Abstract
Channel confluences are common in urban rivers and caused complex hydrodynamic conditions in the downstream section, significantly influencing the distribution of pollutants and the microbial community. So far, the principles of bacterial community assembly and their linkages with environmental factors are poorly understood. In the present study, the hydrodynamic and pollution conditions were investigated in a typical channel confluence of an urban river in the Yangtze River delta area, China, and their impacts on the bacterial community structure in the water and sediment were characterized using 16S rRNA gene high-throughput sequencing technology. Based on the results, the flow velocity was the crucial factor influencing the dispersal of nutrients, organic compounds, and bacterial communities in the river water. Moreover, the sediments exhibited higher α-diversity and bacterial richness for nitrogen and sulfur cycling than the water. In addition to flow velocity, the contents of total organic carbon, total phosphorus, and heavy metals determined the sediment bacterial communities at varying depths. The predictive analysis of functional gene category indicated differences between the water and sediment communities in metabolic potentials and pathogen risk and provided guidance for water pollution control and the eco-remediation of urban rivers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In surface water, the dynamic interchange of material between water and sediment, driven by hydrodynamic diffusion, is a critical process influencing biogeochemical processes (Yang et al. 2019). In this context, sediments can provide a record of the upstream inputs of pollutants and a natural medium for microbe growth (Sun et al. 2012). Microbial communities not only function in the transformation of nutrients, the biodegradation of organics, and the conversion of energy but also are shaped by the hydrodynamic conditions, such as the velocity distribution of cross-section (Li et al. 2020b; Shao et al. 2011; Wu et al. 2017). For instance, Zhang et al. (2022c), by incorporating the bacteria-based index of biotic integrity (Ba-IBI), the path model, and the random forest regression model, found that the primary environmental factors affecting the sediment bacterial communities in river bends were flow velocity and ammonium concentration. Cai et al. (2019) observed that the microbial community assembly in black-odor rivers, which lose ecosystem functions, was mainly controlled by sulfur metabolism under oxygen-limited conditions. In another study, the transport of microorganisms and chemicals within and between water and sediments was identified as the rate-limiting step for the nitrogen dynamics in urban channel confluences using omics-based modeling (Hui et al. 2021). In addition, dam-induced environmental variations, especially hydrological and nutrient variables, potentially influence the microbial food web, intensifying the accumulation of organic carbon, ammonium, and inorganic phosphorus in river systems (Yang et al. 2021a, b). Given that the ecological selections of the microbial community assembly are governed by deterministic and stochastic processes (Li et al. 2020a, 2020c), it is necessary to identify the strong environmental factors that directionally shape the microbial community and specific functions. Such an approach can be used to support the self-purification capacity of water bodies.
The Yangtze-River-Delta is a well-developed mega-region; it contained around 11% of the permanent population in China, and economics of scale accounted for 20% of gross domestic product of the country by the end of 2016 (Wang et al. 2020a). Although the across-the-board Action Plan for the Prevention and Control of Water Pollution has been implemented since 2015, the improvement of the surface water quality remains a major challenge for urban sustainable development in this region, with the goal of ultimately eliminating black-odor water bodies in urban and rural areas by 2025 (GOJPG 2021).
As the junctions of different river systems, urban channel confluences exhibit sharp shifts in the pollutant distribution and flow dynamics on the cross section after the stream mixing (Li et al. 2020a; Hui et al. 2022). The confluence-induced heterogeneity in the microbial community would be mainly created by the diverse pollutant transport and transformation in overlying water and surface sediments, which is critical for the evaluation and improvement of aquatic ecosystem health (Burton and Johnston 2010). However, the principles of microbial community assembly and their linkages with hydrodynamic conditions and pollution levels in urban channel confluences are still poorly understood. In this sense, in situ investigations on the ecological function potentials of microbial communities are needed to provide solid evidence for guiding the eco-remediation of polluted urban rivers.
In this study, a typical cross confluence of an urban channel near the Yangtze River estuary was selected as the study site, with the following main objectives: (1) to characterize the differentiation of the hydrodynamic conditions, contaminant distributions, and bacterial communities in the same downstream section; (2) to analyze the correlation between bacterial community and environmental factors in water and sediment phases; (3) to investigate the metabolic potentials of bacterial communities in nutrient cycling and pollutant degradation.
Materials and methods
Site description and sample collection
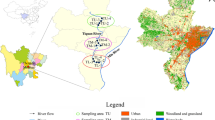
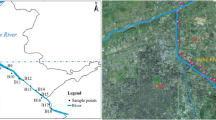
The urban channel cross confluence is located in Suzhou City in the Yangtze-River-Delta, China (121°5′28″E, 31°25′53″N). Channel A is a local important shipping transport waterway, and the joining of Channel B and C changed the hydrodynamic conditions in the downstream section (Fig. 1a and b). There are numerous ports, agricultural land, and residential areas distributed along channels A, B, and C in the upstream, representing point and non-point effluent sources for inputs of nutrients and contaminants.
Two mixed water samples (W1 and W2, 5–20 cm in depth) were collected at the points near the right riverbank (P1) and the main channel (P2) in July 2020. The results of underwater sonar scanning (M9, SonTek, USA) indicated an average flow velocity (u) of 0.18 m/s in P2 and of 0.0 m/s in P1 (Fig. 1b), which created a sedimentation zone near the riverbank. Two groups of sediment samples (S1 and S2) were collected using a piston sampler (XDB0204, Pusen, China) at sediment depths of 0–10 cm (S1-1 and S2-1), 10–30 cm (S1-2 and S2-2), and 30–50 cm (S1-3 and S2-3) in P1 and P2, respectively. To avoid random sampling, the triple parallel sediments were sampled in the triangle (2 m side length) at the same depth. All samples were stored at − 80 °C for the extraction of bacterial DNA and freeze-dried for physicochemical analysis.
Physicochemical analysis
The dissolved oxygen (DO) concentration was determined in situ using a HACH HQ30d portable DO meter (Hach Co., Loveland, CO, USA). The concentrations of ammonia nitrogen (NH4+-N), total nitrogen (TN), total phosphorus (TP), and total organic carbon (TOC) in water samples were measured using the procedure described in Standard Methods (APHA 1998).
The TOC contents in the sediments were analyzed by the thermodilution method, applying sulfuric acid and potassium dichromate; sediment total phosphorus (TP) was determined using the Murphy-Riley method after digestion with H2SO4–HClO4, and the sulfide contents were determined using methylene blue spectrophotometry (Bao 2008). Sediment total nitrogen (TN) was analyzed with a continuous-flow automated analyzer after digestion with H2SO4–H2O2 (Soon and Kalra 1995). The typical heavy metals, including cadmium (Cd), arsenic (As), lead (Pb), chromium (Cr), and copper (Cu), were quantified via inductively coupled plasma (ICP) spectra, following the sample pretreatment described in the literature (Ning et al. 2014).
3D-EEM fluorescence analysis
A three-dimensional excitation-emission matrix (3D-EEM) fluorescence spectrophotometer (FSS, Edinburgh Instrument Ltd., UK) was employed to analyze dissolved organic matter (DOM) in the water samples, using corresponding scanning emission (Em) spectra from 260 to 550 nm by varying the excitation (Ex) wavelength from 200 to 400 nm at 10-nm increments; the scanning speed was set at 2,000 nm·min−1. Two alternative fluorescence indices are proposed for characterizing DOM, with the following calculations (Ohno 2002; Huguet et al. 2009):
where IEm435–480 and IEm300–345 are the fluorescence intensities at the Em of 435–480 and 300–345 nm using an Ex of 254 nm, respectively. A large HIX value indicates an increase in the humification degree of DOM (Ohno 2002).
where IEm380 and IEm430 are the fluorescence intensities at the Em of 380 and 430 nm using an Ex of 310 nm, respectively. The BIX < 0.8 indicates that terrestrial origin inputs (such as anthropogenic activity) dominate the DOM, while the BIX > 1.0 suggests the dominant role of aquatic microbe sources (such as plankton, bacteria, and algae) (Huguet et al. 2009).
DNA extraction, PCR amplification, and sequencing
Microbial DNA was extracted from all samples using the E.Z.N.A.® soil DNA Kit (Omega Bio-tek, Norcross, GA, USA) according to the manufacturer’s protocols. The V3–V4 hypervariable regions of the bacteria 16S rRNA gene were amplified with primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) by the thermocycler PCR system (GeneAmp 9700, ABI, USA). The PCR reactions were conducted using the program in our previous report (Qian et al. 2018). Purified amplicons were pooled in equimolar concentrations and paired-end sequenced (2 × 300) on an Illumina MiSeq platform (Illumina, San Diego, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
Data analysis
The sequencing of 16S rRNA gene amplicons from 6 mixed water and 18 sediment samples produced 1,226,620 reads with an average length of 418 bp. The obtained sequences could be assigned to 7773 operational taxonomic units (OTUs) at a 97% similarity cutoff using UPARSE (version 7.1). The taxonomy of each 16S rRNA gene sequence was analyzed by the RDP Classifier algorithm (http://rdp.cme.msu.edu/) against the Silva (SSU123) 16S rRNA database using a confidence threshold of 70%.
The α-diversity indices (OTUs richness, Ace, Chao, Shannon, and Simpson) of the 24 samples were calculated by Mothur. Composition analysis was conducted at the phylum and family levels. Principal component analysis (PCA) of environmental parameters was performed with the CANOCO 5 software. The Z-score method was used for normalization calculations, and the logarithmic value was displayed according to the logarithmic value Log, so that the change of the color bar was used to visually represent the changes in variables among different samples. Comparison of the relative abundances of the most abundant family among samples was performed using the heatmap package of R 3.2.4 (https://www.r-project.org/). Correlations between the environmental parameters and bacterial communities were measured using the Spearman correlation analysis (significance P < 0.05) in SPSS 20.0. The software Gephi was applied to visualize and render all networks. According to the annotation of the 16S rDNA sequence classification by the KEGG database, the functional prediction of the output microbial community was conducted using Functional Annotation of Prokaryotic Taxa (FAPROTAX) (Qu et al. 2020).
Results and discussion
Physicochemical characteristics of samples
The main water quality parameters and 3D-EEM images of samples W1 and W2 are shown in Fig. 2. With the combined pollution of organics and nutrients (TOC and TN), the DO concentration of channel A was below the Standard V (DO ≥ 3 mg/L) in the Environmental Quality Standard for Surface Water of China (GB3838-2002), which was similar to the typical polluted urban channel located in the Yangtze-River-Delta region (Yang et al. 2021a). There were four characteristic peaks, located at Ex/Em of 255–280/320–335 nm (peak T1), 230–235/330–335 nm (peak T2), 230–240/425–440 nm (peak A), and 305–340/420–430 nm (peak C) in 3D-EEM. According to previous studies (Huguet et al. 2009; Qian et al. 2022), the protein-like substances related to microbial activity (peak T2) dominated in W1 and W2, followed by fulvic acid compounds (peak A). With the different u values in P1 and P2, W1 exhibited a relatively higher BIX and a lower HIX than W2. This indicates that aquatic microbe sources contributed more to the pollution of P1 near the riverbank than terrestrial origin inputs. The higher humification degree of DOM in the main channel (P2) was in agreement with terrestrial origin inputs from upstream runoff.
Figure 3 displays the pollutant contents of sediments in P1 and P2 at various depths. The TOC contents in group S1 increased as increasing sampling depth, and the opposite pattern was found for group S2. Due to the terrestrial origin inputs and low flow velocity, the TOC level (3.5–5.7 g/kg) in the sediments was significantly higher 0.4–3.4 g/kg in the previous reports of this region (Wu et al. 2019; Zhang et al. 2022b). According to the Standard for Contaminated Sediments in Freshwater Ecosystems of USEPA (Cieniawski et al. 2002), the TN, TP, and sulfide contents of S1 and S2 were heavily polluted, with pollution levels above the median values in local urban rivers, namely, as 1.0–4.9 g/kg for TN, 0.4–2.0 g/kg for TP, and 0.02–0.2 g/kg for sulfide (Wu et al. 2019; Lei et al. 2020). In addition, the As, Cd, and Pb levels of S2 were higher than those of S1, and lower levels of total Cr and Cu were found in P2 close to the main channel. Notably, the average contents of total Cr, As, Cd, Pb, and Cu in the sediments were 1.03, 2.40, 3.10, 1.89, and 1.98 times those of the local background values (65.7 g/kg for total Cr, 8.8 g/kg for As, 0.12 g/kg for Cd, 22.8 g/kg for Pb, 20.4 g/kg for Cu) (Wang et al. 2020b), respectively (Vareda et al. 2019). Because of the considerable ecological risk of heavy metals, the channel cleanout or in situ stabilization of the polluted sediments should be conducted in the channel confluences (Ning et al. 2014; Li et al. 2020b).
Bacterial community diversity in water and sediments
The α-diversity indices of water and sediment samples are listed in Table S1. The Ace, Chao, Shannon, and Simpson indices revealed significantly lower levels of bacterial community richness and α-diversity in water samples (average Ace 1837.5, Chao 1613.0, Shannon index 4.85, and Simpson index 0.03) compared to sediments (average Ace 4510.2, Chao 4433.8, Shannon index 6.53, and Simpson index 0.01). Because of the substrate gradients, good biocompatibility, and high enrichment capacity, the sediments could provide diverse niches for microbial growth under anthropogenic interference, facilitating the formation of integrated microecosystems (Crump et al. 2012; Meziti et al. 2016). For the same channel section, considerable differences between W1 and W2 or between S1 and S2 groups in terms of biodiversity were not found.
Figure 4a shows the results of PCA in the water and sediment samples. The dispersion degrees of W1 and W2 spots were significantly larger than those in the sediments (group S1 and S2) in both PCA1 (resolution 67.45%) and PCA2 (14.31%) levels. As shown in Fig. 4b, the shared OTUs between W1 and W2 accounted for 78.0% in W1 and 74.6% in W2, respectively, and group S1 shared more than 90% of OTUs with S2. Liu et al. (2018) investigated the integrated biogeography of sedimentary bacterial communities in the Yangtze River and found that the estimated immigration rate (m) of sediment communities (0.1463–0.1640) was considerably lower than that for water communities (0.1814–0.1997). The relatively stable sediment communities were mainly attributed to the serious dispersal limits (Liu et al. 2018). The flow of river water would not only drive the transports of nutrients, organic matter, and suspend solids in different environmental but also enhance microbial community coalescence and further control habitat biodiversity and metabolic processes (Li et al. 2015; Meziti et al. 2016; Zhang et al. 2022a). The slow u in P1 resulted in a higher bacterial community difference in group S1 than that in group S2, in terms of the longer PAC1 (56.27%) distance in Fig. 4c. The 3638 shared OTUs between S1-1 and S1-3 were less than the 4346 OTUs between S2-1 and S2-3 (Fig. 4d), whereas the surface sediments (4009 OTUs shared by S1-1 and S2-1) exhibited a higher similarity than the deep ones (3535 OTUs shared by S1-3 and S2-3).
As seen in Fig. 5, the dominant bacterial phyla in the water samples were Proteobacteria (relative abundance 23.9% in W1, 38.1% in W2), Actinobacteriota (46.0% in W1, 33.3% in W2), Bacteroidota (12.5% in W1, 10.9% in W2), Cyanobacteria (10.8% in W1, 10.5% in W2), and Chloroflexi (1.5% in W1, 2.3% in W2), which were different from those in the sediment groups, where Proteobacteria (28.0% in S1, 19.8% in S2), Chloroflexi (23.4% in S1, 27.9% in S2), Acidobacteriota (9.6% in S1, 11.1% in S2), Desulfobacterota (7.5% in S1, 5.9% in S2), and Firmicutes (6.1% in S1, 3.9% in S2) were dominant. Notably, slow-growth nitrite-oxidizing bacteria, such as Nitrospirota, were only detected in the sediments (2.1–2.6%). According to Lei et al. (2020), considering phylogenetic turnover deviation, dispersal and variable selection are the most crucial factors dominating microbial community assemblies in water and sediments of urban rivers, respectively. The differences in the spatial distribution of substrates (such as oxygen, nutrients, and organics) and mass transfer resistance result in the heterogeneity of microbial niche in various environmental media (Hollister et al. 2010; Gao et al. 2021). The excess inputs of pollutants with low-flow velocity would promote the enrichment of functional bacteria in the sediments, which are responsible for labile organic matter degradation (Chloroflexi, Acidobacteriota, and Firmicutes) (Ji et al. 2022; Wang et al. 2022), nitrogen cycling (Proteobacteria, Nitrospirota, and Bacteroidota) (Yang et al. 2021b; Deveautour et al. 2022), and ferric or sulfate reduction (Desulfobacterota) (Cai et al. 2019) under anoxic conditions; similar findings have been widely reported for polluted lake bays (Haller et al. 2011; Wu et al. 2017) and urban rivers (Wu et al. 2019; Zhang et al. 2022b). In addition, the different hydrodynamic conditions also influenced the abundances of dominant bacterial phyla in the sediments (Fig. S1). For example, the relative abundance of Chloroflexi in S2-1 (26.7%) was significantly higher than the 18.6% in S1-1, due to abundant substrate in the center of the urban channel. Moreover, the fraction of Desulfobacterota in S1-3 (7.1%) was 1.5 times higher than that in S2-3 (4.8%), which indicated that sulfur biotransformation (such as dissimilated sulfate reduction) preferably occurs in the deeper layer of the static flow field (P1), with a high oxidation–reduction potential (Cai et al. 2019).
Comparison of the bacterial community assembly in the water and sediments at the family level provided detailed information of ecological niche and biogeochemical processes. As shown in Fig. 6, the predominant bacterial family in the water samples was Sporichthyaceae (30.9% in W1, 17.9% in W2), followed by Ilumatobacteraceae (9.6% in W1, 8.9% in W2), norank_o__Chloroplast (7.3% in W1, 6.6% in W2), and Comamonadaceae (5.3% in W1, 13.7% in W2); the other families accounted for less than 5%. In contrast, the total relative abundances of the abovementioned families were only 0.8% in S1 and 1.5% in S2. The most abundant taxa were Steroidobacteraceae (9.5%, Proteobacteria-affiliated denitrifiers) (Liu et al. 2019) in S1 and Anaerolineaceae (6.9%, Chloroflexi-affiliated anaerobe) (Qian et al. 2017) in S2. In particular, the fractions of Nitrosomonadaceae as ammonia-oxidizing bacteria were stable at 1.0–1.5% in group S2 at various depths, whereas those in group S1 experienced a decrease from 1.1 to 0.4% with increasing depth (Fig. 6b).
Correlation analysis of bacterial communities and environmental variables
As shown in Fig. 7a, 14 of the top 16 bacterial families (including Rhodocyclaceae, Halieaceae and Comamonadaceae) in the water samples (W1 and W2) exhibited positive correlations (red lines) with the u value, as the same as TOC, DO, TN, NH4+-N, and TP concentrations in water. The correlations between sediment bacterial communities and water environmental factors were more complex (Fig. 7b), and 7 (such as Pseudomonadaceae, Anaerolineae, and Geothermobacteraceae) and 11 (such as Desulfosarcinaceae, Gemmatimonadaceae. and Vicinamibacteraceae) of the top 18 bacterial families in the surface sediments (S1-1 and S2-1) showed positive and negative correlations with the u value, respectively. As described in previous reports (Li et al. 2015, 2020a; Lei et al. 2020), the hydrodynamic conditions determined the water microbial communities by significantly influencing the DO level and the oxidation–reduction potential. Thus, the combination of aeration and push flow can improve the hydrodynamic conditions and self-purification capacity of rivers in eco-remediation engineering (Sun et al. 2022).
According to the results of the correlation analysis (Fig. 7c), the assembly of sediment bacterial communities was mainly dependent on the u value and the toxic substance (heavy metals) rather than the contents of organics (TOC) and nutrients (TN and TP). Several previous reports have demonstrated that heavy metals transported along rivers are associated with colloids (Ning et al. 2014), and the complex flow field in the channel confluences benefits the settling of heavy metals into the sediments. In the present study, the higher u value combined with shipping disturbance promoted the growth of Pseudomonadaceae, Nitrosomonadaceae, norank_c__P9X2b3D02, and norank_o__RBG-13–54-9, but suppressed that of Steroidobacteraceae, Sutterellaceae, and SC-I-84 in the sediments. The families Steroidobacteraceae, Sutterellaceae, SC-I-84, and Clostridiaceae exhibited a high resistance capacity to Cr and Cu and were sensitive to the presence of Cd, Pb, and As. Pseudomonadaceae, Nitrosomonadaceae, and norank_c__P9X2b3D02 showed the opposite responses to these heavy metals, indicating that changes in the specific bacterial community can be used as indicators of excess exposure to heavy metals, such as Pseudomonadaceae for Cd (Wang et al. 2021) and Steroidobacteraceae for Cd, Cu, Pb, and Ni (Nyoyoko 2022). Phosphorous is often considered a critical growth-limiting substrate of sediment microbes in freshwater bodies (Shao et al. 2011), and TP and TOC showed more significant influences on the bacterial families than the TN contents (Fig. 7c). In polluted urban rivers, Spirochaetaceae (hydrolytic microorganisms) (Avila et al. 2019) and Rhodocyclaceae (denitrifying phosphate accumulating organisms, DPAOs)(Zhang et al. 2021) exhibited different growth trends, which could be explained by the phosphate production via organic solid hydrolysis and the phosphate release of DPAOs under anoxic conditions. In this context, the effects of different P forms on microbial growth in sediments deserve further elaboration.
Predictive analysis of functional gene category
According to high-throughput sequencing of the samples, predictive analyses of the top 30 functional gene categories involved in carbon (15 categories), sulfur (3 categories), nitrogen (6 categories), and chlorine (1 category) cycles; human diseases (2 categories); and cellular processes (3 categories) were conducted using the FAPROTAX tool. As shown in Fig. 8 and Table S2, the water had a greater metabolic potential for carbon cycling (organics metabolism and degradation) than the sediment in terms of dark_hydrogen_oxidation, methanol_oxidation, oxygenic_photoautotrophy, cyanobacteria, photoautotrophy, phototrophy, and chloroplasts. Both water and sediments exhibited comparable gene abundance in aerobic_chemoheterotrophy, chemoheterotrophy, methylotrophy, methanotrophy, and hydrocarbon_degradation. Similar to fermentation under anoxic conditions (Perez-Esteban et al. 2022), sulfur-related metabolism preferably occurred in the sediments, including dark_sulfide_oxidation, dark_oxidation_of_sulfur_compounds, and respiration_of_sulfur_compounds, whereas the transformations between organic sulfur and inorganic sulfide were considered as the critical reason for the black-odor phenomena in polluted water bodies (Cai et al. 2019; Qin et al. 2019; Jiang et al. 2021). Similar to the findings of Cai et al. (2021), in this study, there were no significant differences in the distribution of functional genes at the varying depths. Although the gene abundance of nitrite_ammonification and nitrite_respiration in the water was significantly lower than that in the sediments, W2 exhibited gene levels of nitrate_respiration, nitrate_reduction, nitrogen_respiration, and nitrogen_fixation similar to those of the surface sediments (S1-1 and S2-1). Because of the weak homogenization of static water, environmental selection resulted in lower metabolic potentials of W1 than W2 for nitrification processes (Massara et al. 2017; Li et al. 2020c). Compared to the sediments, the water samples (especially W2) had higher abundances of genes for human disease vector and cellular process (animal_parasites_or_symbionts). The inputs of upstream runoff were regarded as the main origin of pathogens in urban rivers (Sun et al. 2022). Considering the widespread residential areas, agricultural land, and ports in the upstream and busy shipping transport in the channel, it was necessary to strictly control the unorganized discharging of livestock and poultry farming, distributed domestic wastewater, and ship sewage.
Conclusion
The results of this study provide evidence for the bacterial community distribution in the downstream section of urban channel confluences, closely associated with the pollution levels and hydrodynamic conditions. In comparison to the water, the sediments exhibited a higher bacterial diversity and richness. The larger u value in the main channel (P2) resulted in a stronger homogenizing dispersal compared to P1, which was close to the riverbank; the latter showed a higher dispersion degree in the bacterial communities. The slow water flow not only promoted the settling of sediments in the static water zone with the lower DO level but also diversified the sediment bacterial community at different depths, thereby influencing nitrogen metabolism and sulfur reduction processes. In addition to the u value, the TP and heavy metal contents also impacted the bacterial community in the same section. According to the predictive analysis of functional gene categories, the water had a greater metabolic potential for carbon cycling, and urban rivers potentially contain human pathogens. Regarding engineering, the combination of upstream runoff control and eco-remediation technologies (such as channel cleanout, plug flow aeration, and ship sewage treatment) is an effective approach for improving water quality.
Data availability
All data generated or analyzed during this study are included in this published article.
References
American Public Health Association (APHA) (1998) Standard methods for examination of water and wastewater. 20th ed
Avila MP, Brandao LPM, Brighenti LS, Tonetta D, Reis MP, Staehr PA, Asmala E, Amado AM, Barbosa FAR, Bezerra-Neto JF, Nascimento AMA (2019) Linking shifts in bacterial community with changes in dissolved organic matter pool in a tropical lake. Sci Total Environ 672:990–1003
Bao SJ, CAP (2008) Soil agro-chemistrical analysis. China Agric Press 585:586
Burton GA, Johnston EL (2010) Assessing contaminated sediments in the context of multiple stressors. Environ Toxicol Chem 29(12):2625–2643
Cai W, Li Y, Shen Y, Wang C, Wang P, Wang L, Niu L, Zhang W (2019) Vertical distribution and assemblages of microbial communities and their potential effects on sulfur metabolism in a black-odor urban river. J Environ Manage 235:368–376
Cai W, Li Y, Hu J, Cheng H (2021) Exploring the microbial ecological functions in response to vertical gradients in a polluted urban river. Clean-Soil Air Water 49(9):2100004
Cieniawski S, Macdonald DD, Ingersoll CG (2002) A guidance manual to support the assessment of contaminated sediments in freshwater ecosystems. US Environmental Protection Agency, Great Lakes National Program Office
Crump BC, Amaral-Zettler LA, Kling GW (2012) Microbial diversity in arctic freshwaters is structured by inoculation of microbes from soils. ISME J 6(9):1629–1639
Deveautour C, Rojas-Pinzon PA, Veloso M, Rambaud J, Duff AM, Wall D, Carolan R, Philippot L, Richards KG, O’Flaherty V, Brennan F (2022) Biotic and abiotic predictors of potential N2O emissions from denitrification in Irish grasslands soils: a national-scale field study. Soil Biol Biochem 168:108637
Gao Y, Zhang W, Li Y (2021) Microbial community coalescence: does it matter in the Three Gorges Reservoir? Water Res 205:117638
General Office of Jiangsu Provincial Government (GOJPG) (2021) Jiangsu Province's “14th Five-Year Plan” for ecological environmental protection (2021–2025)
Haller L, Tonolla M, Zopfi J, Peduzzi R, Wildi W, Pote J (2011) Composition of bacterial and archaeal communities in freshwater sediments with different contamination levels (Lake Geneva, Switzerland). Water Res 45(3):1213–1228
Hollister EB, Engledow AS, Hammett AJ, Provin TL, Wilkinson HH, Gentry TJ (2010) Shifts in microbial community structure along an ecological gradient of hypersaline soils and sediments. ISME J 4(6):829–838
Huguet A, Vacher L, Relexans S, Saubusse S, Froidefond JM, Parlanti E (2009) Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org Geochem 40(6):706–719
Hui C, Li Y, Zhang W, Yang W, Wang W, Gao Y, Niu L, Wang L, Zhang H (2021) Coupling genomics and hydraulic information to predict the nitrogen dynamics in a channel confluence. Environ Sci Technol 55(8):4616–4628
Hui C, Li Y, Liao Z, Zhang W, Zhang H, Niu L, Wang L (2022) Confluences characteristics determine the influence scope of microbial community from confluence hydrodynamic zone on river network. J Hydrol 612:128288
Ji B, Shi Y, Yılmaz M (2022) Microalgal-bacterial granular sludge process for sustainable municipal wastewater treatment: simple organics versus complex organics. J Water Proc Eng 46:102613
Jiang M, Sheng Y, Liu Q, Wang W, Liu X (2021) Conversion mechanisms between organic sulfur and inorganic sulfur in surface sediments in coastal rivers. Sci Total Environ 752:141829
Lei M, Li Y, Zhang W, Niu L, Wang L, Zhang H (2020) Identifying ecological processes driving vertical and horizontal archaeal community assemblages in a contaminated urban river. Chemosphere 245:125615
Li Y, Wang C, Zhang W, Wang P, Niu L, Hou J, Wang J, Wang L (2015) Modeling the effects of hydrodynamic regimes on microbial communities within fluvial biofilms: combining deterministic and stochastic processes. Environ Sci Technol 49(21):12869–12878
Li Y, Hui C, Zhang W, Wang C, Niu L, Zhang H, Wang L (2020a) Integrating microbial community assembly and fluid kinetics to decouple nitrogen dynamics in an urban channel confluence. Environ Sci Technol 54(18):11237–11248
Li C, Quan Q, Gan Y, Dong J, Fang J, Wang L, Liu J (2020b) Effects of heavy metals on microbial communities in sediments and establishment of bioindicators based on microbial taxa and function for environmental monitoring and management. Sci Total Environ 749:141555
Li W, Zhuang JL, Zhou YY, Meng FG, Kang D, Zheng P, Shapleigh JP, Liu YD (2020c) Metagenomics reveals microbial community differences lead to differential nitrate production in anammox reactors with differing nitrogen loading rates. Water Res 169:115279
Liu T, Zhang AN, Wang J, Liu S, Jiang X, Dang C, Ma T, Liu S, Chen Q, Xie S, Zhang T, Ni J (2018) Integrated biogeography of planktonic and sedimentary bacterial communities in the Yangtze River. Microbiome 6(1):16
Liu Q, Liu HC, Zhou YG, Xin YH (2019) Stenotrophobium rhamnosiphilum gen. nov., sp. Nov., isolated from a glacier, proposal of Steroidobacteraceae fam nov. in Nevskiales and emended description of the family Nevskiaceae. Int J Syst Evol Microbiol 69(5):1404–1410
Massara TM, Malamis S, Guisasola A, Baeza JA, Noutsopoulos C, Katsou E (2017) A review on nitrous oxide (N2O) emissions during biological nutrient removal from municipal wastewater and sludge reject water. Sci Total Environ 596–597:106–123
Meziti A, Tsementzi D, Kormas KAR, Karayanni H, Konstantinidis KT (2016) Anthropogenic effects on bacterial diversity and function along a river-to-estuary gradient in Northwest Greece revealed by metagenomics. Environ Microbiol 18(12):4640–4652
Ning D, Huang Y, Pan R, Wang F, Wang H (2014) Effect of eco-remediation using planted floating bed system on nutrients and heavy metals in urban river water and sediment: a field study in China. Sci Total Environ 485–486:596–603
Nyoyoko VF (2022) Proteobacteria response to heavy metal pollution stress and their bioremediation potential. Cost Effective Technologies for Solid Waste and Wastewater Treatment. Elsevier, London, pp 147–159
Ohno T (2002) Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ Sci Technol 36(4):742–746
Perez-Esteban N, Vinardell S, Vidal-Antich C, Pena-Picola S, Chimenos JM, Peces M, Dosta J, Astals S (2022) Potential of anaerobic co-fermentation in wastewater treatments plants: a review. Sci Total Environ 813:152498
Qian F, Wang J, Shen Y, Wang Y, Wang S, Chen X (2017) Achieving high performance completely autotrophic nitrogen removal in a continuous granular sludge reactor. Biochem Eng J 118:97–104
Qian F, Gebreyesus AT, Wang J, Shen Y, Liu W, Xie L (2018) Single-stage autotrophic nitrogen removal process at high loading rate: granular reactor performance, kinetics, and microbial characterization. Appl Microbiol Biotechnol 102(5):2379–2389
Qian F, Luo J, Yin H, Liu F, Gao S, Gu X (2022) Carbonaceous composite membranes for peroxydisulfate activation to remove sulfamethoxazole in a real water matrix. Chemosphere 288:132597
Qin SS, Zhu MX, Yang GP, Wang D (2019) Atypical diagenesis of sulfur and iron in sediments of the river-dominated Bohai Sea (China). J Mar Syst 189:116–126
Qu Z, Liu B, Ma Y, Sun H (2020) Differences in bacterial community structure and potential functions among Eucalyptus plantations with different ages and species of trees. Appl Soil Ecol 149:103515
Shao K, Gao G, Qin B, Tang X, Wang Y, Chi K, Dai J (2011) Comparing sediment bacterial communities in the macrophyte-dominated and algae-dominated areas of eutrophic Lake Taihu. China Can J Microbiol 57(4):263–272
Soon YK, Kalra YP (1995) A comparison of plant tissue digestion methods for nitrogen and phosphorus analyses. Can J Soil Sci 75:243–245
Sun MY, Dafforn KA, Brown MV, Johnston EL (2012) Bacterial communities are sensitive indicators of contaminant stress. Mar Pollut Bull 64(5):1029–1038
Sun J, Lin Z, Ning D, Wang H, Zhang Z, He Z, Zhou J (2022) Functional microbial community structures and chemical properties indicated mechanisms and potential risks of urban river eco-remediation. Sci Total Environ 803:149868
Vareda JP, Valente AJM, Duraes L (2019) Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: a review. J Environ Manage 246:101–118
Wang S, Xu L, Ge S, Jiao J, Shu YJEI (2020) Driving force heterogeneity of urban PM2.5 pollution: Evidence from the Yangtze River Delta. China. Ecol Indic 113:106210
Wang Y, Duan X, Wang L (2020b) Spatial distribution and source analysis of heavy metals in soils influenced by industrial enterprise distribution: case study in Jiangsu Province. Sci Total Environ 710:134953
Wang M, Zhao S, Wang L, Chen S, Li S, Lei X, Sun X, Qin L (2021) Salt stress-induced changes in microbial community structures and metabolic processes result in increased soil cadmium availability. Sci Total Environ 782:147125
Wang Y, Li X, Li K, Huang Y, Yang H, Zhu P, Chi Z, Xu Y, Li Q (2022) Signature of dissolved organic matter and microbial communities based on different oxygen levels response during distillers dried grains with solubles plus sugarcane pith co-fermentations. Bioresour Technol 349:126868
Wu H, Li Y, Zhang J, Niu L, Zhang W, Cai W, Zhu X (2017) Sediment bacterial communities in a eutrophic lake influenced by multiple inflow-rivers. Environ Sci Pollut Res Int 24(24):19795–19806
Wu H, Li Y, Zhang W, Wang C, Wang P, Niu L, Du J, Gao Y (2019) Bacterial community composition and function shift with the aggravation of water quality in a heavily polluted river. J Environ Manage 237:433–441
Yang Y, Li S, Gao Y, Chen Y, Zhan A (2019) Environment-driven geographical distribution of bacterial communities and identification of indicator taxa in Songhua River. Ecol Indic 101:62–70
Yang N, Wang L, Lin L, Li Y, Zhang W, Niu L, Zhang H, Wang L (2021a) Pelagic-benthic coupling of the microbial food web modifies nutrient cycles along a cascade-dammed river. Front Environ Sci Eng 16(4):1–13
Yang Z, Zhou Q, Sun H, Jia L, Zhao L, Wu W (2021b) Metagenomic analyses of microbial structure and metabolic pathway in solid-phase denitrification systems for advanced nitrogen removal of wastewater treatment plant effluent: a pilot-scale study. Water Res 196:117067
Zhang Q, Zhang C, Zhu Y, Yuan C, Zhao T (2021) Effect of bacteria-to-algae volume ratio on treatment performance and microbial community of a novel heterotrophic nitrification-aerobic denitrification bacteria-chlorella symbiotic system. Bioresour Technol 342:126025
Zhang S, Li K, Hu J, Wang F, Chen D, Zhang Z, Li T, Li L, Tao J, Liu D, Che R (2022a) Distinct assembly mechanisms of microbial sub-communities with different rarity along the Nu River. J Soils Sediments 22(5):1530–1545
Zhang H, Yang L, Li Y, Wang C, Zhang W, Wang L, Niu L (2022b) Pollution gradients shape the co-occurrence networks and interactions of sedimentary bacterial communities in Taihu Lake, a shallow eutrophic lake. J Environ Manage 305:114380
Zhang W, Yang G, Wang H, Li Y, Niu L, Zhang H, Wang L (2022c) Predicting bend-induced heterogeneity in sediment microbial communities by integrating bacteria-based index of biotic integrity and supervised learning algorithms. J Environ Manage 304:114267
Acknowledgements
Authors acknowledge the support from Jiangsu Graduate Student Workstation (Suzhou Hongyu Environment Technology Co., LTD.), China.
Funding
This study was supported by the Natural Science Foundation of Jiangsu Province, China (No. BK20211339); Natural Science Foundation of Jiangsu High Education, China (No. 21KJB610016); Suzhou Social Development Science and Technology Innovation Project, China (No. SS202114); and Suzhou Construction Technology Project, China (No. SZJ202129503).
Author information
Authors and Affiliations
Contributions
Xin Jin: writing—original draft, data curation. Jing Jiang: writing—original draft. Lei Zhang: data curation, writing—review and editing. Guangyu Shi: writing—review and editing. Xueyan Li: Supervision, writing—review and editing. Longfei Zhang: data curation. Xuyu Chen: data curation. Feiyue Qian: resources, conceptualization, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Robert Duran
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xin Jin and Jing Jiang contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jin, X., Jiang, J., Zhang, L. et al. Analysis of bacterial community distribution characteristics in the downstream section of a cross confluence in a polluted urban channel. Environ Sci Pollut Res 30, 43677–43689 (2023). https://doi.org/10.1007/s11356-023-25462-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25462-2