Abstract
Combination of the treatment of effluents with high organic loads and the production of electricity is the driving forces stimulating the development of microbial fuel cells (MFC). The increase in electricity production in MFCs requires not only the optimization of the operational parameters but also the inhibition of the metabolic pathways, which compete with electricity production, such as methanogenesis. The presence of both sulphate and sulphide ions in conventional anaerobic reactors hampers the growth of methanogenic archaea and justifies the use of sulphate and therefore sulphate-reducing bacteria (SRB) in the anodic half-cell of MFC. Most importantly, the literature on the subject reveals that SRB are able to directly transfer electrons to solid electrodes, enabling the production of electrical energy. This technology is versatile because it associates the removal of both sulphate and the chemical oxygen demand (COD) with the production of electricity. Therefore, the current work revises the main aspects related to the inoculation of MFC with SRB focusing on (i) the microbial interactions in the anodic chamber, (ii) the electron transfer pathways to the solid anode, and also (iii) the sulphate and COD removal yields along with the electricity production efficiencies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Following an increasing awareness of the effects of fossil fuels on global warming, new and alternative energy-producing technologies have been intensively researched. Another important concept in modern society is the circular economy, which has changed the perception of residues and effluents, now regarded as valuable resources (Goglio et al. 2019; Manzano-Agugliaro et al. 2013). Both principles have induced the development of microbial fuel cells (MFC) aiming at producing electricity from the anaerobic oxidation of organic matter (Pandey et al. 2016). A large-scale utilization of MFCs is still beyond reach, but energy recovery during wastewater treatment is most likely to happen in the near future (Logan 2009).
An MFC layout comprises two electrically connected chambers. In the anodic half-cell, a biologically mediated oxidation reaction produces electrons, which are externally transferred to the cathodic half-cell where they are used in either a chemical or biological semi-reaction of reduction. An MFC also contains a proton- or cation-exchange membrane and an external resistance (Logan et al. 2006). The growing interest in such technology does not only derive from its potential to produce electricity but also as a more environmentally-friendly technology than anaerobic digestion or any other common effluent treatment process (Pandey et al. 2016).
In addition to organic matter, industrial wastewaters may also contain a series of inorganic pollutants, such as sulphate ions, which need to be removed during effluent treatment. Sulphate-bearing effluents have been produced by several industries, such as electroplating, pulp and paper, pigments, rubber, explosives, fertilizers, and mining-metallurgy (Lens et al. 1998; Sarti et al. 2008). Despite its low toxicity, sulphate levels in wastewaters are regulated and a maximum contaminant level-between 250 and 500 mg L−1 is set by several countries. Sometimes the content of sulphate is regulated through a limit on total dissolved solids (TDS) (Nascimento 1998). Among the alternatives to remove sulphate from wastewaters, biotechnological processes applying sulphate-reducing bacteria (SRB) have been extensively investigated (Bertolino et al. 2012; Bertolino et al. 2015; Hu et al. 2020; Kaksonen et al. 2006; Kaksonen and Puhakka 2007; Luo et al. 2020). These microorganisms utilize sulphate as final electron acceptors resulting in the production of sulphide ions (Liamleam and Annachhatre 2007). Recently, it has been reported that sulphate-reducing bacteria (SRB) are electroactive bacteria (EAB), i.e. SRB are able to transfer electrons directly to an electrode rather than to a redox couple, which is an important mechanism in MFC (Kang et al. 2014; Zhou et al. 2013). This justifies the interest in coupling sulphate reduction and electricity production in these devices.
The first studies addressing sulphate reduction in the context of MFCs speculated the electroactive nature of SRB inoculated in the anodic half-cell (Agostino and Rosenbaum 2018; Hu et al. 2019; Liang et al. 2013a; Zheng et al. 2014). However, another application has also been proposed for the role of SRB. In this latter mechanism, the bacteria accept electrons directly from cathodes to convert carbon dioxide into organic substances (Agostino and Rosenbaum 2018; Luo et al. 2020). Therefore, electro-autotrophic SRB may also function as biocathodes in the production of sulphide ions, and this electrochemical device is known as microbial electrolysis cells (MEC) (Gacitúa et al. 2018; Luo et al. 2014a). Despite the several studies investigating the SRB role in MEC, the current work focuses only on anodic half-cells inoculated with SRB, which play an indisputable role on the generation of electricity (Hu et al. 2019). Specifically, the syntrophic interactions of the microbial consortium, the electron-transfer pathways in the anodic hall-cell, the oxidation of the carbon source, sulphate reduction, and the production of electricity in MFC will be revised.
Microbial fuel cells
Usually, heterogenic bacteria produce energy-rich compounds, such as ATP (used to carry out biological work) via the oxidation of nutrient molecules. The electrons produced during cellular metabolism are transferred to a chemical acceptor such as O2, NO3−, and SO4−2 during cellular respiration (Nelson and Cox 2012; Tortora et al. 2010). Some bacteria in particular can utilize ferric iron as the final electron acceptor, which is found in nature mostly as an insoluble compound. Therefore, it was proposed that ferric iron reduction occurred outside the cell (Lovley 2006). Based on the observation of this phenomenon, the direct electron transfer to solid electrodes by microorganisms was hypothesized. Accordingly, a technology could be devised by which bacteria would transfer electrons from an electron donor to an artificial solid device, producing electricity (Madigan et al. 2015). These microorganisms are classified as either exoelectrogenic or electroactive (Logan 2009).
Electroactive microorganisms have been identified in a wide variety of ecosystems, such as soil, sediment, seawater, and freshwater and also in samples collected from several environments having a diverse microbial community (sewage, activated sludge, industrial and domestic effluents) (Logan 2009). Specifically, these bacterial strains belong to the genera Geobacter sp., Rhodoferax sp., Shewanella sp., Pseudomonas sp., Arcobacter sp., Clostridium sp., Ochrobactrum sp., and Desulfovibrio sp. (Mathuriya 2014; Santoro et al. 2017). Such microorganisms can be technologically applied in a bio-electrochemical reactor, in which electrons are transported to (and from) a solid material (electrode), such as a MFC (Zhou et al. 2013). Such devices can therefore be applied to oxidize reduced species in liquid effluents and produce electricity simultaneously (Friedman et al. 2013). The main argument supporting the development of such technology is to improve sustainability (reducing treatment costs and energy consumption) of effluent treatment operations, particularly those containing moderate to high organic loadings (Santoro et al. 2017).
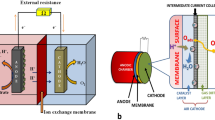
In a typical MFC (Fig. 1), the anodic and cathodic chambers are separated by an ion exchange membrane. In the anodic chamber, the microbial cells in the biofilm oxidize a substrate to produce (i) protons (H+), (ii) oxidized species, such as CO2, and (iii) electrons, which are then transferred to the solid electrode. In addition, as the proton concentration increases in the anodic chamber, they are transferred to the cathodic compartment through the membrane. The electrons in the anode are shuttled to the negative electrode (cathode) wherein species such as oxygen, protons, ferricyanide ions, and hydrogen peroxide are reduced (He et al. 2017).
Some advantages of applying MFC in wastewater treatment are as follows (He et al. 2017; Mathuriya 2014; Santoro et al. 2017): (i) direct conversion of chemical energy in wastewaters into electricity; (ii) smaller production of biomass, even when compared with conventional anaerobic processes; (iii) aeration is eliminated; and (iv) it can be potentially used in places short of electricity supply. In addition, COD removal in MFC may attain values over 80% (Logan 2009; Santoro et al. 2017).
Extensive studies have been carried out in lab-scale in order to improve the energy efficiency of MFCs so that a large-scale use in wastewater treatment becomes feasible, in the treatment of both domestic (Lovley 2008; Tice and Kim 2014) and industrial wastewaters, in particular those containing toxic metals (Luo et al. 2014b; Tao et al. 2014), azo dyes (Khan et al. 2015), residues produced by the oil industry (Jiang et al. 2013; Li et al. 2015), acid mine drainage (Cheng et al. 2007; Hai et al. 2016), phenol and compounds (Feng et al. 2015), and pyridine-related products (Mathuriya 2014). Its potential incorporation to existing industrial operations will require the following: (i) improvement in energetic efficiency, (ii) relative stability in energy production, and also (iii) a reduction in capital expenditure (Pant et al. 2011).
With respect to the electricity production and also the columbic efficiency (the fraction of electrons produced during oxidation, which effectively are transferred to the cathode thus producing electricity) of MFC, the microbial community plays a key role, requiring the presence of a biofilm containing exoelectrogenic species. Nevertheless, during the conversion of the organic matter, there is competition between these electroactive bacteria and methanogenic archaea (which are not electroactive) for the organic substrate, which results in a considerable reduction in electricity production and also in the columbic efficiency (Isosaari and Sillanpää 2017).
The presence of sulphide ions reduces methanogenesis in MFC because H2S can inhibit the growth of methanogenic archaea, justifying the inoculation of the latter with SRB (Chou et al. 2014; Chou et al. 2013; Isosaari and Sillanpää 2017; Liang et al. 2013a; Sangcharoen et al. 2015; Su et al. 2012; Weng and Lee 2015; Zhao et al. 2009). Moreover, as stated, SRB are capable of transferring electrons directly to the anode (Liang et al. 2013b; Zhao et al. 2009; Zhou et al. 2013). A second hypothesis is that there is a synergic pathway involving SRB and SOB (sulphide-oxidizing bacteria), whereby SOB oxidizes the sulphide produced by SRB to elemental sulphur, transferring electrons directly to the anode material (Chou et al. 2014; Chou et al. 2013; Weng and Lee 2015; Zhao et al. 2009). Nevertheless, this latter mechanism requires sulphide diffusion (and its oxidation) in the anode, implying that the direct electron transport mechanism plays a key role in electricity production (Murugan et al. 2018). Such mechanism will be detailed in the following paragraphs.
When applied in electrochemical system aiming at effluent treatment, the participation of other microbial species is mandatory to ensure a diverse microbial community, which is required to produce the syntrophic interactions required to degrade complex organic substrates and to generate electrical energy (Kokko et al. 2016). These interactions are discussed next.
Microbiology and biochemistry of bioelectricity generation
It has been extensively demonstrated that several microbial interactions are present in anaerobic reactors (Bertolino et al. 2012; Bertolino et al. 2015; Chernicharo 1997; Hu et al. 2020; Kaksonen et al. 2006; Kaksonen and Puhakka 2007). This consortium of microorganisms is responsible for the high efficiency of wastewater treatment by anaerobic processes aiming at oxidizing organic matter. In this treatment, macromolecules are first hydrolysed by hydrolytic microorganisms producing soluble species, which will be consumed by other bacteria. Fermentation will occur through the action of acidogenic bacteria on the low molecular weight (LMW) species produced. The presence of acetogenic bacteria is also important to maintain the H2 pressure in non-inhibitory concentrations. Usually, these fermentation products are consumed by methanogenic archaea, but SRB can play the same role when sulphate is present (Muyzer and Stams 2008).
The oxidation reactions of the LMW species and also hydrogen gas are represented by Eqs. (1), (2), (3), (4), (5), and (6). The electrons produced are used in the reduction of either oxygen (aerobic conditions) or other species such as NO3−, SO42-, CO2, and Fe3+ (anaerobic respiration), and in electrochemical systems, the electron transfer is mediated by two solid electrodes (Dong and Stams 1995; Kokko et al. 2016; Logan 2009; Lovley 2006; Madigan et al. 2015). In both anaerobic reactors and MFC, the degradation of these intermediary compounds reduces the Gibbs-free energy of the entire anaerobic organic matter oxidation process (Kokko et al. 2016).
SRB and other EAB can only oxidize low molecular weight substrates such as hydrogen gas, acetate, lactate, ethanol, and glycose, which are converted to H+ and electrons (Das and Mangwani 2010), whereas they lack the ability to degrade more complex molecules (Lovley 2006). However, the biochemical reactions occurring in the anodic cell in an MFC are similar to those observed in anaerobic reactors, as demonstrated by Kumar et al. (2017b), through molecular biology techniques. These authors found in the anode chamber of an MFC hydrolytic bacteria such as Aminobacterium sp. and fermentative microorganisms such as Clostridium sp., which convert monomers to organic acids according to Eqs. (7) and (8). The authors also detected SRB, such as Desulfovibrio sp. whose metabolism is represented by Eqs. (9), (10), (11), (12), (13), and (14), and also other exoelectrogenic bacteria, such as Aeromonas taiwanensis which use acetate as in Eq. (15). In addition, this study still found SOB, such as Tetrathiobacter kashmirensis and Desulfovibrio sulfodismutans, which oxidize sulphides to elemental sulphur (Eqs. (16), (17), and (18)). Furthermore, methanogenesis and denitrification processes compete with electricity generation, and these biochemical reactions should be inhibited so that a significant production of electricity is achieved (Das and Mangwani 2010; Kokko et al. 2018; Kumar et al. 2017a; Madigan et al. 2015). A general overview of the microbial interactions and metabolic products in an MFC is summarized in Fig. 2.
ΔE0 (V) | ||
|---|---|---|
3 CH3CH(OH)COO− ➔ CH3COO− + 2 CH3CH2COO− + HCO3− + H+ | 0.58 | (7) |
CH3CH2COO− + 3 H2O ➔ CH3COO− + HCO3− + H+ + 3 H2 | − 0.79 | (8) |
CH3COO− + SO4−2 ➔ 2 HCO3− + HS− | 0.49 | (9) |
CH3CH2COO− + 0.75 SO4−2 ➔ CH3COO− + HCO3− + 0.75 HS− + 0.25 H+ | 0.39 | (10) |
CH3CH2CH2COO− + 0.5 SO4−2 ➔ 2 CH3COO− + 0.5 HS− + 0.5 H+ | 0.29 | (11) |
CH3CH(OH)COO− + 0.5 SO4−2 ➔ CH3COO− + HCO3− + 0.5 HS− + 0.5 H+ | 0.83 | (12) |
4 H2 + SO4−2 + H+ ➔ HS− + 4 H2O | 1.57 | (13) |
SO4−2 + 4 HCOO− + H+ ➔ HS− + 4 HCO3− | 1.52 | (14) |
CH3COO− + 2 H2O ➔ 2 CO2 (g) + 7 H+ + 8e− | 0.30 | (15) |
H2S ➔ S0 + 2 H+ + 2e− | − 0.17 | (16) |
S−2 ➔ S0 + 2e− | 0.45 | (17) |
HS− ➔ S0 + 2e− + H+ | 0.06 | (18) |
Microbial consortium in the anodic chamber of a microbial fuel cell. 1, hydrolytic bacteria; 2, fermentative bacteria; 3, syntrophic bacteria; 4, exoelectrogens; 5, sulphate-reducing bacteria (SRB); 6, sulphide-oxidizing bacteria (SOB); 7, methanogens; 8, denitrifies. Circle indicates reactions that precede electricity production; diamond indicates electricity production reactions; square indicates reactions that compete with electricity production (This figure was devised based on references Madigan et al. 2015; Kumar et al. 2017b)
Therefore, the profile of the microbial species found in the anodic half-cell will be defined by parameters such as (i) composition of the effluent, (ii) electrode material, and (iii) operating conditions (Kokko et al. 2018).
Although the main biochemical reactions occurring in the anodic chamber are understood, the syntrophic and competitive interactions between different microbial strains should be investigated further on. In addition, new ways of inhibiting the metabolic pathways competing with the production of electricity are yet to be discovered (Bratkova et al. 2019).
Some authors report that in the anodic chamber of an MFC, sulphate ions may compete with the anode as the final electron acceptor during the metabolism of SRB (Habermann and Pommer 1991; Kokko et al. 2016). However, these microorganisms can still contribute to the generation of electricity by transferring electrons via the oxidation of organic matter to the electrode to the detriment of sulphate (Hu et al. 2019; Kang et al. 2014; Loukanov et al. 2019; Miran et al. 2018). Moreover, in terms of electricity production, sulphate reduction does not represent the main role of SRB in the anodic half-cell. Uppermost is the SRB role in (i) the oxidation of the organic substrate and (ii) contribution to biofilm formation as exopolysaccharides and filamentous proteins are excreted by them, in addition to the several electron transfer pathways already proposed for this group of microorganisms (Bratkova et al. 2019; Kokko et al. 2018; Lee et al. 2012; Zhao et al. 2008). Understanding the mechanisms related to electron transfer by SRB to anodes will improve electricity production in MFC and also expand its applications (Murugan et al. 2018).
Electron transfer pathways by SRB
Most studies investigating the role of SRB in MFC proposed somewhat simplified mechanisms for the electron transfer to anodes (Murugan et al. 2018). According to these mechanisms, SRB are generally able to transfer electrons throughout four possible pathways (Blázquez et al. 2019; Kumar et al. 2017b; Miran et al. 2018; Zhou et al. 2013), which are (i) syntrophic interaction with SOB; (ii) via either the outer membrane or periplasmic cytochromes when there is direct contact of the cell with the electrode; (iii) synthesis of nanowires, i.e. electron-conducting pilli produced by bacteria attached to the electrode surface; and (iv) nanoparticles of metal sulphides, such as FeS, transfer electrons via the external membrane of the microbial cells (Chou et al. 2013; Hu et al. 2019; Kang et al. 2014; Lee et al. 2012; Logan 2009; Miran et al. 2017; Murugan et al. 2018; Sangcharoen et al. 2015; Santoro et al. 2017; Zhao et al. 2008). Figure 3 describes schematically electron transfers to the anode in an SRB-containing MFC.
Schematic representation of electron transfer in microbial fuel cells based on sulphate-reducing bacteria. SRB, sulphate-reducing bacteria; SOB, sulphide-oxidizing bacteria; Me, metal (This figure was devised based on reference Miran et al. 2017)
The first pathway proposed for to the production of electricity in MFCs was the autotrophic oxidation (by SOB) of the sulphide produced during sulphate reduction (Blázquez et al. 2019; Chou et al. 2013; Kang et al. 2014; Kumar et al. 2017b; Lee et al. 2012; Sangcharoen et al. 2015; Zhao et al. 2008). An alternative pathway is abiotic sulphide oxidation to elemental sulphur (Sangcharoen et al. 2015).
The direct transfer of electrons to an extracellular solid acceptor (electrode) by SRB is reported in several studies (Eaktasang et al. 2016; Eaktasang et al. 2013; Hu et al. 2019; Loukanov et al. 2019; Miran et al. 2017; Miran et al. 2018). Eaktasang et al. (2016) revealed evidences of electrically conductive nanoscale filaments produced by SRB in MFC using scanning electron microscopy (SEM) and atomic force microscopy (AFM) techniques. Loukanov et al. (2019) reported the formation of nanowires in the solid electrode using SEM-EDS techniques, and Hu et al. (2019) determined the conductive properties of these nanostructures by AFM. In addition, Kang et al. (2014) demonstrated that Desulfovibrio desulfuricans is able to transfer electrons to the anode directly via cytochrome C-type proteins. This was accomplished by producing and isolating this recombinant protein and by using the latter to effectively produce electricity in an MFC. Fourier-transform infrared spectroscopy (FTIR) analyses have proved that the microorganisms bind to the anode surface via hydrogen and peptide bonds. The latter are bonds between amino groups belonging to the cytochrome C, located on the outer membrane of the microorganism, with carboxylic groups present on the carbon-anode surface (Kang et al. 2014). Therefore, the direct transfer of electrons from the periplasmatic region to the extracellular solid acceptor is enabled.
More recently, it was proposed that other conductive metabolites such as iron sulphides (shown in Fig. 3 as MeySx) also mediate the transfer of electrons to the anode (Hu et al. 2018; Murugan et al. 2018). Specifically, Murugan et al. (2018) proposed that the presence of iron in the growth medium doubled the values of the anodic current in electrochemical studies, which was justified by the contribution of iron sulphides to the formation of cell aggregates on the electrode surface. In addition, FeS nanoparticles on the cell surface enabled electron transfers through the bacteria outer membrane to the extracellular medium, which is a more effective and faster pathway than the diffusion of sulphide ions and their oxidation on the anode surface (Murugan et al. 2018). It must be emphasized that sulphides are semi-conductors and may contribute to transfer of charges in the system (Eaktasang et al. 2013).
In addition to these studies discussing the electroactive features of SRB, several studies covering sulphate removal associated to electricity generation in MFC have been published (Bratkova et al. 2019; Chou et al. 2013; Cooney et al. 1996; Habermann and Pommer 1991; Kang et al. 2014; Kumar et al. 2017a; Kumar et al. 2019; Lee et al. 2012; Lee et al. 2014; Liang et al. 2013b; Miran et al. 2017; Niyom et al. 2018; Sangcharoen et al. 2015; Zhao et al. 2008). Some of these works are revised next.
Sulphate reduction in microbial fuel cells
The first reports of the use of SRB in MFCs appeared in the 1990s (Habermann and Pommer 1991; Lee et al. 2012). However, sulphate removal was a controversial hypothesis because it would compete with the anode surface for the electrons available in the anodic half-cell. A decade later, a new theory proposed that the sulphide produced during sulphate reduction was oxidized to elemental sulphur in the anode (Rabaey et al. 2006). As a consequence of this second theory, the number of papers addressing sulphate reduction in the anodic half-cell started to increase, as listed in Table 1.
It can be seen in Table 1 that the current density values are low. Habermann and Pommer (1991) stated that low currents and an unstable production of energy would limit the application of this technology in an industrial scale. However, the main advantage of the use of MFCs would be a significant COD removal associated with the production of electricity, which was proposed for the first time by these authors, who reported 75% COD removal from treating a landfill leachate in an MFC.
Habermann and Pommer (1991) also verified sulphate reduction to sulphide by SRB in the anodic chamber, but, according to the authors, the species must have been re-oxidized to sulphate, justifying the low sulphate removal observed when glucose was used as an energy source. However, this hypothesis is unlikely because elemental sulphur oxidation to sulphate is kinetically slow in the absence of sulphur-oxidation bacteria. The highest current density, as compared with other studies, could have been related to the electrode impregnation with Ni, Co, and Fe (Cooney et al. 1996) or even due to the long acclimatization periods, as these experiments took over 5 years to conclude.
A comparison of the power output values of these studies is challenging, due to the different MFC configurations and the differences in operating conditions. Logan (2012) states that a complete description of the layout and operating conditions of the different MFCs is required to properly assess their performance.
Carbonaceous materials are the main types of electrodes, which include carbon felts, cloths, sheets, and bars. Such low-cost materials are biocompatible, good electrical conductors, corrosion resistant, besides having a high surface area (Santoro et al. 2017). Current density values ranged from 0.002 to 0.09 mA cm−2 in studies using activated carbon as the anode (Table 1). On the other hand, Cooney et al. (1996) and Zhao et al. (2008) reported significantly higher current densities (1.7 mA cm−2 and 1.3 mA cm−2), respectively, when platinum impregnated cathodes and lactate (carbon source) were selected. Thus, the electrode material is the key component in the production of electricity in MFCs using electroactive SRB. Therefore, low-cost and recyclable alternatives must be investigated for this parameter in order to devise a feasible technology (Goglio et al. 2019).
In all the studies listed in Table 1, sulphate and COD removals were greater than 60% irrespective of the carbon source, suggesting therefore that this is not a limiting factor in MFC inoculated with SRB. Nevertheless, it must be added that both the pH and COD/SO4−2 ratio are key parameters in the performance of both sulphate and COD removals in anaerobic reactors (Bertolino et al. 2012; Bertolino et al. 2015; Isosaari and Sillanpää 2017; Kaksonen and Puhakka 2007). Theoretically, a COD/SO4−2 ratio of 0.67 would enable SRB growth and, consequently, sulphate removal (Isosaari and Sillanpää 2017). However, quite a few studies have proposed that COD/SO4−2 ratios around 2 are required if the sulphate reduction is to improve (Bertolino et al. 2012; Bertolino et al. 2015; Bratkova et al. 2019; Miran et al. 2018). Working with COD/SO4−2 ratios below 2 may justify the results presented in Table 1, in which the sulphate removal yields were low (Niyom et al. 2018; Sangcharoen et al. 2015). Similar finding was observed when the COD removal was also low (Lee et al. 2012; Niyom et al. 2018; Sangcharoen et al. 2015). On the other hand, a correlation between sulphate and COD removal with electricity production was not established so far, and this is one of the challenges to be faced by the scientific community.
An important parameter in sulphate reduction in MFC is pH because it defines the concentration of H2S in the reactor, which inhibits sulphate reduction and bacterial growth. The maximum sulphide S2− or H2S concentration tolerated by SRB was proposed to be 230 mg L−1 at pH 7 (Cooney et al. 1996). Liang et al. (2013b) determined the effect of pH on the performance of a MFC inoculated with SRB and reported the best results when the pH was in the 6.5–8.5 range.
The configuration of the MFC, single or double chamber, could not be related to either the production of energy or to sulphate removal, similarly to what was observed with carbonic substrate.
Recently, studies in which a MFC is used for sulphate reduction associated to other technological important process are being published (Eaktasang et al. 2013; Kumar et al. 2019; Miran et al. 2018; Zhao et al. 2008). For instance, Miran et al. (2018) reported significant sulphate removal in a MFC treating azo compounds. Another promising application is to associate sulphate reduction in the anode to algae growth in the cathode, as Kumar et al. (2019) observed that increasing the lipid content in the cathodic half-cell improved the production of electricity. This is an interesting proposition because the algal biomass can be converted subsequently to biodiesel. Furthermore, an advantage of bio-electrochemical systems over conventional biological sulphate removal methods is the oxidation of sulphide ions to elemental sulphur because the accumulation of sulphide in conventional systems inhibits SRB growth, in addition to causing corrosion and malodour issues (Eaktasang et al. 2013; Zhao et al. 2008).
New studies should be focused on the selection of the most appropriate electrode material, and on the COD/SO4−2 ratio so that the maximum COD and SO4−2 removals and also energy production are achieved.
Conclusions and perspectives
The pathways of electron transfer to a solid electrode by SRB are well established. These microorganisms transfer electrons directly to the anode surface either by using cytochrome C-type periplasmic proteins or by producing conducting nanowires. In addition, electron transfer may also occur via active metabolites such as FeS. Sulphide oxidation by SOB is also widely regarded as an electricity producing pathway in these systems. In any case, a microbial consortium is essential for sulphate and COD removal, as in any anaerobic system, and also for electricity production.
Regarding the operating conditions: electrode type and COD/SO4−2 ratio are the main parameters controlling sulphate reduction and the simultaneous production of electricity. The success of the technology will be achieved when these parameters were optimized
Summarizing, the development of the MFC technology requires a multidisciplinary approach to find alternatives sources of electrical energy. It symbolizes the confluence of chemical, physical, and life sciences and is a meeting point for basic and applied research.
References
Agostino V, Rosenbaum MA (2018) Sulfate-reducing electroautotrophs and their applications in bioelectrochemical systems. Frontiers in Energy Research 6:55–65. https://doi.org/10.3389/fenrg.2018.00055
Bertolino SM, Rodrigues ICB, Guerra-Sá R, Aquino SF, Leão VA (2012) Implications of volatile fatty acid profile on the metabolic pathway during continuous sulfate reduction. J Environ Manag 103:15–23. https://doi.org/10.1016/j.jenvman.2012.02.022
Bertolino SM, Silva LAM, Aquino SF, Leão VA (2015) Comparison of UASB and fluidized-bed reactors for sulfate reduction. Brazilian Journal of Chemical Engineering 32:59–71. https://doi.org/10.1590/0104-6632.20150321s00003158
Blázquez E, Baeza JA, Gabriel D, Guisasola A (2019) Treatment of real flue gas desulfurization wastewater in an autotrophic biocathode in view of elemental sulfur recovery: microbial communities involved. Sci Total Environ 657:945–952. https://doi.org/10.1016/j.scitotenv.2018.12.037
Bratkova S, Alexieva Z, Angelov A, Nikolova K, Genova P, Ivanov R, Gerginova M, Peneva N, Beschkov V (2019) Efficiency of microbial fuel cells based on the sulfate reduction by lactate and glucose. Int J Environ Sci Technol 16:6145–6156. https://doi.org/10.1007/s13762-019-02223-8
Cheng S, Dempsey BA, Logan BE (2007) Electricity generation from synthetic acid-mine drainage (AMD) water using fuel cell technologies. Environmental Science & Technology 41:8149–8153. https://doi.org/10.1021/es0712221
Chernicharo CAL (1997) Reatores Anaeróbios. Princípios de Tratamento Biológico de Aguas Residuárias. Editora da UFMG, Belo Horizonte - MG
Chou TY, Whiteley CG, Lee DJ, Liao Q (2013) Control of dual-chambered microbial fuel cell by anodic potential: implications with sulfate reducing bacteria. Int J Hydrog Energy 38:15580–15589. https://doi.org/10.1016/j.ijhydene.2013.04.074
Chou TY, Whiteley CG, Lee DJ (2014) Anodic potential on dual-chambered microbial fuel cell with sulphate reducing bacteria biofilm. Int J Hydrog Energy 39:19225–19231. https://doi.org/10.1016/j.ijhydene.2014.03.236
Cooney MJ, Roschi E, Marison IW, Comminellis C, von Stockar U (1996) Physiologic studies with the sulfate-reducing bacterium Desulfovibrio desulfuricans: evaluation for use in a biofuel cell. Enzym Microb Technol 18:358–365. https://doi.org/10.1016/0141-0229(95)00132-8
Das S, Mangwani N (2010) Recent developments in microbial fuel cells: a review. J Sci Ind Res 69:727–731 http://hdl.handle.net/123456789/10294
Dong X, Stams AJM (1995) Evidence for H2 and formate formation during syntrophic butyrate and propionate degradation. Anaerobe 1:35–39. https://doi.org/10.1016/S1075-9964(95)80405-6
Eaktasang N, Min H-S, Kang C, Kim HS (2013) Control of malodorous hydrogen sulfide compounds using microbial fuel cell. Bioprocess Biosyst Eng 36:1417–1425. https://doi.org/10.1007/s00449-012-0881-3
Eaktasang N, Kang CS, Lim H, Kwean OS, Cho S, Kim Y, Kim HS (2016) Production of electrically-conductive nanoscale filaments by sulfate-reducing bacteria in the microbial fuel cell. Bioresour Technol 210:61–67. https://doi.org/10.1016/j.biortech.2015.12.090
Feng C, Huang L, Yu H, Yi X, Wei C (2015) Simultaneous phenol removal, nitrification and denitrification using microbial fuel cell technology. Water Res 76:160–170. https://doi.org/10.1016/j.watres.2015.03.001
Friedman SE, Miller EK, Lipson AD, Angenent TL (2013) Potentiostatically poised electrodes mimic iron oxide and interact with soil microbial communities to alter the biogeochemistry of Arctic peat soils. Minerals 3:318–336. https://doi.org/10.3390/min3030318
Gacitúa MA, Muñoz E, González B (2018) Bioelectrochemical sulphate reduction on batch reactors: effect of inoculum-type and applied potential on sulphate consumption and pH. Bioelectrochemistry 119:26–32. https://doi.org/10.1016/j.bioelechem.2017.08.006
Goglio A, Tucci M, Rizzi B, Colombo A, Cristiani P, Schievano A (2019) Microbial recycling cells (MRCs): a new platform of microbial electrochemical technologies based on biocompatible materials, aimed at cycling carbon and nutrients in agro-food systems. Sci Total Environ 649:1349–1361. https://doi.org/10.1016/j.scitotenv.2018.08.324
Habermann W, Pommer EH (1991) Biological fuel cells with sulphide storage capacity. Appl Microbiol Biotechnol 35:128–133. https://doi.org/10.1007/BF00180650
Hai T, Wen-Cheng P, Chang-Feng C, Jian-Ping X, Wen-Jun H (2016) Remediation of acid mine drainage based on a novel coupled membrane-free microbial fuel cell with permeable reactive barrier system. Polish Journal of Environmental Studies 25:107-112. https://doi.org/10.15244/pjoes/60891
He L, Du P, Chen Y, Lu H, Cheng X, Chang B, Wang Z (2017) Advances in microbial fuel cells for wastewater treatment. Renew Sust Energ Rev 71:388–403. https://doi.org/10.1016/j.rser.2016.12.069
Hu J, Zeng C, Liu G, Luo H, Qu L, Zhang R (2018) Magnetite nanoparticles accelerate the autotrophic sulfate reduction in biocathode microbial electrolysis cells. Biochem Eng J 133:96–105. https://doi.org/10.1016/j.bej.2018.01.036
Hu J, Zeng C, Liu G, Lu Y, Zhang R, Luo H (2019) Enhanced sulfate reduction accompanied with electrically-conductive pili production in graphene oxide modified biocathodes. Bioresour Technol 282:425–432. https://doi.org/10.1016/j.biortech.2019.03.023
Hu K, Xu D, Chen Y (2020) An assessment of sulfate reducing bacteria on treating sulfate-rich metal-laden wastewater from electroplating plant. J Hazard Mater 393:122376. https://doi.org/10.1016/j.jhazmat.2020.122376
Isosaari P, Sillanpää M (2017) Use of sulfate-reducing and bioelectrochemical reactors for metal recovery from mine water. Separation & Purification Reviews 46:1–20. https://doi.org/10.1080/15422119.2016.1156548
Jiang Y, Ulrich AC, Liu Y (2013) Coupling bioelectricity generation and oil sands tailings treatment using microbial fuel cells. Bioresour Technol 139:349–354. https://doi.org/10.1016/j.biortech.2013.04.050
Kaksonen AH, Puhakka JA (2007) Sulfate reduction based bioprocesses for the treatment of acid mine drainage and the recovery of metals. Engineering in Life Sciences 7:541–564. https://doi.org/10.1002/elsc.200720216
Kaksonen AH, Plumb JJ, Robertson WJ, Riekkola-Vanhanen M, Franzmann PD, Puhakka JA (2006) The performance, kinetics and microbiology of sulfidogenic fluidized-bed treatment of acidic metal- and sulfate-containing wastewater. Hydrometallurgy 83:204–213. https://doi.org/10.1016/j.hydromet.2006.03.025
Kang CS, Eaktasang N, Kwon D-Y, Kim HS (2014) Enhanced current production by Desulfovibrio desulfuricans biofilm in a mediator-less microbial fuel cell. Bioresour Technol 165:27–30. https://doi.org/10.1016/j.biortech.2014.03.148
Khan MZ, Singh S, Sultana S, Sreekrishnan TR, Ahammad SZ (2015) Studies on the biodegradation of two different azo dyes in bioelectrochemical systems. New J Chem 39:5597–5604. https://doi.org/10.1039/C5NJ00541H
Kokko ME, Mäkinen AE, Puhakka JA (2016) Anaerobes in bioelectrochemical systems. In: Hatti-Kaul R, Mamo G, Mattiasson B (eds) Anaerobes in biotechnology. Springer International Publishing, Cham, pp 263–292. https://doi.org/10.1007/10_2015_5001
Kokko M, Epple S, Gescher J, Kerzenmacher S (2018) Effects of wastewater constituents and operational conditions on the composition and dynamics of anodic microbial communities in bioelectrochemical systems. Bioresour Technol 258:376–389. https://doi.org/10.1016/j.biortech.2018.01.090
Kumar SS, Basu S, Bishnoi NR (2017a) Effect of cathode environment on bioelectricity generation using a novel consortium in anode side of a microbial fuel cell. Biochem Eng J 121:17–24. https://doi.org/10.1016/j.bej.2017.01.014
Kumar SS, Malyan SK, Basu S, Bishnoi NR (2017b) Syntrophic association and performance of Clostridium, Desulfovibrio, Aeromonas and Tetrathiobacter as anodic biocatalysts for bioelectricity generation in dual chamber microbial fuel cell. Environ Sci Pollut Res 24:16019–16030. https://doi.org/10.1007/s11356-017-9112-4
Kumar SS, Basu S, Gupta S, Sharma J, Bishnoi NR (2019) Bioelectricity generation using sulphate-reducing bacteria as anodic and microalgae as cathodic biocatalysts. Biofuels 10:81–86. https://doi.org/10.1080/17597269.2018.1426161
Lee D-J, Lee C-Y, Chang J-S (2012) Treatment and electricity harvesting from sulfate/sulfide-containing wastewaters using microbial fuel cell with enriched sulfate-reducing mixed culture. J Hazard Mater 243:67–72. https://doi.org/10.1016/j.jhazmat.2012.09.071
Lee D-J, Liu X, Weng H-L (2014) Sulfate and organic carbon removal by microbial fuel cell with sulfate-reducing bacteria and sulfide-oxidising bacteria anodic biofilm. Bioresour Technol 156:14–19. https://doi.org/10.1016/j.biortech.2013.12.129
Lens PNL, Visser A, Janssen AJH, Pol LWH, Lettinga G (1998) Biotechnological treatment of sulfate-rich wastewaters. Crit Rev Environ Sci Technol 28:41–88. https://doi.org/10.1080/10643389891254160
Li J-J, Gao M-M, Zhang G, Wang X-H, Wang S-G, Song C, Xu Y-Y (2015) Perchlorate reduction in microbial electrolysis cell with polyaniline modified cathode. Bioresour Technol 177:74–79. https://doi.org/10.1016/j.biortech.2014.11.065
Liamleam W, Annachhatre AP (2007) Electron donors for biological sulfate reduction. Biotechnol Adv 25:452–463. https://doi.org/10.1016/j.biotechadv.2007.05.002
Liang F, Deng H, Zhao F (2013a) Sulfur pollutants treatment using microbial fuel cells from perspectives of electrochemistry and microbiology. Chin J Anal Chem 41:1133–1139. https://doi.org/10.1016/S1872-2040(13)60669-6
Liang F, Xiao Y, Zhao F (2013b) Effect of pH on sulfate removal from wastewater using a bioelectrochemical system. Chem Eng J 218:147–153. https://doi.org/10.1016/j.cej.2012.12.021
Logan BE (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol 7:375–381. https://doi.org/10.1038/nrmicro2113
Logan BE (2012) Essential data and techniques for conducting microbial fuel cell and other types of bioelectrochemical system experiments. ChemSusChem 5:988–994. https://doi.org/10.1002/cssc.201100604
Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environmental Science & Technology 40:5181–5192. https://doi.org/10.1021/es0605016
Loukanov A, Angelov A, Takahashi Y, Nikolov I, Nakabayashi S (2019) Carbon nanodots chelated with metal ions as efficient electrocatalysts for enhancing performance of microbial fuel cell based on sulfate reducing bacteria. Colloids Surf A Physicochem Eng Asp 574:52–61. https://doi.org/10.1016/j.colsurfa.2019.04.067
Lovley DR (2006) Bug juice: harvesting electricity with microorganisms. Nat Rev Microbiol 4:497–508. https://doi.org/10.1038/nrmicro1442
Lovley DR (2008) The microbe electric: conversion of organic matter to electricity. Curr Opin Biotechnol 19:564–571. https://doi.org/10.1016/j.copbio.2008.10.005
Luo H, Fu S, Liu G, Zhang R, Bai Y, Luo X (2014a) Autotrophic biocathode for high efficient sulfate reduction in microbial electrolysis cells. Bioresour Technol 167:462–468. https://doi.org/10.1016/j.biortech.2014.06.058
Luo H, Liu G, Zhang R, Bai Y, Fu S, Hou Y (2014b) Heavy metal recovery combined with H2 production from artificial acid mine drainage using the microbial electrolysis cell. J Hazard Mater 270:153–159. https://doi.org/10.1016/j.jhazmat.2014.01.050
Luo H, Bai J, He J, Liu G, Lu Y, Zhang R, Zeng C (2020) Sulfate reduction and elemental sulfur recovery using photoelectric microbial electrolysis cell. Sci Total Environ 728:138685. https://doi.org/10.1016/j.scitotenv.2020.138685
Madigan MT, Martinko JM, Bender KS, Buckley DH, Stahl DA (2015) Brock biology of microorganisms, 14th edn. Pearson, Boston
Manzano-Agugliaro F, Alcayde A, Montoya FG, Zapata-Sierra A, Gil C (2013) Scientific production of renewable energies worldwide: an overview. Renew Sust Energ Rev 18:134–143. https://doi.org/10.1016/j.rser.2012.10.020
Mathuriya AS (2014) Eco-affectionate face of microbial fuel cells. Crit Rev Environ Sci Technol 44:97–153. https://doi.org/10.1080/10643389.2012.710445
Miran W, Jang J, Nawaz M, Shahzad A, Jeong SE, Jeon CO, Lee DS (2017) Mixed sulfate-reducing bacteria-enriched microbial fuel cells for the treatment of wastewater containing copper. Chemosphere 189:134–142. https://doi.org/10.1016/j.chemosphere.2017.09.048
Miran W, Jang J, Nawaz M, Shahzad A, Lee DS (2018) Sulfate-reducing mixed communities with the ability to generate bioelectricity and degrade textile diazo dye in microbial fuel cells. J Hazard Mater 352:70–79. https://doi.org/10.1016/j.jhazmat.2018.03.027
Murugan M, Miran W, Masuda T, Lee DS, Okamoto A (2018) Biosynthesized iron sulfide nanocluster enhanced anodic current generation by sulfate reducing bacteria in microbial fuel cells. ChemElectroChem 5:4015–4020. https://doi.org/10.1002/celc.201801086
Muyzer G, Stams AJM (2008) The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol 6:441–454. https://doi.org/10.1038/nrmicro1892
Nascimento MRL (1998) Remoção e recuperação de urânio de águas ácidas de mina por resina de troca iônica. Dissertação, Universidade Federal de São Carlos
Nelson DL, Cox MM (2012) Lehninger principles of biochemistry, 6th edn. W. H. Freeman, New York
Niyom W, Komolyothin D, Suwannasilp BB (2018) Important role of abiotic sulfide oxidation in microbial fuel cells treating high-sulfate wastewater. Engineering Journal 22:23–37. https://doi.org/10.4186/ej.2018.22.4.23
Pandey P, Shinde VN, Deopurkar RL, Kale SP, Patil SA, Pant D (2016) Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl Energy 168:706–723. https://doi.org/10.1016/j.apenergy.2016.01.056
Pant D, Singh A, Van Bogaert G, Gallego YA, Diels L, Vanbroekhoven K (2011) An introduction to the life cycle assessment (LCA) of bioelectrochemical systems (BES) for sustainable energy and product generation: relevance and key aspects. Renew Sust Energ Rev 15:1305–1313. https://doi.org/10.1016/j.rser.2010.10.005
Rabaey K, van de Sompel K, Maignien L, Boon N, Aelterman P, Clauwaert P, de Schamphelaire L, Pham HT, Vermeulen J, Verhaege M, Lens P, Verstraete W (2006) Microbial fuel cells for sulfide removal. Environmental Science & Technology 40:5218–5224. https://doi.org/10.1021/es060382u
Sangcharoen A, Niyom W, Suwannasilp BB (2015) A microbial fuel cell treating organic wastewater containing high sulfate under continuous operation: performance and microbial community. Process Biochem 50:1648–1655. https://doi.org/10.1016/j.procbio.2015.06.013
Santoro C, Arbizzani C, Erable B, Ieropoulos I (2017) Microbial fuel cells: from fundamentals to applications. A review J Power Sources 356:225–244. https://doi.org/10.1016/j.jpowsour.2017.03.109
Sarti A, Silva AJ, Côrtes RS, Foresti E (2008) Remoção de sulfato de águas residuárias industriais em reator anaeróbio de leito fixo operado em bateladas sequenciais. Engenharia Sanitaria e Ambiental 13:15–22. https://doi.org/10.1590/S1413-41522008000100003
Su W, Zhang L, Tao Y, Zhan G, Li D, Li D (2012) Sulfate reduction with electrons directly derived from electrodes in bioelectrochemical systems. Electrochem Commun 22:37–40. https://doi.org/10.1016/j.elecom.2012.04.030
Tao H-C, Lei T, Shi G, Sun X-N, Wei X-Y, Zhang L-J, Wu W-M (2014) Removal of heavy metals from fly ash leachate using combined bioelectrochemical systems and electrolysis. J Hazard Mater 264:1–7. https://doi.org/10.1016/j.jhazmat.2013.10.057
Tice RC, Kim Y (2014) Energy efficient reconcentration of diluted human urine using ion exchange membranes in bioelectrochemical systems. Water Res 64:61–72. https://doi.org/10.1016/j.watres.2014.06.037
Tortora GJ, Funke BR, Case CL (2010) Microbiology: an introduction,10th edition, 10th edn. Pearson, Boston
Weng H-L, Lee D-J (2015) Performance of sulfate reducing bacteria-microbial fuel cells: reproducibility. J Taiwan Inst Chem Eng 56:148–153. https://doi.org/10.1016/j.jtice.2015.04.028
Zhao F, Rahunen N, Varcoe JR, Chandra A, Avignone-Rossa C, Thumser AE, Slade RCT (2008) Activated carbon cloth as anode for sulfate removal in a microbial fuel cell. Environmental Science & Technology 42:4971–4976. https://doi.org/10.1021/es8003766
Zhao F, Rahunen N, Varcoe JR, Roberts AJ, Avignone-Rossa C, Thumser AE, Slade RCT (2009) Factors affecting the performance of microbial fuel cells for sulfur pollutants removal. Biosens Bioelectron 24:1931–1936. https://doi.org/10.1016/j.bios.2008.09.030
Zheng Y, Xiao Y, Yang Z-H, Wu S, Xu H-J, Liang F-Y, Zhao F (2014) The bacterial communities of bioelectrochemical systems associated with the sulfate removal under different pHs. Process Biochem 49:1345–1351. https://doi.org/10.1016/j.procbio.2014.04.019
Zhou M, Wang H, Hassett DJ, Gu T (2013) Recent advances in microbial fuel cells (MFCs) and microbial electrolysis cells (MECs) for wastewater treatment, bioenergy and bioproducts. J Chem Technol Biotechnol 88:508–518. https://doi.org/10.1002/jctb.4004
Funding
This study was funded by the Universidade Federal de Ouro Preto, Universidade Federal de São João del-Rei and the agencies CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais), FINEP (Financiadora de Estudos e Projetos), and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico). The CNPq scholarship to V. Leão is particularly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Weiming Zhang
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rodrigues, I.C.B., Leão, V.A. Producing electrical energy in microbial fuel cells based on sulphate reduction: a review. Environ Sci Pollut Res 27, 36075–36084 (2020). https://doi.org/10.1007/s11356-020-09728-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09728-7