Abstract
Rivers are critical ecosystems for protecting and harboring high biodiversity. Tropical rivers particularly are unique for facing extreme climatic events under the current accelerated disruption from human activities. The Bobos-Nautla river basin is exposed to climatic events and disturbances from anthropogenic impacts that stress aquatic organisms. We assessed the health condition of this river system using a non-conventional biomonitor, Corydalus sp., with a set of early-warning biomarkers including lipid peroxidation levels (LPO) and antioxidant activity, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and the neurotransmitter acetylcholinesterase (AChE) considering their spatial and temporal variations. Biomarkers and water quality parameters were analyzed, and the integrated biomarker response (IBR) was assessed as a stress index. Biomarkers showed no significant spatial differences; however, a high-stress period during the rainy season was detected, evidenced by the highest LPO levels; this period is related to the leaching of allochthonous materials from agricultural and urban zones. The peak IBR value during the rainy season confirmed the seasonality of biomarkers. A slight increase in IBR was recorded in lowlands, seemingly associated with agricultural land and human settlements. A principal component analysis showed nutrient enrichment during the rainy season and depletion during the cold-dry season, together with a peak activity of antioxidant enzymes. These results highlight the importance of climatic events such as the rainy season on the health condition of Corydalus sp., which is highly sensitive to the complex mixtures of pollutants that enter the waterbody during extreme climatic events, promoting oxidative stress. Our results also showed the ability of Corydalus sp. to recover and return to a basal level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tropical rivers are remarkably complex due to high biodiversity and habitat heterogeneity (Boyero et al. 2009). These ecosystems are strongly influenced by intense convective and cyclonic rain, as the tropics are the region where cyclones often reach their maximum strength (Syvitski et al. 2014). In addition, tropical systems are currently facing substantial changes in landscape (native vegetation is being replaced by agricultural land and human settlements due to deforestation), promoting the input of xenobiotics into streams during the rainy season (Ezemonye and Enete 2004; Uçkun 2017) that may cause various adverse effects on the aquatic biota.
Rivers in the Bobos-Nautla basin, located on the slope of the Gulf of Mexico, are exposed to climatic events typical of tropical zones. Additionally, anthropogenic disturbances such as deforestation, intensive agriculture, and human settlements produce changes in the landscape, which increase the vulnerability of aquatic ecosystems and their biota to the input of xenobiotics (Vázquez et al. 2010). The sustainable management of aquatic ecosystems requires the detection of disturbances produced by xenobiotics that enter water bodies and affect ecosystems. However, in Mexico, as in other developing countries, water quality is monitored only through a few physicochemical analyses of water samples (Bae and Park 2014). These measurements neither reflect the synergistic or antagonistic effects of mixtures of pollutants nor evaluate the impact of xenobiotics on non-target aquatic organisms. Thus, biomonitoring is an approach involving the evaluation of impacts on aquatic biota, which is more suitable and allows setting strategies for the conservation of aquatic ecosystems (Hyne and Maher 2003).
Currently, there are several early-warning biomarkers for environmental assessment based on the different responses of organisms (measured in body fluids, cells, or tissues) that indicate cellular or biochemical changes associated with environmental stressors (Bae and Park 2014). Exposure to xenobiotics can exert oxidative stress by contributing pro-oxidants that increase free-radical levels above the antioxidant capacity of the aquatic biota (Valavanidis et al. 2006). A considerable number of pollutants occur as complex mixtures in natural environments; thus, no single biomarker leads to an accurate diagnosis of environmental degradation (Cazenave et al. 2009). Nonetheless, it is possible to use non-specific biomarkers, such as lipid peroxidation (LPO), to identify the impact of various pro-oxidants produced by mixtures of xenobiotics that may cause adverse effects on organisms (Otitoloju and Olagoke 2011). Studies using biomarkers of oxidative stress have been carried out mainly in fish, birds, and some invertebrates such as molluscs or arthropods (Hyne and Maher 2003; Oost et al. 2003; Hook et al. 2014).
Very few studies monitoring aquatic ecosystems have focused on aquatic invertebrates. To date, there is little information on non-conventional biomonitors (macroinvertebrates) of potential use as indicators of toxic effects of environmental pollutants in tropical regions (García and Sotelo-Mundo 2017). The rainy season produces marked effects in tropical zones, including runoff driven by precipitation. In turn, runoff introduces peak levels of xenobiotics (agrochemicals and heavy metals) into aquatic ecosystems (Schäfer et al. 2012). As far as we know, there are no studies assessing the oxidative stress biomarkers on a sentinel aquatic invertebrate of the Bobos-Nautla rivers, which face climatic stressors and land-use changes in their basin. Therefore, it is essential to assess the responses of biomarkers of sentinel organisms along a river with different land uses across its basin and in different seasons (Hering et al. 2006). Thus, the Bobos-Nautla rivers provide a suitable setting for examining spatial and temporal variations in oxidative biomarkers and AChE along a disturbance gradient in different seasons. Corydalus sp. spends a prolonged part of its life cycle as larvae in the aquatic environment (> 1 year) and is located at the top of the trophic web; both traits make Corydalus sp. a potential biomonitor organism, as it meets the features of a non-conventional biomonitor described by Birk et al. (2012) and Larramendy (2017). Thus, Corydalus sp. might be a suitable candidate to evaluate the effects of pollutants that act together with natural and induced stressors in complex natural systems.
The aim of this study was to determine the health status of Corydalus sp. using a set of early-warning biomarkers such as lipid peroxidation (LPO); the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx); and an indicator of neurotoxicity (activity of acetylcholinesterase, AChE). In addition, the relationship between these biomarkers and some physicochemical parameters indicative of water quality and the differences in biomarker responses across study sites and sampling periods was analyzed along a river running through an area of mountain rainforest in the Gulf of Mexico slope, to obtain information about spatial and seasonal variations in aquatic environmental quality.
Materials and methods
Study area
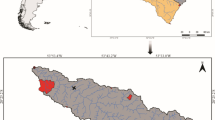
The Bobos River belongs to the Bobos-Nautla basin (Fig. 1), an area that stretches across 2933 km2, including the Bobos and Nautla sub-basins that drain into the Gulf of Mexico (Cotler-Ávalos 2010) in the state of Veracruz, Mexico. The Bobos river comprises several tributaries, among them the rivers Alseseca and Jalacingo, which include waterfalls over 30 m high. The local climate varies from warm to very warm, with a mean annual temperature above 18 °C, and subhumid, with a mean annual precipitation of 2730 mm. Monthly precipitation averages 72 mm in the dry season and 180 mm in the rainy season, but can be as high as 420 mm per month in the hurricane season (SMN-CNA 2019).
Land use in the upper basin includes natural vegetation (coniferous forest, mountain rainforest, tropical forest, etc.); in the middle basin, agriculture (induced pasture, banana and citrus plantations, etc.); and human settlements < 250,000 inhabitants (INEGI 2010) in the lower basin. Samples were collected in 11 study sites from the main Bobos River upstream (PI, HU, and TZ), midstream (FI, MA, PA, and RG), tributaries (JL and TM), and in Nautla lowlands (PL) (Fig. 1). Some sampling sites were selected in the natural protected area (“Filobobos river and its surroundings”) located in the Bobos River midstream. Study sites were selected considering the ecological gradients of lotic systems as well as vegetation, physiographic features, and areas with different land use (natural vegetation, agriculture, and human settlements).
Species studied
The genus Corydalus in the New World includes some species with a broad distribution, a characteristic that makes them potentially suitable for use as sentinel organisms (Brown and Fitzpatrick 2006; Short et al. 2006). In Mexico, 11 species of the the subfamily Corydalinae have been recorded, and the genus Corydalus is widely distributed across the entire Mexican territory (Contreras-Ramos and Rosas 2014). In spite of the diversity of Corydalus species, there are no keys for the taxonomic determination of Corydalus larvae for the country. Our study area is home to two species that inhabit different altitudinal ranges: C. luteus (below 800 m a.s.l.), and C. magnus (≥ 1500 m a.s.l.) (Contreras-Ramos and Rosas 2014). The segment of the river studied is located below 800 m a.s.l., and we hereby use the generic name Corydalus sp. due to the lack of taxonomic keys for Corydalus larvae.
Fieldwork
Water samples and biological materials were collected at each study site in August and December 2013 and in February and June 2014. For each station, the altitude (m a.s.l.) and geographic coordinates were obtained with a GPS Sport Trak Magellan® model. The environmental factors recorded in situ were temperature (°C) and air velocity (m s−1), with an EXTECH® anemometer, and water temperature (°C), dissolved oxygen (DO, mg L−1), turbidity (NTU), salinity (UPS), and conductivity (mS cm−1) with a Quanta® multiparametric probe. In each study site, 500-mL water samples were collected in duplicate; these were transported in the dark and refrigerated for laboratory testing.
Ten specimens of Corydalus sp. (Linnaeus, 1758) larvae (belonging to instar X) were collected from each season and study site for the evaluation of early-warning biomarkers in tissues; to note, Corydalus sp. was not found in some sampling time points and study sites: PL in August, February, and June; MA in December; and EN in June. In each study site, specimens were collected with a kick-net of 500 μm mesh size, used in rapids of streams. The organisms collected were kept in river water for 30 min for purging, as recommended by Barata et al. (2005). Afterward, these were transported in liquid nitrogen. Once in the laboratory, larvae were transferred to a REVCO ultra-freezer to be preserved at − 70 °C.
Laboratory processing
Water samples were tested for total nitrogen (TN, mg L−1), nitrites (NO2, mg L−1), nitrates (NO3, mg L−1), ammonia nitrogen (NH3, mg L−1), sulfates (SO4, mg L−1), orthophosphates (O-PO4, mg L−1), total phosphorus (TP, mg L−1), color (Pt-Co, units), and total suspended solids (TSS, mg L−1) with a HACH® DLR3900 spectrophotometer; hardness (CaCO3, mg L−1) was determined by titration. Biochemical oxygen demand (BOD5, mg L−1), alkalinity (CaCO3, mg L−1), chlorides (Cl, mg L−1), and total and fecal coliforms (MPN/100 mL) were determined according to the techniques of the American Public Health Association (APHA 2005).
In the laboratory, Corydalus sp. larvae (specimens belonging to instar X, head capsule size 13 mm ± 1 and body length 55 mm ± 2.5) were selected in order to get enough tissue to conduct biomarker assessments. In each period and study site, ten individuals were pooled and dissected, then samples of muscle tissue were excised for biomarker testing (see Supplementary material 1): lipid peroxidation (LPO), through the quantification of malondialdehyde (nmol MDA/mg protein) according to Buege and Aust (1978), and proteins, with the method of Bradford (1976) (mg protein/g tissue). Antioxidant activity was evaluated through superoxide dismutase (SOD) activity (total SOD units/mg protein) by the technique of Sun et al. (1988); catalase (CAT, 1k/min), by the method of Cohen et al. (1970); and glutathione peroxidase (GPx, nmol/g protein) according to Lawrence and Burk (1976). Additionally, acetylcholinesterase activity (AChE, μmol/mg min) was evaluated in gills following Hestrin (1949). Each biomarker was measured in two replicates.
Data processing
The normality and homoscedasticity of biomarker data (LPO, SOD, CAT, GPx, and AChE) were tested (Shapiro-Wilk tests). Variables that failed the normality test were log transformed prior to analysis. Statistical differences between sites and sampling time points were examined with two-way ANOVAs followed by Tukey’s multiple comparison test (α = 0.05). Biomarker values are shown as the mean (± standard error) for each study site, and for each study period. Standard errors (SE) for site means were calculated for all study periods; standard errors for means of study periods were calculated for all study sites.
For calculating the integrated biomarker response (IBR) index, biomarker data were standardized as proposed by Beliaeff and Burgeot (2002), and the IBR assessment was conducted following Devin et al. (2013). First, the standardized biomarkers are plotted as vectors; then, the IBR calculates the area of the star-shaped polygon that results from connecting the ends of these vectors. Since biomarker vectors may occupy different positions in the polygon, the median of all potential permutations of such vectors in the star plot is calculated. A two-way ANOVA was performed with the spatial and temporal IBR data, followed by a post hoc Tukey’s test for differences between groups.
A factor analysis (FA) was carried out to summarize the physicochemical parameters into a smaller number of significant variables taking into consideration the first two factors. Those variables that reached significant loadings (loading value between ≥ 0.5 and ≤ − 0.5) in the first two factors were considered as significant according to the criteria of Schumm and Stevens (2006).
Finally, a principal components analysis (PCA) was performed for the ordination of study sites using the variables selected with the FA. PCA is a powerful tool for pattern recognition and detection of correlations (or co-variances) among a set of variables or samples. In our study, a PCA was conducted to detect seasonal and spatial patterns related to biomarker responses and physicochemical factors. All variables were log transformed. Pearson’s correlation coefficient (р < 0.05) was considered in the analysis. All analyses were carried out using the statistical software R version 3.5.1.
Results
Early-warning biomarkers
LPO values showed spatial variations, with the highest levels (0.034 nmol MDA/mg prot) in upstream Bobos River and tributary streams, while midstream Bobos recorded the lowest mean LPO values (< 0.010 nmol MDA/mg prot); however, these differences were not statistically significant (df = 10, F = 0.97, p ≥ 0.05) (Fig. 2a and Table 1).
Values for the biomarkers assessed in Corydalus sp.: a) LPO, b) SOD, c) CAT, d) GPx, and e) AChE, among seasons and study sites. Horizontal bars on plot indicate significant differences (two-way ANOVA) by multiple comparisons between months (Tukey’s multiple comparison). Data are presented as the mean ± standard error for each study site (standard error was obtained from the results of the replicates of each biomarker in the laboratory, n = 2; each replicate was of a pool of 10 organisms)
We found seasonal differences (df = 3, F = 24.06, p ≤ 0.0001). The highest LPO levels, up to twofold higher relative to the dry season, occurred in August (rainy season) (\( \overline{\mathrm{x}} \)= 0.026 ± 0.010 nmol MDA/mg prot) (Fig. 2a and Table 1), and were significantly different from the rest of the study periods in December, February, and June (p ≤ 0.0001) relative to the dry season (cold and warm periods). A second LPO peak was observed in December (\( \overline{\mathrm{x}} \) = 0.014 ± 0.005 nmol MDA/mg prot), which was significantly different from values recorded in February (p ≤ 0.05) and June (p ≤ 0.001); the lowest LPO (\( \overline{\mathrm{x}} \) = 0.005 ± 0.004 nmol MDA/mg prot) was observed in this last month.
Antioxidant SOD activity showed the highest value in sites located in the tributaries, reaching up to 0.035 SOD U/mg prot; in contrast, downstream sites recorded the lowest SOD activity with a mean of 0.006 SOD U tot/mg prot (Fig. 2b); these differences were not statistically significant (df = 10, F = 2.05, p ≥ 0.05).
Seasonal variations in SOD activity were significantly different (df = 3, F = 7.90, p ≤ 0.0001) (Fig. 2b and Table 1). The response showed the lowest activity in August (\( \overline{\mathrm{x}} \) = 0.009 ± 0.007 tot SOD U/mg prot) and the highest in June (\( \overline{\mathrm{x}} \) = 0.024 ± 0.005 tot SOD U/mg prot) (p ≤ 0.001). Also, SOD activity was important in December and February, although the differences versus August were not significant (p ≥ 0.05). However, SOD showed significant differences between December and June (p < 0.05) and between February and June (p ≤ 0.001).
CAT activity showed spatial variations, with a peak of 0.239 CAT 1k/mg prot in upstream and midstream zones. This activity decreased notoriously downstream, with a minimum activity of 0.007 CAT 1k/mg prot recorded in one downstream site; however, these differences were not significant (df = 10, F = 2.90, p ≥ 0.05) (Fig. 2c).
Seasonally, CAT activity showed the highest value in June (\( \overline{\mathrm{x}} \) = 0.163 ± 0.062 CAT 1k/mg prot), which was significantly different from the values recorded in August and December (p < 0.001) (Fig. 2c and Table 1).
GPx activity showed non-significant spatial variations (Fig. 2d) (df = 10, F = 1.34, p ≥ 0.05), with peak activity upstream and in the tributaries (5.9 GPx nmol/g protein) and the lowest in one tributary site (2.9 GPx nmol/g protein). Seasonally significant differences were identified (df = 3, F = 4.38, p ≥ 0.05), with the highest GPx activity observed in December (\( \overline{\mathrm{x}} \) = 5.1 ± 2.3 GPx nmol/g protein) and February (\( \overline{\mathrm{x}} \) = 5 ± 2.3 GPx nmol/g protein) (Fig. 2d and Table 1). These were significantly different from the response in August (\( \overline{\mathrm{x}} \) = 2.8 ± 1.8 GPx nmol/g protein) (p < 0.05); in addition, GPx activity was significantly different in February (p < 0.05) than in June, which was the lowest for the whole study period.
AChE activity was relatively uniform across study sites, with non-significant differences (df = 10, F = 0.84, p ≥ 0.05) (Fig. 2e). The highest activity was observed in the headwaters (4 × 10−6 AChE nmol/mg prot min), while reduced activity levels were recorded in the rest of the stream and its tributaries, as well as downstream (3 × 10−6 AChE μmol/mg min). AChE showed temporal differences (df = 3, F = 50.85, p ≤ 0.0001), with the highest activity recorded in August (Fig. 2e, Table 1), and significant differences versus December, February, and June (\( \overline{\mathrm{x}} \) = 5 × 10−6 ± 5 × 10−7 AChE μmol/mg min) (p < 0.0001). The lowest AChE activity was observed in June (\( \overline{\mathrm{x}} \) = 2 × 10−6 ± 2 × 10−7 AChE nmol/mg prot min), which was significantly different from December (p < 0.001) and February (p < 0.001).
Integrated biomarker response
IBR showed spatial and temporal variations. Spatially, the highest IBR values were observed in headwaters, tributaries, and downstream sites (Fig. 3); the differences between all study sites were not significant (df = 10, F = 0.74, p ≤ 0.0001).
Mean IBR showed seasonal variations (df = 3, F = 3.29, p ≤ 0.05) (Fig. 3). The peak IBR (\( \overline{\mathrm{x}} \) = 9.6 ± 3.8 IBR) was recorded in August, being significantly different versus December (\( \overline{\mathrm{x}} \) = 6.8 ± 5.5 IBR) (p < 0.001), February (\( \overline{\mathrm{x}} \) = 5.5 ± 1.4 IBR) (p < 0.001), and June (\( \overline{\mathrm{x}} \) = 4.3 ± 1.6 IBR) (p < 0.0001).
Environmental factors and biomarkers
According to the FA, 9 of the 21 variables reached significance in their loadings on the first two factors, thus being the variables that best explain the variations in the data (Table 2).
The PCA reached 92.5% of the total variance in the first two axes (Dim) (75.5% in Dim 1; 17% in Dim 2). The first axis (Dim 1) evidenced a seasonal pattern, with samples in the bottom left quadrant corresponding to the rainy season (August), and samples at the far right and top left, to the cold and warm dry periods (Fig. 4a). Heavy rains occurred in August; this period was characterized by the highest concentration of ammonia, orthophosphates, total nitrogen, and total phosphorus, as well as color (mean value of 62 U Pt-Co) (Fig. 4a). The response of Corydalus sp. biomarkers associated with this period included high LPO levels and high AChE activity, leading to the highest IBR (Fig. 4b). On the other hand, the cold and warm dry periods (December and June) (upper left) showed the highest number of total and fecal coliforms and the highest nitrate concentration (Fig. 4a). The antioxidant enzymes SOD and CAT showed a high activity in this period (Fig. 4b); thus, it is possible to associate the dry season with peak antioxidant efficiency and the lowest LPO level in Corydalus sp. (Fig. 4b). Finally, the cold-dry period samples (December 2013 and February 2014) clustered in the bottom right quadrant, also associated with total nitrogen and the highest GPx activity in Corydalus sp. (Fig. 4b).
Discussion
Biomarkers are early-warning indicators commonly used to assess the health condition of species exposed to environmental stressors associated with human activities (Adams et al. 2001; Jiri et al. 2018). Oxidative stress biomarkers reveal damages caused by reactive oxygen species (ROS) (Hyne and Maher 2003). In addition, organisms have developed an antioxidant defense system involving enzymes that detoxify ROS, thereby neutralizing oxidative stress (Barata et al. 2005), which can be used to assess health condition. In this study, the spatial and temporal variations in oxidative stress responses of Corydalus sp. inhabiting the Bobos River were assessed. Our results showed marked seasonal variations in the study biomarkers, while spatial differences seemingly produced a lesser impact on biomarker responses.
LPO levels in Corydalus sp. were up to twofold higher in August (rainy season) than in the dry season (cold and warm dry periods). The high LPO levels may have resulted from the input of multiple organic and inorganic allochthonous materials during the rainy season (Wantzen et al. 2008) that form complex mixtures of pollutants. Several agrochemicals are employed in the agricultural area of the Río Bobos-Nautla basin (Table 3), and are applied at the beginning and end of the wet season (Cochón et al. 2007; Santos et al. 2011). In August, the typical disturbance caused by the rainy season was further exacerbated by the occurrence of an extraordinary hurricane event in the region—the tropical storm “Barry” (Quiroga-Cuellar and Torres 2013). Tropical storms have been associated with peaks of xenobiotic concentrations in water bodies in agricultural basins (Ezemonye and Enete 2004; Lavado et al. 2006; Uçkun 2017), as in the case of the Bobos river (INEGI 2010). Our results showed significant disruptions in the aquatic habitat in this season that may lead to a high mortality of the species inhabiting these ecosystems (Brönmark and Hansson 2017). However, the response of Corydalus sp. also depends on its adaptation, tolerance, resilience, and capacity to recolonize sites where the local population might be removed by current carryover, or where high mortality decimates the local population (Allan and Castillo 2007).
Antioxidant enzymes (SOD, CAT, and GPx) in Corydalus sp. also varied seasonally, with an overall decrease in enzyme activity in the rainy season (when LPO reached high values), suggesting the exhaustion of the antioxidant enzyme response. Paital and Chainy (2013) detected a similar pattern in crabs, with a decrease in antioxidant activity during the rainy season, contrasting with the increased activity recorded in summer.
According to Regoli et al. (2002), the response of antioxidant enzymes such as SOD and CAT is critical in the antioxidant defense of invertebrates. CAT activity in Corydalus sp. showed a sharp decline in the cold season (December) in all study sites; thus, this response may be likely related to a particular physiological condition of Corydalus sp. larvae in this period that may cause oxidative stress. Sahoo et al. (2016) found low CAT levels in late instar larvae of Antheraea mylitta (Lepidoptera: Saturniidae). The Corydalus sp. larvae used in our study were in one of the latest instars (Carr and topping 1983; Mangan 1994; Roell and Orth 1991), and the low CAT levels observed might be related to this physiological condition, as observed in the Lepidoptera Lymantria dispar (Perić-Mataruga et al. 1997). An increase in respiration may translate into a higher ROS production; nevertheless, further studies are necessary to determine the effect of larval development and metamorphosis on antioxidant performance.
Contrary to the findings of Mathews et al. (1997) and Berra et al. (2004), who reported the absence of GPx in some insect species, in our study, GPx activity in Corydalus sp. reached values above 7 GPx nmol/g protein. Furthermore, the response of GPx in this study also decreased in the rainy season (June and August); these results show that GPx plays a role in the antioxidant defense of these organisms. Sanz et al. (2010) recorded values above 20 GPx nmol/g protein in several species of Perloidea (Insecta: Perlidae) from Sierra Nevada, Granada, Spain; they attributed these values to low glutathione reductase levels.
The high LPO levels in Corydalus sp. in the rainy season (August), together with the low activity of the antioxidant enzymes SOD, CAT, and GPx, could be interpreted as an early warning of the input of xenobiotic agents that act as stressors to this species. The activity of antioxidant enzymes depends on the intensity of oxidative stress, and may either increase, decrease, or remain unaffected (Lushchak 2014). Although antioxidant enzymes may respond by upregulating their biosynthesis throughout the life cycle, leading to ROS inactivation (Lushchak 2014), the decrease in antioxidant responses observed during the wet season can be interpreted as the result of excess ROS that regulate the suppression of antioxidant enzyme activity (Livingstone 2003).
We summarized the global response of biomarkers by means of the IBR. This index showed higher values in August, thus confirming the period of stress in the wet season.
LPO values and antioxidant enzymes likely returned to a baseline or steady state of oxidative stress once the effect of the rainy season ceased; according to Lushchak (2014), the possibility of returning to a baseline state decreases under constant stress. Our results show that oxidative stress is not permanent, as the stressors to which Corydalus sp. was exposed during the rainy season and the LPO levels decreased subsequently and remained relatively stable in December and February, when the antioxidant enzyme activity was reinstated. This allows organisms to return to a baseline level and reach a balance between ROS and the antioxidant defenses (Lushchak 2011). In this regard, Xuereb et al. (2009) recommended identifying basal levels in order to make a proper use of biomarkers. These authors found no seasonal differences in biomarkers; by contrast, we did find significant seasonal differences in this study. Biomarkers of oxidative stress attained basal levels during the dry season across all our study sites. These basal levels can be used in future studies to assess biomarker responses in Corydalus sp. in more heavily impacted rivers.
Despite the heterogeneity of the landscape in the river basin, we found no significant differences (р ≥ 0.05) in biomarkers between our study sites. Rodríguez-Romero et al. (2017) assessed a water quality index (WQI) for the Bobos River and found no significant differences between sites, concluding that no specific alterations in water quality occur along the course of the river. Additionally, the Bobos River is considered as a fast-responding river (Levy et al. 2018), i.e., during the rainy season, runoff rapidly transports materials into the river channel and, during the dry season, the river returns to its base flow level with no noticeable changes in its WQI (Rodríguez-Romero et al. 2017, 2018). This is consistent with the response of biomarkers observed in this study, which showed no significant spatial variations but significant seasonal variations that reveal a stress period.
The slightly (although not significantly) higher LPO levels found upstream can be attributed to the particular geological features of the basin. In this same area, Rodríguez-Romero et al. (2017) recorded high water hardness that inhibited mitochondrial activity in spores of the fern Cyathea costaricensis. Hence, high Ca2+ levels in the upper basin of the Bobos River may potentially represent a stressful environment for Corydalus sp. However, the effects of high Ca2+ concentrations on the native aquatic biota inhabiting the upper basin of the Bobos River have not been investigated yet.
In the present study, AChE activity in Corydalus sp. peaked in the rainy season, when xenobiotics (including pesticides) enter water bodies with runoff. In the study area, this macroinvertebrate is potentially exposed to a variety of pesticides from agricultural practices (refer to Table 3). Consistent with our results, Day and Scott (1990) measured AChE levels in several aquatic invertebrates, finding increases in AChE activity in the Plecoptera Claassenia sp. after 24-h exposure to fenitrothion. These authors point out that this increase may be because (a) the insecticides used in these experiments were not toxic at the concentrations tested or (b) AChE does not respond to a measurable extent to sublethal levels of organophosphates. AChE is the main target of anticholinergic pesticides used to control invertebrate pests (Berra et al. 2004). Aquatic invertebrates exhibit a widely variable sensitivity to organophosphate insecticides (Day and Scott 1990). Some pest species have developed at least four detoxification strategies (Siegfried and Scharf 2001), including increased esterase production. This is the case of the hemipteran Helicoverpa armigera in Australia, which detoxifies pyrethroids through scavenging and hydrolysis, as observed in natural populations of this species (Gunning et al. 1996, 1998). Resistance to organophosphate insecticides in de Diptera Culex sp. (which has aquatic larvae) has been widely reported, correlated with highly active carboxylesterases (Soderlund and Bloomquist 1990; Devonshire and Field 1991). The Corydalus sp. populations inhabiting the Bobos River are probably able to develop detoxification strategies (similar to those of H. armigera or Culex sp.) in the presence of anticholinergic pesticides; however, no toxicological information is currently available on the effect of pesticides on Corydalus sp.
The PCA allowed an integrated analysis of abiotic factors across the different periods and study sites; it showed the impact of seasonality on environmental conditions—driven by the incorporation of materials to the water body—which trigger a number of responses in Corydalus sp. The PCA also showed recovery once the impact of the rainy season ceased in the Bobos-Nautla river.
Conclusions
The health status of Corydalus sp. in the Bobos-Nautla river varies according to seasonal events in the basin. An adverse or stressful effect was recorded during the rainy season, which may jeopardize the colonization and survival of Corydalus sp. In contrast, low-rainfall periods bring about favorable conditions for antioxidant defense, allowing to neutralize the pro-oxidants to which Corydalus sp. is exposed (a healthier state that can be regarded as the basal level for the biomarkers studied). These findings suggest that Corydalus sp. is suitable for use as a non-conventional biomonitor for the early detection of water quality impairment, due to the high availability of this organism. Our results are a first attempt at defining basal levels for five widely used enzymatic biomarkers (LPO, SOD, CAT, GPx, and AChE) in a riverine benthic macroinvertebrate. The biomarkers used in this study indicate that the Bobos River shows a good health status, where seasonal variations prevail over local impacts by point or diffuse pollution along the stream. The Bobos-Nautla river is one of the few systems in Mexico that still maintain a favorable condition; hence, the early warnings identified in this study call for actions to be taken for the conservation and improvement of the environment where organisms such as Corydalus sp. inhabit.
References
Adams SM, Giesy JP, Tremblay LA, Eason CT (2001) The use of biomarkers in ecological risk assessment: recommendations from the Christchurch conference on biomarkers in ecotoxicology. Biomarkers 6:1–6. https://doi.org/10.1080/135475001452724
Allan JD, Castillo MM (2007) Stream ecology. Structure and function of running waters, 2nd edn. Springer, Dordrecht
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health, Washington DC, Association
Bae MJ, Park YS (2014) Biological early warning system based on the responses of aquatic organisms to disturbances: a review. Sci Total Environ 466–467:635–649. https://doi.org/10.1016/j.scitotenv.2013.07.075
Barata C, Lekumberri I, Vila-Escalé M et al (2005) Trace metal concentration, antioxidant enzyme activities and susceptibility to oxidative stress in the tricoptera larvae Hydropsyche exocellata from the Llobregat river basin (NE Spain). Aquat Toxicol 74:3–19. https://doi.org/10.1016/j.aquatox.2005.04.002
Barata C, Solayan A, Porte C (2004) Role of B-esterases in assessing toxicity of organophosphorus (chlorpyrifos, malathion) and carbamate (carbofuran) pesticides to Daphnia magna. Aquat Toxicol 66:125–139. https://doi.org/10.1016/j.aquatox.2003.07.004
Beliaeff B, Burgeot T (2002) Integrated biomarker response: a useful tool for ecological risk assessment. Environ Toxicol Chem 21:1316–1322
Berra E, Forcella M, Giacchini R et al (2004) Evaluation of enzyme biomarkers in freshwater invertebrates from Taro and Ticino river, Italy. Ann Limnol 40:169–180. https://doi.org/10.1051/limn/2004015
Birk S, Bonne W, Borja A et al (2012) Three hundred ways to assess Europe’s surface waters: an almost complete overview of biological methods to implement the Water Framework Directive. Ecol Indic 18:31–41. https://doi.org/10.1016/j.ecolind.2011.10.009
Boyero L, Ramírez A, Dudgeon D, Pearson RG (2009) Are tropical streams really different? J North Am Benthol Soc 28:397–403
Brönmark C, Hansson L-A (2017) The biology of lakes and ponds. Oxford University Press
Brown AV, Fitzpatrick LC (2006) Life history and population energetics of the Dobson Fly, Corydalus cornutus. Ecology 59:1091–1108. https://doi.org/10.2307/1938225
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods in enzymology. Elsevier, In, pp 302–310
Carr RI, Topping MS (1983) Aspects of the life history of the hellgrammite in southwestern Missouri, Corydalus cornutus. Trans Missouri Acad Sci 17:196
Cazenave J, Bacchetta C, Parma MJ et al (2009) Multiple biomarkers responses in Prochilodus lineatus allowed assessing changes in the water quality of Salado River basin (Santa Fe, Argentina). Environ Pollut 157:3025–3033. https://doi.org/10.1016/j.envpol.2009.05.055
Cochón AC, Della Penna AB, Kristoff G et al (2007) Differential effects of paraquat on oxidative stress parameters and polyamine levels in two freshwater invertebrates. Ecotoxicol Environ Saf 68:286–292. https://doi.org/10.1016/j.ecoenv.2006.11.010
Cohen G, Dorothy D, Judith M (1970) Measurement of catalase activity in tissue extracts. Anal Biochem 34:30–38
Contreras-Ramos A, Rosas MV (2014) Biodiversidad de Megaloptera y Raphidioptera en México. Rev Mex Biodivers 85:257–263. https://doi.org/10.7550/rmb.32049
Cotler-Ávalos H (2010) Las Cuencas Hidrográficas de México: Diganóstico y Priorización. Las cuencas hidrográficas México diagnóstico y priorización:3–5
Day KE, Scott IM (1990) Use of acetylcholinesterase activity to detect sublethal toxicity in stream invertebrates exposed to low concentrations of organophosphate insecticides. Aquat Toxicol 18:101–113. https://doi.org/10.1016/0166-445X(90)90021-G
Devin S, Giambérini L, Burgeot T et al (2013) The integrated biomarker response revisited: optimization to avoid misuse. Environ Sci Pollut Res 21:2448–2454. https://doi.org/10.1007/s11356-013-2169-9
Devonshire AL, Field LM (1991) Gene amplification and insecticide resistance. Annu Rev Entomol 36:1–21
El-Gendy KS, Radwan MA, Gad AF et al (2019) Use of multiple endpoints to investigate the ecotoxicological effects of abamectin and thiamethoxam on Theba pisana snails. Ecotoxicol Environ Saf 167:242–249. https://doi.org/10.1016/j.ecoenv.2018.10.027
Ezemonye LIN, Enete E (2004) The earthworm–Aporrectodea longa as ndicator of heavy metal pollution in soil. Afr J Env Pollut Heal 3:11–21
Fulton MH, Key PB (2001) Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ Toxicol Chem 20:37–45. https://doi.org/10.1002/etc.5620200104
García CF, Sotelo-Mundo RR (2017) Chapter 9. Freshwater prawns Macrobrachium borellii and Palaemonetes argentinus (Crustacea: Palaemonidae) as valid indicators of organophosphate pesticide exposure. A biochemical assessment. In: Ecotoxicology and Genotoxicology. pp 196–211
Giddings JM, Hall LW, Solomon KR (2000) Ecological risks of diazinon from agricultural use in the Sacramento-San Joaquin River Basins, California. Risk Anal 20:545–572. https://doi.org/10.1111/0272-4332.205052
Gunning RV, Moores GD, Devonshire AL (1996) Esterases and esfenvalerate resistance in Australian Helicoverpa armigera (Hubner) lepidoptera:noctuidae. Pestic Biochem Physiol 54:12–23. https://doi.org/10.1006/pest.1996.0004
Gunning RV, Moores GD, Devonshire AL (1998) Insensitive acetylcholinesterase and resistance to organophosphates in Australian Helicoverpa armigera. Pestic Biochem Physiol 62:147–151. https://doi.org/10.1006/pest.1998.2380
Hering D, Johnson RK, Kramm S et al (2006) Assessment of European streams with diatoms, macrophytes, macroinvertebrates and fish: a comparative metric-based analysis of organism response to stress. Freshw Biol 51:1757–1785
Hestrin S (1949) The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. J Biol Chem 180:249–261
Hook SE, Gallagher EP, Batley GE (2014) The role of biomarkers in the assessment of aquatic ecosystem health. 10:327–341. https://doi.org/10.1002/ieam.1530
Hyne RV, Maher WA (2003) Invertebrate biomarkers: links to toxicosis that predict population decline. Ecotoxicol Environ Saf 54:366–374. https://doi.org/10.1016/S0147-6513(02)00119-7
INEGI (Instituto Nacional de Estadística Geografía e Informática) (2010) Carta Geológica-Minera Altotonga E14-B16, 1:50,000: México
Jiri Z, Tazvivinga A, Greenfield R, Van Vuren JHJ (2018) Oxidative stress biomarkers in oreochromis niloticus as early warning signals in assessing pollution from acid mine drainage and diffuse sources of pollutants in a subtropical river. Water SA 44:318–327. https://doi.org/10.4314/wsa.v44i2.17
Kim RO, Kim BM, Jeong CB et al (2016) Effects of chlorpyrifos on life cycle parameters, cytochrome P450S expression, and antioxidant systems in the monogonont rotifer Brachionus koreanus. Environ Toxicol Chem 35:1449–1457. https://doi.org/10.1002/etc.3288
Larramendy ML (2017) Ecotoxicology and genotoxicology: non-traditional aquatic models
Lavado R, Ureña R, Martin-Skilton R, Torreblanca A, del Ramo J, Raldúa D, Porte C (2006) The combined use of chemical and biochemical markers to assess water quality along the Ebro River. Environ Pollut 139(2):330–339
Lawrence RA, Burk RF (1976) Reprint of “Glutathione peroxidase activity in selenium-deficient rat liver.” Biochem Biophys Res Commun 425:503–509. doi: https://doi.org/10.1016/j.bbrc.2012.08.016
Levy MC, Lopes AV, Cohn A et al (2018) Land use change increases streamflow across the arc of deforestation in Brazil. Geophys Res Lett 45:3520–3530. https://doi.org/10.1002/2017GL076526
Livingstone DR (2003) Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Rev Med Vet (Toulouse) 154:427–430
Lushchak VI (2014) Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact 224:164–175. https://doi.org/10.1016/j.cbi.2014.10.016
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30. https://doi.org/10.1016/j.aquatox.2010.10.006
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Mac Loughlin C, Canosa IS, Silveyra GR et al (2016) Effects of atrazine on growth and sex differentiation, in juveniles of the freshwater crayfish Cherax quadricarinatus. Ecotoxicol Environ Saf 131:96–103. https://doi.org/10.1016/j.ecoenv.2016.05.009
Mangan BP (1994) Pupation ecology of the Dobsonfly Corydalus cornutus (Corydalidae: Megaloptera) along a large river. J Freshw Ecol 9:57–62. https://doi.org/10.1080/02705060.1994.9664427
Mathews MC, Summers CB, Felton GW (1997) Ascorbate peroxidase: a novel antioxidant enzyme in insects. Arch Insect Biochem Physiol 34:57–68. doi: 10.1002/(SICI)1520-6327(1997)34:1<57: AID-ARCH5>3.0.CO;2-T
Novais SC, de Coen W, Amorim MJB (2012) Gene expression responses linked to reproduction effect concentrations (EC10,20,50,90) of dimethoate, atrazine and carbendazim, in enchytraeus albidus. PLoS One 7: https://doi.org/10.1371/journal.pone.0036068
Oost D, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental assessment: a review. Environ Toxicol Pharmacol Fish bioaccumulation and biomarkers in environmental risk assessment. Environ Toxicol Pharmacol 13:57–149. https://doi.org/10.1016/S1382-6689(02)00126-6
Otitoloju A, Olagoke O (2011) Lipid peroxidation and antioxidant defense enzymes in Clarias gariepinus as useful biomarkers for monitoring exposure to polycyclic aromatic hydrocarbons. Environ Monit Assess 182:205–213. https://doi.org/10.1007/s10661-010-1870-0
Paital B, Chainy GBN (2013) Seasonal variability of antioxidant biomarkers in mud crabs (Scylla serrata). Ecotoxicol Environ Saf 87:33–41. https://doi.org/10.1016/j.ecoenv.2012.10.006
Perić-Mataruga V, Blagojević D, Spasić M et al (1997) Effect of the host plant on the antioxidative defence in the midgut of Lymantria dispar L. caterpillars of different population origins. J Insect Physiol 43:101–106. https://doi.org/10.1016/S0022-1910(96)00018-2
Quiroga-Cuellar ER, Torres MA (2013) Daños por Tormenta Tropical Barry Daños Precipitaciones. Evaluación Riesgos Nat.
Regoli F, Pellegrini D, Winston GW et al (2002) Application of biomarkers for assessing the biological impact of dredged materials in the Mediterranean: the relationship between antioxidant responses and susceptibility to oxidative stress in the red mullet (Mullus barbatus). Mar Pollut Bull 44:912–922. https://doi.org/10.1016/S0025-326X(02)00120-0
Rodríguez-Romero A, Rico-Sánchez A, Mendoza-Martínez E et al (2018) Impact of changes of land use on water quality, from tropical forest to anthropogenic occupation: a multivariate approach. Water 10:1518. https://doi.org/10.3390/w10111518
Rodríguez-Romero AJ, Rico-Sánchez AE, Catalá M et al (2017) Mitochondrial activity in fern spores of Cyathea costaricensis as an indicator of the impact of land use and water quality in rivers running through cloud forests. Chemosphere 189:435–444. https://doi.org/10.1016/j.chemosphere.2017.09.094
Roell MJ, Orth DJ (1991) Production of dobsonfly (Corydalus cornutus) larvae in the New River of West Virginia. J Freshw Ecol 6:1–9. https://doi.org/10.1080/02705060.1991.9665270
Sahoo A, Sahu S, Dandapat J, Samanta L (2016) Pro-oxidative challenges and antioxidant protection during larval development of non-mulberry silkworm, Antheraea mylitta (Lepidoptera: Saturniidae). Ital J Zool 83:3–14. https://doi.org/10.1080/11250003.2015.1103319
Santos MJG, Morgado R, Ferreira NGC et al (2011) Evaluation of the joint effect of glyphosate and dimethoate using a small-scale terrestrial ecosystem. Ecotoxicol Environ Saf 74:1994–2001. https://doi.org/10.1016/j.ecoenv.2011.06.003
Sanz A, Trenzado CE, López-Rodríguez MJ et al (2010) Study of antioxidant defense in four species of Perloidea (Insecta, Plecoptera). Zool Sci 27:952–958. https://doi.org/10.2108/zsj.27.952
Schäfer RB, van den Brink PJ, Liess M (2012) Impacts of pesticides on freshwater ecosystems. In: Schafer RB, van den Brink PJ, Liess M (eds) Ecological impacts of toxic chemicals (Open Access). Bentham Science Publishers, pp 111–137
Schumm WR, Stevens J (2006) Applied multivariate statistics for the social sciences. Routledge
Short RA, Stanley EH, Harrison JW, Epperson CR (2006) Production of Corydalus cornutus (Megaloptera) in four streams differing in size, flow, and temperature. J North Am Benthol Soc 6:105–114. https://doi.org/10.2307/1467220
Siegfried BD, Scharf ME (2001) Mechanisms of organophosphate resistance in insects. In: Biochemical sites of insecticide action and resistance. Springer, Berlin Heidelberg, pp 269–291
SMN-CNA (2019) Información estadística climatológica. https://smn.cna.gob.mx/es/climatologia/informacion-climatologica/informacion-estadistica-climatologica. Accessed 19 Feb 2019
Soderlund DM, Bloomquist JR (1990) Molecular mechanisms of insecticide resistance. Pesticide resistance in arthropods. Springer US, In, pp 58–96
Sun YI, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Syvitski JPM, Cohen S, Kettner AJ, Brakenridge GR (2014) How important and different are tropical rivers? — an overview. Geomorphology 227:5–17. https://doi.org/10.1016/j.geomorph.2014.02.029
Uçkun AA (2017) Ecotoxicological evaluation of pesticide pollution in Ataturk Dam Lake (Euphrates River), Turkey. Turk J Fish Aquat Sci 17:313–321
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64:178–189. https://doi.org/10.1016/j.ecoenv.2005.03.013
Van Dijk TC, Van Staalduinen MA, Van der Sluijs JP (2013) Macro-invertebrate decline in surface water polluted with Imidacloprid. PLoS One:8. https://doi.org/10.1371/journal.pone.0062374
Vázquez SM, Carvajal CI, Aquino AM (2010) Áreas Naturales Protegidas. Atlas del patrimonio natural, histórico y cultural de Veracruz, In, pp 249–274
Wantzen KM, Yule CM, Mathooko JM, Pringle CM (2008) Organic matter processing in tropical streams. Tropical stream ecology. Elsevier, In, pp 43–64
Xuereb B, Chaumot A, Mons R et al (2009) Acetylcholinesterase activity in Gammarus fossarum (Crustacea Amphipoda). Intrinsic variability, reference levels, and a reliable tool for field surveys. Aquat Toxicol 93:225–233. https://doi.org/10.1016/j.aquatox.2009.05.006
Acknowledgments
We wish to thank Consejo Nacional de Ciencia y Tecnología Py 1931 and the Secretaría de Investigación y Posgrado at Instituto Politécnico Nacional for the grants to carry out this research through SIP project 20195670. The first author thanks CONACyT for the master scholarship. We thank Ma. Teresa Camacho García for her technical assistance in laboratory assays. María Elena Sánchez-Salazar and Salvador Sánchez-Colón edited the English manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Rico-Sánchez, A.E., Rodríguez-Romero, A.J., Sedeño-Díaz, J.E. et al. Assessment of seasonal and spatial variations of biochemical markers in Corydalus sp. (Megaloptera: Corydalidae), a non-conventional biomonitor, in a mountain cloud forest in Mexico. Environ Sci Pollut Res 27, 30755–30766 (2020). https://doi.org/10.1007/s11356-020-09161-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09161-w