Abstract
The requirement for a low-cost option for wastewater treatment and simultaneous bioenergy and resource recovery from the wastewater to make treatment sustainable has prompted the researchers to seek innovative technologies. Microbial fuel cell (MFC) is one of the bio-based novel technologies that converts the chemical energy of substrate into electrical energy using electrochemically active bacteria as biocatalyst. With the forefront energy crisis, the MFC has gained widespread popularity due to its capability to harvest direct electricity, while simultaneously treating wastewater. To make this technology scalable, significant efforts and modifications have been attempted by the researchers, including improved design, hybrid concepts, use of low-cost materials for the basic components (electrodes, membrane), establishing innovative low-cost catalysts, identifying several microorganisms as exoelectrogens and methods of pre-treatment of mixed anaerobic sludge to enrich electrogens, etc. This review summarises some of these recent advances pertaining to the MFC and few upscaling applications of MFC. Furthermore, a concise future scope is elaborated in the view of common challenges in the field of MFC for wastewater treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The bio-electrochemical systems (BES) are a group of engineered systems that have been in the limelight for wastewater treatment and resource recovery. While several variants of BES exists today, the first-generation systems, namely the microbial fuel cells (MFCs) have been profoundly explored for recovery of bioelectricity as well as other valuables, while facilitating treatment of wastewater. The concept of recovering electricity by utilising microbial colonies that are abundantly found in nature, and have the ability to consume waste-based or other sources of oxidisable organic matters, was first demonstrated by M. C. Potter in 1911 (Potter 1911). The investigation documented the production of electrical energy using selected pure culture strains of bacteria and fungi (Escherichia coli and Saccharomyces). This thought was promulgated further, when almost 25 years later, Cohen stacked up 35 cells to derive 2 mA of current generation (Cohen 1931). In the year 1976, Suzuki et al., demonstrated a stable hydrogen generation with a suitable design for MFC (Karube et al. 1976).
Further to this, the application of synthetic mediators for shuttling electrons between microbes and electrode led to the design of MFC, which has undergone minimal architectural changes till the date (Allen and Bennetto 1993). However, a paradigm shift in the operational procedure occurred when Kim and Al discovered electroactive bacteria that did not require any mediators to shuttle electrons (Kim and Al 1999). This drastically reduced the economic demand of BES technology by eliminating the need or expensive external mediators. Subsequent to this, the last two decades have witnessed a sizeable amount of research on BES design, optimization of physical, chemical and biological operating conditions, optimization of microbial metabolism, appropriate selection of exoelectrogens and construction materials to enhance the electron transport vis-á-vis enhanced efficacy of the different BES variants (Logan et al. 2006; Ghangrekar and Shinde 2008). Presently, there are five major variants of BES that have been developed over the last two decades. Among these, MFC and microbial electrolysis cell (MEC) are the two variants of first-generation BES, while the other types, namely microbial carbon-capture cell (MCC), microbial desalination cell (MDC), and the microbial electrosynthesis cell (MES) can be thought as the derived variants of the two fundamental types of BES.
It is estimated that domestic wastewater contains higher energy than the energy required for its treatment. This states that theoretically, wastewater treatment can be a self-sustained process. One of the most recent approaches of energy generation from wastewater is via the use of MFC, which has the capability of bioelectricity generation through oxidation of organic matter (Li et al. 2014). Unlike other processes such as methane and hydrogen production via anaerobic digestion and biofermentation, respectively, that require two stages for electricity generation, MFC offers an advantage of onestep electricity production. Apart from electricity production, MFCs are energy saving technology that does not require aeration (for air–cathode MFC), thus contributing to reduced energy input. Compared to the conventional activated sludge process, MFC contributes to far less sludge production (He 2013 Li et al. 2014). Moreover, the anode in MFC acts as an electron sink and makes the process of organic matter degradation independent of other terminal electron acceptors, which are essential in the conventional anaerobic digestion (Logan 2009).
Although, the BES technology has a niche over the conventional treatments and has opened up an entirely new field of transdisciplinary research augmenting biotechnology, energy science, materials chemistry, surface chemistry, chemical engineering, and electrochemistry; however, the majority of investigations are still imited to lab-scale. Similar to the conventional fuel cells and electrolysis cells, which are used for electricity generation and other industrial applications, respectively, the MFC and MEC also have similar bottlenecks associated with the usage of electrodes, catalysts, and membranes. In addition to these, in the majority of cases biological reactions drive the waste treatment processes and the electricity generation, hence the direct energy that can be harvested is very low for grid-based utilisation.
Moreover, the different problems related to scaling-up, such as high cost of electrodes, high diffusion losses for a larger sized reactor, inadequate electrode surface area to volume ratio, higher fabrication cost leading to smaller size of membrane panels, even while using low-cost ceramic membranes, etc., need to be addressed. These are the universal bottlenecks of BES variants as well as bottlenecks pertaining to the scaling-up of MFC, a first-generation BES. The present technological development and research findings are driven towards finding solutions to overcome or mitigate these constraints to move towards scaling-up of this state-of-the-art technology that enables wastewater treatment and consequential one-step valuables and/or energy recovery. The discussions in this review paper are primarily focused on the advancements in the field of MFC and the decadal developments as well as addressing future research requirements for the possible field-scale application of this technology.
Description of Microbial Fuel Cell and Other Bio-Electrochemical Systems
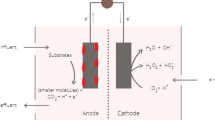
A MFC can have a dual chamber configuration having an aqueous cathode or a single chamber with an air exposed cathode, which directly use oxygen from air (Jadhav et al. 2017). The dual chamber MFC has a separator in between the anodic and the cathodic chambers, which is usually a cation/proton exchange membrane (CEM/PEM) (Fig. 1a). The anodic and the cathodic chambers have anode and cathode electrodes, respectively, which are connected through an external load using conductive wires. The materials popularly used for anode and the cathode are carbon felt, carbon fibre, carbon cloth, and stainless steel mesh modified suitably to sustain biofilm in case of anode, and facilitate gas diffusion in case of air cathode. Other suitable materials such as biochar (Huggins et al. 2014), activated carbon granules (Neethu et al. 2020b), graphite granules (Zhang et al. 2011), polymer-based electrodes (Yuan and Kim 2008) have also been developed to be used as electrodes in MFC. The anodic chamber houses the bacterial inoculum that utilises the organic substrate for metabolism and in the process releases electrons (Eq. 1) (Logan et al. 2006):
These electrons are transferred to the anode of the MFC via electrically conductive nanowires and mediators. The electrons thus passed on to the anode travel through an external circuit to reach the cathode. On the cathode surface any suitable chemical species, generally O2, act as a terminal electron acceptor. When O2 is used as an electron acceptor, then the cathodic reaction is termed as oxygen reduction reaction (ORR) owing to the reduction of oxygen to H2O2 (Eq. 2) or H2O (Eq. 3) by accepting two or four electrons, respectively, from the cathode and utilising the H+ that migrates through the CEM/PEM from the anodic to cathodic side (Rabaey and Verstraete 2005) (Fig. 1a):
In case of single chamber MFC, the cathode has a gas diffusion layer that is exposed to the air and a catalyst layer that is exposed to the anolyte either directly or juxtaposed to an ion exchange membrane that faces the anolyte and anode (Fig. 1b). The oxygen diffuses through the gas diffusion layer and reaches the catalyst layer, where it reacts with the migrated proton to form H2O or H2O2 (Noori et al. 2016; Dong et al. 2018). In the case of both dual chamber as well as single chamber MFC usual practice is to employ CEM/PEM. However, the use of PEM, such as Nafion, also allows transport of other cations (Na+, K+, NH4+, Ca2+, Mg2+) as well, apart from the proton (H+). Typically in MFCs, the concentration of other cations is 105 times higher than protons. This cation transport to the cathodic chamber results in a drop of anolyte pH in the anodic chamber and an increase of catholyte pH, leading to reduced cathode potential (Rozendal et al. 2006). Hence, in certain cases, anion exchange membranes are also used for the operation of MFC and the OH− generated during cathodic H2O dissociation migrates to the anodic chamber, where it balances this pH imbalance created due to the generation of H+ during the anaerobic respiration (Rozendal et al. 2007; Pandit et al. 2012).
Apart from the pH imbalance due to the application of PEM/CEM in MFC, there are several bottlenecks that have to be addressed prior to the commercialization of this technology. One major issue is the slower interaction of the bulk media and the anodic biofilm in case of larger reactors. The larger reactor size also limits the flow of ions ‘from’ and ‘to’ the surface of the anodic biofilm due to slow diffusion and mass transport rates of ions. The larger size of MFCs with higher bulk volume also increases the susceptibility of substrate and proton scavenging biochemical and chemical reactions. In addition to this, scale-up also necessitates the usage of low cost electrode materials while achieving high conductivity, chemical stability, biocompatibility (in case of anode and biocathode), having low charge transfer resistance, and favouring higher oxygen reduction reaction (ORR) catalytic activity (in case of cathode) (Wei et al. 2011; Yuan and He 2015).
Hence, in light of the above discussion, it is imperative that for scaling-up of MFC and to make it sustainable, research should be focussed on several of issues a few out of them being: development of low cost electrodes and membranes; novel reactor design to maintain electrode area to volume ratio; novel hydraulic design to reduce the diffusional and mass transport losses; minimise the unwanted scavenging reactions; and developing novel power management systems for boosting the power harvested from MFC. In the subsequent sections of this review, the research developments towards achieving these goals have been discussed. In addition to these, the performance of MFC in the field of contaminant removal has also been highlighted.
Recent Developments in Microbial Fuel Cells for Enhancing Performance
Suppression of Methanogenesis from Mixed Anaerobic Sludge Used as an Anodic Inoculum
In the anodic chamber of MFC, exoelectrogens transfer the electrons released during the oxidation of organic matter to the anode. However, the use of mixed anaerobic sludge as inoculum in the anodic chamber leads to substrate and proton loss due to the presence of acetoclastic and hydrogenotrophic methanogens. Interestingly, the hydrogenotrophic methanogens exist in syntropy with the exoelectrogens in nature, wherein the electrons generated during the metabolic activity of exoelectrogens are transferred to the hydrogenotrophic methanogen by means of hydrogen ion (Kim et al. 2005; Schröder and Harnisch 2017). Instead of transfer of protons to the cathode via cation exchange membrane, the available hydrogenotrophic methanogens in the mixed sludge consume hydrogen and CO2 and produce methane. Thus, the presence of methanogens leads to methanogenesis instead of electrogenesis reflecting in reduced current production in MFC. On the other hand, the acetoclastic methanogens compete with the exoelectrogens for the substrate in the form of acetate when both of the species co-exist in the anodic chamber of MFC (Kim et al. 2005). This points to the fact that the methanogens are unwanted species in the anodic chamber of MFC and the presence of methanogens reduces power production both by reducing substrate availability as well as by scavenging H+ and e− (Nath and Ghangrekar 2020). Hence to improve the performance of MFC by suppressing the methanogenesis several strategies have been devised by the researchers (Table 1).
The strategies applied for suppression of methanogenesis in the inoculum sludge in anodic chamber of MFC can be classified into two different categories, viz., physical or chemical techniques. The physical suppression techniques include the application of heat treatment, ultrasonication, and regulation of anode potential (Ghangrekar and Shinde 2008; More and Ghangrekar 2010; Grüning et al. 2015). Among chemical techniques, the application of 2-BES has been a popular choice due to the effective suppression of methanogenesis in the anodic chamber. Other compounds and chemical species that have been experimented for suppression of methanogens include antibiotics (Ray et al. 2017), algal fatty acids (Rajesh et al. 2015), and metal ions such as Al (Bagchi and Behera 2019). The suppression of methanogenesis has also been achieved by regulation of anode potential as well as external resistance. It has been observed in the past investigations that the population of methanogens get attenuated at positive anode potentials or potentials near to zero (Zhao et al. 2019; Nath et al. 2021). Regulation of external load also is an indirect manifestation of the anode potential regulation and has been demonstrated to be successful in enriching electrogens in the anodic chamber of MFC in past investigations (Rabaey et al. 2005).
In an investigation, marine algae Chaetoceros was employed to inhibit the methanogenesis in the mixed anaerobic sludge used as an anodic inoculum. This can be achieved by the impact of hexadecatrionic acid, which is present in this marine algae, on the cell membrane of the methanogens (Rajesh et al. 2015). Application of biogenic compounds for suppression of methanogenesis was also demonstrated by employing extracted peptaibiotics from Trichoderma sp. for suppression of methanogenesis in the anodic chamber of MFC (Ray et al. 2017). The MFC inoculated with mixed anaerobic sludge pre-treated with the peptaibiotics exhibited a 2 times increase in power density as compared to the control MFC with similar anode and cathode catalyst configurations and inoculated with mixed anaerobic sludge without any pre-treatment. Extending this work, another research demonstrated that the plant extracts had a profound effect on the power density of MFC when applied in different concentrations (Neethu et al. 2020a). However, different follow-up investigations revealed that the effects of plant extract are manifold and not limited to the suppression of methanogenesis. The plant extracts, on one hand, cause changes in the cell morphology and stimulates gene expressions that trigger conductive nanowire growth in the methanogens, while on the other hand acts as an electron shuttle for the extracellularly projected electrons (Nath and Ghangrekar 2020).
These investigations indicate that the methanogenesis suppression and promotion of exoelectrogenesis can be achieved by low cost facile biogenic methods. Even certain wastewater characteristics can cause intrinsic suppression of methanogenesis as it is a well-established fact that methanogens are susceptible to high organic fluctuations, high ammonia concentration, and are vulnerable to acidic and alkaline pH. This was observed in a work done by Bhowmick et al., in which the high ammonia content of the fish processing wastewater caused inhibition of the methanogenesis and enhanced the power density of MFC, while treating fish processing wastewater (Bhowmick et al. 2020).
Cathode Catalysts for Microbial Fuel Cell
As elaborated in "Description of microbial fuel cell and other bio-electrochemical systems", the terminal electron acceptor is reduced at the cathode of MFC by incoming electron from the anode. The ORR kinetics on the cathode is directly dependent on the cathode material and unmodified carbon electrodes with lower content of active sites lead to sluggish ORR. This sluggish ORR causes overpotential losses, which directly affects the power generation of the MFC. This has prompted researchers to develop the electrocatalytic material for enhancing ORR at the cathode and harvest a higher amount of electricity from the MFC. Over the years the research in the BES domain has led to the development of a wide array of cathode catalysts (Table 2), which includes metal-based, carbon-based catalysts, and biocatalysts (Rismani-Yazdi et al. 2008; Khilari and Pradhan 2017).
Platinum is the popularly used benchmark cathode catalyst in MFC, which is giving excellent performance in terms of power generation due to enhanced ORR (Logan et al. 2005). When Pt was used as a cathode catalyst at a loading of 0.1 mg cm−2 the catalysed MFC was capable of generating the maximum power density of 340 mW cm−2 (Cheng et al. 2006), thus reflecting improved ORR of catalysed MFC even at very low Pt loading. However, high cost and catalyst poisoning limits the use of Pt for practical and scaled-up MFCs. In this regard, considerable efforts have been made to develop low-cost cathode catalysts using metals, metal oxides, combinations of various metal oxides, and metal-based composites to cut down the cost of the MFC fabrication and to make it more practicable (Yuan et al. 2016).
Over the years, for the replacement to Pt, metals such as Co (Lefebvre et al. 2009), Ni (Ghasemi et al. 2013), Pd (Das et al. 2020b), and Pt-based catalysts like Pt–Co (Huang et al. 2006), Pt–Ni (Yang et al. 2004), Pt–Pd (Quan et al. 2015), exhibited resilient ORR activity when used as cathode catalyst in MFC (Table 2). In one of the investigations, V2O5 micro-flowers were effectively used as cathode catalyst in an air cathode MFC (Noori et al. 2016). The electrochemical analysis demonstrated enhanced ORR that caused 6.06 W m−3 of maximum power density along with 80% of chemical oxygen demand (COD) removal from fish market wastewater. Improved electrochemical performance of MFC using V2O5 might be linked to a reduction in the charge transfer resistance in addition to the highly porous structure, which aided in superior oxygen penetration. Similarly, other metal oxides, viz. lead oxide (Morris et al. 2007), manganese oxide (Liu et al. 2010), cobalt oxide, iron oxide (Bhowmick et al. 2019b), tungsten oxide (Das and Ghangrekar 2020), etc., have been widely used as cathode catalyst in MFCs.
The use of carbon-supported metallic catalyst is another class of cathode catalyst that has gained popularity due to its superior electrochemical performance. Carbon-based materials, such as carbon nano tubes (CNT), activated carbon (AC), graphene, graphitic carbon, etc., have excellent electrocatalytic properties (Yang et al. 2013; Ben Liew et al. 2014). The graphene and CNT-based catalysts are capable of forming a 3D structure with an improved surface area. Moreover, catalyst modification using the hydrothermal technique caused improved conductivity and electron transfer efficiency due to 3D aerogel and hydrogel microstructures formation in N and S doped graphene (Verma et al. 2020). Hence, these materials are becoming a choice for the development of novel high performance cathode catalysts in MFC (Wang et al. 2011a; Ma et al. 2015). In an investigation by Yuan et al., the application of amino-functionalized multi-walled CNT as a support matrix for iron phthalocyanine has demonstrated four-electron pathway for oxygen reduction, thus reflecting high oxygen reduction potential. The corresponding maximum power density of 601 mW m−2 obtained by MFC was even better than the MFC having Pt/C cathode (Yuan et al. 2011). Similarly, other carbon-based metal catalysts used are: (1) carbon-supported Cu-Sn (Noori et al. 2018a), nickel-phthalocyanine/MnOx (Tiwari et al. 2017); (2) CNT supported MnO2 (Lu et al. 2011), Co and Fe (Türk et al. 2018); (3) graphene supported MnO2 (Khilari et al. 2013), Co–Ni (Hou et al. 2016), Pt–Co (Yan et al. 2013); (4) graphitic carbon-supported Ag–Fe2O4 (Ma et al. 2015); (5) AC supported silver (Pu et al. 2014), Cu2O (Zhang et al. 2015), MnO2 (Singh and Chandra 2013), Rh (Bhowmick et al. 2019a).
Carbonaceous catalysts are much cheaper than metal-based electrocatalysts and are proven to be effective as cathode catalyst in MFC. One of the major benefits of carbon-based catalyst is low-cost, as these catalysts can be synthesised using pyrolysis of the waste products as well. The AC had also been used as cathode catalyst in MFC derived from various precursors, such as coal, coconut shell, peat, hardwood, etc. Coal-derived AC has resulted in a maximum power density of 1620 mW m−2, additionally the n value of 3.6 indicated a nearly four-electron pathway of oxygen reduction (Watson et al. 2013). Yet, the catalytic activity towards ORR is still lower, due to which modifications are required in pure AC matrix (Zhang et al. 2014; Lv et al. 2018).
Doping of nitrogen in carbon matrix is one of the effective ways of improving the ORR catalysis in AC catalyst. Over the years, researchers have explored pre-treatment methods for effective nitrogen doping in the carbon matrix. The combination of acidic and alkaline pre-treatment using H2SO4 and KOH, respectively, has resulted in an increase in nitrogen content to 8.65% in the AC catalyst. The H2SO4 pre-treatment with KMnO4 produced oxygen-rich group in AC and successive KOH treatment caused activation of AC. When the pre-treated AC was pyrolyzed with external N in the form of cyanamide, the amines group in cyanamide reacted with oxygen-rich group of pre-treated AC. This caused the formation of C3N4 deposition over AC that facilitated the increase in N content. The successive acid–alkali pre-treatment aided in development of oxygen-rich groups, which directly assisted in increased N content due to C3N4 deposition. The improved ORR activity might be due to the occurrence of pyridinic-N (5.56%), which is capable of reducing the energy barrier to ORR (Zhang et al. 2014). In another case, nitrogen doped rice straw-derived carbon in three step process (hydrothermal carbonization, freezing followed by heat treatment in NH3) was used as a cathode catalyst in air–cathode MFC. Three step process successfully synthesised nitrogen-doped carbon with 5.57% of nitrogen and when used in MFC, a maximum power density of 2300 mW m−2 was harvested from the system (Liu et al. 2015). Similarly, Fe–N doped AC proved to be an excellent cathode catalyst compared to AC with nearly twice the maximum power density (2437 mW m−2) than plain AC (Pan et al. 2016).
Biochar, which is derived from waste-based precursors, such as sewage sludge, alfalfa leaf, corncob, watermelon rind, etc., has proved to be a cheaper option of cathode catalyst in comparison with metal-based electrocatalysts. In addition, to the properties such as high surface area, presence of pyrrolic, graphitic, and pyridinic nitrogen, and presence of phosphorus make it an attractive contender for low-cost cathode catalyst (Chakraborty et al. 2020c). Microalgae used for the tertiary treatment of wastewater have an inherent higher content of nitrogen and phosphorus in its cells. In one of the recent investigations, the biochar synthesised from the microalgae using heat treatment revealed the uniform presence of nitrogen and phosphorous. The MFC having cathode modified with microalgae derived biochar catalyst successfully achieved a maximum power density of 12.86 W m−3, while simultaneously achieving the maximum COD removal efficiency of 79.5% in the anodic chamber (Chakraborty et al. 2020a).
Low-Cost Ceramic-Based Proton Exchange Membranes for Microbial Fuel Cell
The PEM/CEM is one of the most essential components in a dual chamber MFC as it separates catholyte and anolyte, thus maintaining aerobic and anaerobic conditions in respective chambers. It is estimated that the use of membrane is associated with about 38% of the total capital cost in BES (Rozendal et al. 2008). Earlier, Nafion was by far the most widely used PEM in BES for the lab-based investigations owing to its excellent proton conductivity. However, in the later stages, it was revealed that oxygen leakage from cathode to anode, substrate crossover, and biofouling were a few of the major drawbacks associated with the use of Nafion as a PEM (Chae et al. 2008). From the view of upscaling, low mechanical stability and high cost of Nafion further reduces its acceptance for commercial application in scaled-up MFCs.
Ceramic is one of the low-cost membrane materials, made by using naturally available clay soil, which has considerably better mechanical and structural properties than polymeric membranes. Ceramic materials, such as terracotta, red soil, black soil, mullite, pyrophyllite, etc., have been used successfully as ion exchange membranes in MFC (Winfield et al. 2016). The first application in this regard was reported by Park and Zeikus with porcelain septum as PEM in MFC. The single chamber air cathode MFC with porcelain septum as PEM was capable of generating a maximum power density of 788 mW m−2 with Mn4+ and Fe3+ coated graphite as anode and cathode, respectively (Park and Zeikus 2003). The implemented single chamber MFC configuration is superior to dual chamber configuration in terms of scalability, due to non-requirement of the external aeration. In another investigation earthen pot (wall of 4 mm thick) manufactured from locally available soil with kaolinite, illite, and smectite was used as a medium for proton exchange. The earthen pot MFC achieved the coulombic efficiency (CE) of 64.5%, using stainless steel mesh cathode and KMnO4 as cathodic electrolyte (Behera et al. 2010a). The same research group revealed better organic matter removal in MFC using clayware separator compared to the Nafion as a separator. The MFC with clayware ceramic separator was capable of generating a power density of 14.59 W m−3, which ascertains the efficacy of clayware ceramic separator in electricity generation as well as wastewater treatment (Jana et al. 2010). The advantage of the ceramic material is that the same can act as a separator as well as the chamber made from it acts as the anodic chamber (Fig. 2). The configuration can be used as a single chamber MFC by exposing the cathode to air or as a dual chamber MFC by immersing the cylinder in a bigger container having catholyte.
Though ceramic membranes are successfully employed as a separator in MFC, naturally available clay has limited proton exchange capability. However, some minerals such as vermiculite, montmorillonite, illite, kaolinite, and halloysite possess high availability of cation exchangeable sites (Carroll 1959). One of the early investigations of blending montmorillonite and kaolinite in different ratios in natural clay for the development of scalable cation exchange membrane was undertaken by Ghadge and Ghangrekar (Fig. 3a). Blending of 20% montmorillonite in natural clay and using these membranes in MFC not only exhibited a maximum power density of 7.5 W m−3 but also these membranes demonstrated better cation exchange and reduced oxygen diffusion and substrate crossover compared to other ratios of montmorillonite and kaolinite (Ghadge and Ghangrekar 2015). Establishment of ceramic separator having cation exchange capacity comparable with Nafion and suitable for application in MFC as a separator, has actually worked as a breakthrough to see few pilot-scale MFC demonstration plants.
In another case, four types of ceramic membranes, namely mullite, earthenware, pyrophyllite, and alumina, were used as cation exchange membrane in MFC with human urine as anolyte. All the modifications resulted in faster start-up, whereas the highest power density was observed using pyrophyllite (6.93 W m−3). Though ceramic is a cheaper option, long term operation revealed increased internal resistance, biofouling of the porous membrane, and deposition of uric salts, which might affect the power output in long term operation (Pasternak et al. 2016).
Biochar produced from pyrolysis of organic fraction can be another option for PEM as biochar has a high surface area and more surface functional groups in addition to cation exchange property (Chakraborty et al. 2020b). In this regard, Neethu et al., fabricated a composite PEM using coconut shell derived carbon and natural clay (Fig. 3b), which unveiled high ion exchange capability and lower charge transfer resistance and oxygen crossover. Moreover, the capital cost of the composite membrane (45 $ m−2) was 38-folds lower than Nafion, thus giving it an edge over the polymeric membranes for practical use (Neethu et al. 2019). Owing to the low-cost and comparable performance to the polymeric membranes, ceramic-based membranes are becoming a popular choice amongst the researchers for its application as PEM in MFC (Table 3). Moreover, ceramic-based membranes provide superior mechanical and chemical stability and make ceramic-based PEMs an economical and viable choice for upscaling of MFCs in pilot and field trials.
Complex Wastewater Treatment Using Microbial Fuel Cell
Apart from the domestic wastewater, MFCs have been successfully implemented for the treatment of complex industrial wastewaters as well as wastewaters laden with emerging contaminants (ECs). The degradation process in the anodic chamber of MFC using exoelectrogenic bacteria is of anaerobic nature, which does not require external energy, thus it can offer a low-cost solution for the treatment of industrial wastewaters. The anode in MFC acts as a continual electron sink for generated electrons (Logan 2009), thus making the process independent of dissolved terminal electron acceptors in anodic chamber. This makes MFCs capable of treating complex organics from wastewater, which cannot be easily accomplished by means of conventional anaerobic processes (Table 4).
Over the past decade treatment of industrial wastewaters ranging from high strength alcohol distillery wastewater to toxic recalcitrant pharmaceutical wastewater had been accomplished in MFC (Pandey et al. 2016). The treatment of carbohydrate rich and high organic contained cassava wastewater with initial COD and cyanide concentrations of 16,000 and 86 mg L−1, respectively, was attained in MFC demonstrating COD removal of 88% after 120 h. The presence of cyanide had no detrimental impact on the performance of MFC, which achieved a maximum power density of 1771 mW m−2 (Kaewkannetra et al. 2011). Rice milling wastewater is another agro processing wastewater, which was successfully treated with 96.5% of COD removal in MFC. The novelty of the research lies in the application of clayware as a membrane, thus providing a cheaper solution to Nafion (Behera et al. 2010b). Easily biodegradable wastewater emerging from dairy, food processing, and slaughterhouse have been treated with COD removal efficiency in excess of 80% using MFC (Katuri et al. 2012; Mahdi Mardanpour et al. 2012; Jayashree et al. 2016).
Paper recycling wastewater, which typically contains high values of cellulose, was treated in MFC to attain 76% of COD removal, while simultaneously generating a maximum power density of 501 mW m−2. In addition, cellulose removal (96%) in the same setup, indicated the capability of MFC to degrade complex substrate, such as cellulose, in addition to organic matter (Huang and Logan 2008). In another case, biodegradation of diesel with n-alkane markers from C-8 to C-25 in MFC (81%) was 2.6 times higher than control setup (without electrical circuit). The sulphate concentrations in MFC was higher than the control setup, which suggested that in MFC, anode was preferred over sulphate as terminal electron acceptor (Morris et al. 2009). Though 21 days of retention time was required to achieve the aforementioned removal, it suggests that MFC can be used for in-situ bioremediation of contaminated sites. Although, electricity production is an attractive proposition when using MFC, enhanced pollutant removal and capability of eliminating complex organics present in industrial wastewaters gives this technology an edge over the conventional systems.
Industrial wastewater often contains a high concentration of sulphide. During the anaerobic treatment of wastewater, sulphate is reduced to sulphide by the sulphate reducing bacteria. The conversion of sulphate to sulphide can be inhibitory to the methanogens, which causes a reduction in the methane yield (McCartney and Oleszkiewicz 1991). The anaerobic treatment in the anodic chamber of MFC can be considered as a potential technology for treating sulphate laden wastewater. In the anodic chamber of MFC, to remove the sulphate, sulphate reducing bacteria should work along with electrogens. In one of the investigations, the MFC coupled with upflow anaerobic sludge blanket (UASB) reactor demonstrated 98% of sulphide removal, while simultaneously generating 101 mW L−1 of maximum power density (Rabaey et al. 2006). Various other researchers have also successfully applied MFCs for treating sulphate laden wastewater with simultaneous electricity production (Zhao et al. 2009; Ghangrekar et al. 2010; Lee et al. 2015; Chatterjee et al. 2017). In addition, it has also been reported that sulphate reducing bacteria are capable of direct electron transfer to the anode, which is beneficial for the performance of MFC (Kang et al. 2014).
Removal of phosphorus is one of the key parameters in controlling the eutrophication of surface water. Chemical precipitation is one of the most widely used forms for phosphate removal along with biological processes. The use of MFC gives a unique option of phosphate recovery, unlike other biological processes. Phosphate removal in the MFC is via precipitation at the cathode in the form of struvite. High localised alkalinity near the cathode leads to deposition of struvite at the cathode from where it can be scrapped and recovered. In one of the early investigations, iron phosphate from the dried sludge was reduced to orthophosphate at the cathode of MFC. Later, following the addition of ammonium hydroxide and manganese dichloride, the struvite precipitates were obtained at pH of 10 (Fischer et al. 2011). In another case, it was observed that simultaneous wastewater treatment and struvite recovery was possible on the aqueous face of the air cathode in a single chamber MFC. The precipitation of struvite predominantly on the cathode suggested that localised alkaline pH contributed to the chemical precipitation. The MFC contributed to 70–82% of phosphorus removal of which, 4.6–27% phosphorus was recovered as struvite (Ichihashi and Hirooka 2012). However, it was later revealed that electricity generation is reduced with an increase in struvite precipitation on the cathode, which might be due to the reduced approachability of the reacting species to the cathode surface owing to mass transfer limitation (Hirooka and Ichihashi 2013). Hence to prevent the reduced electricity generation in MFC, a two-stage system comprising of separate chamber for struvite precipitation can be an attractive option for the successful long term stable operation of MFC.
Improved lifestyle and recent developments triggered the higher occurrence of ECs in domestic wastewater apart from the organic matter and nutrients. Commonly used biological secondary processes for wastewater treatment are not effective for the removal of EC. However, MFC offers a unique advantage of simultaneous organic matter and EC removal, which cannot be achieved in conventional biological secondary treatment processes. Over the years the use of MFC has been explored for the removal of ECs, such as dyes, pharmaceuticals, pesticides, polyaromatic hydrocarbons, surfactants, etc., apart from the removal of organic matter and nutrients while simultaneously harvesting the bioelectricity (Solanki et al. 2013; Kronenberg et al. 2017; Zhang et al. 2018; Sathe et al. 2020). Current generated from MFC is a direct function of a suitable environment and substrate availability for exoelectrogenic microorganisms in the anodic chamber of MFC. Thus, the current response from MFC can also be a reliable and timely indication of possible toxicity induced by ECs, based on which suitable dilution can be considered.
Azo dyes are generally unaffected in aerobic conditions; however, undergo reductive biotransformation to aromatic amines under anaerobic conditions. Hence the anodic treatment in the MFC can successfully transform the azo dyes into aromatic amines. A single chamber MFC accomplished > 90% of the acid orange transformation into aromatic amines at a loading rate of 70–210 g m−3 d−1. A second stage aerobic bioreactor ensured degradation of aromatic amines into simpler non-toxic metabolites (Fernando et al. 2014). Several investigators have also reported the successful application of BES-based two-stage treatment processes for the degradation of dyes (Sultana et al. 2015; Yuan et al. 2017).
Staking of MFC is considered as a solution for upscaling of MFC in field-scale application. It has been observed that stacking not only has a positive impact on the reduction of overpotential losses but also has beneficial effect on the decolourisation of azo dyes. A stacked MFC with three modules demonstrated 80.3% decolourization of azo dye, which was 1.7 times higher than decolourization in a single module of MFC (Kong et al. 2018). Similarly, a dual chamber configuration of MFC can also be used for simultaneous treatment of organic matter in the anodic chamber and dye degradation in the cathodic chamber. Wherein, the in situ synthesised •OH at the cathode via bio-electro-Fenton oxidation contributed to 95% of Rhodamine B decolourization using Fe2O3 and non-catalysed carbon felt cathode (Zhuang et al. 2010). This proves the capability of simultaneous wastewater treatment and dye degradation using MFC.
Heavy metals are generally not affected in the secondary treatment processes and demand tertiary treatment for their removal from the wastewater. In MFC, during the anaerobic degradation of organic matter, the released electrons are transferred to the cathode via an external circuit, which can be used for the reduction of heavy metals at the cathode. Heavy metals such as Cr(VI), As(III), Cu(II), Fe(III), etc. have redox potential higher than the anode potential of MFC and hence can be directly reduced at the cathode from where the deposited metals can be recovered. It was witnessed that Cr(VI) was completely removed from the catholyte with an initial concentration of 100 mg L−1 in 150 h of contact time. The MFC simultaneously generated a maximum power density of 133 mW m−2 (Wang et al. 2008). In another investigation, it was revealed that cuprous oxide and metal copper deposits were detected on the cathode of MFC used for removing copper from catholyte. At an acidic pH (4.7) more than 99% of copper was removed with an initial concentration of 200 mg L−1 (Tao et al. 2011). Other heavy metals that have been reduced at the cathode of MFC include As(III) (Wang et al. 2014), Hg(II) (Wang et al. 2011b), V(V) (Zhang et al. 2009). Apart from these, noble metals (gold and silver) have also been successfully reduced at the cathode of MFC in the past investigations (Choi and Hu 2013; Ali et al. 2019). However, other metals with negative redox potentials cannot be reduced by the incoming electrons. In such cases, the reduction can be accomplished via supplementing external power as in the case of MEC.
Though MFC is still in the lab-scale stage in terms of the treatment of complex industrial wastewater and EC removal, successful implementation of MFC for sewage treatment has been reported in recent investigations as elaborated in "Up-scaling of microbial fuel cell". The addition of a few antibiotics such as penicillin caused improved electricity generation; whereas tobramycin addition had resulted in a negative impact on power generation (Wen et al. 2011; Wu et al. 2014). This indicated that the optimum concentration and loading rate for each EC will be different and there is a need to optimise the operating parameters for given conditions. Also, there is a need of identifying the by-products emerging from the degradation of target pollutants based on which follow-up treatment can be decided.
Modelling of Microbial Fuel Cell
Similar to other biochemical and electrochemical reactors, modelling of biochemical and electrochemical reactions of MFC has been undertaken in the past research. In an investigation done by Zeng et al. (2010), the cathodic reaction was found to be the limiting factor for the performance of MFC. In a different approach, it was stated that by modelling the MFC as a potentiostat and accounting for the different losses by incorporating appropriate factors, one could model the power supply pattern of a bigger-sized MFC with a steady state electrical model (Serra et al. 2020). In a different work, the direct effect of extrinsic parameters such as change in substrate concentration, effect of resistance on biofilm growth, and effect of anode surface area on the biofilm growth and in turn the power of MFC was modelled (Karamzadeh et al. 2020). It was observed that higher resistance induced growth of biofilm that has low conductivity. The investigation also emphasised that utilising low strength substrate increased the mass transport limitation of the reactant species (Karamzadeh et al. 2020). In addition to controllable extrinsic factors, effect of environmental factors also has been modelled for MFC. For example, by integrating a machine learning approach with statistical and mechanistic models, the developed algorithm could predict the qualitative trend of power production with temperature changes (Yewale et al. 2020).
In a more fundamental work, the correlation of substrate and mediator concentration with current output was established. The current response in regards to the varying substrate strength is delayed in comparison to the reducing intermediates; whereas, a pronounced effect of varying mediator concentration was observed on current output (Zhang and Halme 1995). While it is imperative to understand the effect of the different substrates and other operational parameters, it is also important to understand the flow dynamics and the mass transfer modelling in MFC. This would be beneficial towards identifying the mass transport and the diffusion limitations that can be further translated to effective hydraulic designs and reduction of dead pocketing inside the individual MFC. A simulation study using computational fluid dynamics approach emphasised the negative correlation of the boundary layer thickness around anode fibres on power density (Alvarez et al. 2020). The same investigation also indicated the existence of an optimum boundary layer thickness for three-dimensional graphite fibre brush anode for deriving maximum power density.
Coupling computational fluid dynamics models with other relevant modelling approaches have also been explored in past. The research conducted by Zhao et al. (2016) coupled computational fluid dynamics with the Butler–Volmer equation to simulate heterogeneous species distribution and the convective flow conditions. The model could well predict the current generation trend. The investigation further emphasised the scope of further improvement of the model performance by incorporating factors such as dynamic cathodic potential conditions, biomass decay, consideration of more diverse bacterial speciation, and complex hydrodynamic conditions (Zhao et al. 2016). In another investigation coupling Anaerobic Digestion Model 1 with complex computational model of MFC achieved a holistic model for predicting power and substrate utilisation pattern with considerable accuracy (Picioreanu et al. 2008).
As mentioned previously, Alvarez et al. (2020) demonstrated the effect of boundary layer thickness and in turn, the anode geometry is a limiting factor for the electrical performance of MFC. The effect of anodic processes, anode configuration, and geometry has been extensively investigated in the past. For example, modelling the anodic oxidation rate of a substrate (electron donor) by deriving a combined Nernst–Monod equation demonstrated a limiting value of anodic biofilm that can support an optimum value of current density (Marcus et al. 2007). The investigation further emphasised that if the biofilm is considered to be a conductive solid matrix, then the electron donor oxidation and consequently the current flux achievable is greater for higher values of biofilm conductivity. Higher values of this biofilm conductivity (> 10−3 mS cm−1) can enhance the development of an active biomass layer to more than tens of micrometre away from the anode surface (Marcus et al. 2007). In a different investigation, the one-dimensional model proposed by Marcus et al. (2007) was further implemented to multi-dimensional geometry and it was concluded that the current flux was directly proportional to the availability of nutrients in the biofilm matrix (Merkey and Chopp 2012). The current flux reduced due to enhancement of microbial array in a three-dimensional direction that led to the development of nutrient decreasing concentration gradient in the inner layers of the biofilm.
Several other approaches towards modelling of MFCs, such as the implementation of artificial intelligence methods (Garg et al. 2014), equivalent circuit modelling (Sindhuja et al. 2016), neural network modelling (Ma et al. 2019), etc. have been implemented. Further detailed discussion on the same can be found in past review work focussing in detail on this subject (Jadhav et al. 2020). The modelling of bio-electrochemical processes occurring in MFC can aid in designing further experiments without actually executing them and also in simulating scaling up situations before actual construction. This would save considerable time and resources and would be a sustainable approach towards understanding the performance of MFC. However, further investigations are required for coming up with a more robust model that can be implemented to different configurations and has provisions of user defined input for different operating, extrinsic and process parameters pertaining to the MFC operation.
Up-Scaling of Microbial Fuel Cell
From the above discussion, it can be witnessed that MFC is a promising technology for wastewater treatment and has the potential of upscaling. Of the various operating parameters, temperature affects the performance of MFC. Similar to that of conventional anaerobic digestion the mesophilic temperature range is found to be optimum for MFCs as well (Behera et al. 2011). Past investigations have demonstrated that there is only a 1.1 times increase in the maximum power density in MFC operated at mesophilic temperature of (30 °C) compared to an ambient temperature of 23 °C (Ahn and Logan 2010). Although at a lower temperature the electrical performance of MFC deteriorates, for the practical implementations, and for maintaining the low cost of treatment, it might not be economically viable to employ a temperature control mechanism for anolyte. Moreover, a temperature control mechanism at a pilot or field-scale setup would increase the capital as well as operating costs.
The advent of low-cost ceramic-based PEMs and Pt-free catalysts aided in minimising the capital cost of MFCs. Though successful lab-based applications have been demonstrated, very few investigations have been reported on possible scaled-up or pilot scale operations of MFC. In an early investigation, Feng et al. tried a 250-L horizontal plug flow typed MFC for domestic wastewater treatment. Despite of high COD removal efficiency (79%), the system could harvest only 0.47 W m−3 of maximum power density possibly due to higher internal resistance than typical lab-scaled setups (Feng et al. 2014). A 90-L MFC, consisting of 5 modules operated for more than 6 months, was used for the treatment of brewery wastewater. Based on the results of electricity generation, it was estimated that the net electrical energy harvested was 0.021 to 0.034 kWh m−3 (Dong et al. 2015).
With an effort to reduce the fabrication cost of MFC, ceramic separator was used as PEM in a 45-L pilot scale MFC. Following the optimization, it was observed that MFC achieved a maximum current of 42 mA at an organic loading rate of 4.5 g COD L−1 d−1, with an internal resistance of 12.4 Ω (Ghadge et al. 2016b). The main advantage of a ceramic separator is the capability to handle more hydraulic pressure than polymeric membrane, which can offer an advantage for its application for field-scale MFCs. The ceramic membranes have been widely used for field-scale applications of MFC by Ieropoulos and group for the PEE-power urinals. The first trial of 330-L MFC was conducted at Glastonbury Music Festival in England which consisted of 12 MFC modules (total 432 MFCs in stack). The assembly generated an average power of 300 mW, which was stored in supercapacitors for powering LEDs (Ieropoulos et al. 2016). In the subsequent trial, efforts were made to increase the capacity of urine treatment using cascade type MFC modules, which produced an average power of 424 mW. In the field-scale trial, the COD removal of 48% was achieved at the hydraulic retention time of 700 min (Walter et al. 2018). In the recent investigation, 6-chambered stacked MFC with a working volume of 720-L was used for the treatment of sewage (Fig. 4). The cathode was coated with Co0.5Zn0.5Fe2O4 and Sn5Cu84 as cathode catalyst; whereas, goethite was used as anode catalyst in the MFC. The electrical energy harvested (maximum power generation of 61 mW) was stored in supercapacitors for illumination at night. The overall hydraulic retention time of 18 h resulted in a COD removal efficiency of 78.4%, which improved to 87.3% following a doubling of hydraulic retention time (Das et al. 2020a).
In order to prove that MFC can be a scalable technology for wastewater treatment, initial pilot and field-scale investigations act as a benchmark based on which future course of action in this direction can be finalised. Based on the available literature on scale-up MFCs, current issues that still need to be addressed include high capital cost, overpotential losses, high internal resistance, and operational issues related to long-term operation, such as stability of cathode catalyst and membrane fouling. Thus, continual development pertaining to these issues is essential to make this technology a commercial treatment system for wastewater treatment.
Future Scope
The MFC has been a widely researched technology in the last two decades owing to its ability to treat wastewater, while simultaneously generating renewable energy in the form of bioelectricity. The majority of the past investigations have been in the lab-scale setup (up to few litres). Based on this it can be concluded that, although successfully operated in the lab-scale, MFC is still not fully matured to be used for the field-scale operations. Although MFC has the capability of bioelectricity generation, the harvested electricity is far less than that required for direct applications. Presently, MFC research is associated with a variety of limitations, such as activation losses, mass transport and diffusional losses, ohmic losses, and other concerns pertaining to scavenging reactions, which limits electricity production. The high value of internal resistance in MFC causes a potential drop, thus drastically deteriorating the power output of the MFC. Oxygen is the most practical terminal electron acceptor in MFC; conversely, the fugitive diffusion of oxygen into the anodic chamber can affect the performance of anaerobic microorganisms, thus affecting the rate of electricity generation (Pham et al. 2006). High capital cost is another drawback associated with the use of MFC for field-scale applications. To reduce the capital cost, ceramic-based CEM and carbon-based cathode catalysts can be a suitable option as elaborated in "Low-cost ceramic-based proton exchange membranes for microbial fuel cell" and "Cathode catalysts for microbial fuel cell", respectively. In addition to this, diffusion barriers considerably reduce the power generation of large MFCs; hence to enhance the power generation, stacking arrangement of multiple small size MFCs can be practiced (Ieropoulos et al. 2016).
Also, the anode and cathode surface area provided with specific electrochemical properties of the material selected plays a very important role. The ratio of area of the electrode and volume of the anodic chamber provided for desired organic loading rate is crucial. It has been reported that Butler–Volmer kinetics dominates the anode surface area required than the area required for polarisation and biofilm formation because the activation loss is the predominant factor that affects the rate of electrochemical transformation and power output from fuel cell (Ghadge et al. 2016a). Therefore, anode surface area is governed by Butler–Volmer kinetics and based on this necessary anode surface area should be provided to harvest more electricity from the MFC to optimise the benefit from this system.
The air–cathode configuration of MFC is practicable for field-sale applications, the design is still associated with practical difficulties, such as salt depositions and biofouling of cathode (An et al. 2017). The fouling problem can be mitigated by incorporating the catalytic material which also has the biocidal properties, such as silver nano particles (Noori et al. 2018b), to have long term stable performance of the MFC. Further to the task of maintaining long term stable performance, reducing cost of the electrodes, PEM/CEM, and catalysts, without compromising desired electrochemical properties, is still a challenging task associated with this technology, which make the system comparably expensive and unpredictable than conventional systems. Future research in the aforementioned issues needs to be performed to make MFC a promising technology for wastewater treatment in the upcoming years.
Conclusion
The application of MFC for onsite wastewater treatment and concomitant power recovery is a state-of-the-art technology that has the potential to become the central theme of future waste treatment and valorisation facilities. The present scaled-up studies of MFC exhibit immense potential in terms of onsite electricity usage for small devices, area illumination as well as for holistic treatment of wastewater. Moreover, the ability to remove aromatic and other complex organic pollutants both by anodic biodegradation and cathode-based oxidation and low sludge generation owing to anaerobic metabolism pathway are clear advantages over conventional biological processes. These findings point towards the fact that in near future, MFC-based treatment units could become a game changer in the domain of waste treatment.
Data availability
All data generated or analysed during this study are included in this article.
Code availability
Not applicable.
References
Ahn Y, Logan B (2010) Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour Technol 101(2):469–475. https://doi.org/10.1016/j.biortech.2009.07.039
Ajayi FF, Weigele PR (2012) A terracotta bio-battery. Bioresour Technol 116:86–91. https://doi.org/10.1016/j.biortech.2012.04.019
Ali J, Wang L, Waseem H, Sharif HMA, Djellabi R, Zhang C, Pan G (2019) Bioelectrochemical recovery of silver from wastewater with sustainable power generation and its reuse for biofouling mitigation. J Clean Prod 235:1425–1437. https://doi.org/10.1016/j.jclepro.2019.07.065
Allen RM, Bennetto HP (1993) Microbial fuel-cells—electricity production from carbohydrates. Appl Biochem Biotechnol 39:27–40. https://doi.org/10.1007/BF02918975
An J, Li N, Wan L et al (2017) Electric field induced salt precipitation into activated carbon air-cathode causes power decay in microbial fuel cells. Water Res 123:369–377. https://doi.org/10.1016/j.watres.2017.06.087
Bagchi S, Behera M (2019) Methanogenesis suppression in microbial fuel cell by aluminium dosing. Bioelectrochemistry 129:206–210. https://doi.org/10.1016/j.bioelechem.2019.05.019
Baranitharan E, Khan M, Prasad D, Salihon J (2013) Bioelectricity generation from palm oil mill effluent in microbial fuel cell using polacrylonitrile carbon felt as electrode. Water Air Soil Pollut 224:1533. https://doi.org/10.1007/s11270-013-1533-1
Behera M, Jana PS, Ghangrekar MM (2010a) Performance evaluation of low cost microbial fuel cell fabricated using earthen pot with biotic and abiotic cathode. Bioresour Technol 101(4):1183–1189. https://doi.org/10.1016/j.biortech.2009.07.089
Behera M, Jana PS, More TT, Ghangrekar MM (2010b) Rice mill wastewater treatment in microbial fuel cells fabricated using proton exchange membrane and earthen pot at different pH. Bioelectrochemistry 79(2):228–233. https://doi.org/10.1016/j.bioelechem.2010.06.002
Behera M, Murthy S, Ghangrekar M (2011) Effect of operating temperature on performance of microbial fuel cell. Water Sci Tech 89(12):3985–3994. https://doi.org/10.2166/wst.2011.704
Ben Liew K, Daud WRW, Ghasemi M, Leong J, Lim S, Ismail M (2014) Non-Pt catalyst as oxygen reduction reaction in microbial fuel cells: a review. Int J Hydrogen Energy 39:4870–4883. https://doi.org/10.1016/j.ijhydene.2014.01.062
Bhowmick GD, Das S, Adhikary K, Ghangrekar MM, Mitra A (2019a) Using rhodium as a cathode catalyst for enhancing performance of microbial fuel cell. Int J Hydrogen Energy 44(39):22218–22222. https://doi.org/10.1016/j.ijhydene.2019.06.063
Bhowmick GD, Das S, Verma HK, Neethu B, Ghangrekar MM (2019b) Improved performance of microbial fuel cell by using conductive ink printed cathode containing Co3O4 or Fe3O4. Electrochim Acta 310:173–183. https://doi.org/10.1016/j.electacta.2019.04.127
Bhowmick GD, Neethu B, Ghangrekar MM, Banerjee R (2020) Improved performance of microbial fuel cell by in situ methanogenesis suppression while treating fish market wastewater. Appl Biochem Biotechnol 192:1060–1075. https://doi.org/10.1007/s12010-020-03366-y
Carroll D (1959) Ioon exchange in clays and other minerals. Bull Geol Soc Am 70(6):749–779. https://doi.org/10.1130/0016-7606(1959)70[749:IEICAO]2.0.CO;2
Chae KJ, Choi M, Ajayi FF, Park W, Kim I (2008) Mass transport through a proton exchange membrane (Nafion) in microbial fuel cells. Energy Fuels 22(1):169–176. https://doi.org/10.1021/ef700308u
Chakraborty I, Bhowmick GD, Ghosh D, Dubey BK, Pradhan D, Ghangrekar MM (2020a) Novel low-cost activated algal biochar as a cathode catalyst for improving performance of microbial fuel cell. Sustain Energy Technol Assessments 42:100808. https://doi.org/10.1016/j.seta.2020.100808
Chakraborty I, Das S, Dubey BK, Ghangrekar MM (2020b) Novel low cost proton exchange membrane made from sulphonated biochar for application in microbial fuel cells. Mater Chem Phys 239:122025. https://doi.org/10.1016/j.matchemphys.2019.122025
Chakraborty I, Sathe SM, Dubey BK, Ghangrekar MM (2020c) Waste-derived biochar: Applications and future perspective in microbial fuel cells. Bioresour Technol 312:123587. https://doi.org/10.1016/j.biortech.2020.123587
Chatterjee P, Ghangrekar MM, Rao S, Kumar S (2017) Biotic conversion of sulphate to sulphide and abiotic conversion of sulphide to sulphur in a microbial fuel cell using cobalt oxide octahedrons as cathode catalyst. Bioprocess Biosyst Eng 40:759–768. https://doi.org/10.1007/s00449-017-1741-y
Cheng S, Liu H, Logan BE (2006) Power densities using different cathode catalysts (Pt and CoTMPP) and polymer binders (Nafion and PTFE) in single chamber microbial fuel cells. Environ Sci Technol 40(1):364–369. https://doi.org/10.1021/es0512071
Choi C, Hu N (2013) The modeling of gold recovery from tetrachloroaurate wastewater using a microbial fuel cell. Bioresour Technol 133:589–598. https://doi.org/10.1016/j.biortech.2013.01.143
Cohen B (1931) The bacterial culture as an electrical half-cell. J Bacteriol 21:18–19
Das S, Ghangrekar MM (2020) Tungsten oxide as electrocatalyst for improved power generation and wastewater treatment in microbial fuel cell. Environ Technol 41(19):2546–2553. https://doi.org/10.1080/09593330.2019.1575477
Das I, Ghangrekar MM, Satyakam R, Srivastava P, Khan S, Pandey H (2020a) On-site sanitary wastewater treatment system using 720-L stacked microbial fuel cell: case study. J Hazardous Toxic Radioact Waste. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000518
Das S, Chakraborty I, Rajesh PP, Ghangrekar MM (2020b) Performance evaluation of microbial fuel cell operated with Pd or MnO2 as cathode catalyst and chaetoceros pretreated anodic inoculum. J Hazardous Toxic Radioact Waste. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000501
Das I, Das S, Dixit R, Ghangrekar M (2020c) Goethite supplemented natural clay ceramic as an alternative proton exchange membrane and its application in microbial fuel cell. Ionics 26:3061–3072. https://doi.org/10.1007/s11581-020-03472-1
Dong Y, Qu Y, He W, Du Y, Liu J, Han X, Feng Y (2015) A 90-liter stackable baffled microbial fuel cell for brewery wastewater treatment based on energy self-sufficient mode. Bioresour Technol 195:66–72. https://doi.org/10.1016/j.biortech.2015.06.026
Dong H, Liu X, Xu T, Wang Q, Chen X, Chen S, Zhang H, Liang P, Huang X, Zhang X (2018) Hydrogen peroxide generation in microbial fuel cells using graphene-based air-cathodes. Bioresour Technol 247:684–689. https://doi.org/10.1016/j.biortech.2017.09.158
Feng Y, Wang X, Logan BE, Lee H (2008) Brewery wastewater treatment using air-cathode microbial fuel cells. Appl Microbiol Biotechnol 78:873–880. https://doi.org/10.1007/s00253-008-1360-2
Feng Y, He W, Liu J, Wang X, Qu Y, Ren N (2014) A horizontal plug flow and stackable pilot microbial fuel cell for municipal wastewater treatment. Bioresour Technol 156:132–138. https://doi.org/10.1016/j.biortech.2013.12.104
Fernando E, Keshavarz T, Kyazze G (2014) Complete degradation of the azo dye Acid Orange-7 and bioelectricity generation in an integrated microbial fuel cell, aerobic two-stage bioreactor system in continuous flow mode at ambient temperature. Bioresour Technol 156:155–162. https://doi.org/10.1016/j.biortech.2014.01.036
Fischer F, Bastian C, Happe M, Mabillard E, Schmidt N (2011) Microbial fuel cell enables phosphate recovery from digested sewage sludge as struvite. Bioresour Technol 102(10):5824–5830. https://doi.org/10.1016/j.biortech.2011.02.089
Gajda I, Greenman J, Melhuish C, Ieropoulos I (2015) Simultaneous electricity generation and microbially-assisted electrosynthesis in ceramic MFCs. Bioelectrochemistry 104:58–64. https://doi.org/10.1016/j.bioelechem.2015.03.001
Garg A, Vijayaraghavan V, Mahapatra SS, Tai K, Wong CH (2014) Performance evaluation of microbial fuel cell by artificial intelligence methods. Expert Syst Appl 41(4):1389–1399. https://doi.org/10.1016/j.eswa.2013.08.038
Ghadge AN, Ghangrekar MM (2015) Development of low cost ceramic separator using mineral cation exchanger to enhance performance of microbial fuel cells. Electrochim Acta 166(1):320–328. https://doi.org/10.1016/j.electacta.2015.03.105
Ghadge AN, Jadhav DA, Ghangrekar MM (2016a) Wastewater treatment in pilot-scale microbial fuel cell using multielectrode assembly with ceramic separator suitable for field applications. Environ Prog Sustain Energy 35(6):1809–1817. https://doi.org/10.1002/ep.12403
Ghadge AN, Ghangrekar MM, Scott K (2016b) Maximum anode chamber volume and minimum anode area for supporting electrogenesis in microbial fuel cells treating wastewater. J Renew Sustain Energy. https://doi.org/10.1063/1.4961587
Ghangrekar MM, Shinde VB (2008) Simultaneous sewage treatment and electricity generation in membrane-less microbial fuel cell. Water Sci Technol 8(1):37–43. https://doi.org/10.2166/wst.2008.339
Ghangrekar MM, Murthy S, Behera M, Duteanu N (2010) Effect of sulfate concentration in the wastewater on microbial fuel cell performance. Environ Eng Manag J 9:1227–1234
Ghasemi M, Daud WRW, Rahimnejad M, Reyazi M, Fatemi A, Jafari Y, Somalu M, Manzour A (2013) Copper-phthalocyanine and nickel nanoparticles as novel cathode catalysts in microbial fuel cells. Int J Hydrogen Energy 38(22):9533–9540. https://doi.org/10.1016/j.ijhydene.2013.01.177
Grüning A, Beecroft NJ, Avignone-Rossa C (2015) Low-potential respirators support electricity production in microbial fuel cells. Microb Ecol 70:266–273. https://doi.org/10.1007/s00248-014-0518-y
Guo F, Fu G, Zhang Z, Zhang C (2013) Mustard tuber wastewater treatment and simultaneous electricity generation using microbial fuel cells. Bioresour Technol 136:425–430. https://doi.org/10.1016/j.biortech.2013.02.116
He Z (2013) Microbial fuel cells: now let us talk about energy. Env Sci and Tech 47(1):332–333. https://doi.org/10.1021/es304937e
Hirooka K, Ichihashi O (2013) Phosphorus recovery from artificial wastewater by microbial fuel cell and its effect on power generation. Bioresour Technol 137:368–375. https://doi.org/10.1016/j.biortech.2013.03.067
Hou Y, Yuan H, Wen Z, Cui S, Guo X, He Z, Chen J (2016) Nitrogen-doped graphene/CoNi alloy encased within bamboo-like carbon nanotube hybrids as cathode catalysts in microbial fuel cells. J Power Sources 307:561–568. https://doi.org/10.1016/j.jpowsour.2016.01.018
Huang L, Logan BE (2008) Electricity generation and treatment of paper recycling wastewater using a microbial fuel cell. Appl Microbiol Biotechnol 80:349–355. https://doi.org/10.1007/s00253-008-1546-7
Huang Q, Yang H, Tang Y, Lu T, Akins D (2006) Carbon-supported Pt-Co alloy nanoparticles for oxygen reduction reaction. Electrochem Commun 8(8):1220–1224. https://doi.org/10.1016/j.elecom.2006.05.027
Huggins T, Wang H, Kearns J, Jenkins P, Ren Z (2014) Biochar as a sustainable electrode material for electricity production in microbial fuel cells. Bioresour Technol 157:114–119. https://doi.org/10.1016/j.biortech.2014.01.058
Ichihashi O, Hirooka K (2012) Removal and recovery of phosphorus as struvite from swine wastewater using microbial fuel cell. Bioresour Technol 114:303–307. https://doi.org/10.1016/j.biortech.2012.02.124
Ieropoulos IA, Stinchcombe A, Gajda I, Forbes S, Jimenez I, Pasternak G, Sanchez-Herranz D, Greenman J (2016) Pee power urinal-microbial fuel cell technology field trials in the context of sanitation. Environ Sci Water Res Technol 2:336–343. https://doi.org/10.1039/c5ew00270b
Ismail Z, Habeeb A (2017) Experimental and modeling study of simultaneous power generation and pharmaceutical wastewater treatment in microbial fuel cell based on mobilized biofilm bearers. Renew Energy 101:1256–1265. https://doi.org/10.1016/j.renene.2016.10.008
Jadhav DA, Ghosh Ray S, Ghangrekar MM (2017) Third generation in bio-electrochemical system research—a systematic review on mechanisms for recovery of valuable by-products from wastewater. Renew Sustain Energy Rev 76:1022–1031. https://doi.org/10.1016/J.RSER.2017.03.096
Jadhav DA, Alessandro A, Chendake A, Pandit S, Pant D (2020) Modeling and optimization strategies towards performance enhancement of microbial fuel cells. Bioresour Technol 320:124256. https://doi.org/10.1016/j.biortech.2020.124256
Jana PS, Behera M, Ghangrekar MM (2010) Performance comparison of up-flow microbial fuel cells fabricated using proton exchange membrane and earthen cylinder. Int J Hydrogen Energy 35(11):5681–5686. https://doi.org/10.1016/j.ijhydene.2010.03.048
Jayashree C, Tamilarasan K, Rajkumar M, Arulazhagan P, Yogalakshmi K, Srikanth M, Banu J (2016) Treatment of seafood processing wastewater using upflow microbial fuel cell for power generation and identification of bacterial community in anodic biofilm. J Environ Manage 180:351–358. https://doi.org/10.1016/j.jenvman.2016.05.050
Kaewkannetra P, Chiwes W, Chiu TY (2011) Treatment of cassava mill wastewater and production of electricity through microbial fuel cell technology. Fuel 90(8):2746–2750. https://doi.org/10.1016/j.fuel.2011.03.031
Kang CS, Eaktaang N, Kwon D, Kim H (2014) Enhanced current production by Desulfovibrio desulfuricans biofilm in a mediator-less microbial fuel cell. Bioresour Technol 165:27–30. https://doi.org/10.1016/j.biortech.2014.03.148
Karamzadeh M, Kadivarian H, Kadivarian M, Kazemi A (2020) Modeling the influence of substrate concentration, anode electrode surface area and external resistance in a start-up on the performance of microbial fuel cell. Bioresour Technol Rep 12:100559. https://doi.org/10.1016/j.biteb.2020.100559
Karube I, Matsunaga T, Tsuru S, Suzuki S (1976) Continous hydrogen production by immobilized whole cells of Clostridium butyricum. BBA - Gen Subj 444(2):338–343. https://doi.org/10.1016/0304-4165(76)90376-7
Katuri KP, Enright AM, O’Flaherty V, Leech D (2012) Microbial analysis of anodic biofilm in a microbial fuel cell using slaughterhouse wastewater. Bioelectrochemistry 87:164–171. https://doi.org/10.1016/j.bioelechem.2011.12.002
Kaur A, Boghani H, Michie I, Dinsdale R, Guwy A, Premier G (2014) Inhibition of methane production in microbial fuel cells: operating strategies which select electrogens over methanogens. Bioresour Technol 173:75–81. https://doi.org/10.1016/j.biortech.2014.09.091
Khilari S, Pradhan D (2017) Role of cathode catalyst in microbial fuel cell. In: Microbial fuel cell: a bioelectrochemical system that converts waste to Watts. pp 141–163. https://doi.org/10.1007/978-3-319-66793-5_8
Khilari S, Pandit S, Ghangrekar MM, Das D, Pradhan D (2013) Graphene supported α-MnO2 nanotubes as a cathode catalyst for improved power generation and wastewater treatment in single-chambered microbial fuel cells. RSC Adv 3:7902–7911. https://doi.org/10.1039/c3ra22569k
Kim BH, Al E (1999) US Patent: Mediator-less biofuel cell. 5976719
Kim JR, Min B, Logan BE (2005) Evaluation of procedures to acclimate a microbial fuel cell for electricity production. Appl Microbiol Biotechnol 68:23–30. https://doi.org/10.1007/s00253-004-1845-6
Kong F, Ren H, Pavlostathis SG, Wang A, Nan J, Ren N (2018) Enhanced azo dye decolorization and microbial community analysis in a stacked bioelectrochemical system. Chem Eng J 354:351–362. https://doi.org/10.1016/j.cej.2018.08.027
Kronenberg M, Trably E, Bernet N, Patureau D (2017) Biodegradation of polycyclic aromatic hydrocarbons: Using microbial bioelectrochemical systems to overcome an impasse. Environ Pollut 231(1):509–523. https://doi.org/10.1016/j.envpol.2017.08.048
Lee D, Lee C, Chang J, Liao Q, Su A (2015) Treatment of sulfate/sulfide-containing wastewaters using a microbial fuel cell: single and two-anode systems. Int J Green Energ 12(10):998–1004. https://doi.org/10.1080/15435075.2014.910780
Lefebvre O, Ooi WK, Tang Z et al (2009) Optimization of a Pt-free cathode suitable for practical applications of microbial fuel cells. Bioresour Technol 100(20):4907–4910. https://doi.org/10.1016/j.biortech.2009.04.061
Li W, Yu H, He Z (2014) Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. RSC Adv 7:911–924. https://doi.org/10.1039/C3EE43106A
Liu XW, Sun XF, Huang YX, Sheng G, Zhou K, Zeng R, Dong F, Wang S, Xu A, Tong Z, Yu H (2010) Nano-structured manganese oxide as a cathodic catalyst for enhanced oxygen reduction in a microbial fuel cell fed with a synthetic wastewater. Water Res 44(18):5298–5305. https://doi.org/10.1016/j.watres.2010.06.065
Liu L, Xiong Q, Li C, Feng Y, Chen S (2015) Conversion of straw to nitrogen doped carbon for efficient oxygen reduction catalysts in microbial fuel cells. RSC Adv 5:89771–89776. https://doi.org/10.1039/c5ra15235f
Logan BE (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol 7:375–381. https://doi.org/10.1038/nrmicro2113
Logan BE, Murano C, Scott K, Gray N, Head I (2005) Electricity generation from cysteine in a microbial fuel cell. Water Res 39(5):942–952. https://doi.org/10.1016/j.watres.2004.11.019
Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: Methodology and technology. Environ Sci Technol 40(17):5181–5192. https://doi.org/10.1021/es0605016
Lu N, Zhou S, Zhuang L, Zhang J, Ni J (2009) Electricity generation from starch processing wastewater using microbial fuel cell technology. Biochem Eng J 43(3):246–251. https://doi.org/10.1016/j.bej.2008.10.005
Lu M, Kharkwal S, Ng HY, Li SFY (2011) Carbon nanotube supported MnO2 catalysts for oxygen reduction reaction and their applications in microbial fuel cells. Biosens Bioelectron 26(12):4728–4732. https://doi.org/10.1016/j.bios.2011.05.036
Lv K, Zhang H, Chen S (2018) Nitrogen and phosphorus co-doped carbon modified activated carbon as an efficient oxygen reduction catalyst for microbial fuel cells. RSC Adv 8:848–855. https://doi.org/10.1039/c7ra12907f
Ma M, You S, Gong X, Dia Y, Zou J, Fu H (2015) Silver/iron oxide/graphitic carbon composites as bacteriostatic catalysts for enhancing oxygen reduction in microbial fuel cells. J Power Sources 283:74–83. https://doi.org/10.1016/j.jpowsour.2015.02.100
Ma F, Yin Y, Pang S, Liu J, Chen W (2019) A data-driven based framework of model optimization and neural network modeling for microbial fuel cells. IEEE Access 7:162036–162049. https://doi.org/10.1109/ACCESS.2019.2951943
Mahdi Mardanpour M, Nasr Esfahany M, Behzad T, Sedaqatvand R (2012) Single chamber microbial fuel cell with spiral anode for dairy wastewater treatment. Biosens Bioelectron 38(1):264–269. https://doi.org/10.1016/j.bios.2012.05.046
Marcus KA, Torres CI, Rittmann BE (2007) Conduction-based modeling of the biofilm anode of a microbial fuel cell. Biotechnol Bioeng 98(6):1171–1182. https://doi.org/10.1002/bit.21533
McCartney DM, Oleszkiewicz JA (1991) Sulfide inhibition of anaerobic degradation of lactate and acetate. Water Res 25(2):203–209. https://doi.org/10.1016/0043-1354(91)90030-T
Merkey BV, Chopp DL (2012) The performance of a microbial fuel cell depends strongly on anode geometry: a multidimensional modeling study. Bull Math Biol 74(4):834–857. https://doi.org/10.1007/s11538-011-9690-0
More TT, Ghangrekar MM (2010) Improving performance of microbial fuel cell with ultrasonication pre-treatment of mixed anaerobic inoculum sludge. Bioresour Technol 101(2):562–567. https://doi.org/10.1016/j.biortech.2009.08.045
Morris JM, Jin S, Wang J, Zhu C, Urynowicz M (2007) Lead dioxide as an alternative catalyst to platinum in microbial fuel cells. Electrochem Commun 9(7):1730–1734. https://doi.org/10.1016/j.elecom.2007.03.028
Morris JM, Jin S, Crimi B, Pruden A (2009) Microbial fuel cell in enhancing anaerobic biodegradation of diesel. Chem Eng J 146(2):161–167. https://doi.org/10.1016/j.cej.2008.05.028
Nath D, Ghangrekar MM (2020) Plant secondary metabolites induced electron flux in microbial fuel cell: investigation from laboratory-to-field scale. Sci Rep 10:17185. https://doi.org/10.1038/s41598-020-74092-y
Nath D, Chakraborty I, Ghangrekar MM (2021) Methanogenesis inhibitors used in bio-electrochemical systems: A review revealing reality to decide future direction and applications. Bioresour Technol 319:124141. https://doi.org/10.1016/j.biortech.2020.124141
Neethu B, Bhowmick GD, Ghangrekar MM (2019) A novel proton exchange membrane developed from clay and activated carbon derived from coconut shell for application in microbial fuel cell. Biochem Eng J 148:170–177. https://doi.org/10.1016/j.bej.2019.05.011
Neethu B, Bhowmick GD, Fathima A, Ghangrekar MM (2020a) Anodic inoculum pre-treatment by extracts of Azadirachta indica leaves and Allium sativum peels for improved bioelectricity recovery from microbial fuel cell. Int J Hydrogen Energy 45(43):23391–23400. https://doi.org/10.1016/j.ijhydene.2020.06.086
Neethu B, Bhowmick GD, Ghangrekar MM (2020b) Improving performance of microbial fuel cell by enhanced bacterial-anode interaction using sludge immobilized beads with activated carbon. Process Saf Environ Prot 143:285–292. https://doi.org/10.1016/j.psep.2020.06.043
Noori MT, Ghangrekar MM, Mukherjee CK (2016) V2O5 microflower decorated cathode for enhancing power generation in air-cathode microbial fuel cell treating fish market wastewater. Int J Hydrogen Energy 41(5):3638–3645. https://doi.org/10.1016/j.ijhydene.2015.12.163
Noori MT, Bhowmick GD, Tiwari BR, Ghangrekar OM, Ghangrekar MM, Muckerjee CK (2018a) Carbon supported Cu-Sn bimetallic alloy as an excellent low-cost cathode catalyst for enhancing oxygen reduction reaction in microbial fuel cell. J Electrochem Soc 165:F621. https://doi.org/10.1149/2.0271809jes
Noori MT, Tiwari BR, Mukherjee CK, Ghangrekar MM (2018b) Enhancing the performance of microbial fuel cell using Ag–Pt bimetallic alloy as cathode catalyst and anti-biofouling agent. Int J Hydrogen Energy 43(42):19650–19660. https://doi.org/10.1016/j.ijhydene.2018.08.120
Pan Y, Mo X, Li K, Pu L, Yang T (2016) Iron-nitrogen-activated carbon as cathode catalyst to improve the power generation of single-chamber air-cathode microbial fuel cells. Bioresour Technol 206:285–289. https://doi.org/10.1016/j.biortech.2016.01.112
Pandey P, Shinde VN, Deopurkar RL, Kale P, Patil S, Pant D (2016) Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl Energy 168:706–723. https://doi.org/10.1016/j.apenergy.2016.01.056
Pandit S, Ghosh S, Ghangrekar MM, Das D (2012) Performance of an anion exchange membrane in association with cathodic parameters in a dual chamber microbial fuel cell. Int J Hydrogen Energy 37(11):9383–9392. https://doi.org/10.1016/j.ijhydene.2012.03.011
Park DH, Zeikus JG (2003) Improved fuel cell and electrode designs for producing electricity from microbial degradation. Biotechnol Bioeng. 81(3):348–355. https://doi.org/10.1002/bit.10501
Pasternak G, Greenman J, Ieropoulos I (2016) Comprehensive study on ceramic membranes for low-cost microbial fuel cells. Chemsuschem 9(1):88–96. https://doi.org/10.1002/cssc.201501320
Pham TH, Rabaey K, Aelterman P, Clauwaert P, Schamphelaire L, Boon N, Verstraete W (2006) Microbial fuel cells in relation to conventional anaerobic digestion technology. Eng Life Sci 6(3):285–292. https://doi.org/10.1002/elsc.200620121
Picioreanu C, Katuri KP, Head IM, van Loosdrecht MC, Scott K (2008) Mathematical model for microbial fuel cells with anodic biofilms and anaerobic digestion. Water Sci Technol 57(7):965–971. https://doi.org/10.2166/wst.2008.095
Potter MC (1911) Electrical effects accompanying the decomposition of organic compounds. Proc R Soc London Ser b, Contain Pap a Biol Character 84:260–276. https://doi.org/10.1098/rspb.1911.0073
Pu L, Li K, Chen Z, Zhang P, Zhang X, Fu Z (2014) Silver electrodeposition on the activated carbon air cathode for performance improvement in microbial fuel cells. J Power Sources 268:476–481. https://doi.org/10.1016/j.jpowsour.2014.06.071
Puig S, Serra M, Coma M, Cabre M, Balaguer M, Colprim J (2011) Microbial fuel cell application in landfill leachate treatment. J Hazard Mater 185:763–767. https://doi.org/10.1016/j.jhazmat.2010.09.086
Quan X, Mei Y, Xu H, Sun B, Zhang X (2015) Optimization of Pt-Pd alloy catalyst and supporting materials for oxygen reduction in air-cathode microbial fuel cells. Electrochim Acta 165:72–77. https://doi.org/10.1016/j.electacta.2015.02.235
Rabaey K, Verstraete W (2005) Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol 26(6):291–298. https://doi.org/10.1016/j.tibtech.2005.04.008
Rabaey K, Clauwaert P, Aelterman P, Verstraete W (2005) Tubular microbial fuel cells for efficient electricity generation. Environ Sci Technol 39(20):8077–8082. https://doi.org/10.1021/es050986i
Rabaey K, Van De Sompel K, Maignien L, Boon N, Aelterman P, Clauwaert P, De Schamphelaire L, Pham HT, Vermeulen J, Verhaege M, Lens P, Verstraete W (2006) Microbial fuel cells for sulfide removal. Environ Sci Technol 40(17):5218–5224. https://doi.org/10.1021/es060382u
Rajesh PP, Noori MT, Ghangrekar MM (2014) Controlling methanogenesis and improving power production of microbial fuel cell by lauric acid dosing. Water Sci Technol 70:1363–1369. https://doi.org/10.2166/wst.2014.386
Rajesh PP, Jadhav DA, Ghangrekar MM (2015) Improving performance of microbial fuel cell while controlling methanogenesis by Chaetoceros pretreatment of anodic inoculum. Bioresour Technol 180:66–71. https://doi.org/10.1016/j.biortech.2014.12.095
Rajesh PP, Jadhav DA, Ghangrekar MM (2018) Pre-treatment of anodic inoculum with nitroethane to improve performance of a microbial fuel cell. Water Sci Technol 77(10):2491–2496. https://doi.org/10.2166/wst.2018.206
Ray G, Noori MT, Ghangrekar MM (2017) Novel application of peptaibiotics derived from Trichoderma sp. for methanogenic suppression and enhanced power generation in microbial fuel cells. RSC Adv 7:10707–10717. https://doi.org/10.1039/c6ra27763b
Raychaudhuri A, Behera M (2020) Comparative evaluation of methanogenesis suppression methods in microbial fuel cell during rice mill wastewater treatment. Environ Technol Innov 17:100509. https://doi.org/10.1016/j.eti.2019.100509
Rismani-Yazdi H, Carver SM, Christy AD, Tuovinen OH (2008) Cathodic limitations in microbial fuel cells: an overview. J Power Sources 180(2):683–694. https://doi.org/10.1016/j.jpowsour.2008.02.074
Rismani-Yazdi H, Carver SM, Christy AD, Yu Z, Bibby K, Peccia J, Tuovinen OH (2013) Suppression of methanogenesis in cellulose-fed microbial fuel cells in relation to performance, metabolite formation, and microbial population. Bioresour Technol 129:281–288. https://doi.org/10.1016/j.biortech.2012.10.137
Rivera-Alvarez I, Brown RK, Keskin-Pyttel D, Steffens J, Farber P, Schröder U (2020) Correlating theoretical boundary layer thickness to the power output of a microbial fuel cell with a complex anode geometry operated at varying flow rates. J Power Sources 470:228428. https://doi.org/10.1016/j.jpowsour.2020.228428
Rozendal RA, Hamelers HVM, Buisman CJN (2006) Effects of membrane cation transport on pH and microbial fuel cell performance. Environ Sci Technol 40(17):5206–5211. https://doi.org/10.1021/es060387r
Rozendal RA, Hamelers HVM, Molenkamp RJ, Buisman CJN (2007) Performance of single chamber biocatalyzed electrolysis with different types of ion exchange membranes. Water Res 41(9):1984–1994. https://doi.org/10.1016/j.watres.2007.01.019
Rozendal RA, Hamelers HVM, Rabaey K, Keller J, Buisman C (2008) Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol 28(8):450–459. https://doi.org/10.1016/j.tibtech.2008.04.008
Sathe SM, Bhowmick GD, Dubey BK, Ghangrekar MM (2020) Surfactant removal from wastewater using photo-cathode microbial fuel cell and laterite-based hybrid treatment system. Bioprocess Biosyst Eng 43:2075–2084. https://doi.org/10.1007/s00449-020-02396-4
Schröder U, Harnisch F (2017) Life Electric—nature as a blueprint for the development of microbial electrochemical technologies. Joule 1(2):244–252. https://doi.org/10.1016/j.joule.2017.07.010
Serra PMD, Espírito-Santo A, Magrinho M (2020) A steady-state electrical model of a microbial fuel cell through multiple-cycle polarization curves. Renew Sustain Energy Rev 117:109439. https://doi.org/10.1016/j.rser.2019.109439
Sindhuja M, Kumar NS, Sudha V, Harinipriya S (2016) Equivalent circuit modeling of microbial fuel cells using impedance spectroscopy. J Energy Storage 7:136–146. https://doi.org/10.1016/j.est.2016.06.005
Singh I, Chandra A (2013) Need for optimizing catalyst loading for achieving affordable microbial fuel cells. Bioresour Technol 142:77–81. https://doi.org/10.1016/j.biortech.2013.05.034
Solanki K, Subramanian S, Basu S (2013) Microbial fuel cells for azo dye treatment with electricity generation: a review. Bioresour Technol 131:564–571. https://doi.org/10.1016/j.biortech.2012.12.063
Sonu K, Syed Z, Sogani M (2020) Up-scaling microbial fuel cell systems for the treatment of real textile dye wastewater and bioelectricity recovery. Int J Environ Stud 77(4):692–702. https://doi.org/10.1080/00207233.2020.1736438
Sultana S, Khan MD, Sabir S, Gani KM, Oves M, Khan MZ (2015) Bio-electro degradation of azo-dye in a combined anaerobic–aerobic process along with energy recovery. New J Chem 39:9461–9470. https://doi.org/10.1039/C5NJ01610J
Tamakloe RY, Opoku-Donkor T, Donkor M, Agamasu H (2015) Comparative study of double-chamber microbial fuel cells (DC-MFCs) using Mfensi clay as ionexchange-partition: effect of electrodes. Afr J Sci Technol Innov Dev 7(3):207–210. https://doi.org/10.1080/20421338.2015.1043706
Tao HC, Liang M, Li W, Zhang LJ, Ni JR, Wu WM (2011) Removal of copper from aqueous solution by electrodeposition in cathode chamber of microbial fuel cell. J Hazard Mater 189:186–192. https://doi.org/10.1016/j.jhazmat.2011.02.018
Tiwari B, Ghangrekar MM (2018) Electricity production during distillery wastewater treatment in a microbial fuel cell equipped with low cost PVA-Nafion-borosilicate membrane. J. Clean Energy Technol 6(2):155–158
Tiwari BR, Noori MT, Ghangrekar MM (2017) Carbon supported nickel-phthalocyanine/MnOx as novel cathode catalyst for microbial fuel cell application. Int J Hydrogen Energy 42(36):23085–23094. https://doi.org/10.1016/j.ijhydene.2017.07.201
Türk KK, Kruusenberg I, Kibena-Põldsepp E, Bhowmick GD, Kook M, Tammeveski K, Matisen L, Merisalu M, Sammelselg V, Ghangrekar MM, Mitra A, Banerjee R (2018) Novel multi walled carbon nanotube based nitrogen impregnated Co and Fe cathode catalysts for improved microbial fuel cell performance. Int J Hydrogen Energy 43(51):23027–23035. https://doi.org/10.1016/j.ijhydene.2018.10.143
Verma R, Chakraborty I, Chowdhury S, Ghangrekar MM, Balasubramanian R (2020) Nitrogen and sulfur codoped graphene macroassemblies as high-performance electrocatalysts for the oxygen reduction reaction in microbial fuel cells. ACS Sustain Chem Eng 8(44):16591–16599. https://doi.org/10.1021/acssuschemeng.0c05909
Walter XA, Merino-Jiménez I, Greenman J, Ieropoulos I (2018) PEE POWER® urinal II—urinal scale-up with microbial fuel cell scale-down for improved lighting. J Power Sources 392:150–158. https://doi.org/10.1016/j.jpowsour.2018.02.047
Wang G, Huang L, Zhang Y (2008) Cathodic reduction of hexavalent chromium [Cr (VI)] coupled with electricity generation in microbial fuel cells. Biotechnol Lett 30:1959. https://doi.org/10.1007/s10529-008-9792-4
Wang H, Wu Z, Plaseied A, Jenkins P, Simpson L, Engtrakul C, Ren Z (2011a) Carbon nanotube modified air-cathodes for electricity production in microbial fuel cells. J Power Sources 196(18):7465–7469. https://doi.org/10.1016/j.jpowsour.2011.05.005
Wang Z, Lim B, Choi C (2011b) Removal of Hg2+ as an electron acceptor coupled with power generation using a microbial fuel cell. Bioresour Technol 102(10):6304–6307. https://doi.org/10.1016/j.biortech.2011.02.027
Wang X, Liu C, Yuan Y, Li F (2014) Arsenite oxidation and removal driven by a bio-electro-Fenton process under neutral pH conditions. J Hazard Mater 275:200–209. https://doi.org/10.1016/j.jhazmat.2014.05.003
Watson VJ, Nieto Delgado C, Logan BE (2013) Influence of chemical and physical properties of activated carbon powders on oxygen reduction and microbial fuel cell performance. Environ Sci Technol 47(12):6704–6710. https://doi.org/10.1021/es401722j
Wei J, Liang P, Huang X (2011) Recent progress in electrodes for microbial fuel cells. Bioresour Technol 102(20):9335–9344. https://doi.org/10.1016/j.biortech.2011.07.019
Wen Q, Kong F, Zheng H, Cao D, Ren Y, Yin J (2011) Electricity generation from synthetic penicillin wastewater in an air-cathode single chamber microbial fuel cell. Chem Eng J 168:572–576. https://doi.org/10.1016/j.cej.2011.01.025
Winfield J, Gajda I, Greenman J, Ieropoulos I (2016) A review into the use of ceramics in microbial fuel cells. Bioresour Technol 215:296–303. https://doi.org/10.1016/j.biortech.2016.03.135
Wu W, Lesnik KL, Xu S, Wang L, Liu H (2014) Impact of tobramycin on the performance of microbial fuel cell. Microb Cell Fact 13(91):1–7. https://doi.org/10.1186/s12934-014-0091-6
Yan Z, Wang M, Huang B, Liu R, Zhao J (2013) Graphene supported Pt-Co alloy nanoparticles as cathode catalyst for microbial fuel cells. Int J Electrochem Sci 8:149–158
Yang H, Vogel W, Lamy C, Alonso-Vante N (2004) Structure and electrocatalytic activity of carbon-supported Pt-Ni alloy nanoparticles toward the oxygen reduction reaction. J Phys Chem B 108(30):11024–11034. https://doi.org/10.1021/jp049034+
Yang Z, Nie H, Chen X, Chen X, Huang S (2013) Recent progress in doped carbon nanomaterials as effective cathode catalysts for fuel cell oxygen reduction reaction. J Power Sources 236:238–249. https://doi.org/10.1016/j.jpowsour.2013.02.057
Yewale A, Methekar R, Agrawal S (2020) Multiple model-based control of multi variable continuous microbial fuel cell (CMFC) using machine learning approaches. Comput Chem Eng 140:106884. https://doi.org/10.1016/j.compchemeng.2020.106884
Yuan H, He Z (2015) Graphene-modified electrodes for enhancing the performance of microbial fuel cells. Nanoscale 7:7022–7029. https://doi.org/10.1039/C4NR05637J
Yuan Y, Kim S (2008) Improved performance of a microbial fuel cell with polypyrrole/carbon black composite coated carbon paper anodes. Bull Korean Chem Soc 29(7):1344–1348. https://doi.org/10.5012/bkcs.2008.29.7.1344
Yuan Y, Zhao B, Jeon Y, Zhong S, Zhou S, Kim S (2011) Iron phthalocyanine supported on amino-functionalized multi-walled carbon nanotube as an alternative cathodic oxygen catalyst in microbial fuel cells. Bioresour Technol 102(10):5849–5854. https://doi.org/10.1016/j.biortech.2011.02.115
Yuan Y, Yuan T, Wang D, Tang J, Zhou S (2013) Sewage sludge biochar as an efficient catalyst for oxygen reduction reaction in an microbial fuel cell. Bioresour Technol 144:115–120. https://doi.org/10.1016/j.biortech.2013.06.075
Yuan H, Hou Y, Abu-Reesh IM, Chen J, He Z (2016) Oxygen reduction reaction catalysts used in microbial fuel cells for energy-efficient wastewater treatment: A review. Mater Horizons 3:382–401. https://doi.org/10.1039/C6MH00093B
Yuan GE, Li Y, Lv J, Zhang G, Yang F (2017) Integration of microbial fuel cell and catalytic oxidation reactor with iron phthalocyanine catalyst for Congo red degradation. Biochem Eng J 120:118–124. https://doi.org/10.1016/j.bej.2017.01.005
Zeng Y, Choo YF, Kim BH, Wu P (2010) Modelling and simulation of two-chamber microbial fuel cell. J Power Sources 195(1):79–89. https://doi.org/10.1016/j.jpowsour.2009.06.101
Zhang XC, Halme A (1995) Modelling of a microbial fuel cell process. Biotechnol Lett 17(8):809–814. https://doi.org/10.1007/BF00129009
Zhang B, Zhao H, Shi C, Zhou S, Ni J (2009) Simultaneous removal of sulfide and organics with vanadium(V) reduction in microbial fuel cells. J Chem Technol Biotechnol 84(12):1780–1786. https://doi.org/10.1002/jctb.2244
Zhang GD, Zhao QL, Jiao Y, Zhang J, Jiang J, Ren N, Kim B (2011) Improved performance of microbial fuel cell using combination biocathode of graphite fiber brush and graphite granules. J Power Sources 196(15):6036–6041. https://doi.org/10.1016/j.jpowsour.2011.03.096
Zhang B, Wen Z, Ci S, Mao S, Chen J, He Z (2014) Synthesizing nitrogen-doped activated carbon and probing its active sites for oxygen reduction reaction in microbial fuel cells. ACS Appl Mater Interfaces 6(10):7464–7470. https://doi.org/10.1021/am5008547
Zhang X, Li K, Yan P, Liu Z, Pu L (2015) N-type Cu2O doped activated carbon as catalyst for improving power generation of air cathode microbial fuel cells. Bioresour Technol 187:299–304. https://doi.org/10.1016/j.biortech.2015.03.131
Zhang Q, Zhang L, Wang H, Jiang Q, Zhu X (2018) Simultaneous efficient removal of oxyfluorfen with electricity generation in a microbial fuel cell and its microbial community analysis. Bioresour Technol 250:658–665. https://doi.org/10.1016/j.biortech.2017.11.091
Zhao F, Rahunen N, Varcoe JR, Roberts AJ, Avignone-Rossa C, Thumser AE, Slade RCT (2009) Factors affecting the performance of microbial fuel cells for sulfur pollutants removal. Biosens Bioelectron 24(7):1931–1936. https://doi.org/10.1016/j.bios.2008.09.030
Zhao L, Li J, Battaglia F, He Z (2016) Investigation of multiphysics in tubular microbial fuel cells by coupled computational fluid dynamics with multi-order Butler-Volmer reactions. Chem Eng J 296:377–385. https://doi.org/10.1016/j.cej.2016.03.110
Zhao N, Treu L, Angelidaki I, Zhang Y (2019) Exoelectrogenic anaerobic granular sludge for simultaneous electricity generation and wastewater treatment. Environ Sci Technol 53(20):12130–12140. https://doi.org/10.1021/acs.est.9b03395
Zhong K, Li M, Yang Y, Zhang H, Zhang B, Tang J, Yan J, Su M, Yang Z (2019) Nitrogen-doped biochar derived from watermelon rind as oxygen reduction catalyst in air cathode microbial fuel cells. Appl Energy 242:516–525. https://doi.org/10.1016/j.apenergy.2019.03.050
Zhuang L, Zhou S, Yuan Y, Liu M, Wang Y (2010) A novel bioelectro-Fenton system for coupling anodic COD removal with cathodic dye degradation. Chem Eng J 163:160–163. https://doi.org/10.1016/j.cej.2010.07.039
Zhuang L, Chen Q, Zhou S, Yuan Y, Yuan H (2012) Methanogenesis control using 2-Bromoethanesulfonate for enhanced power recovery from sewage sludge in air-cathode microbial fuel cells. Int J Electrochem Sci 7:6512–6523
Funding
This work did not receive any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sathe, S.M., Chakraborty, I. & Ghangrekar, M.M. Wastewater Treatment and Concomitant Bioelectricity Production Using Microbial Fuel Cell: Present Aspects, Up-Scaling and Future Inventiveness. Trans Indian Natl. Acad. Eng. 6, 633–651 (2021). https://doi.org/10.1007/s41403-021-00245-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41403-021-00245-8