Abstract
A potential ecological risk assessment was conducted based on the analyzing results of the typical pollutants (Pb, Cu, Cr, Cd, and Zn) contents in the sediments of Yangtze River within the Wanzhou section using the index number techniques of single factor and Hakanson method for the quality status of the sediments. The results indicted that cadmium (Cd) had the largest pollution index and was the main pollution factor among the metals. The ecological risk sequence of the metals was Cd > Zn >Pb > Cu >Cr, while the sequence of the potential ecological risk posed by the metals was Cd > Pb > Cu> Zn >Cr. The index range of potential ecological risk was from 101.39 to 184.31, and the average index of potential ecological risk factors (RI) was 152.35. The Yangtze River within the Wanzhou section has a middle potential ecological risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As an important part of environment pollution, heavy metal pollution in soil and water has become an important environmental issue in many countries [1]. Heavy metals added to an aquatic system by natural and manmade sources during their transport are distributed among different compartments of aquatic ecosystems, such as water, sediment, and biota [2]. During the transportation, heavy metals are firstly dissolved in water, then adsorbed, and finally accumulated on the bottom sediments acting as a sink [3]. In addition, heavy metals of anthropogenic origin are generally introduced into the environment as inorganic complexes or hydrated ions, which are easily adsorbed on the surface of sediment particles through relatively weak physical and chemical bonds [4]. In the bottom sediments, the distribution of heavy metals is affected by mineralogical and chemical composition of suspended material, anthropogenic influences, and situational processes such as deposition, sorption, enrichment in organism, etc. [5]. Thus, in the natural conditions of the aquatic system, suspended and bed sediments play an important role in buffering higher metal concentrations of water particularly by adsorption and coprecipitation [6–8].
It is now widely accepted and realized that the role of aquatic sediments as a sink or a source of pollutants cannot be fully assessed by measuring total heavy metal concentrations. The toxicity and fate of the water borne metal are dependent on its chemical form; therefore, quantifications of the different forms of metals are more meaningful than the estimation of its total metal concentrations [7]. The heavy metal pollution in sediment has been studied by many groups. Fytianos and Lourantou [9] investigated the speciation of trace elements in sediment samples of the Volvi and the Koronia lakes in N. Chandra Sekhar [10] studied fractionation and bioaccumulation of sediment bound heavy metals in the Kolleru Lake by edible fish and reported that fish of the Kolleru Lake are contaminated with metals. Singh studied distribution and fractionation of heavy metals in Gomti River sediments and reported that cadmium lead pose high risk to the environment [11]. At the same time, several studies have also been carried out on the accumulation effects of metals in environment under different drainage basins in China [12–14]. However, previous studies focused mainly on the metal contaminations in soils caused by farming, industry and urban development, and little efforts have been performed on the metal contamination of soils in the ecologically sensitive areas, especially for the Chinese Three Gorges areas.
The objective of the present study is to evaluate the quality status of the sediments in the Wanzhou section of the Yangtze River for the Three Gorge Area’s environmental protection. As a most important and sensitive city, Wanzhou is situated in the center of the Three Gorges reservoir area and has become a most important area of pollutant influx for research after the construction of the Three Gorges dam since there are more than 2,000 sewage draining exiting in upper reach of the Yangtze River every year. In the present study, the contents of typical pollutants (Pb, Cu, Cr, Cd, and Zn) in sediments were analyzed, and the ecological risk of metal contaminants was assessed using the index number techniques of single factor and Hakanson ecological risk.
Materials and Methods
Site Description and Sediments Sampling
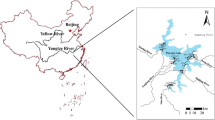
A national control cross-section (Shaiwang Dike) and two civic control cross-sections (Nanmenkou and Yangtze River Bridge) are situated in the Wanzhou section of the Yangtze River. At the same time, Zhuxi River as a chief branch of the Yangtze River within the Wanzhou section has a basin area of 228.8 km2 and intersects with the Yangtze River in Nanmenkou. At present, Zhuxi River has been polluted seriously and confirmed as one of 12 serious secondary grade rivers by the national environmental protection department of China (Fig. 1).
According to the channel transverse, hydrological condition, sewage outfall distribution, and branch status, experimental samples are collected from eight cross-sections (the Yangtze River Bridge, Panpanshi, Nanmenkou, Tianxianhu Dike, Wanzhou Bridge, Wanyi Bridge, the Second Bridge of the Yangtze River, and Shaiwang Dike) in the Wanzhou section of the Yangtze River and Zhuxi River. The samples are collected within 2–5 cm of top layers and 5–10 cm of bottom layers separately. The sampling depth is about 30 cm, and three bottom-mud samples are collected in each cross-section. All the samples are preserved in polyethylene bags.
Analysis Method
Firstly, the samples are air-dried in a dark and ventilative place. Then, the gravels, shells, weeds, and so on are winkled out [15]. Finally, the samples are spread on an organic glass, pressed to pass 20-item selections completely [16]. The griddled samples are divided to 5–10 g by four-cent method and skived by agate mortar then passed the 100 nylon griddle by discarding sundries. The griddled samples are put in a labeled palm jar and preserved frozenly.
Samples Analysis
A Perkin Elmer Instruments (Shelton, USA) model Analyst 200 flame atomic absorption spectrometer was used for the determination of heavy metal in the sediments sample. Accurately known mass of approximately 2.0 g of Sediments sample was placed in PTFE vessel and 20 ml of 1:1 (v/v) nitric acid solution were added. Afterwards, the vessel was closed and put into a pressurized digestion system. The thermal heating was carried out in a stove at 150°C for 6 h. The resultant solution was allowed to cool. After cooling at room temperature, these solutions were adjusted to pH with a 10% (w/v) sodium hydroxide. The solution was made up to required volume with deionized water into a 50-mL volumetric flask. The same procedure was used for the blank solutions. Spiked samples were also prepared in order to calculate the recovery of the digestion procedure.
Standard reference materials obtained from the Center of National Standard Reference Material of China are analyzed as part of the quality assurance and quality control (QA/QC) procedures. Rigorous QA/QC protocols include inserting of “blind” standard reference materials for determination of the accuracy of the methods and analytical duplicates to allow estimation of the precision of the method. Satisfactory recoveries are obtained for Cd (93–98%), Cr (98–103%), Pb (94–106%), Zn (96–102%), and Zn (96–103%).
Theory Base of Evaluation on Potential Ecological Risk
In the present study, Hakanson’s ecological risk method [3, 17] is used to evaluate the potential ecological risk of metal contaminants in sediments. In 1980, Lars Hakanson reported an ecological risk index for aquatic pollution control; therefore, Hakanson’s method has been often used in ecological risk assessment as a diagnostic tool to penetrate one of many possible avenues towards a potential ecological risk index, i.e., to sort out which drainage area, reservoir, and substances should be given special attention. The method is based on the hypothesis that a sediment ecological risk index for toxic substances in limnic systems should account for the following requirements: (1) the potential ecological risk index (RI) increases with the metal pollution increase in sediments; (2) the ecological harms of different heavy medals in sediments have cooperativity, and the potential ecological risk of the cooperative harm is more serious, especially for Cu, Zn, Pb, Cd, and Cr; and (3) toxicity response of each heavy metal element is different, and those metals whose biologic toxicity are strong have larger proportion in RI.
On the premise mentioned above, the index is calculated as the following equations:
In which, \(C_f^i \) is the pollution coefficient of single metal; \(C_D^i \) is the measured concentration of sample; \(C_R^i \) is the background concentration of sediments; C H is the polluted coefficient of many metals; \(E_f^i \) is the potential ecological risk factor of single metal; \(T_f^i \) is the biological toxicity factor of different metals; and RI is the potential ecological risk index of many metals.
According to the bibliography [18–19] and characters of metal pollution in the canal (Wanzhou section), the highest pre-industrial background concentration of global sediments is selected as the reference ratio in this study. At the same time, seven biological toxicity factors of heavy metals (\(T_f^i \)) are defined, which are shown in Table 1.
Results and Discussion
Evaluation on Heavy Metal Pollution in Sediments
The chemical speciations and average contents of the heavy metals are presented in Table 2. From Table 2, high Cd and Zn concentrations are found in the acid soluble fraction (F1). The Cd and Zn contents of F1 are in the ranges of 39–69% and 37–62%, respectively. Regarding Pb, the proportion of the reducible fraction (F2; 43–72%) is significantly higher than that of other metals. The residual fraction (F4) of Cr and Cu takes a predominant part for all samples. The oxidizable fraction (F3) only takes a minor role for all five heavy metals. At the same time, it also can be seen that Zn has the highest average content in all the sediments, and the average content sequence of the metals in the sediments is Zn > Pb > Cu > Cr > Cd. Although Cd has the lowest average content, compared with the background concentration shown in Table 1, it has the highest increasing rate.
According to Hakanson’s ecological risk index method, the heavy metal polluted elements in sediment samples are analyzed and evaluated. In this study, only five polluted elements (Cu, Pb, Zn, Cd, and Cr) are investigated, which are less than eight elements required for Hakanson’s method. Therefore, the integrated pollution degree (C H ) is defined as following: C H < 5, low-pollution; 5 ≤ C H < 10, middle-pollution; 10 ≤ C H < 20, high-pollution; C H ≥ 20, higher-pollution.
The pollution index and integrated pollution index are used to evaluate pollution of the sediment heavy metals in the Wanzhou section of the Yangtze Rive, and the evaluation results are shown in Table 3. As shown in Table 3, it is evident that Cd has the largest pollution index value, suggesting that Cd is the most important pollution factor among heavy metals in the Yangtze River within Wanzhou section, and the pollution factor sequence of the heavy metals is Cd > Zn > Pb > Cu > Cr. In addition, the results also indicate that the heavy metals contents in sediments are up to middle-pollution in the 3, 4, 5, and 6 sampling sites and moreover up to high-pollution in the 1, 2, 7, and 8 sampling sites. It also can be seen that the heavy metal pollution in the Wanzhou section of the Yangtze River is much serious, and the integrated pollution indexes (C H ) of the 1, 2 sampling sites which are situated in the upper stream are higher than those of the 7, 8 sampling sites which are situated in the lower stream and the 3, 4, 5, and 6 cross-sections of the Zhuxi River. This result suggests that the reason for heavy metal pollution is the upper streams’ pollution source; and the Zhuxi River has little relation with heavy metal pollution in the Wanzhou section of the Yangtze River, though it has always been seen as a serious pollution factor.
Evaluation on Heavy Metal Ecological Risk in Sediments
According to Hakanson’s method [20], the contents of Cd, Pb, Cu, Cr, and Zn in marsh sediments are measured, and pollution degree and potent ecological risk are analyzed by the use of potent ecological risk index method [21]. The evaluated standards for the analyzing potential ecological risk factor (\(E_f^i \)) and potential ecological RI are given in Table 4.
The results of evaluation on potential risk of heavy metal pollution in sediments are summarized in Table 5. From Table 5, it can be seen that the order of potential ecological risk factor of heavy metal in sediments of the Wanzhou section of the Yangtze River is Cd > Pb > Cu > Zn > Cr; Cd is the most important one and its risk factor is up to the appreciable grade in all the cross-sections, and the potential ecological risk grades of Cd in almost all the cross-sections reaches middle grade except for the two cross-sections. The results indicate that the range of Cd pollution in the Wanzhou section of theYangtze Rive is wide.
Conclusions
The contents of Cd, Pb, Cu, and Zn in the sediments of the Yangtze River within the Wanzhou section were analyzed, and the potential ecological risk of the sediments was assessed using the single factor index method and Hakanson’s ecological risk index method in this study. The sediments of the Yangtze River within the Wanzhou section are high pollution and those of the Zhuxu River are middle pollution. The heavy metal pollution is from the upper streams. The Zhuxi River within Wanzhou section has low heavy metal contents, though it is often thought as a serious pollution factor. The range of potential ecological RI is from 101.39 to 184.31, and the average RI is 152.35. Most cross-sections of the Yangtze River within the Wanzhou section are in middle potential ecological risk.
References
Heikens A, Peijenburg WJGM, Hendriks AJ (2001) Bioaccumulation of heavy metals in terrestrial invertebrates. Environ Pollut 113:385–393
Moore JW, Ramamoorthy S (1985) Heavy metals in natural waters, Applied monitoring and impact assessment. J Hydrol 80(1–2):192–193
Hakanson L (1980) An ecological risk index for aquatic pollution control—a sediment ecological approach. Water Res 14:975–1001
Pempkowiase J, Sikora A, Biemacka E (1999) Speciation of heavy metals in marine sediments vs. their bioaccumulation by Mussels. Chemosphere 39(2):313–321
Jain CK, Singhal DC, Sharma MK (2005) Metal pollution assessment of sediment and water in the river Hindon, India. Environ Monit Assess 105(1–3):193–207
Jain CK, Sharma MK (2002) Adsorption of cadmium on bed sediments of river Hindon: Adsorption models and kinetics. Water, Air Soil Pollut 137:1–19
Jain CK, Singhal DC, Sharma MK (2004) Adsorption of zinc on bed sediment of River Hindon: Absorption models and kinetics.2004. J Hazard Mater 114(1–3):231–239
Gyula L, Erno F, Ilona M et al (2003) Ecotoxicological studies and risk assessment on cyanide contamination in Tisza river. Toxicol Lett 140–141:333–342
Fytianos K, Lourantou A (2004) Speciation of elements in sediment samples collected at lakes Volvi and Koronia, N. Greece. Environ Int 30(1):11–17
Chandra Sekhar K, Chary NS, Kamala CT et al (2004) Fractionation studies and bioaccumulation of sediment bound heavy metals in Kolleru lake by edible fish. Environ Int 29(7):1001–1008
Singh KP, Mohan D, Singh VK, Malik A (2005) Studies on distribution and fractionation of heavy metals in Gomti river sediments – a tributary of the Ganges. India. J Hydrol 312:14–27
Zhou X, Zheng J, Zhang S et al (2005) Heavy Metals Distribution of Soils in Water level fluctuating Zone of the Three Gorges Reservoir. Environmental Monitoring in China 27:470–473
Li Z, Ningjing H, Sherriff B et al (2006) Ecological risk assessment for heavy metal contamination of a new Consideration Agricultural soils. Chinese Journal of Geochemistry 25:123–129
Teng Y, Ni S, Zheng C et al (2006) Environmental geochemistry and ecological risk of vanadium pollution in Panzhihua mining and smelting area, Sichuan, China. Chinese Journal of Geochemistry 25:378–384
Chen CS (2005) Ecological risk assessment for aquatic species exposed to contaminants in Keelung River, Taiwan. Chemosphere 61:1142–1158
Joo AY (2004) Soil ecotoxicity assessment using cadmium sensitive plants. Environmental Pollution 127:21–26
Liu EF, Ji S, Yang LY (2007) Chemical fractionation and pollution characteristics of heavy metals in the sediment of nansihu Lake and its main inflow rivers. China. Environ Sci 28(6):1377–1382
Hakan P, Duran K, Savas A et al (2004) Ecological risk assessment using trace elements from surface sediments of Izmit Bay (Northeastern Marmara Sea) Turkey. Mar Pollut Bull 48:946–953
Sekhar C, Chary NS, Kamala CT et al (2005) Environmental Pathway and Risk Assessment Studies of the Musi River’s Heavy Metal Contamination—A Case Study. Hum Ecol Risk Assess 11(6):1217–1222
Johansson H, Lindstrom M, Hakanson L (2001) On the modelling of the particulate and dissolved fractions of substances in aquatic ecosystems—sedimentological and ecological interactions. Ecol Model 137(2):225–240
Puente A, Juanes JA, García A et al (2008) Ecological assessment of soft bottom benthic communities in northern Spanish estuaries. Ecological Indicators 8(4):373–388
Acknowledgements
This research project is funded and supported by The Light of Spring Plans Project of the Ministry of Education of China, and The Chongqing Science and Research Commission. The authors thank the members of the Steering Group for their offer of assistance and guidance to the research program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, C., Guo, J., Pan, J. et al. Potential Ecological Risk Assessment of Heavy Metal Pollution in Sediments of the Yangtze River Within the Wanzhou Section, China. Biol Trace Elem Res 129, 270–277 (2009). https://doi.org/10.1007/s12011-008-8300-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-008-8300-y