSystems 1, e00117–16
Fischer H, ., Pusch M, . (1999): Use of the [(14)C]leucine incorporation technique to measure bacterial production in river sediments and the epiphyton. Appl Environ Microbiol 65, 4411-4418
Fu C, Guo J, Jie P, Qi J, Zhou W (2009) Potential ecological risk assessment of heavy metal pollution in sediments of the Yangtze River within the Wanzhou section, China. Biol Trace Elem Res 129:270–277
Fuhrman JA (2009) Microbial community structure and its functional implications. Nature 459:193–199
Gibbons SM, Jones E, Bearquiver A, Blackwolf F, Roundstone W, Scott N, Hooker J, Madsen R, Coleman ML, Gilbert JA (2014) Human and environmental impacts on river sediment microbial communities. PLoS One 9:e97435
Gibson JF, Stein ED, Baird DJ, Finlayson CM, Zhang X, Hajibabaei M (2015) Wetland ecogenomics-the next generation of wetland biodiversity and functional assessment. Wetland Sci Pract 32:27–32
Gillan DC, Roosa S, Kunath B, Billon G, Wattiez R (2015) The long-term adaptation of bacterial communities in metal-contaminated sediments. A metaproteogenomic study. Environ Microbiol 17:1991–2005
Gourvendu S, Marzinelli EM, Naing NN, Zhili H, Yuting L, Lauren T, Suparna M, Han P, Joshi UM, Sheela R (2015) Ecogenomics reveals metals and land-use pressures on microbial communities in the waterways of a megacity. Environ Sci Technol 49:1462
He Z, Piceno Y, Deng Y, Xu M, Lu Z, Desantis T, Andersen G, Hobbie SE, Reich PB, Zhou J (2012) The phylogenetic composition and structure of soil microbial communities shifts in response to elevated carbon dioxide. ISME J 6:259–272
Hu A, Yang X, Chen N, Hou L, Ma Y, Yu C-P (2014) Response of bacterial communities to environmental changes in a mesoscale subtropical watershed, Southeast China. Sci Total Environ 472:746–756
Ibekwe AM, Leddy MB, Bold RM, Graves AK (2012) Bacterial community composition in low-flowing river water with different sources of pollutants. FEMS Microbiol Ecol 79:155–166
Ibekwe AM, Ma J, Murinda SE (2016) Bacterial community composition and structure in an Urban River impacted by different pollutant sources. Sci Total Environ 566-567:1176–1185
Isabwe A, Yang JR, Wang Y, Liu L, Chen H, Yang J (2018) Community assembly processes underlying phytoplankton and bacterioplankton across a hydrologic change in a human-impacted river. Sci Total Environ 630:658–667
Jing Z, Rapp G, Gao T (1997) Geoarchaeological aids in the investigation of early shang civilization on the floodplain of the Lower Yellow River, China. World Archaeol 29:36–50
Jingxin Z, Yaobin Z, Xie Q, Shuo C (2013) Effects of ferric iron on the anaerobic treatment and microbial biodiversity in a coupled microbial electrolysis cell (MEC)--anaerobic reactor. Water Res 47:5719–5728
Jones SE, Newton RJ, Mcmahon KD (2010) Evidence for structuring of bacterial community composition by organic carbon source in temperate lakes. Environ Microbiol 11:2463–2472
Jroundi F, Martinez-Ruiz F, Merroun ML, Gonzalez-Muñoz MT (2020) Exploring bacterial community composition in Mediterranean deep-sea sediments and their role in heavy metal accumulation. Sci Total Environ 712:135660
Jung J, Philippot L, Park W (2016) Metagenomic and functional analyses of the consequences of reduction of bacterial diversity on soil functions and bioremediation in diesel-contaminated microcosms. Sci Rep 6:23012
Kritzberg ES, Cole JJ, Pace ML, Granéli W, Bade DL (2004) Autochthonous versus allochthonous carbon sources of bacteria: results from whole-lake 13C addition experiments. Limnol Oceanogr 49:588–596
Langille MGI, Jesse Z, Caporaso JG, Daniel MD, Dan K, Reyes JA, Clemente JC, Burkepile DE, Thurber RL, Vega, Rob K (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821
Larson JL, Zak DR, Sinsabaugh RL (2002) Extracellular Enzyme activity beneath temperate trees growing under elevated carbon dioxide and ozone. Soil Sci Soc Am J 66:1848–1856
Lewis GP, Mitchell JD, Andersen CB, Haney DC, Liao MK, Sargent KA (2007) Urban influences on stream chemistry and biology in the big brushy creek watershed, south Carolina. Water Air Soil Pollut 182:303–323
Li D, Qi R, Yang M, Zhang Y, Yu T (2011) Bacterial community characteristics under long-term antibiotic selection pressures. Water Res 45:6063–6073
Li X, Meng D, Li J, Yin H, Liu H, Liu X, Cheng C, Xiao Y, Liu Z, Yan M (2017) Response of soil microbial communities and microbial interactions to long-term heavy metal contamination. Environ Pollut 231:908–917
Li S, Wu J, Huo Y, Zhao X, Xue L (2021) Profiling multiple heavy metal contamination and bacterial communities surrounding an iron tailing pond in Northwest China. Sci Total Environ 752:141827
Liu Q, Yang Y, Mei X, Liu B, Chen C, Xing D (2018a) Response of the microbial community structure of biofilms to ferric iron in microbial fuel cells. Sci Total Environ 631:695–701
Liu S, Wang C, Wang P, Chen J, Wang X, Yuan Q (2018b) Variation of bacterioplankton community along an urban river impacted by touristic city: with a focus on pathogen. Ecotoxicol Environ Saf 165:573–581
Logue JB, Lindström ES (2010) Species sorting affects bacterioplankton community composition as determined by 16S rDNA and 16S rRNA fingerprints. ISME J 4:729–738
Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime JP, Hector A, Hooper DU, Huston MA, Raffaelli D, Schmid B, Tilman D, Wardle DA (2001) Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294:804–808
Mejias Carpio IE, Ansari A, Rodrigues DF (2018) Relationship of biodiversity with heavy metal tolerance and sorption capacity: a meta-analysis approach. Environ Sci Technol 52:184–194
Miettinen IT, Vartiainen T, Martikainen PJ (1997) Phosphorus and bacterial growth in drinking water. Appl Environ Microbiol 63:3242–3245
Mishra A, Malik A (2013) Recent advances in microbial metal bioaccumulation. Crit Rev Environ Sci Technol 43:1162–1222
Moynihan EL, Richards KG, Brennan FP, Tyrrel SF, Ritz K (2015) Enteropathogen survival in soil from different land-uses is predominantly regulated by microbial community composition. Appl Soil Ecol 89:76–84
Obernosterer I, Catala P, Lebaron P, West NJ (2011) Distinct bacterial groups contribute to carbon cycling during a naturally iron fertilized phytoplankton bloom in the Southern Ocean. Limnol Oceanogr 56:2391–2401
Paul MJ, Meyer JL (2001) Streams in the urban landscape. Annu Rev Ecol.EvolSyst 32: 333–365
Phillips RL, Zak DR, Holmes WE, White DC (2002) Microbial community composition and function beneath temperate trees exposed to elevated atmospheric carbon dioxide and ozone. Oecologia 131:236–244
Price JR, Ledford SH, Ryan MO, Toran L, Sales CM (2017) Wastewater treatment plant effluent introduces recoverable shifts in microbial community composition in receiving streams. Sci Total Environ 613–614:1104–1116
Rickard TA (1934) Man and metals. A history of mining in relation to the development of civilization. Geologiska Föreningen i Stockholm Förhandlingar 56:640–641
Roberto AA, Van Gray JB, Leff LG (2018) Sediment bacteria in an urban stream: Spatiotemporal patterns in community composition. Water Res 134:353–369
Saito R, Smoot ME, Ono K, Ruscheinski J, Wang P-L, Lotia S, Pico AR, Bader GD, Ideker T (2012) A travel guide to Cytoscape plugins. Nat Methods 9:1069–1076
Sanchez-Peinado MD, Gonzalez-Lopez J, Martinez-Toledo MV, Pozo C, Rodelas B (2010) Influence of linear alkylbenzene sulfonate (LAS) on the structure of alphaproteobacteria, actinobacteria, and acidobacteria communities in a soil microcosm. Environ Sci Pollut Res 17:779–790
Seiler C, Berendonk TU (2012) Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front Microbiol 3:399
Stacy A, Abraham N, Jorth P, Whiteley M (2016) Microbial community composition impacts pathogen iron availability during polymicrobial infection. PLoS Pathog 12:e1006084
Staley C, Gould TJ, Wang P, Phillips J, Cotner JB, Sadowsky MJ (2015) Species sorting and seasonal dynamics primarily shape bacterial communities in the Upper Mississippi River. Sci Total Environ 505:435–445
Steffen MM, Li Z, Effler TC, Hauser LJ, Boyer GL, Wilhelm SW (2012) Comparative metagenomics of toxic freshwater cyanobacteria bloom communities on two continents. PLoS One 7:e44002
Sun MY, Dafforn KA, Johnston EL, Brown MV (2013) Core sediment bacteria drive community response to anthropogenic contamination over multiple environmental gradients. Environ Microbiol 15:2517–2531
Tang X, Chao J, Gong Y, Wang Y, Wilhelm SW, Gao G (2017) Spatiotemporal dynamics of bacterial community composition in large shallow eutrophic Lake Taihu: High overlap between free-living and particle-attached assemblages. Limnology and Oceanography 62:1366–1382
Teng Y, Jie Y, Sun Z, Wang J, Rui Z, Zheng J (2011) Environmental vanadium distribution, mobility and bioaccumulation in different land-use Districts in Panzhihua Region, SW China. Environ Monit Assess 176:605–620
Valls M, De LV (2003) Exploiting the genetic and biochemical capacities of bacteria for the remediation of heavy metal pollution. FEMS Microbiol Rev 26:327–338
Vetterli A, Hyytiäinen K, Ahjos M, Auvinen P, Paulin L, Hietanen S, Leskinen E (2015) Seasonal patterns of bacterial communities in the coastal brackish sediments of the Gulf of Finland, Baltic Sea. Estuar Coast Shelf Sci 165:86–96
Wang XQ, Liu ZC, Miao JL, Zuo N (2015) Relationship between nutrient pollutants and suspended sediments in upper reaches of Yangtze River. Water Sci Eng 8:121–126
Wang H, Marshall CW, Cheng M, Xu H, Li H, Yang X, Zheng T (2017) Changes in land use driven by urbanization impact nitrogen cycling and the microbial community composition in soils. Sci Rep 7:44049
Wang L, Zhang J, Li H, Yang H, Peng C, Peng Z, Lu L (2018) Shift in the microbial community composition of surface water and sediment along an urban river. Sci Total Environ 627:600–612
Wang P, Zhao J, Xiao H, Yang W, Yu X (2019) Bacterial community composition shaped by water chemistry and geographic distance in an anthropogenically disturbed river. Sci Total Environ 655:61–69
Wenger SJ, Roy AH, Jackson CR, Bernhardt ES, Carter TL, Filoso S, Gibson CA, Hession WC, Kaushal SS, Martí E, Meyer JL, Palmer MA, Paul MJ, Purcell AH, Ramírez A, Rosemond AD, Schofield KA, Sudduth EB, Walsh CJ (2009): Twenty-six key research questions in urban stream ecology: an assessment of the state of the science. Journal of the North American Benthological Society 28:1080–1098
Winter C, Matthews B, Suttle CA (2013) Effects of environmental variation and spatial distance on Bacteria, Archaea and viruses in sub-polar and arctic waters. ISME J 7:1507–1518
Xia N, Xia X, Zhu B, Zheng S, Zhuang J (2013) Bacterial diversity and community structure in the sediment of the middle and lower reaches of the Yellow River, the largest turbid river in the world. Aquat Microb Ecol 71:43–U168
Xie Y, Wang J, Wu Y, Chen R, Song C, Yang J, Yu H, Giesy JP, Zhang X (2016) Using in situ bacterial communities to monitor contaminants in river sediments ☆. Environ Pollut 212:348–357
Yang Y, Wan MX, Shi WY, Peng H, Qiu GZ, Zhou JZ, Liu XD (2007) Bacterial diversity and community structure in acid mine drainage from Dabaoshan Mine, China. Aquat Microb Ecol 47:141–151
Zhang J, Wang LH, Yang JC, Liu H, Dai JL (2015) Health risk to residents and stimulation to inherent bacteria of various heavy metals in soil. Sci Total Environ 508:29–36
Zhang C, Nie S, Liang J, Zeng G, Wu H, Hua S, Liu J, Yuan Y, Xiao H, Deng L, Xiang H (2016) Effects of heavy metals and soil physicochemical properties on wetland soil microbial biomass and bacterial community structure. Sci Total Environ 557:785–790
Funding
This work was financially supported by the National Key Plan for Research and Development of China (2016YFC0502203), the Key Program of National Natural Science Foundation of China (No.91647206), the National Science Funds for Creative Research Groups of China (No. 51421006), and the National Natural Science Foundation of China (No. 51479065, 51579073).
Author information
Authors and Affiliations
Contributions
Chao Wang and Peifang Wang: methodology and funding acquisition; Sheng Liu: data analyzing and writing original draft; Juan Chen and Xun Wang: manuscript reviewing and field sampling; Qiusheng Yuan and Jingjie Ma: field sampling. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 1303 kb).
Rights and permissions
About this article
Cite this article
Wang, C., Liu, S., Wang, P. et al. How sediment bacterial community shifts along the urban river located in mining city. Environ Sci Pollut Res 28, 42300–42312 (2021). https://doi.org/10.1007/s11356-020-12031-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-12031-0